Abstract
Background—Dyslipidemia is one of the prominent risk factors for cardiovascular disease, which is the leading cause of death worldwide. Dyslipidemia has various causes, including metabolic capacity, genetic problems, physical inactivity, and dietary habits. This study aimed to determine the association between dyslipidemia and exposure to heavy metals in adults. Methods—Using data from the seventh Korean National Health and Nutrition Examination Survey (2016–2017), 5345 participants aged ≥20 years who were tested for heavy metal levels were analyzed in this study. Multiple logistic regression was conducted to assess the factors affecting the prevalence of dyslipidemia. Results—The risks of dyslipidemia among all and male participants with mercury (Hg) levels of ≥2.75 μg/L (corresponding to the Korean average level) were 1.273 and 1.699 times higher than in those with levels of <2.75 μg/L, respectively. The factors that significantly affected the dyslipidemia risk were age, household income, body mass index, and subjective health status in both males and females. Conclusions—In adult males, exposure to Hg at higher-than-average levels was positively associated with dyslipidemia. These results provide a basis for targeted prevention strategies for dyslipidemia using lifestyle guidelines for reducing Hg exposure and healthy behavioral interventions.
Keywords: dyslipidemia, mercury, Hg, exposure, heavy metals
1. Introduction
The increasing popularity of Western diets in Korea is increasing the importance of dyslipidemia [1,2]. Dyslipidemia is one of the common risk factors and predictors for cardiovascular disease, which is associated with high mortality rates due to myocardial infarction or stroke [3,4,5]. In addition, abnormal cholesterol metabolism with dyslipidemia is a public health issue since it can aggravate the status of patients with chronic disease [6,7,8]. These characteristics make the aggressive management of dyslipidemia necessary. There are several risk factors for dyslipidemia, including metabolic capacity, genetic problems, physical inactivity, and dietary habits [9,10], and its progression is a complex process due to dietary nutrition and metabolism being influenced by genetic factors. Modifications to the metabolism of lipid proteins are also involved in dyslipidemia [11]. However, dyslipidemia is not always the consequence of lifestyle, aging, and genetic problems since it has recently been reported that dyslipidemia may be associated with exposure to various environmental hazard factors, including heavy metals.
Heavy metals, including cadmium (Cd), lead (Pb), and mercury (Hg), are prevalent toxic substances that accumulate easily and are widely distributed in the environment. Exposure to heavy metals is suspected to alter energy metabolism, including that of lipid proteins. A study involving a mouse model showed that chronic Cd exposure induced the alteration of lipid metabolism [12]. Reportedly, cadmium interferes with antioxidant activity in normal cells and affects the overall metabolism [13]. Furthermore, cross-sectional studies showed a significant relationship between metabolic diseases and exposure to Cd [14]. The accumulation of Hg induced metabolic inactivity, including dyslipidemia by oxidative stress in humans [15], and a significant interrelation between lipid contents and the blood Hg level in older people was demonstrated [16]. Lead ions can disrupt cell metabolism by replacing other ions such as Ca2+, Mg2+, Fe2+, and Na+ in the human body [17]. It has also been shown that higher levels of heavy metals, including Pb, are related to altered levels of total cholesterol in adolescents [18].
Despite the potential of a close association between dyslipidemia and heavy metals, the actual impact of this association tends to be underestimated due to difficulties in proving it. However, there has been increasing concern about the potential for this health-related problem because the accumulation of heavy metals is associated with various diseases. Thus, this study aimed to determine the association between dyslipidemia and exposure to heavy metals in Korean adults based on the Korean National Health and Nutrition Examination Survey (KNHANES).
2. Methods
2.1. Data Source and Participants
This study analyzed data obtained from the seventh KNHANES (2016–2017) conducted by the Korea Centers for Disease Control and Prevention. The KNHANES uses a complex, stratified, multistage, and probability cluster sampling design as a nationwide population-based survey of the health and nutritional status of Koreans. The overall response rate for the seven KNHANES was 76.6%. The study analyzed 5345 of the 16,277 total KNHANES respondents aged ≥20 years who were tested for heavy metals. The study was approved by the university’s Institutional Review Board (no. 1044396-202004-HR-089-01). Ethical issues regarding plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancy have been completely observed by the author.
2.2. Study Variables: Demographic Factors
The following demographic characteristics of the participants were analyzed: sex, age, marital status, education level, occupation, household income, and residential area. Marital status was defined as being or not being currently married. Education level was classified into elementary school or below, middle school, high school, and university or above. Occupation was categorized into five groups: white-collar (WC) worker, including managers, professionals, and office workers; pink-collar (PC) workers, including service and sales workers; blue-collar (BC) workers, including technicians, as well as device and machine operators; agribusiness and low-level (AL) workers, including skilled workers in agriculture and fisheries, and laborers; unemployed. Household income was divided into four quartiles. Residential area was categorized into urban areas (administrative divisions of a city) and rural areas (areas not classified as administrative divisions of a city).
2.3. Study Variables: Health-Related Factors
The health-related factors included health behavior factors, such as drinking status, smoking status, body mass index (BMI), and the prevalence of dyslipidemia, as well as mental health factors, such as the subjective health status and stress level. Smoking status was categorized into current smoker (current smoking) and nonsmoker (former smoker or never). The drinking status was classified based on alcohol consumption into current drinking and not drinking.
The BMI was calculated as the weight in kilograms divided by the square of the height in meters and was categorized into normal weight (18.5–25.0 kg/m2), overweight (25.0–29.9 kg/m2), obesity (≥30 kg/m2), and underweight (<18.5 kg/m2). The presence of dyslipidemia was defined based on having been diagnosed with dyslipidemia by a physician. The subjective health status was divided into five categories: very good, good, moderate, poor, and very poor. For the analysis, these responses were condensed into the three categories of good (combining very good and good), moderate, and poor (combining poor and very poor). Stress levels were grouped into low, moderate, and high (combining very high and high).
