Abstract
Background & Aims
Data on the clinical characteristics of patients with inflammatory bowel diseases (IBDs) with coronavirus disease 2019 (COVID-19) are scarce. The aim of our systematic review was to investigate symptoms and diagnostic–therapeutic management of IBD patients with COVID-19.
Methods
We searched PubMed, Embase, Web of Science, and MedRxiv up to July 29, 2020, to identify all studies reporting clinical information on adult and pediatric IBD patients with confirmed COVID-19.
Results
Twenty-three studies met our inclusion criteria, including 243,760 IBD patients. COVID-19 was diagnosed in 1028 patients (509 with Crohn’s disease [49.5%], 428 with ulcerative colitis [41.6%], 49 with indeterminate colitis [4.8%], and 42 with missing data [4.1%]), accounting for a cumulative prevalence of 0.4%. Viral infection occurred more frequently in males than in females (56.5% vs 39.7%), and the mean age ranged from 14 to 85 years. The most common symptoms were fever (48.3%), cough (46.5%), and diarrhea (20.5%), and a COVID-19 diagnosis was achieved mainly through polymerase chain reaction analysis of nasopharyngeal swabs (94.4%) and chest computed tomography scans (38.9%). Hydroxychloroquine (23.9%), lopinavir/ritonavir (8.2%), steroids (3.2%), and antibiotics (3.1%) were the most used drugs. Overall, approximately a third of patients were hospitalized (30.6%), and 11.4% of them required admission to the intensive care unit. In total, 29 COVID-19–related deaths were reported (3.8%), and increasing age and the presence of comorbidities were recognized as risk factors for COVID-19 and negative outcomes.
Conclusions
Diarrhea occurs more frequently in IBD patients with COVID-19 than in the non-IBD population. Further studies are needed to define the optimal diagnostic–therapeutic approach in IBD patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Crohn’s Disease, Ulcerative Colitis, Inflammatory Bowel Disease
Abbreviations used in this paper: aOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CD, Crohn’s disease; IBD, inflammatory bowel disease; ICU, intensive care unit; NOS, Newcastle–Ottawa Scale; OR, odds ratio; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UC, ulcerative colitis
What You Need to Know.
Background
Little data are available on the clinical characteristics of inflammatory bowel disease (IBD) patients with COVID-19 and their diagnostic-therapeutic management is not well established.
Findings
IBD patients with COVID-19 have symptoms similar to IBD patients except for a higher percentage of diarrhea. The diagnostic-therapeutic approach does not differ between IBD and non-IBD patients with COVID-19.
Implications for patient care
Fecal test for new coronavirus detection could allow to differentiate infected patients from those with IBD re-exacerbation. In addition, IBD medications could play a role in the treatment of COVID-19.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new β-coronavirus that was identified in China after the onset, in December 2019, of some pneumonia cases of unknown etiology.1 Viral infection can be asymptomatic or cause the coronavirus disease 2019 (COVID-19), which is characterized by a wide range of clinical manifestations including respiratory and gastrointestinal symptoms up to severe events such as pneumonia, acute respiratory distress syndrome, and death.2 The high transmission capacity and the rapid virus spread worldwide have led the World Health Organization to declare a pandemic state and national and international authorities to impose several precautions and prohibitions to limit the contagion up to the total lockdown.3 , 4 As of June 12, 2020, there were 7,410,510 cases of COVID-19 that have been ascertained globally, with a total of 418,294 deaths.5 Since the beginning of the health emergency, particular attention has been paid to the management of patients with chronic inflammatory bowel diseases (IBDs) because they frequently are treated with immunosuppressive drugs and therefore potentially are exposed to a greater infectious risk than the general population.6 In addition, hospitals also profoundly have been reorganized to address the growing number of infected patients, to adapt to social distancing measures, and to prevent the infection risk, postponing or canceling nonessential activities and replacing outpatient visits with virtual clinics.7 , 8 The British Society of Gastroenterology, the European Crohn’s and Colitis Organization, and the International Organization for the Study of Inflammatory Bowel Disease promptly provided empiric recommendations for the management of patients with Crohn’s disease (CD) and ulcerative colitis (UC).9, 10, 11 However, knowledge of SARS-CoV-2 evolves daily and some doubts persist on the optimal approach in subjects treated with immunosuppressants, biologics, or small molecules. The aim of our study was to provide a systematic overview of the literature data on IBD patients with COVID-19 to report the clinical characteristics of disease, to identify any risk factors for severe/complicated disease, and to investigate the diagnostic–therapeutic management of IBD patients in this emergency setting.
Methods
We conducted a systematic review in accordance with the Cochrane Handbook12 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for reporting of systematic reviews incorporating network meta-analysis.13
Data Sources and Search Strategy
We searched PubMed, Embase, Web of Science, and MedRxiv up to July 29, 2020, to identify all studies reporting information on IBD patients with COVID-19. The following medical subject heading terms were combined with the Boolean operators “AND” or “OR”: “COVID-19,” “coronavirus disease 2019,” “SARS-CoV-2,” “severe acute respiratory syndrome coronavirus 2,” “new coronavirus,” “Crohn’s disease,” “CD,” “ulcerative colitis,” “UC,” “inflammatory bowel disease,” “IBD.” The search was restricted to human studies, although no language or time restrictions were applied. Titles and abstracts were scrutinized independently by all 3 authors (F.D., S.D., and L.P.B.) to identify eligible studies. Subsequently, full-text articles were examined for inclusion, and any disagreements were resolved through collegial discussion. Finally, the reference lists of the selected manuscripts were hand-searched to identify studies missed by the electronic search.
