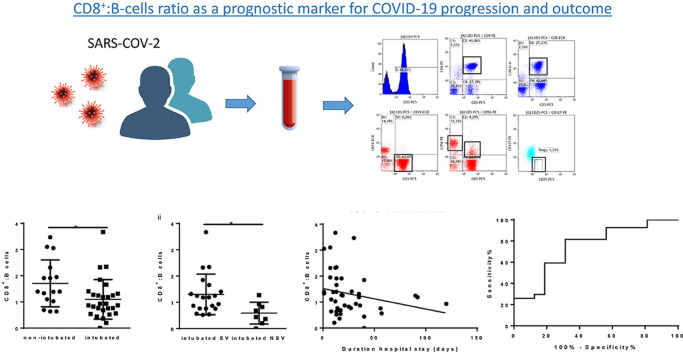
Abstract
Infection with SARS-COV-2 may result in severe pneumonia potentially leading to mechanical ventilation and intensive care treatment. The aim of the present study was to analyze the immune responses in critically ill coronavirus 2019 (COVID-19) patients requiring mechanical ventilation and assess their potential use as markers of clinical progression and outcome. Confirmed COVID-19 patients were grouped into those requiring mechanical ventilation (intubated) and non-intubated. Immune phenotyping was performed and cytokine levels were determined. A novel ratio of CD8+:B cells was significantly lower in intubated versus non-intubated (p = 0.015) and intubated non-survivors (NSV) versus survivors (SV) (p = 0.015). The same ratio correlated with outcome, CRP, IL-6 levels and neutrophil count. Receiving operating curve (ROC) analysis for prediction of requirement of mechanical ventilation by the CD8+:B cells ratio revealed an AUC of 0.747 and a p = 0.007. The ratio of CD8+:B cells may serve as a useful prognostic marker for disease severity and outcome.
Keywords: Immune response, COVID-19, Mechanical ventilation, Prognostic marker
Graphical abstract
1. Introduction
The SARS-COV-2 epidemic remains one of the most serious health emergencies since its outbreak in late December 2019 as it has led to thousands of deaths globally. Combating the epidemic has placed health systems across the world under enormous pressure, especially intensive care units, as patients often require mechanical ventilation.
Infection and disease with SARS-COV-2 named coronavirus disease (COVID)-19 is mainly mild in a majority of individuals with symptoms mimicking those of the common cold. However, in some cases SARS-COV-2 leads to severe pneumonia and acute respiratory distress syndrome (ARDS) (Huang et al., 2020). Since the beginning of the SARS-COV-2 pandemic enormous efforts have been made in order to evaluate the clinical characteristics of COVID-19 patients with severe disease and comprehend their immune responses in an attempt to ultimately link them to clinical progression and outcome. It is thus becoming merely acceptable that severe COVID-19 may be separated into phases of clinical progression with specific features (Jamilloux et al., 2020). The initial phase involves mainly viral entry and infection and is accompanied by a pro-inflammatory state followed by the second phase characterised by an increase in inflammatory parameters such as ferritin and CRP and an initial cytokine induction. In the third phase severe lymphocytopenia occurs in patients prior to the second wave of cytokines, and the last phase of the disease, which has detrimental effects on patients such as rapid deterioration of symptoms, phagocytosis, ARDS, and coagulation leading to a need for mechanical ventilation and intensive care unit (ICU) treatment (Jamilloux et al., 2020).
The characterisation of the immune response of COVID-19 patients is therefore under a lot of investigation as it may provide key information regarding disease progression and the immune factors affecting it. The aim of the present study was to analyze the immune responses in patients who required mechanical ventilation and assess their use as markers of COVID-19 progression and outcome.
2. Materials and methods
A written informed consent was obtained from all patients. Ethical approval was obtained from the Ethics committee of Evangelismos Hospital. A total of 44 patients were recruited. All patients had a positive polymerase chain reaction (PCR) test for SARS-COV-2 from nasopharyngeal sample. Patients were grouped into those who did not require mechanical ventilation (non-intubated, n = 16) and those who required mechanical ventilation (intubated, n = 28). Eight patients from the intubated group did not survive and were used for further analysis of intubated non-survivors, (intubated NSV) versus intubated survivors (intubated SV). Blood samples were collected and analyzed at the point of admission into either ward (non-intubated patients) or ICU (intubated patients).
2.1. Immune phenotyping
Patient immune phenotyping was performed in whole blood samples of patients by flow cytometric analysis. The TQprep workstation was used for lysis, lymphocyte preparation and staining prior to flow cytometric analysis in a Navios EX flow cytometer (Beckman Coulter CA, USA). Antibodies used for cell staining are listed in Supplemental table 1. Data were analyzed using the Kaluza flow cytometric analysis software (Beckman Coulter, CA, USA).