2.4. Study Variables: Heavy Metal Testing
Blood Pb and Cd were measured using graphite furnace atomic absorption spectrometry with a Zeeman background correction (AAnalyst 600, Perkin Elmer, Turku, Finland). The blood Hg level was measured using a gold amalgam collection method (DMA 80, Milestone, Bergamo, Italy). The blood Pb, Hg, and Cd levels were dichotomized based on the corresponding Korean average levels found in the third Korean National Environmental Health Survey (KoNEHS) (2015–2017) into the following groups: Pb, <1.60 and ≥1.60 μg/dL; Hg, <2.75 and ≥2.75 μg/L; Cd, <0.36 and ≥0.36 μg/L [19].
2.5. Statistical Analysis
Sampling weights were applied to the participants to avoid bias in the national estimates and thereby ensure that the sample was representative of the Korean population. The chi-square test was used to assess the relationships of dyslipidemia with demographic, health-related factors, and heavy metal exposure. Multiple logistic regression models were analyzed to identify the factors that significantly affected the prevalence of dyslipidemia separately in males and females.
Statistical significance in this study was defined as a p-value of <0.05. The complex sample design was taken into consideration for the data analysis, which was conducted using SPSS software (version 25.0, IBM Corporation, Armonk, NY, USA).
3. Results
3.1. General Characteristics of the Study Population
Table 1 lists the following general characteristics of the study population quantified as unweighted numbers and weighted percentages or weighted means: age, marital status, education level, occupation, household income, residential area, drinking status, smoking status, BMI, prevalence of dyslipidemia, subjective health status, stress level, and exposure to heavy metals (Pb, Hg, and Cd). The 5345 study participants comprised 2424 males and 2921 females with a mean age of 43.7 years. Married participants and those with an education level of university or above predominated among both the males and females. Regarding occupation, those who were unemployed predominated among the total sample and females, with white-collar workers predominating among males. The largest proportion of participants had a household income in the fourth quartile (highest level) and resided in urban areas. Current drinking, nonsmokers, normal BMI, and no dyslipidemia diagnosis also predominated, except for male smokers. Moderate levels predominated for subjective health status and stress level. Regarding heavy metal exposure, the average blood lead, mercury, and cadmium concentrations were 1.60 μg/dL, 2.75 μg/L, and 0.36 μg/L in the third Korean National Environmental Health Survey. In this study, the Pb level was mostly <1.60 μg/dL among all participants and females, and mostly ≥1.60 μg/dL among males; the Hg level was mostly ≥2.75 μg/L among all participants and males, and mostly <2.75 μg/L among females; the Cd level was mostly ≥0.36 μg/L among all participants, males, and females.
Table 1.
General characteristics of the study participants.
| Variables | Items | Total Sample | Male | Female | ||||
|---|---|---|---|---|---|---|---|---|
| Unweighted No. | Weighted% (SE) | Unweighted No. | Weighted% (SE) | Unweighted No. | Weighted% (SE) | |||
| Socio-demographic factors | Age (years) + | 5345 | 43.7 (0.3) | 2424 | 42.8 (0.4) | 2921 | 44.6 (0.4) | |
| Marital status | Yes | 3997 | 69.1 (0.8) | 1696 | 64.9 (1.1) | 2301 | 73.2 (1.0) | |
| No | 1348 | 30.9 (0.8) | 728 | 35.1 (1.1) | 620 | 26.8 (1.0) | ||
| Education | ≤Elementary | 1189 | 18.2 (0.6) | 444 | 13.9 (0.7) | 745 | 22.4 (1.0) | |
| Middle school | 633 | 12.4 (0.6) | 301 | 12.6 (0.8) | 332 | 12.3 (0.8) | ||
| High school | 1529 | 32.6 (0.9) | 696 | 33.3 (1.2) | 833 | 32.0 (1.1) | ||
| ≥University | 1744 | 36.7 (0.9) | 857 | 40.1 (1.3) | 887 | 33.3 (1.0) | ||
| Occupation | WC worker | 1174 | 26.4 (0.8) | 603 | 30.3 (1.2) | 571 | 22.6 (0.9) | |
| PC worker | 591 | 13.0 (0.6) | 216 | 11.5 (0.8) | 375 | 14.4 (0.9) | ||