Selection Criteria
All studies meeting the following criteria were included: (1) adult and/or pediatric patients with a confirmed diagnosis of IBD; (2) studies reporting at least 1 confirmed case of COVID-19; and (3) studies addressing clinical management of IBD patients with COVID-19. Reviews, systematic reviews, meta-analyzes, guidelines, letters, and editorials that did not show original data were excluded from our work. Furthermore, all studies involving non-IBD patients were excluded if the IBD population data could not be distinguished. If some results were reported at multiple time points, the study with the most comprehensive data was included.
Data Extraction and Analysis
Each article was assessed qualitatively. All 3 authors extracted the following data from the selected studies: first author, journal and year of publication, study design, number of participants, patient characteristics (age, sex, concomitant treatments, IBD type), number of IBD patients with confirmed COVID-19, symptoms of COVID-19, diagnostic approach, COVID-19 therapy, hospitalizations, admission to the intensive care unit (ICU), number of deaths, and risk factors associated with COVID-19.
Quality of Studies
The Newcastle–Ottawa Scale (NOS) score was used to measure the quality of nonrandomized clinical trials, and the Jadad score was adopted for randomized clinical trials.14 , 15 The NOS score ranges from 0 to 9. The NOS score is based on 8 items: representativeness of the exposed cohorts, selection of the nonexposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of the study, comparability of cohorts on the basis of the design or analysis, assessment of the outcome, follow-up period is long enough for outcomes to occur, and adequacy of the follow-up evaluation. One point can be assigned to each item, except for cohort comparability (which can be assigned 2 points). A NOS score of 6 or higher was associated with high-quality studies, while scores of 3 or lower or between 4 and 5 indicated low- and moderate-quality studies, respectively. On the other hand, the Jadad score ranges from 0 to 5 and it assesses the following parameters: randomized study, appropriate randomization, double-blind study, appropriate double-blind study, and a description of withdrawals/dropouts. Each parameter is assigned 1 point and a study is defined as a high-quality study if the Jadad score is 3 or higher. All 3 authors graded the studies independently and any disagreements were discussed until their resolution.
Results
Study Characteristics
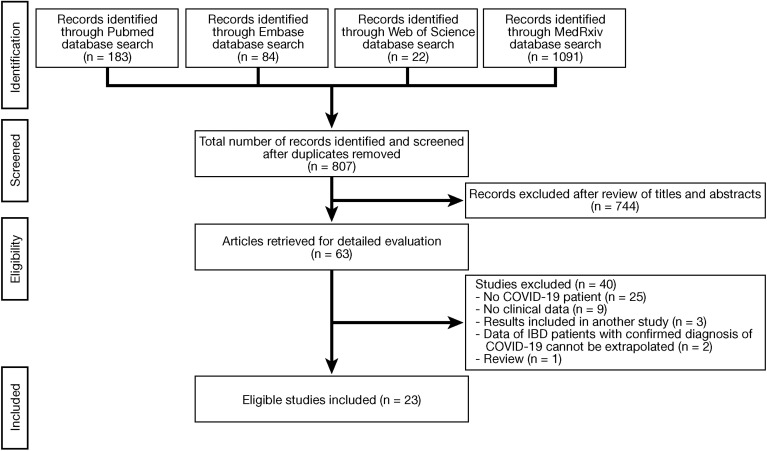
The flow chart of the search process is detailed in Figure 1 . A total of 1380 articles were identified through our search (PubMed, 183; Embase, 84; Web of Science, 22, and MedRxiv, 1091). After removing duplicates and reviewing titles and abstracts, 63 studies were evaluated for full-text analysis. An additional 40 studies were excluded because they did not include COVID-19 patients (n = 25), did not evaluate clinical data (n = 9), results were included in another study (n = 3), data of IBD patients with a confirmed diagnosis of COVID-19 could not be extrapolated (n = 2), or data were not original (n = 1). Finally, 23 studies16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 were included in our systematic review. Most studies were case reports (12 [52.2%]),16 , 18 , 19 , 22 , 27 , 29 , 32, 33, 34, 35, 36 , 38 followed by observational cohort studies (3 prospective [13.0%]17, 21, 25 and 6 retrospective studies [26.1%]20, 24, 26, 28, 31, 37) and case series (2 [8.7%])23 , 30 (Tables 1 and 2 ). Most studies were of moderate quality according to the NOS score (15 [65.2%]),16 , 19 , 20 , 22 , 26, 27, 28 , 30 , 31 , 38 while the remaining studies were classified as high-quality studies (8 [34.8%])17 , 18 , 21 , 23, 24, 25 , 29 (Table 3 ).
Figure 1.
Flow chart of the search process. COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease.
Table 1.