2.2. Cytokine analysis
Serum samples were isolated from whole blood by centrifugation for 15 min at 1000 rcf at 4 °C. Serum aliquots were stored in the −80 °C until further use. Levels of IL-1β, IL-6, IL-8 and IL-10 were measured in serum samples from non-intubated (n = 12) and intubated (n = 26) patients. Cytokine ELISA kits were obtained from Invitrogen (Invitrogen, CA, USA) and assays were carried out according to the manufacturer's instructions. Measurements were performed using the Triturus automated sampler according to the manufacturer's instructions (Grifols, Barcelona, Spain). In case where cytokine levels were undetectable, a value of 0 was assigned. Normal range values for cytokines as stated in technical bulletin of the ELISA kit used were as follows: 1.1–14.3 pg/ml for IL-6, 7.9–12.9 pg/ml for IL-10, and 34.8–666.4 pg/ml for IL-8. IL-1β was reported as undetectable in healthy human samples and one positive sample showed a value of 8.7 pg/ml.
2.3. Statistical analysis
Results are reported as absolute numbers, medians, or means and standard deviations, as appropriate. No imputation was made for missing data. Statistical analysis was performed using the GraphPad Prism 8.0 software for Windows. Data were tested for normality using the Shapiro-Wilks test. Unpaired t-test or Mann-Whitney was used in the case of data displaying normality or not respectively. A paired t-test was used for comparisons between variables within the same group in the event of normally distributed data and a Wilcoxon test was used for comparison of data within the same group without normality. Spearman correlation was used for correlation of data. A p value less than p < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics and monitoring
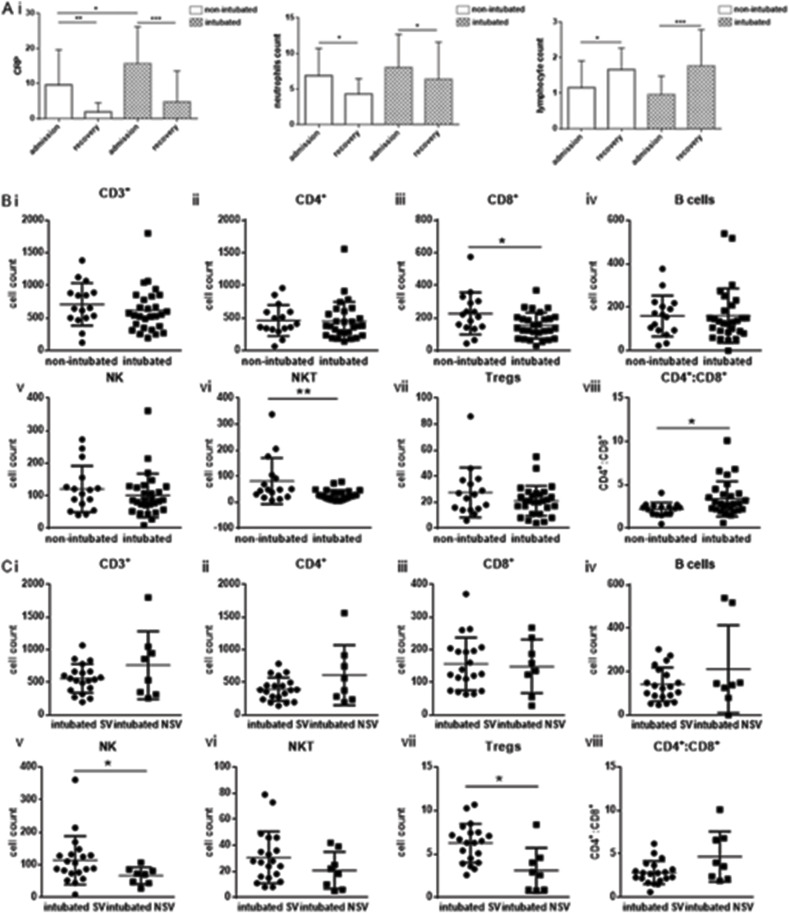
Patient characteristics and clinical data on admission are provided in Supplemental table 1. The mean age of the total population of the cohort was 61.00 ± 13.03 and 75% were male. The most frequent symptoms were fever, cough and dyspnea. The two groups of patients recruited in the study showed differences in the prevalence of cardiovascular disease and Pao2/Fio2 ratio (Supplemental table 1). Statistically significant differences were observed for levels of C-reactive protein, aspartate aminotransferase (AST), alanine transaminase (ALT), gamma-glutamyltransferase (GGT) and lactate dehydrogenase (LDH) (Supplemental table 1). A better outcome was observed for the non-intubated patients estimated by the duration of hospitalisation (days) which was lower in the non-intubated patients and survival (Supplemental table 1). C-reactive protein, neutrophil and lymphocyte levels were monitored for all patients. CRP and neutrophil levels were markedly decreased at the recovery stage of both groups of patients with a simultaneous increase in lymphocyte counts (Fig. 1 A).