| BC worker | 494 | 11.3 (0.6) | 416 | 19.7 (1.2) | 78 | 3.1 (0.4) | ||
| AL worker | 618 | 10.9 (0.6) | 272 | 10.4 (0.7) | 346 | 11.4 (0.8) | ||
| Unemployed | 1930 | 38.3 (0.9) | 634 | 28.0 (1.2) | 1296 | 48.4 (1.1) | ||
| Household income | Lowest | 906 | 13.8 (0.7) | 369 | 12.4 (0.9) | 537 | 15.3 (0.9) | |
| Lower middle | 1315 | 23.1 (0.9) | 589 | 22.6 (1.1) | 726 | 23.7 (1.1) | ||
| Upper middle | 1495 | 30.0 (1.0) | 716 | 31.6 (1.2) | 779 | 28.4 (1.2) | ||
| Highest | 1614 | 33.0 (1.2) | 740 | 33.5 (1.5) | 874 | 32.6 (1.4) | ||
| Residential area | Urban | 4382 | 86.5 (1.6) | 1990 | 86.7 (1.6) | 2392 | 86.2 (1.6) | |
| Rural | 963 | 13.5 (1.6) | 434 | 13.3 (1.6) | 529 | 13.8 (1.6) | ||
| Health behaviors | Drinking | No | 907 | 15.6 (0.6) | 302 | 11.5 (0.8) | 605 | 19.8 (0.9) |
| Yes | 4368 | 84.4 (0.6) | 2088 | 88.5 (0.8) | 2280 | 80.2 (0.9) | ||
| Smoking | No | 2853 | 57.6 (0.8) | 486 | 25.1 (1.2) | 2367 | 89.7 (0.7) | |
| Yes | 1906 | 42.4 (0.8) | 1628 | 74.9 (1.2) | 278 | 10.3 (0.7) | ||
| BMI | Normal | 3216 | 60.3 (0.9) | 1380 | 55.8 (1.2) | 1836 | 64.9 (1.1) | |
| Overweight | 1502 | 27.7 (0.8) | 813 | 34.1 (1.1) | 689 | 21.3 (0.9) | ||
| Obesity | 279 | 5.4 (0.4) | 105 | 5.1 (0.5) | 174 | 5.6 (0.5) | ||
| Underweight | 341 | 6.6 (0.4) | 124 | 5.0 (0.5) | 217 | 8.2 (0.7) | ||
| Dyslipidemia | No | 3988 | 84.1 (0.6) | 1818 | 85.9 (0.8) | 2170 | 82.4 (0.9) | |
| Yes | 841 | 15.9 (0.6) | 330 | 14.1 (0.8) | 511 | 17.6 (0.9) | ||
| Mental health | Subjective health status | Good | 1610 | 33.0 (0.8) | 826 | 36.9 (1.1) | 784 | 29.1 (1.1) |
| Moderate | 2628 | 51.5 (0.8) | 1138 | 49.3 (1.2) | 1490 | 53.7 (1.2) | ||
| Poor | 881 | 15.5 (0.7) | 346 | 13.8 (0.9) | 535 | 17.1 (0.9) | ||
| Stress level | Low | 789 | 14.2 (0.6) | 383 | 15.4 (0.8) | 406 | 13.1 (0.7) | |
| Moderate | 2909 | 56.7 (0.8) | 1317 | 56.3 (1.2) | 1592 | 57.0 (1.1) | ||
| High | 1455 | 29.1 (0.8) | 624 | 28.3 (1.1) | 831 | 29.9 (1.0) | ||
| Heavy metal exposure | Pb | <1.60 μg/dL | 2681 | 53.6 (1.0) | 910 | 42.1 (1.3) | 1771 | 65.0 (1.1) |
| ≥1.60 μg/dL | 2664 | 46.4 (1.0) | 1514 | 57.9 (1.3) | 1150 | 35.0 (1.1) | ||
| Hg | <2.75 μg/L | 2407 | 44.7 (1.0) | 839 | 34.6 (1.2) | 1568 | 54.8 (1.4) | |
| ≥2.75 μg/L | 2938 | 55.3 (1.0) | 1585 | 65.4 (1.2) | 1353 | 45.2 (1.4) | ||
| Cd | <0.36 μg/L | 689 | 15.9 (0.7) | 403 | 19.4 (1.1) | 286 | 12.5 (0.8) | |
| ≥0.36 μg/L | 4656 | 84.1 (0.7) | 2021 | 80.6 (1.1) | 2635 | 87.5 (0.8) | ||
Note: WC—white-collar, PC—pink-collar, BC—blue-collar, AL—agribusiness and low-level, BMI—body mass index, Pb—lead, Hg—mercury, Cd—cadmium; + weighted mean with SE.
3.2. Weighted Prevalence of Dyslipidemia According to Sex
The characteristics of the weighted population according to the diagnosis of dyslipidemia and sex are presented in Table 2. Age, marital status, education, occupation, household income, drinking status, BMI, subjective health status, and exposure to Pb, Hg, and Cd differed significantly between the dyslipidemia and non-dyslipidemia groups among all participants. Among males, there were significant differences between these two groups in age, marital status, education, smoking status, BMI, subjective health status, and exposure to Pb, Hg, and Cd. Among females, there were significant intergroup differences in age, marital status, education, occupation, household income, drinking status, smoking status, BMI, subjective health status, and exposure to Pb, Hg, and Cd.
Table 2.
Weighted prevalence of dyslipidemia according to sex.
| Characteristics | Total Sample | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Dyslipidemia | Dyslipidemia | p-Value | Non-Dyslipidemia | Dyslipidemia | p-Value | Non-Dyslipidemia | Dyslipidemia | p-Value | ||
| Weighted% (SE) | Weighted% (SE) | Weighted% (SE) | ||||||||
| Age (years) + | 44.56 (0.33) | 59.90 (0.50) | <0.001 | 44.45 (0.41) | 57.18 (0.77) | <0.001 | 44.68 (0.39) | 62.06 (0.57) | <0.001 | |
| Marital status | Yes | 80.3 (0.8) | 19.7 (0.8) | <0.001 | 82.0 (1.1) | 18.0 (1.1) | <0.001 | 78.8 (1.1) | 21.2 (1.1) | <0.001 |