Patient Demographics and Main Characteristics of Case Series and Observational Cohort Studies
| Study | Study design | Study population | COVID-19 | Sex | Mean age, y | Ongoing therapy | Symptoms | Diagnosis | Treatment | Hospitalization | ICU | Mechanical ventilation | Death | Risk factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lukin et al17 | Prospective cohort study | 80 IBD, 160 controls | 26 CD, 38 UC | 135 males (56.3%) | 48.7 | Mesalamine, 20 (25%); anti-TNF, 16 (20%); steroids, 13 (16.2%); UST, 12 (15%); VDZ, 10 (8.0%); combination therapy, 4 (5.0%); thiopurines, 4 (5.0%); methotrexate, 3 (3.7%); TOFA, 1 (1.2%) | Cough, 54 (67.5%); fever, 53 (66.6%); diarrhea, 36 (45.0%); shortness of breath, 23 (28.8%); abdominal pain, 16 (20.0%); nausea, 12 (15%); vomiting, 10 (12.5%); myalgia/fatigue, 7 (8.8%); anorexia, 7 (8.8%); anosmia, 7 (8.8%); dysgeusia, 4 (5.0%) | NPS | / | 17 (21.3%) | 3 (17.6%) | 2 (11.8%) | 0 | Diagnosis of UC was associated with emergency visit or admission |
| Rodríguez-Lago et al20 | Retrospective cohort study | 13 CD, 23 UC, 4 IC | 13 CD, 23 UC, 4 IC | 24 males (60%) | 59 | Mesalamine, 26 (65%); thiopurines, 8 (20%); steroids, 4 (10%); methotrexate, 3 (8%); UST, 3 (8%); combination therapy, 2 (5.0%); anti-TNF, 3 (8%); VDZ, 1 (3%) | Fever, 31 (77%); cough, 27 (67%); diarrhea, 8 (20%) | NPS | Hydroxychloroquine, 25 (63%); lopinavir/ritonavir, 15 (38%); antibiotic, 9 (23%); steroid, 5 (13%); oseltamivir, 1 (3%); tocilizumab, 1 (3%); anakinra, 1 (3%) | 21 (52.5%) | 0 | / | 2 (5%) | / |
| Brenner et al21 | Prospective cohort study | 312 CD (59.4%), 203 UC (38.7%), 7 IC (1.3%), 3 missing (0.6%) | 312 CD (59.4%), 203 UC (38.7%), 7 IC (1.3%), 3 missing (0.6%) | 276 males (52.6%) | 42.9 | Anti-TNF, 176 (33.5%); mesalamine, 117 (22.3%); UST, 55 (10.5%); thiopurines, 53 (10.1%); combination therapy, 52 (9.9%); VDZ, 50 (9.5%); systemic steroids, 37 (7.0%); other, 22 (4.2%); budesonide, 18 (3.4%); TOFA, 8 (1.5); methotrexate, 5 (1.0%) | / | / | Hydroxychloroquine, 98 (18.7%); other, 67 (12.8%); lopinavir/ritonavir, 28 (5.3%); chloroquine, 14 (2.7%); steroid, 12 (2.3%); oseltamivir, 6 (1.1%); tocilizumab, 5 (1.0%); remdesivir, 2 (0.4%) | 161 (30.7%) | 24 (4.6%) | 21 (4.0%) | 16 (3%) | Increasing age, ≥2 comorbidities, systemic corticosteroids, and mesalamine/sulfasalazine use were risk factors for ICU admission/ ventilation/death |
| Taxonera et al23 | Case series | 920 CD (48.0%), 997 UC (52.0%) | 7 CD, 5 UC | 3 males (25.0%) | 47.9 | Mesalamine, 4 (33.3%); thiopurines, 3 (25.0%); anti-TNF, 3 (25.0%); methotrexate, 3 (25.0%); UST, 1 (8.3%); VDZ, 1 (8.3%) | Fever, 9 (75%); diarrhea, 9 (75%); cough, 6 (50%); myalgia, 6 (50%); dyspnea, 5 (41.7%); sore throat, 4 (33.3%); ageusia, 4 (33.3%); fatigue, 4 (33.3%); anosmia, 3 (25.0%); headache, 3 (25.0%); nausea/vomiting, 2 (16.7%) | NPS | Hydroxychloroquine, 8 (66.7%); antibiotic, 6 (5.0%); lopinavir/ritonavir, 4 (33.3%) | 8 (66.7%) | 1 (8.3%) | 1 (8.3%) | 2 (0.1%) | / |
| Gubatan et al24 | Retrospective cohort study | 86 UC (51.2%), 66 CD (39.3%), 16 IC (9.5%) | 3 UC, 2 CD | 2 males (40.0%) | / | Mesalamine, 4 (80.0%); steroids, 1 (20.0%); thiopurines, 1 (20.0%); anti-TNF, 1 (20.0%) | Cough, 4 (80.0%); fever, 3 (60.0%); fatigue, 3 (60.0%); rhinopharyngitis, 3 (60.0%); myalgia, 3 (60.0%); sore throat, 2 (40.0%); dyspnea, 2 (40.0%); pneumonia, 2 (40.0%); diarrhea, 1 (20.0%); abdominal pain, 1 (20.0%); nausea/vomiting, 1 (20.0%) | / | / | / | / | / | 1 | Age >66 y was associated independently with increased risk of COVID-19 |
| Bezzio et al25 | Prospective cohort study | 32 CD, 47 UC | 32 CD, 47 UC | 44 males (55.7%) | 45 | Anti-TNF, 29 (36.7%); mesalamine, 24 (30.4%); VDZ, 15 (18.9%); steroids, 9 (11.4%); thiopurines, 6 (7.6%); UST, 3 (3.8%); investigational drugs, 2 (2.5%); calcineurin inhibitors, 1 (1.3%) | Fever, 71 (89.9%); cough, 52 (65.8%); dysosmia or dysgeusia, 19 (24.0%); arthralgia or myalgia, 18 (22.8); dyspnea, 15 (19.0%); diarrhea, 12 (15.2%); rhinopharyngitis, 13 (16.4%); dysphonia 1 (1.2%); conjunctivitis, 1 (1.2%) | NPS, CT scan | / | 22 (27.8%) | 2 (2.5%) | 2 (2.5%) | 6 (7.6%) | Age >65 y, active IBD, and CCI score >1 were associated significantly with COVID-19–related death |
| Khan et al26 | Retrospective cohort study | 37,857 IBD | 36 IBD (0.1%) | / | 63 | Anti-TNF, 2 (5.5%); thiopurines, 3 (8.3%) | / | Laboratory test | / | / | / | / | / | CCI score was associated significantly with the risk of COVID-19 |