Fig. 1.
Severe lymphopenia in non-intubated and intubated coronavirus disease 2019 patients.
Ai) C-reactive protein, absolute neutrophil count (ii) and lymphocyte count (iii) in non-intubated and intubated patients at admission and recovery. Both groups showed increased CRP, neutrophil count and decreased lymphocyte count at admission which was reversed at recovery. B. Immune phenotyping of patients was performed by flow cytometric analysis of whole blood patient samples in order to determine levels of (i) CD3+ (ii) CD4+, iii) CD8+, iv) B cells, v) Natural Killer cells, vi), Natural Killer-like T cells vii) Tregs and viii) CD4+:CD8+ ratio between non-intubated and intubated and between intubated survivors and intubated non-survivors (C). Statistical analysis was performed by Student's t-test, Mann-Whitney U test or Wilcoxon test where applicable. *p < 0.05, **p < 0.01, ***p < 0.001. Cell populations normal cell count ranges (healthy individuals): CD3+: 1500–1900, CD4+: 663–1477, CD8+: 342–754, B-cells: 150–400, NK cells: 100–350, NKT: NA, Tregs: NA, ratio CD4+:CD8+:1.4–2.6.
3.2. Immune phenotype
A representative flow cytometric analysis of lymphocyte subsets and gating strategy is shown in Supplemental Fig. 1. Immune phenotyping revealed a striking reduction in T lymphocytes (CD3+) for both groups of patients. A severe decrease in all lymphocyte subsets was observed apart from natural killer (NK) cells for both non-intubated and intubated patients. (Fig. 1 B). Subtype analysis showed a statistically significant decrease for CD8+ cells (p = 0.022) and natural killer-like T cells (NKT) (p = 0.006) in intubated versus non-intubated patients. A significant increase of the CD4+:CD8+ cell ratio was observed in intubated patients (p = 0.02). Further analysis of all lymphocyte subtypes between intubated survivors (SV, n = 20) and intubated non-survivors (NSV, n = 8) revealed a significant decrease for NK cells (p = 0.031) as well as T regulatory cells (Tregs) (p = 0.039) (Fig. 1 C).
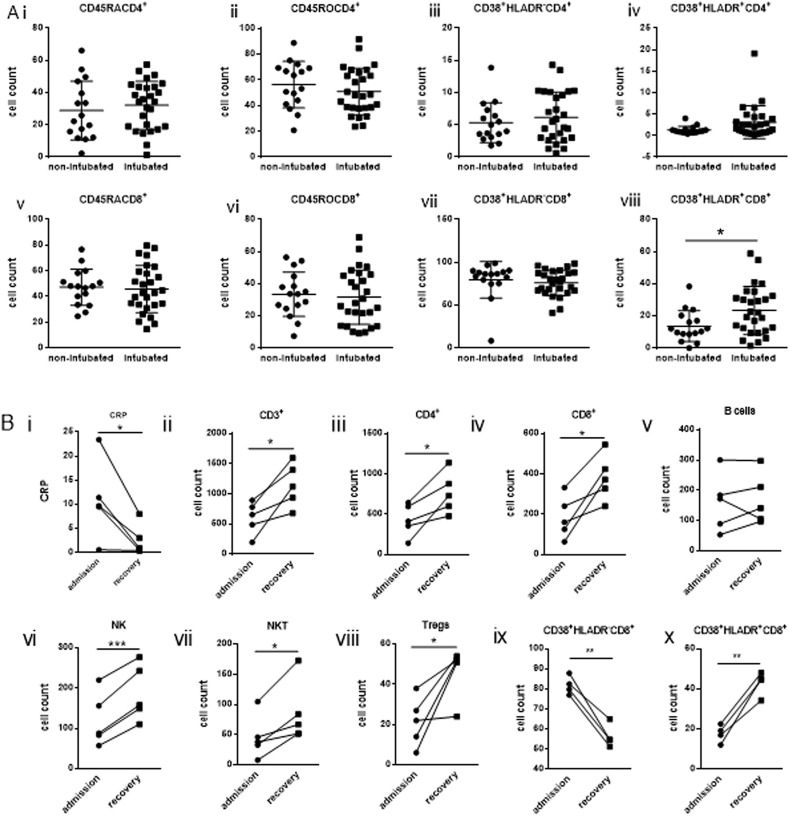
Analysis of levels of naïve (CD45RA+CD4+), memory (CD45RO+CD4+) and activated CD4+ T cells expressing either CD38 (CD38+HLADR−CD4+) or CD38 and HLADR (CD38+HLADR+CD4+) revealed no difference between non-intubated and intubated patients (Fig. 2 A). Variations were not observed for naïve (CD45RA+CD8+), memory (CD45RO+CD8+) or CD38+HLADR−CD8+ activated CD8+ T cells (Fig. 2 A). However, a significant increase was observed for activated CD8+ cells (CD38+HLADR+CD8+) of intubated patients compared to non-intubated (p = 0.025) (Fig. 2 A). No difference was observed in levels of naïve, memory and activated CD4+ and CD8+ T cells between intubated survivors versus non-survivors (Supplemental Fig. 2).