| No | 96.9 (0.6) | 3.1 (0.6) | 96.4 (0.9) | 3.6 (0.9) | 97.7 (0.9) | 2.3 (0.9) | ||||
| Education | ≤Elementary school | 64.2 (2.1) | 35.8 (2.1) | <0.001 | 74.9 (3.4) | 25.1 (3.4) | <0.001 | 59.4 (2.6) | 40.6 (2.6) | <0.001 |
| Middle school | 74.5 (2.3) | 25.5 (2.3) | 81.8 (3.3) | 18.2 (3.3) | 67.4 (3.5) | 32.6 (3.5) | ||||
| High school | 86.8 (1.0) | 13.2 (1.0) | 88.1 (1.3) | 11.9 (1.3) | 85.5 (1.5) | 14.5 (1.5) | ||||
| ≥University | 90.3 (0.8) | 9.7 (0.8) | 87.2 (1.3) | 12.8 (1.3) | 94.0 (0.9) | 6.0 (0.9) | ||||
| Occupation | WC worker | 90.4 (1.0) | 9.6 (1.0) | <0.001 | 87.6 (1.5) | 12.4 (1.5) | 0.550 | 94.1 (1.1) | 5.9 (1.1) | <0.001 |
| PC worker | 85.6 (1.6) | 14.4 (1.6) | 86.4 (2.6) | 13.6 (2.6) | 85.0 (2.1) | 15.0 (2.1) | ||||
| BC worker | 85.2 (1.8) | 14.8 (1.8) | 85.8 (1.8) | 14.2 (1.8) | 82.0 (5.4) | 18.0 (5.4) | ||||
| AL worker | 79.5 (1.9) | 20.5 (1.9) | 86.0 (2.5) | 14.0 (2.5) | 73.7 (2.7) | 26.3 (2.7) | ||||
| Unemployed | 79.3 (1.1) | 20.7 (1.1) | 83.7 (1.8) | 16.3 (1.8) | 77.0 (1.5) | 23.0 (1.5) | ||||
| Household income | Lowest | 78.4 (1.8) | 21.6 (1.8) | <0.001 | 83.3 (2.3) | 16.7 (2.3) | 0.654 | 74.6 (2.6) | 25.4 (2.6) | <0.001 |
| Lower middle | 82.8 (1.3) | 17.2 (1.3) | 85.9 (1.7) | 14.1 (1.7) | 79.9 (1.8) | 20.1 (1.8) | ||||
| Upper middle | 84.7 (1.2) | 15.3 (1.2) | 86.8 (1.6) | 13.2 (1.6) | 82.4 (1.7) | 17.6 (1.7) | ||||
| Highest | 87.2 (1.0) | 12.8 (1.0) | 86.3 (1.5) | 13.7 (1.5) | 88.0 (1.3) | 12.0 (1.3) | ||||
| Residential area | Urban | 84.0 (0.7) | 16.0 (0.7) | 0.597 | 85.4 (0.9) | 14.6 (0.9) | 0.182 | 82.6 (1.0) | 17.4 (1.0) | 0.574 |
| Rural | 84.8 (1.4) | 15.2 (1.4) | 88.6 (2.0) | 11.4 (2.0) | 81.2 (2.3) | 18.8 (2.3) | ||||
| Drinking | No | 73.0 (2.4) | 27.0 (2.4) | <0.001 | 84.9 (3.3) | 15.1 (3.3) | 0.732 | 69.2 (2.8) | 30.8 (2.8) | <0.001 |
| Yes | 85.2 (0.7) | 14.8 (0.7) | 86.0 (0.9) | 14.0 (0.9) | 84.2 (1.0) | 15.8 (1.0) | ||||
| Smoking | No | 83.4 (0.8) | 16.6 (0.8) | 0.195 | 90.6 (1.5) | 9.4 (1.5) | 0.002 | 81.5 (1.0) | 18.5 (1.0) | 0.014 |
| Yes | 84.9 (0.9) | 15.1 (0.9) | 84.4 (1.0) | 15.6 (1.0) | 88.6 (2.3) | 11.4 (2.3) | ||||
| BMI | Normal | 86.3 (0.8) | 13.7 (0.8) | <0.001 | 87.1 (1.1) | 12.9 (1.1) | 0.042 | 85.6 (1.0) | 14.4 (1.0) | <0.001 |
| Overweight | 79.2 (1.2) | 20.8 (1.2) | 83.8 (1.4) | 16.2 (1.4) | 71.8 (2.1) | 28.2 (2.1) | ||||
| Obesity | 77.3 (2.8) | 22.7 (2.8) | 82.7 (4.0) | 17.3 (4.0) | 72.4 (4.0) | 27.6 (4.0) | ||||
| Underweight | 97.3 (1.2) | 2.7 (1.2) | 96.0 (2.8) | 4.0 (2.8) | 98.0 (1.1) | 2.0 (1.1) | ||||
| Subjective health status | Good | 91.3 (0.9) | 8.7 (0.9) | <0.001 | 91.2 (1.2) | 8.8 (1.2) | <0.001 | 91.4 (1.3) | 8.6 (1.3) | <0.001 |
| Moderate | 84.2 (0.9) | 15.8 (0.9) | 86.7 (1.2) | 13.3 (1.2) | 81.9 (1.2) | 18.1 (1.2) | ||||
| Poor | 70.8 (2.0) | 29.2 (2.0) | 72.0 (2.9) | 28.0 (2.9) | 69.9 (2.4) | 30.1 (2.4) | ||||
| Stress level | Low | 83.5 (1.5) | 16.5 (1.5) | 0.191 | 85.9 (2.2) | 14.1 (2.2) | 0.292 | 80.9 (2.1) | 19.1 (2.1) | 0.569 |
| Moderate | 83.4 (0.8) | 16.6 (0.8) | 84.9 (1.2) | 15.1 (1.2) | 81.9 (1.2) | 18.1 (1.2) | ||||
| High | 85.7 (1.1) | 14.3 (1.1) | 88.1 (1.5) | 11.9 (1.5) | 83.5 (1.7) | 16.5 (1.7) | ||||
| Pb exposure | <1.60 μg/dL | 87.5 (0.8) | 12.5 (0.8) | <0.001 | 89.1 (1.2) | 10.9 (1.2) | 0.003 | 86.5 (1.1) | 13.5 (1.1) | <0.001 |
| ≥1.60 μg/dL | 80.9 (1.0) | 19.1 (1.0) | 84.0 (1.1) | 16.0 (1.1) | 75.7 (1.7) | 24.3 (1.7) | ||||
| Hg exposure | <2.75 μg/L | 86.4 (0.9) | 13.6 (0.9) | 0.003 | 90.5 (1.3) | 9.5 (1.3) | <0.001 | 84.1 (1.2) | 15.9 (1.2) | 0.046 |
| ≥2.75 μg/L | 82.6 (0.9) | 17.4 (0.9) | 84.0 (1.1) | 16.0 (1.1) | 80.5 (1.4) | 19.5 (1.4) | ||||
| Cd exposure | <0.36 μg/L | 97.3 (0.8) | 2.7 (0.8) | <0.001 | 97.4 (1.0) | 2.6 (1.0) | <0.001 | 97.3 (1.2) | 2.7 (1.2) | <0.001 |
| ≥0.36 μg/L | 82.7 (0.7) | 17.3 (0.7) | 84.2 (0.9) | 15.8 (0.9) | 81.4 (1.0) | 18.6 (1.0) | ||||
Note: WC—white-collar, PC—pink-collar, BC—blue-collar, AL—agribusiness and low-level, BMI—body mass index, Pb—lead, Hg—mercury, Cd—cadmium; + weighted mean with SE.