| Allocca et al28 | Retrospective cohort study | 6000 IBD | 9 CD, 6 UC | 4 males (26.7%) | 39.1 | Anti-TNF, 8 (53.3%); UST, 3 (20.0%); steroids, 2 (13.3%); thiopurines, 2 (13.3%); mesalamine, 1 (6.7%); VDZ, 1 (6.7%); investigational drugs, 1 (6.7%); calcineurin inhibitors, 1 (6.7%) | / | NPS | / | 5 (33.3%) | 0 | / | 0 | / |
| Turner et al30 | Case series | 4 pediatric CD,a 2 pediatric UC, 1 pediatric IC | 3 CD, 2 UC | 2 males (40.0%) | 16.4 | Mesalamine, 3 (60.0%); thiopurines, 3 (60.0%); anti-TNF, 2 (40.0%); steroids, 1 (20.0%); VDZ, 1 (20.0%) | Cough, 3 (60.0%); fever, 2 (40.0%); fatigue, 1 (20.0%); rhinitis, 1 (20.0%); chest pain, 1 (20.0%); anosmia, 1 (20.0%); ageusia, 1 (20.0%) | / | / | 0 | 0 | 0 | 0 | / |
| Marafini et al31 | Retrospective cohort study | 397 CD, 269 UC, 6 IC | 3 IBD | / | / | / | / | NPS | / | 2 (66.6%) | / | / | 1 | / |
| Singh et al37 | Retrospective cohort study | 196,403 IBD, 19,776 non-IBD | 101 CD, 93 UC, 38 IC | 85 males (36.7%) | 51.2 | / | Cough, 56 (24.1%); fever, 38 (16.4%); dyspnea, 30 (12.9%); nausea, 25 (10.8%); vomiting, 25 (10.8%); fatigue, 20 (8.6%); diarrhea, 19 (8.2%); abdominal pain, 18 (7.7%); sore throat, 14 (6.0%); hypoxemia, 12 (5.2%) | / | / | 56 (24.1%) | / | / | / | The risk of severe COVID-19 was higher in IBD patients who received corticosteroids up to 3 mo before the diagnosis of COVID-19 |
CD, Crohn’s disease; CCI, Charlson Comorbidity Index; COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease; IC, indeterminate colitis; ICU, intensive care unit; NPS, nasopharyngeal swab; TNF, tumor necrosis factor; TOFA, tofacitinib; UC, ulcerative colitis; UST, ustekinumab; VDZ, vedolizumab; -, not applicable; /, not reported.
One CD patient was excluded from the analysis because he was included in the Surveillance Epidemiology of Coronavirus Under Research Exclusion- Inflammatory Bowel Disease trial.
Table 2.
Patient Demographics and Main Characteristics of Case Reports
| Study | Study design | Study population | COVID-19 | Sex | Mean age, y | Ongoing therapy | Symptoms | Diagnosis | Treatment | Hospitalization | ICU | Mechanical ventilation | Death | Risk factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mazza et al16 | Case report | 1 severe UC | 1 | F | 80 | Mesalamine | Fever, diarrhea, anemia, dry cough, pneumonia | NPS, CT scan | Noninvasive ventilation Lopinavir/ritonavir Hydroxychloroquine Steroid |
1 | 0 | 0 | 1 | / |
| Jacobs et al18 | Case report | 1 UC | 1 | F | 33 | TOFA | Fever, chills, cough, myalgias, sore throat, fatigue, night sweats | NPS | TOFA | 0 | 0 | 0 | 0 | / |
| Kunisaki et al19 | Case report | 1 UC | 1 | M | 60 | IFX + azathioprine Mesalamine |
Cough and fever | NPS, CT scan | - | 1 | 0 | 0 | 0 | / |
| Tursi et al22 | Case report | 1 CD | 1 | M | 30 | Mesalamine Adalimumab |
Fever Chest pain |
NPS, CT scan | Noninvasive ventilation | 1 | 0 | 0 | 0 | / |
| Rosen et al27 | Case report | 1 severe UC during pregnancy | 1 | F | 26 | None | Abdominal pain, diarrhea, hematochezia, urgency | NPS | Steroid Antibiotic Hydroxychloroquine Cyclosporine |
1 | 0 | 0 | 0 | / |
| Dolinger et al29 | Case report | 1 pediatric CD | 1 | M | 14 | / | Fever, abdominal pain, erythematous maculopapular facial rash | / | Hydroxychloroquine Antibiotic Infliximab |
1 | 0 | 0 | 0 | / |
| Wolf et al38 | Case report | 1 CD | CD | M | 85 | None | Diarrhea, anorexia, fatigue, cough, weight loss | NPS | Bismuth subsalicylate | 0 | 0 | 0 | 0 | / |
| Di Ruscio et al32 | Case report | 1 severe UC | UC | F | 60 | Infliximab | Fever, cough, dyspnea, diarrhea, abdominal pain, fatigue | NPS, chest radiography, CT scan | Hydroxychloroquine, darunavir/cobicistat, supplemental oxygen | 1 | 0 | 0 | 0 | / |
| Bezzio and Saibeni34 | Case report | 1 severe UC | UC | M | 40 | Oral steroid | Diarrhea, abdominal pain, fever, cough | NPS, chest radiography | Azithromycin, hydroxychloroquine | 1 | 0 | 0 | 0 | / |
| Bezzio et al33 | Case report | 1 severe UC | UC | M | 36 | Mesalamine | Diarrhea, fever, dyspnea, cough | NPS, CT scan | Infliximab | 1 | 0 | 0 | 0 | / |
| Calabrese et al35 | Case report | 1 UC | UC | F | 19 | None | Fever, nausea, vomiting, diarrhea, anosmia, ageusia | NPS, CT scan | Hydroxychloroquine | 1 | 0 | 0 | 0 | / |
| Giulia and Patrizia36 | Case report | 1 severe pediatric CD | CD | F | 17 | Adalimumab | Fever, fatigue, dyspnea | NPS | None | 1 | 0 | 0 | 0 | / |
CD, Crohn’s disease; COVID-19, coronavirus disease 2019; F, female; IBD, inflammatory bowel disease; ICU, intensive care unit; IFX, infliximab; M, male; NPS, nasopharyngeal swab; TOFA, tofacitinib; UC, ulcerative colitis; -, not applicable; /, not reported.