Fig. 2.
Increased activation of CD8+ T cells in critical coronavirus disease 2019 patients.
Levels of Ai) naïve CD4+ (CD45RACD4+) ii) memory CD4+ (CD45ROCD4+) and activated CD4+ expressing either CD38+ (CD38+HLADRCD4+) (iii) or CD38+ and HLADR (CD38+HLADR+CD4+) (iv) as well as v) naïve CD8+ (CD45RACD8+) vi) memory CD8+ (CD45ROCD8+) and activated CD8+ expressing either CD38+ (CD38+HLADRCD8+) (vii) or CD38+ and HLADR (CD38+HLADR+CD8+) (viii) by flow cytometric analysis gated on either CD4+ or CD8+ respectively of non-intubated and intubated patients. B) Levels of cellular subtypes in intubated patients upon recovery. Statistical analysis was performed by Student's t-test, Mann-Whitney U test or Wilcoxon test where applicable. *p < 0.05, **p < 0.01, ***p < 0.001.
Immune phenotyping was repeated for five patients (two non-intubated patients and three intubated) at the recovery stage at which CRP levels were markedly reduced and patients were clinically improved. Although levels of lymphocyte subsets were still low an increase was observed at the recovery stage for all subsets, compared to admission, apart from B cells, the levels of which remained fairly unaltered (Fig. 2 B). Levels of activated CD8+ expressing only CD38 (CD38+HLADR−CD8+) were also shown to decrease whereas CD38+HLADR+CD8+ activation persisted with a further increase (Fig. 2 B).
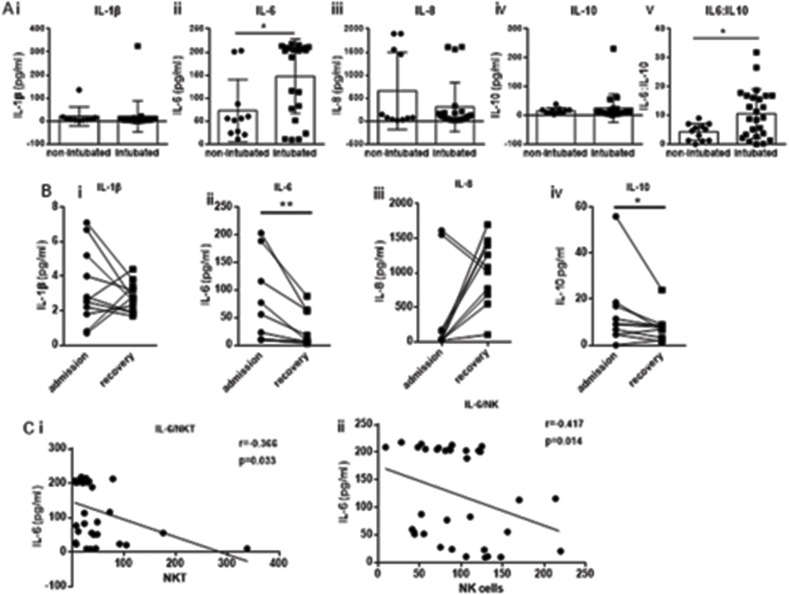
3.3. Cytokine profiling
Cytokine levels were measured at the same time point as the one at which the immune phenotype was determined. A profound increase in IL-6 levels was observed for the intubated group of patients (p = 0.014) with no significant difference in any other cytokine. A significant increase was also observed for the IL-6:IL-10 ratio in intubated patients compared to non-intubated ones (p = 0.023) (Fig. 3 A). Further analysis between intubated survivors versus non-survivors revealed no differences of cytokine levels between the two groups (data not shown).
Fig. 3.
Increased levels of IL-6 in critical coronavirus disease 2019 patients. A) Levels of cytokines were determined in serum samples of non-intubated and intubated coronavirus disease 2019 patients by standard immunoassay. i) IL-1β ii) IL-6, iii) IL-8 and iv) IL-10. v) ratio of IL-6:IL-10. B) The levels of the same cytokines for patients upon recovery. C) A positive correlation of IL-6 with i) Natural Killer cells and ii) Natural Killer-like T cells was observed. Statistical analysis was performed by Student's t-test, Mann-Whitney U test or Wilcoxon test where applicable. *p < 0.05, **p < 0.01. Spearman correlation was performed for correlation analyses. Normal range values for cytokines as stated in technical bulletin of the ELISA kit used were as follows: 1.1–14.3 pg/ml for IL-6, 7.9–12.9 pg/ml for IL-10, and 34.8–666.4 pg/ml for IL-8. IL-1β was reported as undetectable in healthy human samples and one positive sample showed a value of 8.7 pg/ml.