3.3. Factors Affecting Dyslipidemia Risk According to Sex
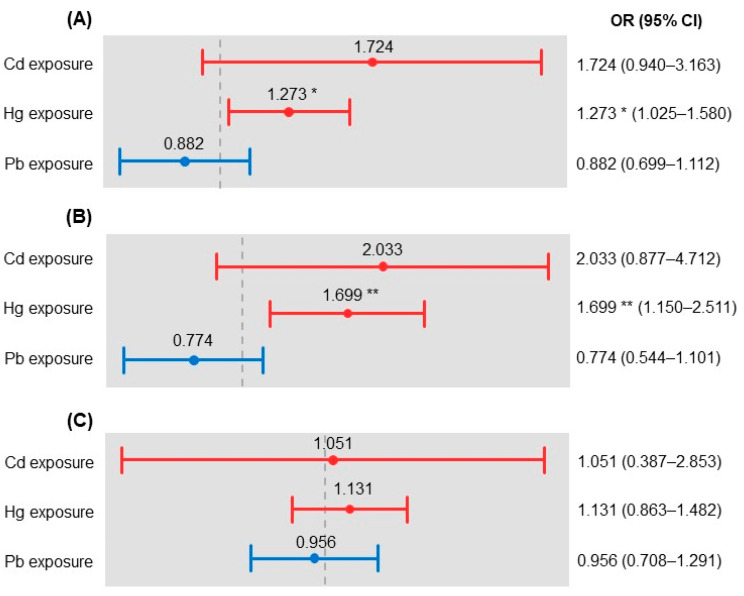
The results of the multiple logistic regression analysis are presented in Table 3. The factors affecting the risk of dyslipidemia were age, household income, BMI, subjective health status, and Hg exposure among all participants. Among the total sample, the odds ratio (OR) for the dyslipidemia risk was 1.068 for age. Regarding household income, the risks of dyslipidemia were 1.571, 1.872, and 1.621 times higher for those in the second, third, and fourth quartiles, respectively, than for those in the first quartile. The ORs for the dyslipidemia risk were 1.558, 2.184, and 0.192 for those who were overweight, obese, and underweight, respectively, compared to those who were of normal weight. The ORs for the dyslipidemia risk were 1.821 and 3.115 for those with moderate and poor subjective health statuses, respectively, compared to those with a good status. Furthermore, the dyslipidemia risk was 1.273 times higher among those with Hg ≥2.75 μg/L than among those with Hg <2.75 μg/L (Figure 1A).
Table 3.
Factors affecting dyslipidemia risk according to sex.
| Characteristics | Total Sample | Male | Female | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Age | 1.068 *** | 1.056–1.080 | 1.066 *** | 1.049–1.084 | 1.079 *** | 1.062–1.097 | |
| Marital status | Yes | 1 | 1 | 1 | |||
| No | 0.748 | 0.456–1.229 | 0.846 | 0.457–1.563 | 0.802 | 0.318–2.027 | |
| Education | ≤Elementary | 1 | 1 | 1 | |||
| Middle school | 0.971 | 0.704–1.340 | 0.934 | 0.525–1.660 | 1.162 | 0.763–1.769 | |
| High school | 0.900 | 0.662–1.224 | 1.124 | 0.691–1.831 | 0.923 | 0.604–1.411 | |
| ≥University | 0.773 | 0.538–1.110 | 1.244 | 0.694–2.230 | 0.620 | 0.358–1.072 | |
| Occupation | WC worker | 1 | 1 | 1 | |||
| PC worker | 0.989 | 0.690–1.419 | 1.226 | 0.706–2.130 | 0.855 | 0.501–1.457 | |
| BC worker | 0.855 | 0.566–1.291 | 0.960 | 0.568–1.625 | 0.996 | 0.468–2.119 | |
| AL worker | 0.800 | 0.548–1.167 | 0.577 | 0.297–1.119 | 1.066 | 0.608–1.867 | |
| Unemployed | 0.878 | 0.643–1.198 | 0.689 | 0.409–1.160 | 1.008 | 0.606–1.676 | |
| Household income | Lowest | 1 | 1 | 1 | |||
| Lower middle | 1.571 * | 1.085–2.275 | 1.210 | 0.679–2.156 | 1.980 ** | 1.243–3.152 | |
| Upper middle | 1.872 ** | 1.283–2.732 | 1.307 | 0.730–2.340 | 2.376 *** | 1.502–3.757 | |
| Highest | 1.621 * | 1.122–2.342 | 1.257 | 0.710–2.223 | 1.744 * | 1.094–2.782 | |
| Drinking | No | 1 | 1 | 1 | |||
| Yes | 1.032 | 0.758–1.405 | 1.109 | 0.582–2.117 | 1.125 | 0.773–1.636 | |
| Smoking | No | 1 | 1 | 1 | |||
| Yes | 0.889 | 0.714–1.107 | 1.101 | 0.699–1.735 | 0.958 | 0.558–1.645 | |
| BMI | Normal | 1 | 1 | 1 | |||
| Overweight | 1.558 *** | 1.261–1.924 | 1.423 * | 1.036–1.956 | 1.567 ** | 1.163–2.111 | |
| Obesity | 2.184 *** | 1.454–3.282 | 2.016 * | 1.043–3.896 | 2.078 ** | 1.258–3.433 | |
| Underweight | 0.192 ** | 0.070–0.533 | 0.183 * | 0.036–0.940 | 0.221 * | 0.066–0.747 | |
| Subjective health status | Good | 1 | 1 | 1 | |||
| Moderate | 1.821 *** | 1.392–2.381 | 1.412 | 0.942–2.116 | 2.141 *** | 1.422–3.225 | |
| Poor | 3.115 *** | 2.281–4.252 | 3.621 *** | 2.313–5.668 | 2.665 *** | 1.688–4.209 | |
| Pb exposure | <1.60 μg/dL | 1 | 1 | 1 | |||
| ≥1.60 μg/dL | 0.882 | 0.699–1.112 | 0.774 | 0.544–1.101 | 0.956 | 0.708–1.291 | |
| Hg exposure | <2.75 μg/L | 1 | 1 | 1 | |||
| ≥2.75 μg/L | 1.273 * | 1.025–1.580 | 1.699 ** | 1.150–2.511 | 1.131 | 0.863–1.482 | |
| Cd exposure | <0.36 μg/L | 1 | 1 | 1 | |||
| ≥0.36 μg/L | 1.724 | 0.940–3.163 | 2.033 | 0.877–4.712 | 1.051 | 0.387–2.853 | |
Note: Bolded numbers represent statistically significant values; WC—white-collar, PC—pink-collar, BC—blue-collar, AL—agribusiness and low-level, BMI—body mass index, Pb—lead, Hg—mercury, Cd—cadmium; * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 1.
Heavy metals affecting dyslipidemia risk according to sex: (A) total sample, (B) male, and (C) female. The grey dotted lines represent OR = 1. * p < 0.05, ** p < 0.01.