Table 3.
Quality of the Studies Included in the Systematic Review According to the Newcastle–Ottawa Scale
| Study | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Score |
|---|---|---|---|---|---|---|---|---|---|
| Mazza et al16 | ★ | ★ | ★ | ★ | 4 | ||||
| Lukin et al17 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★★ | 9 |
| Jacobs et al18 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Kunisaki et al19 | ★ | ★ | ★ | ★ | 4 | ||||
| Rodríguez-Lago et al20 | ★ | ★ | ★ | ★ | ★ | 5 | |||
| Brenner et al21 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Tursi et al22 | ★ | ★ | ★ | ★ | 4 | ||||
| Taxonera et al23 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Gubatan et al24 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Bezzio et al25 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Khan et al26 | ★ | ★ | ★ | ★ | 4 | ||||
| Rosen et al27 | ★ | ★ | ★ | ★ | 4 | ||||
| Allocca et al28 | ★ | ★ | ★ | ★ | 4 | ||||
| Dolinger et al29 | ★ | ★ | ★ | ★ | ★ | ★ | 6 | ||
| Turner et al30 | ★ | ★ | ★ | ★ | 4 | ||||
| Marafini et al31 | ★ | ★ | ★ | ★ | 4 | ||||
| Wolf et al38 | ★ | ★ | ★ | ★ | 4 | ||||
| Di Ruscio et al32 | ★ | ★ | ★ | ★ | 4 | ||||
| Bezzio and Saibeni34 | ★ | ★ | ★ | ★ | 4 | ||||
| Bezzio et al33 | ★ | ★ | ★ | ★ | 4 | ||||
| Calabrese et al35 | ★ | ★ | ★ | ★ | 4 | ||||
| Giulia and Patrizia36 | ★ | ★ | ★ | ★ | 4 | ||||
| Singh et al37 | ★ | ★ | ★ | ★ | ★ | ★ | 6 |
NOTE. Items were as follows: 1, representativeness of the exposed cohort; 2, selection of the nonexposed cohort; 3, ascertainment of exposure; 4, demonstration that the outcome of interest was not present at the start of the study; 5, assessment of the outcome; 6, follow-up period was long enough for outcomes to occur; 7, adequacy of follow-up evaluation (>75% follow-up evaluation, or description for those lost); 8, comparability of cohorts on the basis of the design or analysis. Single stars, 1 point; double stars, 2 points.
Patient Characteristics
The overall study population consisted of 243,760 IBD patients. COVID-19 was diagnosed in 1028 patients (509 CD patients [49.5%], 428 UC patients [41.6%], 49 with indeterminate colitis [4.8%], and 42 with missing data [4.1%]), accounting for a cumulative prevalence of 0.4%. More than half of infected patients were male (581 [56.5%]), 408 were female (39.7%), and in 39 cases sex was not specified (3.8%). The mean age ranged from 14 to 85 years. Elderly patients (>65 y) with COVID-19 were found in 10 studies (43.5%),16 , 17 , 20 , 23, 24, 25, 26 , 31 , 37 , 38 pediatric cases (<18 y) were reported in 4 studies (17.4%),21 , 29 , 30 , 36 and an infected pregnant patient was described in 1 case report (4.3%).27 The ongoing drugs at the time of COVID-19 diagnosis were reported in almost all studies (20 [87.0%])16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 , 30 , 32, 33, 34, 35, 36 , 38 and were as follows: anti–tumor necrosis factor (243 of 790 [30.8%]), mesalamine (203 [25.7%]), thiopurine (83 [10.5%]), vedolizumab (79 [10.0%]), ustekinumab (77 [9.7%]), steroids (68 [8.6%]), combination therapy (biological drug + thiopurine) (59 [7.5%]), other (27 [3.4%]), methotrexate (14 [1.8%]), and tofacitinib (10 [1.3%]). As for COVID-19 symptoms, they were reported in 19 studies (82.6%).16, 17, 18, 19, 20 , 22, 23, 24, 25 , 27 , 29 , 30 , 32, 33, 34, 35, 36, 37, 38 The most frequent symptoms were fever (217 of 449 [48.3%]), cough (209 [46.5%]), and diarrhea (92 [20.5%]), followed by dyspnea (55 [12.2%]), nausea (40 [8.9%]), and abdominal pain (39 [8.7%]) (Table 4 ).
Table 4.
Symptoms of IBD Patients With a Confirmed Diagnosis of COVID-19 in the Overall Population
| Symptoms | N (%) |
|---|---|
| Fever | 217/449 (48.3) |
| Cough | 209/449 (46.5) |
| Diarrhea | 92/449 (20.5) |
| Dyspnea | 55/449 (10.5) |
| Nausea | 40/449 (8.9) |
| Abdominal pain | 39/449 (8.7) |
| Vomiting | 39/449 (8.7) |
| Fatigue | 39/449 (8.7) |
| Myalgia | 35/449 (7.8) |
| Dysgeusia | 23/449 (5.1) |
| Sore throat | 21/449 (4.7) |
| Rhinopharyngitis | 17/449 (3.8) |
| Anosmia | 12/449 (2.7) |
| Hypoxemia | 12/449 (2.7) |
| Anorexia | 8/449 (1.8) |
| Ageusia | 6/449 (1.3) |
| Headache | 3/449 (0.7) |
| Chest pain | 2/449 (0.4) |
| Night sweats | 1/449 (0.2) |
| Dysphonia | 1/449 (0.2) |
| Conjunctivitis | 1/449 (0.2) |
| Hematochezia | 1/449 (0.2) |
| Urgency | 1/449 (0.2) |
| Skin rash | 1/449 (0.2) |
| Weight loss | 1/449 (0.2) |
COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease.