Measurement of cytokine levels for eight patients at two time points (admission and recovery) showed a reduction in levels of IL-1β, IL-6 and IL-10 at the recovery stage (Fig. 3 B). Interestingly, a significant increase of IL-8 levels was observed in all but two patients upon recovery. IL-6 inversely correlated with NKT (r = −0.366, p = 0.033) and NK cell subsets (r = −0.417, p = 0.014) (Fig. 3C).
3.4. CD8+: B cells ratio prognostic value
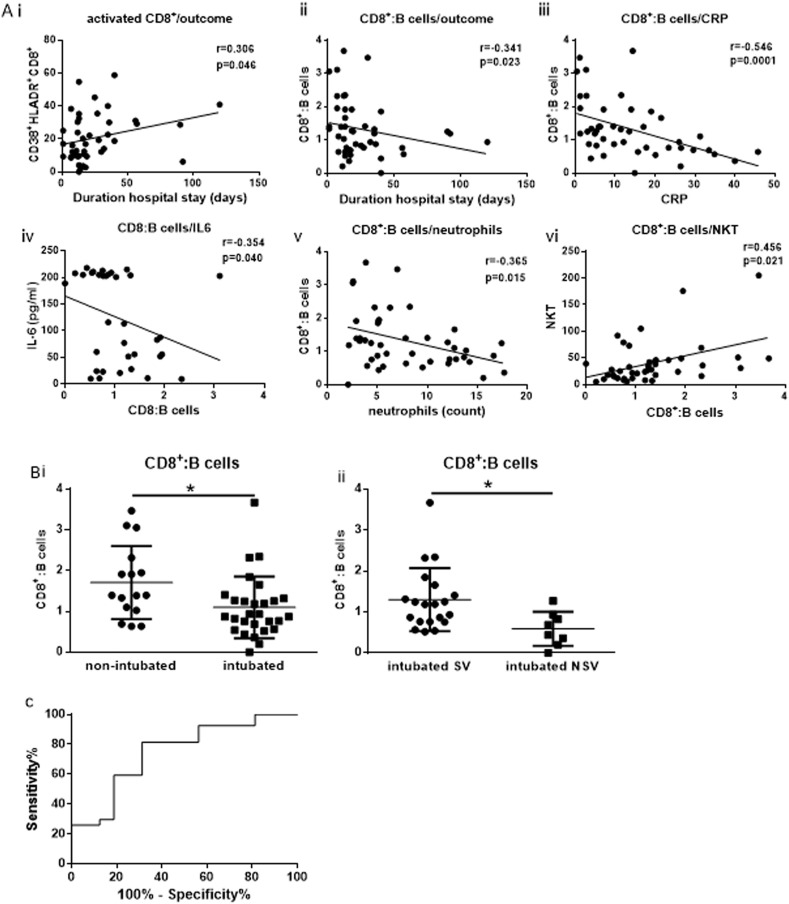
We next examined whether any of the cellular subsets or the cytokines measured exhibited a correlation with outcome (duration of hospitalisation), and important inflammatory parameters used as markers for COVID-19 progression such as CRP. A positive correlation with outcome was observed for the activated CD38+HLADR+CD8+ cells (r = 0.306, p = 0.046) (Fig. 4 A). An inverse correlation was demonstrated between the ratio of CD8+:B cells and outcome (r = −0.341, p = 0.023) as well as with CRP (r = −0.571, p = 0.001) (Fig. 4 A). An inverse correlation was also shown between CD8+: B cells and IL-6 (r = −0.354, p = 0.040) as well as with total count of neutrophils (r = −0.365, p = 0.015). The same ratio correlated positively with NKT cells (r = 0.399, p = 0.007) (Fig. 4A). The above findings for the specific ratio prompted us to examine whether the same ratio varied between the two groups of study patients. The ratio of CD8+:B cells was found significantly lower in intubated patients compared to non-intubated (p = 0.001) (Fig. 4 B). This statistically significant decrease was also observed between intubated NSV versus intubated SV (p = 0.001) (Fig. 4 B). We further performed receiving operating curve (ROC) analysis in order to estimate whether CD8+:B cells ratio holds a prognostic value in predicting poor progression such as requirement of mechanical ventilation (Fig. 4 C). An AUC of 0.7477, and a p = 0.007 were obtained suggesting a potential value of the particular ratio in predicting disease progression and outcome of severe COVID-19. The cut-off point was determined at a value of 1.323 with a sensitivity value of 81.48% and a specificity value of 68.77% and a likelihood ratio of 2.607.