Among males, the significant factors affecting dyslipidemia risk were age, BMI, subjective health status, and Hg exposure. The OR for the dyslipidemia risk was 1.066 for age, and 1.423, 2.016, and 0.183 for those who were overweight, obese, and underweight, respectively, compared to those who were of normal weight. The OR for the dyslipidemia risk for someone with a poor subjective health status was 3.621 compared to a good status. Furthermore, the dyslipidemia risk was 1.699 times higher among those with Hg ≥2.75 μg/L than among those with Hg <2.75 μg/L (Figure 1B).
Among females, the significant factors affecting dyslipidemia risk were age, household income, BMI, and subjective health status. The OR for the dyslipidemia risk was 1.079 for age. Regarding household income, the risks of dyslipidemia were 1.980, 2.376, and 1.744 times higher for those in the second, third, and fourth quartiles, respectively, than for those in the first quartile. The ORs for the dyslipidemia risk were 1.567, 2.078, and 0.221 for those who were overweight, obese, and underweight, respectively, compared to those who were of normal weight. The ORs for the dyslipidemia risk were 2.141 and 2.665 for those with moderate and poor subjective health statuses, respectively, compared to those with a good status (Figure 1C).
4. Discussion
Heavy metals are non-biodegradable environmental chemicals that exert numerous adverse effects on humans. There is increasing interest in the adverse effects of exposure to heavy metals and its association with dyslipidemia. In this study, we focused on finding the association between exposure to heavy metals and dyslipidemia by analyzing multistage, stratified sampling data for adults. Our results showed that a higher Hg level in the blood was associated with dyslipidemia, with this association varying according to sex.
Mercury is one of the major heavy metals that exacerbate metabolic syndrome and cardiovascular disorders, including atherosclerosis [20,21]. A cross-sectional study found that the dysregulation of lipids was related to higher levels of Hg [22]. The present study showed that higher blood levels of Hg are significantly associated with dyslipidemia, which is consistent with the previous findings. Few previous studies have investigated the mechanisms underlying the effects of Hg in dyslipidemia. One possible mechanism involves the homeostasis of lipid metabolism and adipocytes [23]. Adipocytes are involved in lipid metabolism by producing adipokines [24], and Hg reportedly produced functional abnormalities in the adipose tissue of mice [25]. Furthermore, the toxic effects of Hg not only include oxidative stress but also the depletion of antioxidants [26]. Oxidative stress is a major factor that contributes to cell dysfunction and is linked to various diseases, including dyslipidemia [27]. Hg exposure induces the overproduction of reactive oxygen species following cell damage and the oxidation of low-density lipoprotein cholesterol [28]. Thus, it is speculated that pathogenesis is linked to abnormal lipid metabolism, which can develop into dyslipidemia.
A particularly interesting finding of the present study was that dividing heavy metal levels based on the Korean average level in the third KoNEHS revealed that the association of dyslipidemia with Hg exposure might be influenced by sex. There are several possible reasons for this result. Although there have been inconsistencies, some previous studies have found males to be more vulnerable to the adverse effects of Hg than females [29], which could be explained by sex differences in detoxification processes, including the oxidative stress pathway and the availability of glutathione. The primary route for eliminating Hg from the body is excretion in bile after binding to glutathione [30]. The plasma level of glutathione peroxidase has been reported to be higher in females than in males; therefore, Hg might exert greater effects in males [31]. An observational study found an association between altered lipid profiles and blood Hg in Korean male adolescents [32], which is consistent with our findings. Another possible reason is that males are exposed to higher Hg levels compared to females. In this study, 71.3% of males and 49% of females showed blood Hg levels of ≥2.75 μg/L; therefore, the effects of Hg may have been obscured in females.
It is noteworthy that levels of Pb and Cd above the population averages were not significantly related to dyslipidemia despite the ability of these heavy metals to alter lipid metabolism. Indeed, chronic oral exposure to heavy metals, including Pb and Cd, affected the oxidative stress level and did not alter lipid profiles with oxidative stress in a rat model [33]. In this study, although not statistically significant, Cd exposure showed a tendency, especially for males with a prevalence of dyslipidemia. Cd can affect human biological systems at very low doses [34]. In this study, we compared people who were exposed to Cd at higher-than-average levels, which might have obscured the effects of Cd on dyslipidemia. Therefore, the association between Cd and dyslipidemia cannot be completely ruled out.
Dyslipidemia is one of the most well-known risk factors for cardiovascular disease, which is the leading cause of death worldwide. This means that the mortality rate associated with cardiovascular disease could be lowered by identifying the factors related to dyslipidemia and the implementation of comprehensive management [35]. In particular, various factors, including sex, are associated with dyslipidemia [36]. The present study found that age was significantly associated with dyslipidemia in both males and females. Factors including household income and BMI also showed significant associations with dyslipidemia. These relationships are speculated to be due to dietary habits playing an important role in modulating the blood lipid profile. A previous study found that the diet quality differed significantly according to age, sex, and household income in Americans [37], and that subjective health status was associated with dyslipidemia in both sexes. It is therefore necessary to consider these related factors when developing interventions for dyslipidemia.
This study was subject to several limitations. It was not possible to determine the causal relationships between the variables due to the cross-sectional design used to analyze the secondary data. Therefore, we sought to minimize this influence by considering confounding variables, including sociodemographic factors, health behaviors, and mental health factors. Nevertheless, considering that dyslipidemia is a complex metabolic disease involving various risk factors, similar replication studies should continue to be carried out to ensure that there is an association between dyslipidemia and heavy metals. In addition, we could not analyze biomarkers of oxidative stress that are suspected to underlie the effects of Hg on lipid metabolism. Thus, further studies are warranted to identify the underlying mechanisms using a variable for measuring oxidative stress.
5. Conclusions
In adult males, exposure to Hg above the average level for the total population is positively associated with dyslipidemia, while such an association was not found in females. These results provide the basis for targeted prevention strategies against dyslipidemia using lifestyle guidelines for reducing Hg exposure and healthy behavioral interventions. However, further studies are needed to reveal causal relationships and to identify the mechanisms underlying this interrelation at the genetic, epigenetic, and biochemical levels.