Diagnosis and Treatment
Eighteen studies (78.3%)16, 17, 18, 19, 20 , 22 , 23 , 25, 26, 27, 28 , 31, 32, 33, 34, 35, 36 , 38 evaluated the diagnostic approach in IBD patients with COVID-19. The polymerase chain reaction (PCR) analysis of the nasopharyngeal swabs was the most commonly adopted method (17 of 18 [94.4%]).16, 17, 18, 19, 20 , 22 , 23 , 25 , 27 , 28 , 31, 32, 33, 34, 35, 36 , 38 A chest computed tomography was performed in 7 studies (38.9%).16 , 19 , 22 , 25 , 32, 33, 34, 35 In 1 study (5.6%),26 the diagnosis was achieved by laboratory test. The treatment of infected subjects was described in 14 articles (60.1%).16 , 18 , 20, 21, 22, 23 , 27 , 29 , 32, 33, 34, 35, 36 , 38 The most used drugs were hydroxychloroquine (140 of 586 [23.9%]), lopinavir/ritonavir (48 [8.2%]), steroids (19 [3.2%]), antibiotics (18 [3.1%]), and chloroquine (14 [2.4%]) (Table 5 ). Importantly, in 3 case reports the patients were treated with infliximab29 , 33 or tofacitinib.18
Table 5.
Treatment of IBD Patients With a Confirmed Diagnosis of COVID-19 in the Overall Population
| Treatment | N (%) |
|---|---|
| Hydroxychloroquine | 140/586 (23.9) |
| Lopinavir/ritonavir | 48/586 (8.2) |
| Steroid | 19/586 (3.2) |
| Antibiotic | 18/586 (3.1) |
| Chloroquine | 14/586 (2.4) |
| Oseltamivir | 7/586 (1.2) |
| Tocilizumab | 6/586 (1.0) |
| Remdesivir | 2/586 (0.3) |
| Noninvasive ventilation | 2/586 (0.3) |
| Infliximab | 2/586 (0.3) |
| Tofacitinib | 1/586 (0.2) |
| Anakinra | 1/586 (0.2) |
| Cyclosporine | 1/586 (0.2) |
| Other | 70/586 (11.9) |
COVID-19, coronavirus disease 2019; IBD, inflammatory bowel disease.
Prognosis and Risk Factors
The percentage of severe COVID-19 (need for hospitalization, admission to the ICU, or mechanical ventilation) was reported in 21 articles (91.3%).16, 17, 18, 19, 20, 21, 22, 23 , 25 , 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Overall, 302 of 987 patients (30.6%) were hospitalized and only a small part of them stayed in the ICU (28 of 246 [11.4%]). In 17 studies16, 17, 18, 19 , 21, 22, 23 , 25 , 27 , 29 , 30 , 32, 33, 34, 35, 36 , 38 the need for mechanical ventilation was described, with an average value of 3.7% patients (26 of 697). Moreover, the percentage of IBD patients who died from COVID-19 was investigated in all studies except 2,26 , 37 with a total of 29 deaths in 760 cases (3.8%). It is noteworthy that in only 2 studies17 , 37 was there a control group consisting of non-IBD patients with COVID-19. Interestingly, death and ICU admission were numerically lower in the IBD group than in the control group (24% vs 35%, respectively; P = .352).17 Finally, 6 studies (26.1%)17 , 21 , 24, 25, 26 , 37 explored the risk factors in infected IBD patients. The Charlson Comorbidity Index26 (odds ratio [OR], 1.240; 95% CI, 1.106–1.3912; P = .0002) and age older than 66 years24 (OR, 21.30; P = .022) were associated with an increased risk of COVID-19, while patients with a UC diagnosis17 had higher rates of emergency visits or admissions (adjusted OR [aOR], 12.7; P = .009). Age older than 65 years (OR, 19.6; 95% CI, 2.95–130.6; P = .002), active IBD (OR, 8.45; 95% CI, 1.26–56.56; P = .02), and Charlson Comorbidity Index greater than 1 (OR, 16.66; 95% CI, 1.80–153.9; P = .01) were associated with COVID-19–related deaths.25 Similarly, increasing age (aOR, 1.04; 95% CI, 1.01–1.02), 2 or more comorbidities (aOR, 2.9; 95% CI, 1.1–7.8), systemic corticosteroids (aOR, 6.9; 95% CI, 2.3–20.5), and mesalamine/sulfasalazine use (aOR, 3.1; 95% CI, 1.3–7.7) were risk factors for severe COVID-19.21 Interestingly, Singh et al37 confirmed that IBD patients treated with steroids in the previous 3 months had a higher risk of severe COVID-19 than untreated (30.98% vs 19.25%; relative risk, 1.60; 95% CI, 1.01–2.57; P = .04), while no difference was found with the use of other immune-mediated therapies (relative risk, 1.01; 95% CI, 0.62–1.65; P = .97).
Discussion
This was a systematic review reporting the prevalence, clinical characteristics, diagnostic/therapeutic management, and risk factors of IBD patients with a confirmed diagnosis of COVID-19. Twenty-three studies were included showing a COVID-19 prevalence of 0.4% in our IBD cohort. COVID-19 was found in more men than women (56.5% vs 39.7%), and patients of all ages, from children to the elderly, were involved also, as highlighted in the first reports from China on non-IBD individuals.2 , 39 In line with general population data,2 fever (48.3%) and cough (46.5%) were the most frequent symptoms in infected patients with IBD. Interestingly, approximately a fifth of the patients experienced diarrhea. Our previous pooled analysis40 and 2 recent systematic reviews and meta-analyses41 , 42 showed a cumulative prevalence of diarrhea of approximately 7% to 10% in patients with COVID-19. This high disparity could be related to the influence of the underlying disease on the number of evacuations, justifying the greater percentage of diarrhea in CD and UC patients than in the general population. On the other hand, SARS-CoV-2 has been isolated in the duodenum and rectum,43 and a higher concentration of fecal calprotectin, a known inflammatory marker, has been found in infected patients with diarrhea compared with those without diarrhea (123.2 vs 17.3 μg/g; P < .001),44 suggesting that viral gut tropism could worsen inflammatory status and symptoms of IBD patients. Unfortunately, it is extremely challenging to assign the symptom to the underlying disease or to the concomitant infection, making it difficult to interpret data. A COVID-19 diagnosis was achieved mainly through nasopharyngeal swabs (94.4%) and chest computed tomography scans (38.9%).