Fig. 4.
Prognostic value of the CD8+:B cells ratio. A) Correlation analysis revealed a positive correlation of activated CD8+ expressing CD38+ and HLADR (CD38+HLADR + CD8+) and outcome (duration of hospitalisation) (i), an inverse correlation of the ratio of CD8+:B cells with ii) outcome iii) C-reactive protein iv) IL-6 v) neutrophil total count and a positive correlation with Natural Killer-like T cells (vi). B). A significant decrease of the ratio of CD8+:B cells was observed between non-intubated and intubated (i) as well as between intubated survivors versus non-survivors (ii). Statistical analysis was performed by Mann-Whitney U test or Wilcoxon test. *p < 0.05. C) Receiving operating curve analysis of CD8+:B cells in predicting poor prognosis as the requirement of mechanical ventilation. AUC = 0.7477, Standard error = 0.0729, 95% Confidence interval; 0.5922 to 0.9031, p = 0.007. cut-off = 1.323, sensitivity = 81.48%, specificity = 68.77%, likelihood ratio = 2.607.
4. Discussion
Our study involved COVID-19 patients in need of mechanical ventilation and non-intubated patients. An inverse correlation was observed between CD8+:B cells ratio with outcome and CRP (Fig. 4 A). The same ratio also inversely correlated with IL-6 and neutrophil total count whereas a positive correlation was shown for CD8+:B cells with NKT cells. To our knowledge, the specific ratio has not been reported before either in COVID-19 or other viral infections. It combines two different lymphocyte subsets, CD8+ T lymphocytes, which play an important role in cellular immunity, especially antiviral immunity, and B cells which are key players of humoral immunity thus bridging antiviral and humoral immunity. The role of CD8+ T-cells in viral infections especially respiratory viral infections is considered as major both in virus clearance in lung airways (Jozwik et al., 2015), (McMichael et al., 1983)as well as for triggering protection against re-infection by the CD8+ T memory cells (Schmidt and Varga, 2018). Following infection CD8+ T cells trigger the production of various cytokines and become activated. Furthermore, the reduction of CD8+ T cell levels has been associated with disease progression and worse outcome in COVID-19 (Wang et al., 2020b). CD8+ and B cell populations have been shown to contribute significantly to the COVID-19 immune response. Specifically, they have been reported as markers for poor prognosis and outcome following treatments (Wang et al., 2020b), (European Centre for Disease Prevention and Control, 2020). In our study, the ratio of CD8+cells:B cells was found significantly lower in both intubated versus non-intubated as well as between intubated NSV versus SV. It also correlated positively with NKT cells which were found significantly higher in critically ill patients. Data on a larger sample population will help to verify our initial observations however, taken together these data suggest a possible value of the specific ratio as a prognosis marker in patients with severe disease.
An increase of CD4+:CD8+ (Fig. 2) as well as IL-6:IL-10 (data not shown) ratios in intubated patients was observed in line with all up to date reports (Wang et al., 2020a), (Liu et al., 2020), (Dong et al., 2020), (Odak et al., 2020), (Song et al., 2020), (Wang et al., 2020b). Both intubated and non-intubated patients showed a marked reduction of all lymphocyte subsets apart from NK cells the majority of which remained within the normal range for both groups of patients. A significant decrease however, was observed for CD8+ T cells, and NKT cells in intubated versus non-intubated patients (Fig. 1 B). Subsequent analysis between intubated SV versus NSV revealed a significant decrease in NK and Tregs thus identifying a decrease of distinct cells subsets in NSV.
Our study also revealed a significant increase in activated CD38+HLADR+CD8+ cells (Fig. 1 B) which also positively correlated with outcome (Fig. 4). No difference in activation levels of naïve or memory CD4+ or CD8+ was observed between intubated and non-intubated patients or between intubated NSV and SV. The increase in CD38+HLADR+CD8+ cells is in line with previous studies (Song et al., 2020), (Odak et al., 2020) confirming an increase in activation of CD8+ cells in severely ill patients. HLA-DR and CD38 are well-described activation markers that are expressed by activated T cells during the acute phase of chronic and persistent viral infections in humans. The specific receptors define the level of CD8+ activation and expression of both CD38 and HLADR represents the classical activation phenotype which displays higher ability for proliferation, cytotoxicity, and cytokine production (Gonzalez et al., 2017). Our finding is in line with similar studies which have also shown increased levels of CD38+HLADR+CD8+ cells in COVID-19 patients with severe disease further suggesting a state of antigen driven activation of SARS-COV-2 specific CD8+ cell (Mathew et al., 2020) (Chen and John Wherry, 2020). Interestingly an increase in CD38+HLADR+CD8+ has been previously reported as a marker for persistent infection with human immunodeficiency (HIV) (Gonzalez et al., 2017), Epstein-Barr (EBV) and hepatitis C (HCV) viruses (Appay et al., 2002). Although, their function has been shown as important in virus control the maintenance of the specific population in chronic infections seems to result in loss of their functional abilities, increased expression of inhibitory molecules related to immune exhaustion, and to activation-induced cell death (Gonzalez et al., 2017). Furthermore, the specific phenotype has been associated with poor prognosis and worse outcome in patients with influenza virus H7N9 (Wang et al., 2018). The study revealed that persistence of this phenotype was related to fatal cases of H7N9 which shares the same characteristic of lymphopenia with cases of severe COVID-19.
