Abstract
Objective
To describe the design, implementation, and utilization of electronic health record (EHR)–based digital health surveillance strategies used to manage the coronavirus disease 2019 (COVID-19) pandemic and to ensure delivery of high-quality clinical care, such as case identification, remote monitoring, telemedicine services, and recruitment to clinical trials at Mayo Clinic.
Methods
The design and implementation work described in this report was performed at Mayo Clinic, a large multistate integrated health care system with more than 1.5 million annual patient visits that uses the Epic EHR system. Rule-based live registries were designed in the EHR system to classify patients who currently test positive for COVID-19, patients who test positive but have recovered from COVID-19, patients who are thought to have COVID-19 but do not yet meet clinical diagnostic criteria, patients who test negative for COVID-19, and patients who exceed a risk score for serious complications from COVID-19.
Results
By use of registries, custom dashboards and operational reports were developed to provide a daily high-level summary for clinical practice use and up-to-date information to manage individual patients affected by COVID-19, including support of case identification, contact isolation, and other care management tasks.
Conclusion
We developed and implemented a systematic approach to the use of EHR patient registries to manage the COVID-19 pandemic that proved feasible and useful in a large multistate group clinical practice. The key to harnessing the potential of digital surveillance tools to promote patient-centered care during the COVID-19 pandemic was to use the registry data, reports, and dashboards as informatics tools to inform decision-making.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; EHR, electronic health record; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
The coronavirus disease 2019 (COVID-19) pandemic continues to challenge the health system with the need to make operational decisions based on limited data and rapidly evolving clinical practice guidelines.1, 2, 3, 4, 5, 6, 7 Digital health surveillance applications based on electronic health record (EHR) data can help address the challenges posed by COVID-19, including needs for health surveillance, screening, triage, diagnosis, and monitoring. New approaches include adoption of novel digital patient-facing self-triage and self-scheduling tools.8 However, there is little experience with use of live rule-based EHR patient registries to manage a pandemic crisis. A well-executed patient registry can provide a real-time view of the clinical practice, patient outcomes, safety, and comparative effectiveness.9 In this report, we present an approach to the use of EHR patient registries in the management of COVID-19 in a large multistate group practice. As the COVID-19 pandemic continues to have an impact on the health system and clinical practice, we share this experience to provide insight to other institutions that use similar interoperable EHR systems.
Methods
Mayo Clinic is a large academic health system with primary campuses located in Minnesota, Florida, and Arizona as well as multiple Mayo Clinic Health System hospitals and clinics throughout the Midwest in Minnesota and Wisconsin. The clinical practice has been supported by EHR vendor Epic Systems Corporations since 2018. Approximately 1.5 million patients receive care annually. The design and implementation work described in this report was performed at Mayo Clinic in Rochester, Minnesota.
Identification of Patients for COVID-19 Registries
Ascertainment of patients for each COVID-19 registry was accomplished using the results of the diagnostic tests for COVID-19; the International Statistical Classification of Diseases and Related Health Problems, Revision 10 (ICD-10), Clinical Modification for diagnosis; and the ICD-10 Procedure Coding System for medical billing codes for COVID-19 procedures. All the diagnostic tests and laboratory information for COVID-19 are entered in real time by the Mayo Clinic Laboratories in the EHR. External diagnostic test results for COVID-19 are reviewed by clinicians in the EHR before patients are diagnosed with COVID-19.
COVID-19 Diagnostic Tests
The laboratory referral center for Mayo Clinic is Mayo Clinic Laboratories, which specializes in esoteric laboratory testing for health care organizations throughout the United States and around the world. Diagnosis of COVID-19 illness is due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Molecular tests are used to detect genetic material of the SARS-CoV-2 virus by the polymerase chain reaction (PCR). An antigen test is a newer diagnostic COVID-19 test that can detect certain proteins that are part of the SARS-CoV-2 virus. In this report, the current Food & Drug Administration–approved diagnostic tests for COVID-19 used by Laboratory Medicine and Pathology, Mayo Clinic, include both the molecular and antigen techniques.