Surprisingly, although approximately 40% of stool samples have been reported as positive for fecal SARS-CoV-2,42 no test for the presence of viral RNA shedding in the stool was performed in the included studies. To date, no clear evidence is available on the sensitivity of fecal PCR for the diagnosis of COVID-19. However, we hypothesize that fecal PCR may be useful in IBD patients to distinguish disease re-exacerbation from viral superinfection, allowing better patient management and targeted therapy. Hydroxychloroquine (23.9%) and lopinavir/ritonavir (8.2%) were the most frequently administered drugs in our cohort. Hydroxychloroquine is an antimalarial drug that proved to be effective in inhibiting in vitro replication of the new coronavirus.45 However, data supporting its use in infected patients are still limited. A prospective observational study46 conducted on 1376 patients with COVID-19 showed no significant difference in the risk of intubation and death between hydroxychloroquine users and nonusers (hazard ratio, 1.04; 95% CI, 0.82–1.32), raising several doubts on its efficacy.
Similarly, inconclusive data have been reported regarding lopinavir/ritonavir. A randomized controlled trial47 compared lopinavir/ritonavir with standard care (supplemental oxygen, noninvasive and invasive ventilation, antibiotics, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation) for the treatment of hospitalized COVID-19 patients. Lower 28-day mortality (19.2% vs 25.0%; 95% CI, −17.3 to 5.7) and shorter hospital stay (median, 12 vs 14 d; 95% CI, 0–3) and ICU stay (median, 6 vs 11 d; 95% CI, −9 to 0) were found in patients treated with the antiviral compared with the control group, but no significant difference between the 2 groups was detected regarding the primary end point of time to clinical improvement (median, 16 vs 16 d; hazard ratio, 1.31; 95% CI, 0.95–1.80; P = .09). Moreover, it is important to emphasize that in 3 case reports,18 , 29 , 33 clinical remission of IBD patients with COVID-19 was achieved after treatment with infliximab or tofacitinib. These findings certainly are not sufficient to support the use of these drugs, but they provide numerous insights. Accumulating evidence has shown that COVID-19 severity is associated with a cytokine storm syndrome, characterized by an increase in interleukin 2, interleukin 7, granulocyte-colony stimulating factor, interferon-γ–inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α.48 Based on these findings, it is legitimate to hypothesize that the use of biological drugs that selectively inhibit specific cytokines or small molecules that simultaneously block multiple cellular pathways may play a role in the treatment of these patients. In addition, the mortality rate that we found in IBD patients with COVID-19 (3.8%) was lower compared with the general population (∼10%).49 , 50 This could be explained by a lower rate of COVID-19 risk factors (increasing age and comorbidities) in IBD subjects. Importantly, more than half of the patients included in our study were treated with biologics or small molecules at the time of COVID-19 diagnosis, but it is not known if these drugs influenced the prognosis of infected IBD patients. Several ongoing studies are recruiting patients to assess the efficacy and safety of biologics (NCT04344249 and NCT04425538) and small molecules (NCT04373044, NCT04346147, and NCT04362943) for COVID-19 treatment and will allow us to understand if these drugs can be used in this setting.
Our systematic review addressed several practical aspects of managing IBD patients with COVID-19, including moderate- to high-quality studies and reporting data from a relevant number of patients. However, some limitations must be mentioned. First, no randomized clinical trial has been conducted to date in patients with IBD. Second, we excluded all studies reporting data collected in the Surveillance Epidemiology of Coronavirus Under Research Exclusion- Inflammatory Bowel Disease registry, but any overlaps resulting from nonexplicit inclusion in the registry cannot be excluded. Nonetheless, the description of clinical symptoms was missing in the Surveillance Epidemiology of Coronavirus Under Research Exclusion- Inflammatory Bowel Disease database, although most of the evaluated articles provided this important information.
In conclusion, symptoms experienced by IBD patients with COVID-19 are similar to those occurring in the general population, except for a higher percentage of diarrhea. Currently, the diagnostic–therapeutic approach does not differ between IBD and non-IBD patients, but further studies are needed to evaluate whether fecal research of viral RNA and treatment with IBD drugs may play a role in the management of COVID-19 patients.