NKT reduction in severe COVID-19 patients has been reported in another study which also showed a difference for CD8+ and no difference in CD4+ between mild and severe cases (Odak et al., 2020). NKT cells are a distinct type of cells sharing characteristics of both T cells and NK cells (Jiang et al., 2014)They have the ability to produce IL-4 and INF-γ as well as various other cytokines such as IL-13 (Kelly-Rogers et al., 2006) thus affecting the activation of various cell populations including NK cells, CD4+ and CD8+ T cells, macrophages and B cells as well as Tregs (Matsuda et al., 2008). Regulation of neutrophil recruitment by NKT cells has also been reported mainly through IFNγ secretion. The reduction of NKT cells observed in intubated patients was also observed in intubated NSV although it did not reach statistical significance. A reduction was also observed in Tregs in intubated versus non-intubated subjects, however it only reached statistical significance in intubated NSV.
NKT cells also inversely correlated with IL-6 and the CD8+:B cells ratio (Fig. 4). NKT cell population is largely heterogeneous. One specific subset of NK-like T cells, so called invariant NKT cells (iNKT), that recognize glycolipid antigens presented by major histocompatibility class I-like C1d molecules has been under a lot of investigation as α-galactosylceramide (α-GalCer), a known ligand and potent activator of iNKT cells (Kawano et al., 1999) has been reported to trigger protection against Influenza A virus infection. Studies have shown that α-GalCer vaccination in combination with hemagglutinin resulted in an effective cross-protection against various influenza virus strains, including H5N1 and improved survival from influenza induced lethal pneumonia (Kamijuku et al., 2008). Furthermore, administration of α-GalCer during infection with Influenza A virus was shown to augment the early innate immune response in the lungs and contribute to antiviral immunity (Ho et al., 2008). Our study could not specifically examine iNKT cells due to the available antigens for analysis on routine clinical evaluations. However, the specific cell subset could constitute a potential target for novel therapeutic strategies against COVID-19 and possibly warrants further research.
The current study is in line with most similar reports on immune profiling of COVID-19 patients. However, although a trend for reduction of other cellular subsets was obvious a statistical significance was not reached. This could probably be explained by the relatively small number of patients recruited in our study thus constituting a potential limitation. The limited cohort of patients might also account for the relatively weak correlations shown for the CD8:B-cells ratio. However, although fairly weak the correlations are all statistically significant. A larger number of patients might enable confirmation of the potential strength of the correlations. Furthermore, although we measured activation of CD4+ and CD8+ cells no investigation was carried out regarding exhaustion of the same cells. Finally, another potential limitation of our study is that a mild COVID-19 patients group was not included which might have allowed for further distinction of lymphocytes subpopulations as well as cytokine expression.
In summary our results describe the immune response of severe COVID-19 patients and highlight the value of a novel ratio of CD8+:B-cells as a putative marker of poor prognosis. Nevertheless, further research is warranted in order to fully comprehend the transition of the different stages of COVID-19 progression in the context of successful combat of this novel disease.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Maria G. Detsika: Conceptualization, Methodology, data collection and monitoring, Formal analysis, and interpretation, writing and reviewing of manuscript. Kleio Ampelakiotou: sample handling and laboratory measurements, reviewing of manuscript. Eirini Grigoriou: laboratory measurements, reviewing of manuscript. Katherina Psarra: laboratory measurements, reviewing of manuscript. Edison Jahaj: clinical sample isolation and data collection and monitoring. Charis Roussos: reviewing of manuscript. Ioanna Dimopoulou: data collection and monitoring, reviewing of manuscript. Stylianos E. Orfanos: reviewing of manuscript. Alexandra Tsirogianni: Conceptualization, reviewing of manuscript. Anastasia Kotanidou: Conceptualization, Supervision, reviewing of manuscript.