Five molecular methods are currently available at Mayo Clinic in Rochester, Minnesota, for detection of SARS-CoV-2 RNA in upper respiratory tract swab samples (eg, nasopharyngeal swab, nasal swab, throat swab) or lower respiratory tract specimens (eg, sputum, bronchoalveolar lavage fluid, and tracheal secretion). First, a laboratory test targets the nucleocapsid gene of SARS-CoV-2; samples are extracted on the bioMérieux easyMAG or eMAG instrument, followed by PCR amplification on the Roche LightCycler 480. Second, the Roche SARS-CoV-2 real-time PCR assay is performed on the automated cobas 6800 or 8800 instrument, which performs both extraction and amplification. Third, samples may be tested by the Abbott SARS-CoV-2 RealTime assays on the m2000 instrument. Fourth, the fully automated Hologic Panther system is a transcription-mediated amplification assay used to detect SARS-CoV-2 RNA in clinical samples. Finally, the Luminex ARIES system is a rapid (2-hour), sample-to-answer system that is offered to detect SARS-CoV-2 RNA in upper respiratory tract swab specimens. Each of these methods has received emergency use authorization from the Food & Drug Administration; each has a limit of detection in the range of 100 to 500 copies/mL and an analytical specificity approaching 100%. The diagnostic and management procedures for COVID-19 at Mayo Clinic have previously been described.4
COVID-19 Registry Design Considerations
The registry framework was developed through rapid cycles of iterative processes under the oversight of the Mayo Clinic COVID-19 Research Task Force.10 Five live COVID-19 registries were developed with EHR data collected from the COVID-19 diagnostic and serologic testing strategies already implemented by the health system.3,11,12 On the basis of predetermined rules criteria, patients are assigned to the respective COVID-19 registry (Table 1). Additional data collected in each COVID-19 registry include comorbidities, past visit information, immunizations, medications, procedures, general health information, demographics, vitals, social history, laboratory values, flowsheets, health encounter data, and other disease risk scores in the patient’s file. The 5 COVID-19 registries are as follows:
COVID-19 Confirmed Patient Registry to identify patients who tested positive for COVID-19
COVID-19 Negative Patient Registry to identify patients who tested negative for COVID-19
COVID-19 Recovered Patient Registry to identify patients who have recovered from COVID-19
COVID-19 Risk Factor ≥3 Points Registry to identify patients with preexisting health conditions or other risk factors, using a risk scoring system (Table 2) developed for internal use within the health system
COVID-19 Suspected Patient Registry to identify patients who do not yet fulfill all criteria for COVID-19 diagnosis but are thought to have COVID-19
Table 1.
Criteria Rules Used by Mayo Clinic COVID-19 Registries
| COVID-19 registry name | COVID-19 registry criteria rules |
|---|---|
| COVID-19 Confirmed Patient Registry | 1. Active COVID-19 infection or 2. Patient does not have a resolved COVID-19 infection and 3. Patient has had a positive COVID-19 SARS-CoV-2 laboratory test result in the last 60 daysNote: criterion 3 includes the original miscellaneous laboratory results sent out before the PCR test response is available. |
| COVID-19 Risk Factor ≥3 Points Registry | Includes any patient with risk score value ≥3. The points are assigned on the basis of preselected risk factors, such as age and sex. Mayo Clinic COVID-19 risk factor scoring system is developed for internal use and is outlined in Table 2 |
| COVID-19 Negative Patient Registry | 1. Patient has not had a positive or indeterminate SARS-CoV-2 laboratory result and 2. Patient has had at least 1 negative SARS-CoV-2 laboratory result in the past 365 days |
| COVID-19 Recovered Patient Registry | 1. 10 days since first positive result or 10 days since second first positive in the case of a reinfection past the initial 90-day period and 2. Resolved COVID-19 infection record and no active COVID-19 infection record |
| COVID-19 Suspected Patient Registry | 1. Patient is alive and 2. Patient does not have an active infection of COVID-19 (confirmed) and 3. Patient does not have a recent positive result for SARS-CoV-2 laboratory test in past 60 days and 4. Patient is not a member of the COVID-19 confirmed registry and 5. Patient has active infection of COVID-19 rule-out and [8. contact with a person with confirmed or suspected COVID-19 or 9. COVID-19 symptoms or 10. suspected COVID-19 diagnosis] or 6. Patient does not have a resolved infection of COVID-19 rule-out and has either [8. suspected COVID-19 diagnosis or 9. contact with a person who was confirmed or thought to have COVID-19 or 10. communicable disease symptoms] or [7. order for SARS-CoV-2 laboratory test] and [8. suspected COVID-19 diagnosis or 9. contact with a person who was confirmed or thought to have COVID-19 or 10. communicable disease symptoms] |
COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 2.