Footnotes
Conflicts of interest These authors disclose the following: Silvio Danese has served as a speaker, consultant and advisory board member for Schering-Plough, AbbVie, MSD, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alphawasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, Johnson & Johnson, Nikkiso Europe GmbH, and Theravance; and Laurent Peyrin-Biroulet has served as a speaker, consultant, and advisory board member for Merck, AbbVie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC-Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, and Theravance. The remaining author discloses no conflicts.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anon. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 May 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-may-2020 Available from:
- 4.Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020;20:e102–e107. doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anon Coronavirus Disease (COVID-19) Situation Reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available from:
- 6.D’Amico F., Peyrin-Biroulet L., Danese S. Inflammatory bowel diseases and COVID-19: the invisible enemy. Gastroenterology. 2020;158:2302–2304. doi: 10.1053/j.gastro.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese S., Ran Z.H., Repici A. Gastroenterology department operational reorganisation at the time of Covid-19 outbreak: an Italian and Chinese experience. Gut. 2020;69:981–983. doi: 10.1136/gutjnl-2020-321143. [DOI] [PubMed] [Google Scholar]
- 8.Allocca M., Fiorino G., Furfaro F. Maintaining the quality standards of care for inflammatory bowel disease patients during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2020;18:1882–1883. doi: 10.1016/j.cgh.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy N.A., Jones G.-R., Lamb C.A. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2020;69:984–990. doi: 10.1136/gutjnl-2020-321244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Amico F., Danese S., Peyrin-Biroulet L. Inflammatory bowel disease management during the COVID-19 outbreak: a survey from the European Crohn’s and Colitis Organization (ECCO) Gastroenterology. 2020;159:14–19.e3. doi: 10.1053/j.gastro.2020.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin D.T., Abreu M.T., Rai V. Management of patients with Crohn’s disease and ulcerative colitis during the COVID-19 pandemic: results of an international meeting. Gastroenterology. 2020;159:6–13.e6. doi: 10.1053/j.gastro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P.T., Cochrane Collaboration, editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Wiley-Blackwell; Hoboken, NJ: 2020. [Google Scholar]
- 13.Hutton B., Salanti G., Caldwell D.M. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 14.Anon Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from:
- 15.Jadad A.R., Moore R.A., Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Mazza S., Sorce A., Peyvandi F. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020;69:1148–1149. doi: 10.1136/gutjnl-2020-321183. [DOI] [PubMed] [Google Scholar]
- 17.Lukin D.J., Kumar A., Hajifathalian K. Baseline disease activity and steroid therapy stratify risk of COVID-19 in patients with inflammatory bowel disease. Gastroenterology. 2020 May 29 doi: 10.1053/j.gastro.2020.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs J., Clark-Snustad K., Lee S. Case report of a SARS-CoV-2 infection in a patient with ulcerative colitis on tofacitinib. Inflamm Bowel Dis. 2020;26:e64. doi: 10.1093/ibd/izaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunisaki R., Tsukiji J., Kudo M. Potential inhibition of COVID-19-driven pneumonia by immunosuppressive therapy and anti-TNFα antibodies: a case report. J Crohns Colitis. 2020 May 30 doi: 10.1093/ecco-jcc/jjaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Lago I., Ramírez de la Piscina P., Elorza A. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain) Gastroenterology. 2020;159:781–783. doi: 10.1053/j.gastro.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner E.J., Ungaro R.C., Gearry R.B. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tursi A., Angarano G., Monno L. COVID-19 infection in Crohn’s disease under treatment with adalimumab. Gut. 2020;69:1364–1365. doi: 10.1136/gutjnl-2020-321240. [DOI] [PubMed] [Google Scholar]
- 23.Taxonera C., Sagastagoitia I., Alba C. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:276–283. doi: 10.1111/apt.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gubatan J., Levitte S., Balabanis T. SARS-CoV-2 testing, prevalence, and predictors of COVID-19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020;159:1141–1144. doi: 10.1053/j.gastro.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezzio C., Saibeni S., Variola A. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- 26.Khan N., Patel D., Xie D. Impact of anti-TNF and thiopurines medications on the development of COVID-19 in patients with inflammatory bowel disease: a nationwide VA cohort study. Gastroenterology. 2020 May 29 doi: 10.1053/j.gastro.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen M.H., Axelrad J., Hudesman D. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis. 2020;26:971–973. doi: 10.1093/ibd/izaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allocca M., Fiorino G., Zallot C. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. 2020;18:2134–2135. doi: 10.1016/j.cgh.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolinger M.T., Person H., Smith R. Pediatric Crohn’s disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020;71:153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner D., Huang Y., Martín-de-Carpi J. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70:727–733. doi: 10.1097/MPG.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marafini I., Salvatori S., Sena G. Low frequency of COVID-19 in inflammatory bowel diseases. Dig Liver Dis. 2020 Jun 13 doi: 10.1016/j.dld.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Ruscio M., Variola A., Angheben A. A challenging colectomy for acute severe ulcerative colitis complicated by COVID-19. Inflamm Bowel Dis. 2020;26:e120–e122. doi: 10.1093/ibd/izaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezzio C., Manes G., Bini F. Infliximab for severe ulcerative colitis and subsequent SARS-CoV-2 pneumonia: a stone for two birds. Gut. 2020 Jun 17 doi: 10.1136/gutjnl-2020-321760. [DOI] [PubMed] [Google Scholar]
- 34.Bezzio C., Saibeni S. Severe IBD flares and COVID-19: expand the gastroenterology-surgery team to include an infectious disease specialist. Gastroenterology. 2020 Jun 15 doi: 10.1053/j.gastro.2020.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrese E., Zorzi F., Monteleone G. Ref. no. DLD-20-852: onset of ulcerative colitis during SARS-Cov-2 infection. Dig Liver Dis. 2020 Jun 11 doi: 10.1016/j.dld.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giulia B., Patrizia A. SARS-CoV-2 infection in severe pediatric Crohn’s disease. What about anti-tumor necrosis factor α therapy? Dig Liver Dis. 2020 Jul 11 doi: 10.1016/j.dld.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh S., Khan A., Chowdhry M. Risk of severe COVID-19 in patients with inflammatory bowel disease in United States. A Multicenter Research Network Study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.06.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf D.C., Wolf C.H., Rubin D.T. Temporal improvement of a COVID-19-positive Crohn’s disease patient treated with bismuth subsalicylate. Am J Gastroenterol. 2020;115:1298. doi: 10.14309/ajg.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Amico F., Baumgart D.C., Danese S. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao R., Qiu Y., He J.-S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parasa S., Desai M., Thoguluva Chandrasekar V. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 44.Effenberger M., Grabherr F., Mayr L. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020;69:1543–1544. doi: 10.1136/gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J., Cao R., Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]