Acknowledgments
The Korean Centers for Disease Control and Prevention provided the data for this study.
Author Contributions
Conceptualization, P.K., H.Y.S. and K.Y.K.; methodology, P.K. and K.Y.K.; validation, P.K., H.Y.S. and K.Y.K.; formal analysis, P.K., H.Y.S. and K.Y.K.; investigation, P.K., H.Y.S. and K.Y.K.; data curation, P.K., H.Y.S. and K.Y.K.; writing—original draft preparation, P.K., H.Y.S. and K.Y.K.; writing—review and editing, P.K., H.Y.S. and K.Y.K.; visualization, P.K. and H.Y.S.; supervision, K.Y.K.; project administration, K.Y.K.; funding acquisition, K.Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Gachon University research fund of 2020 (GCU-2020-02950001).
Institutional Review Board Statement
The study was approved by the university’s Institutional Review Board (no. 1044396-202004-HR-089-01).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Na W., Chung B.H., Sohn C. A Relationship Between Dietary Patterns and Dyslipidemia in Urban-Dwelling Middle-Aged Korean Men: Using Korean Genome and Epidemiology Study (KoGES) Clin. Nutr. Res. 2019;8:219–228. doi: 10.7762/cnr.2019.8.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim H., Lee H.J., Choue R., Wang Y. Trends in Fast-Food and Sugar-Sweetened Beverage Consumption and Their Association With Social Environmental Status in South Korea. J. Acad. Nutr. Diet. 2018;118:1228–1236.e1. doi: 10.1016/j.jand.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Van Bussel E., Hoevenaar-Blom M.P., Poortvliet R.K., Gussekloo J., Van Dalen J., Van Gool W., Richard E., Van Charante E.M. Predictive Value of Traditional Risk Factors for Cardiovascular Disease in Older People: A Systematic Review. Prev. Med. 2020;132:105986. doi: 10.1016/j.ypmed.2020.105986. [DOI] [PubMed] [Google Scholar]
- 4.Noale M., Limongi F., Maggi S. Epidemiology of Cardiovascular Diseases in the Elderly. Taurine 6. 2020;1216:29–38. doi: 10.1007/978-3-030-33330-0_4. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Li Y., Liu X., Zhang H., Abdulai T., Tu R., Tian Z., Qian X., Jiang J., Qiao D., et al. Prevalence and Influencing Factors of Coronary Heart Disease and Stroke in Chinese Rural Adults: The Henan Rural Cohort Study. Front. Public Health. 2020;7:411. doi: 10.3389/fpubh.2019.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mooradian A.D. Dyslipidemia in Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 7.Shin S., Shin D.W., Cho I.Y., Jeong S.-M., Jung H. Status of Dyslipidemia Management and Statin Undertreatment in Korean Cancer Survivors: A Korean National Health and Nutrition Examination Survey Study. Eur. J. Prev. Cardiol. 2020:2047487320905722. doi: 10.1177/2047487320905722. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi Madani N., Ismail-Beigi F., Poustchi H., Nalini M., Sepanlou S.G., Malek M., Abbasi M.A., Khajavi A., Khamseh M.E., Malekzadeh R. Impaired Fasting Glucose and Major Adverse Cardiovascular Events by Hypertension and Dyslipidemia Status: The Golestan Cohort Study. BMC Cardiovasc. Disord. 2020;20:113. doi: 10.1186/s12872-020-01390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannon B.A., Khan A.N., Teran-Garcia M. Nutrigenetic Contributions to Dyslipidemia: A Focus on Physiologically Relevant Pathways of Lipid and Lipoprotein Metabolism. Nutrients. 2018;10:1404. doi: 10.3390/nu10101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamba V. Update on Screening, Etiology, and Treatment of Dyslipidemia in Children. J. Clin. Endocrinol. Metab. 2014;99:3093–3102. doi: 10.1210/jc.2013-3860. [DOI] [PubMed] [Google Scholar]
- 11.Morita S.-Y. Metabolism and Modification of Apolipoprotein B-Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol. Pharm. Bull. 2016;39:1–24. doi: 10.1248/bpb.b15-00716. [DOI] [PubMed] [Google Scholar]
- 12.He X., Qi Z., Hou H., Gao J., Zhang X.-X. Effects of Chronic Cadmium Exposure at Food Limitation-Relevant Levels on Energy Metabolism in Mice. J. Hazard. Mater. 2020;388:121791. doi: 10.1016/j.jhazmat.2019.121791. [DOI] [PubMed] [Google Scholar]
- 13.Flora S.J.S., Mittal M., Mehta A. Heavy Metal Induced Oxidative Stress & Its Possible Reversal by Chelation Therapy. Indian J. Med. Res. 2008;128:501–523. [PubMed] [Google Scholar]
- 14.Tinkov A.A., Filippini T., Ajsuvakova O.P., Aaseth J., Gluhcheva Y.G., Ivanova J.M., Bjørklund G., Skalnaya M.G., Gatiatulina E.R., Popova E.V., et al. The Role of Cadmium in Obesity and Diabetes. Sci. Total. Environ. 2017;601-602:741–755. doi: 10.1016/j.scitotenv.2017.05.224. [DOI] [PubMed] [Google Scholar]
- 15.Moon S.-S. Additive Effect of Heavy Metals on Metabolic Syndrome in the Korean Population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocrine. 2014;46:263–271. doi: 10.1007/s12020-013-0061-5. [DOI] [PubMed] [Google Scholar]
- 16.You C.-H., Kim B.-G., Kim J.-M., Yu S.-D., Kim Y.-M., Kim R.-B., Hong Y.-S. Relationship Between Blood Mercury Concentration and Waist-to-Hip Ratio in Elderly Korean Individuals Living in Coastal Areas. J. Prev. Med. Public Health. 2011;44:218–225. doi: 10.3961/jpmph.2011.44.5.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Z., Xi S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods. 2019;30:167–176. doi: 10.1080/15376516.2019.1701594. [DOI] [PubMed] [Google Scholar]