Declarations of competing interest
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.virol.2021.01.002.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- Appay V., Dunbar P.R., Callan M., Klenerman P., Gillespie G.M., Papagno L., Ogg G.S., King A., Lechner F., Spina C.A., Little S., Havlir D.V., Richman D.D., Gruener N., Pape G., Waters A., Easterbrook P., Salio M., Cerundolo V., McMichael A.J., Rowland-Jones S.L. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- Chen Z., John Wherry E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Wang M., Liu S., Zhu J., Xu Y., Cao H., Li L. Immune characteristics of patients with coronavirus disease 2019 (COVID-19) Aging and disease. 2020;11:642–648. doi: 10.14336/AD.2020.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
- Gonzalez S.M., Taborda N.A., Rugeles M.T. Role of different subpopulations of CD8(+) T cells during HIV exposure and infection. Front. Immunol. 2017;8:936. doi: 10.3389/fimmu.2017.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L.P., Denney L., Luhn K., Teoh D., Clelland C., McMichael A.J. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur. J. Immunol. 2008;38:1913–1922. doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., Walzer T., Francois B., Seve P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Cui X., Cui C., Zhang J., Zhou F., Zhang Z., Fu Y., Xu J., Chu Z., Liu J., Han X., Liao C., Wang Y., Cao Y., Shang H. The function of CD3+CD56+ NKT-like cells in HIV-infected individuals. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/863625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwik A., Habibi M.S., Paras A., Zhu J., Guvenel A., Dhariwal J., Almond M., Wong E.H.C., Sykes A., Maybeno M., Del Rosario J., Trujillo-Torralbo M.B., Mallia P., Sidney J., Peters B., Kon O.M., Sette A., Johnston S.L., Openshaw P.J., Chiu C. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijuku H., Nagata Y., Jiang X., Ichinohe T., Tashiro T., Mori K., Taniguchi M., Hase K., Ohno H., Shimaoka T., Yonehara S., Odagiri T., Tashiro M., Sata T., Hasegawa H., Seino K.I. Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1:208–218. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- Kawano T., Nakayama T., Kamada N., Kaneko Y., Harada M., Ogura N., Akutsu Y., Motohashi S., Iizasa T., Endo H., Fujisawa T., Shinkai H., Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Canc. Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- Kelly-Rogers J., Madrigal-Estebas L., O'Connor T., Doherty D.G. Activation-induced expression of CD56 by T cells is associated with a reprogramming of cytolytic activity and cytokine secretion profile in vitro. Hum. Immunol. 2006;67:863–873. doi: 10.1016/j.humimm.2006.08.292. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., Kuri-Cervantes L., Pampena M.B., D'Andrea K., Manne S., Chen Z., Huang Y.J., Reilly J.P., Weisman A.R., Ittner C.A.G., Kuthuru O., Dougherty J., Nzingha K., Han N., Kim J., Pattekar A., Goodwin E.C., Anderson E.M., Weirick M.E., Gouma S., Arevalo C.P., Bolton M.J., Chen F., Lacey S.F., Ramage H., Cherry S., Hensley S.E., Apostolidis S.A., Huang A.C., Vella L.A., Unit U.P.C.P., Betts M.R., Meyer N.J., Wherry E.J. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369 doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J.L., Mallevaey T., Scott-Browne J., Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr. Opin. Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J., Gotch F.M., Noble G.R., Beare P.A. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Odak I., Barros-Martins J., Bosnjak B., Stahl K., David S., Wiesner O., Busch M., Hoeper M.M., Pink I., Welte T., Cornberg M., Stoll M., Goudeva L., Blasczyk R., Ganser A., Prinz I., Forster R., Koenecke C., Schultze-Florey C.R. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine. 2020;57:102885. doi: 10.1016/j.ebiom.2020.102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. Front. Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.W., Zhang C., Fan X., Meng F.P., Xu Z., Xia P., Cao W.J., Yang T., Dai X.P., Wang S.Y., Xu R.N., Jiang T.J., Li W.G., Zhang D.W., Zhao P., Shi M., Agrati C., Ippolito G., Maeurer M., Zumla A., Wang F.S., Zhang J.Y. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11:3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., Liu W., Zhu Y., Lin Q., Mao L., Fang M., Zhang H., Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI insight. 2020;5 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhu L., Nguyen T.H.O., Wan Y., Sant S., Quinones-Parra S.M., Crawford J.C., Eltahla A.A., Rizzetto S., Bull R.A., Qiu C., Koutsakos M., Clemens E.B., Loh L., Chen T., Liu L., Cao P., Ren Y., Kedzierski L., Kotsimbos T., McCaw J.M., La Gruta N.L., Turner S.J., Cheng A.C., Luciani F., Zhang X., Doherty P.C., Thomas P.G., Xu J., Kedzierska K. Clonally diverse CD38(+)HLA-DR(+)CD8(+) T cells persist during fatal H7N9 disease. Nat. Commun. 2018;9:824. doi: 10.1038/s41467-018-03243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.