- 18.Poursafa P., Ataee E., Motlagh M.E., Ardalan G., Tajadini M.H., Yazdi M., Kelishadi R. Association of Serum Lead and Mercury Level With Cardiometabolic Risk Factors and Liver Enzymes in a Nationally Representative Sample of Adolescents: The CASPIAN-III Study. Environ. Sci. Pollut. Res. 2014;21:13496–13502. doi: 10.1007/s11356-014-3238-4. [DOI] [PubMed] [Google Scholar]
- 19.Yoo J., Choi W., Jeon H., Kwon Y., Lee N., Jung S., Joo Y., Lee K., Lee C., Yu S. Korean National Environmental Health Survey (KoNEHS) Environmental Health Research Division, Environmental Health Research Department, National Institute of Environmental Research; Incheon, Korea: 2017. pp. 1–64. [Google Scholar]
- 20.Azevedo B.F., Furieri L.B., Peçanha F.M., Wiggers G.A., Vassallo P.F., Simões M.R., Fiorim J., De Batista P.R., Fioresi M., Rossoni L., et al. Toxic Effects of Mercury on the Cardiovascular and Central Nervous Systems. J. Biomed. Biotechnol. 2012;2012:1–11. doi: 10.1155/2012/949048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eom S.-Y., Choi S.H., Ahn S.-J., Kim D.-K., Lim J.-A., Choi B.-S., Shin H.-J., Yun S.-W., Yoon H.-J., Kim Y.-M., et al. Reference Levels of Blood Mercury and Association With Metabolic Syndrome in Korean Adults. Int. Arch. Occup. Environ. Health. 2013;87:501–513. doi: 10.1007/s00420-013-0891-8. [DOI] [PubMed] [Google Scholar]
- 22.Park K., Seo E. Toenail Mercury and Dyslipidemia: Interaction With Selenium. J. Trace Elem. Med. Biol. 2017;39:43–49. doi: 10.1016/j.jtemb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Rizzetti D.A., Corrales P., Piagette J.T., Uranga J.A., Medina-Gomez G., Peçanha F.M., Vassallo D.V., Miguel M., Peçanha F.M. Chronic Mercury at Low Doses Impairs White Adipose Tissue Plasticity. Toxicology. 2019;418:41–50. doi: 10.1016/j.tox.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Zou C., Shao J. Role of Adipocytokines in Obesity-Associated Insulin Resistance. J. Nutr. Biochem. 2008;19:277–286. doi: 10.1016/j.jnutbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Kawakami T., Hanao N., Nishiyama K., Kadota Y., Inoue M., Sato M., Suzuki S. Differential Effects of Cobalt and Mercury on Lipid Metabolism in the White Adipose Tissue of High-Fat Diet-Induced Obesity Mice. Toxicol. Appl. Pharmacol. 2012;258:32–42. doi: 10.1016/j.taap.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Joshi D., Srivastav S.K., Belemkar S., Dixit V.A. Zingiber Officinale and 6-Gingerol Alleviate Liver and Kidney Dysfunctions and Oxidative Stress Induced by Mercuric Chloride in Male Rats: A Protective Approach. Biomed. Pharmacother. 2017;91:645–655. doi: 10.1016/j.biopha.2017.04.108. [DOI] [PubMed] [Google Scholar]
- 27.Guzik T.J., Touyz R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension. 2017;70:660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- 28.Farkhondeh T., Afshari R., Mehrpour O., Samarghandian S. Mercury and Atherosclerosis: Cell Biology, Pathophysiology, and Epidemiological Studies. Biol. Trace Elem. Res. 2019;196:27–36. doi: 10.1007/s12011-019-01899-w. [DOI] [PubMed] [Google Scholar]
- 29.Kern J.K., Geier D.A., Homme K.G., King P.G., Bjørklund G., Chirumbolo S., Geier M.R. Developmental Neurotoxicants and the Vulnerable Male Brain: A Systematic Review of Suspected Neurotoxicants That Disproportionally Affect Males. Acta Neurobiol. Exp. 2017;77:269–296. doi: 10.21307/ane-2017-061. [DOI] [PubMed] [Google Scholar]
- 30.Spiller H.A. Rethinking Mercury: The Role of Selenium in the Pathophysiology of Mercury Toxicity. Clin. Toxicol. 2018;56:313–326. doi: 10.1080/15563650.2017.1400555. [DOI] [PubMed] [Google Scholar]
- 31.Rush J.W.E., Sandiford S.D. Plasma Glutathione Peroxidase in Healthy Young Adults: Influence of Gender and Physical Activity. Clin. Biochem. 2003;36:345–351. doi: 10.1016/S0009-9120(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 32.Cho H.W., Kim S.-H., Park M.J. An Association of Blood Mercury Levels and Hypercholesterolemia Among Korean Adolescents. Sci. Total. Environ. 2020;709:135965. doi: 10.1016/j.scitotenv.2019.135965. [DOI] [PubMed] [Google Scholar]
- 33.Oladipo O.O., Ayo J.O., Ambali S.F., Mohammed B., Aluwong T. Dyslipdemia Induced by Chronic Low Dose Co-Exposure to Lead, Cadmium and Manganese in Rats: The Role of Oxidative Stress. Environ. Toxicol. Pharmacol. 2017;53:199–205. doi: 10.1016/j.etap.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Alissa E.M., Ferns G.A. Heavy Metal Poisoning and Cardiovascular Disease. J. Toxicol. 2011;2011:1–21. doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee E.-J., Kim H.C., Kim J.H., Lee E.Y., Kim B.J., Kim E.M., Song Y., Lim J.H., Kim H.J., Choi S., et al. 2018 Guidelines for the Management of Dyslipidemia. Korean J. Intern. Med. 2019;34:723–771. doi: 10.3904/kjim.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opoku S., Gan Y., Fu W., Chen D., Addo-Yobo E., Trofimovitch D., Yue W., Yan F., Wang Z., Lu Z. Prevalence and Risk Factors for Dyslipidemia Among Adults in Rural and Urban China: Findings from the China National Stroke Screening and Prevention Project (CNSSPP) BMC Public Health. 2019;19:1–15. doi: 10.1186/s12889-019-7827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiza H.A., Casavale K.O., Guenther P.M., Davis C.A. Diet Quality of Americans Differs by Age, Sex, Race/Ethnicity, Income, and Education Level. J. Acad. Nutr. Diet. 2013;113:297–306. doi: 10.1016/j.jand.2012.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.