Mayo Clinic Risk Factor Points Scoring System Used for Management of Coronavirus Disease 2019
| Domains | Variables | Score |
|---|---|---|
| Age | 3 points for age >80 years | 3 |
| 2 points for age between 70 and 79 years | 2 | |
| 1 point for age between 60 and 69 years | 1 | |
| 0 points for age <60 years | 0 | |
| Sex | Male | 1 |
| Preexisting health condition | Congestive heart failure | 1 |
| Preexisting health condition | Congenital heart disease | 1 |
| Preexisting health condition | Chronic lung disease on problem list or Part of the asthma registry | 1 |
| Preexisting health condition | Diabetes mellitus on problem list | 1 |
| Preexisting health condition | Coronary artery disease | 1 |
| Preexisting health condition | Hypertension | 1 |
| Immunity status | Considered immune compromised | 1 |
| Currently receiving chemotherapy or Receiving immunosuppressive medications or Has human immunodeficiency virus infection | ||
| Social determinants of health status | Nursing home resident | 1 |
| Chronic disease management status | Chronic dialysis registry | 1 |
| Chronic disease management status | End-stage liver disease on problem list | 1 |
| Medical vulnerability status | Pregnant | 1 |
COVID-19 Dashboards
The dashboard provided a view of the entire clinical practice by listing current and historical statistics of key practice performance indicators for the health system. The information is summarized in a tabular or graphical view for conducting performance analysis of the entire clinical practice. Dashboards allow quick detection of negative trend development and help the health system to take immediate action. The COVID-19 registries were used to develop tailored COVID-19 dashboards.
COVID-19 Reports
COVID-19 reports are real-time operational reports of current data pertaining to COVID-19 patients. The COVID-19 registries data metrics are used to develop tailored reports for various care teams in the health system. All registry reports were based on the following 4 mutually exclusive groups of patients: COVID-19 confirmed (active cases), COVID-19 recovered (former cases), COVID-19 negative (tested but not positive), and COVID-19 suspected (met some criteria to suggest testing, but diagnosis was not yet finalized).
Results
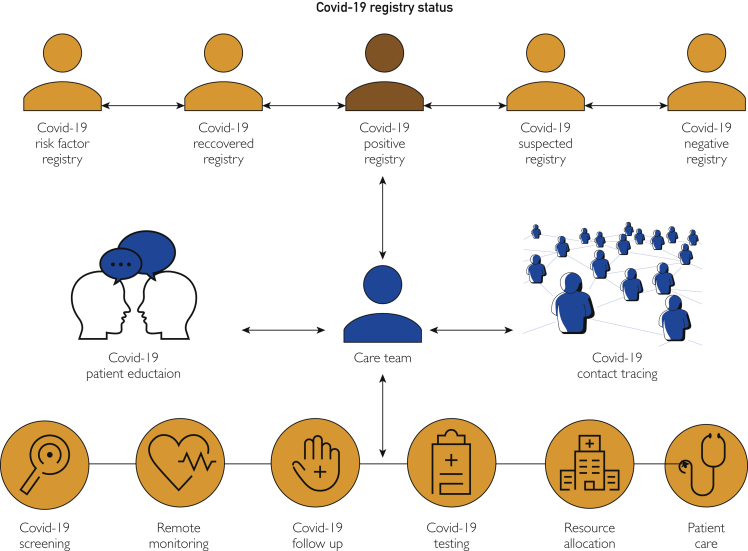
The COVID-19 patient registries were implemented for clinical practice use starting May 5, 2020. As of December 7, 2020, a total of 765,324 COVID-19 tests were completed across the health system, with test results and other information that flowed in real time to the COVID-19 registries. The utilization of the new COVID-19 registries by our health system is depicted in Figure 1.
Figure 1.
Utilization of coronavirus disease 2019 (COVID-19) registries at Mayo Clinic.
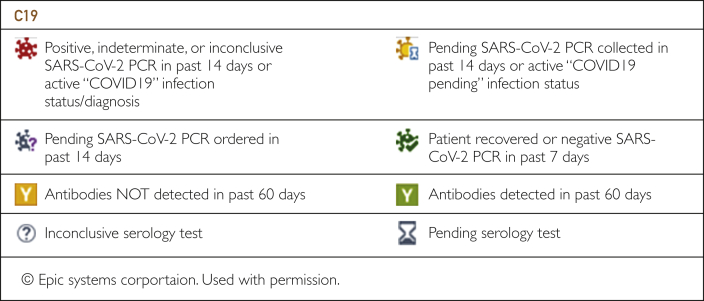
Customized dashboards were implemented using data from COVID-19 registries. All dashboards used to manage patient care were created in Epic so that the information was available in the workflow. Each dashboard was tailored according to the needs of the end users and enabled users to slice data for additional insights, including current trends on testing capacity, bed capacity, ventilator use, and hospitalization statistics. The dashboards enabled clinicians and scientists to visualize data in a self-service capacity for purposes directly relevant to their practices. For example, the surgical services use the COVID-19 serology dashboard (Figure 2) to coordinate operations during the pandemic. To more easily identify patients who had positive or pending COVID-19 test results, the infection column on the status board is updated with specific COVID-19 and COVID-19 rule-out icons. If a patient's infection status includes COVID-19, a red biohazard icon is displayed. If a patient's infection status includes COVID-19 rule-out (COVID-19 test results are pending), a black biohazard icon is displayed.
Figure 2.
Coronavirus disease 2019 (COVID-19) serology dashboard for surgical services. PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
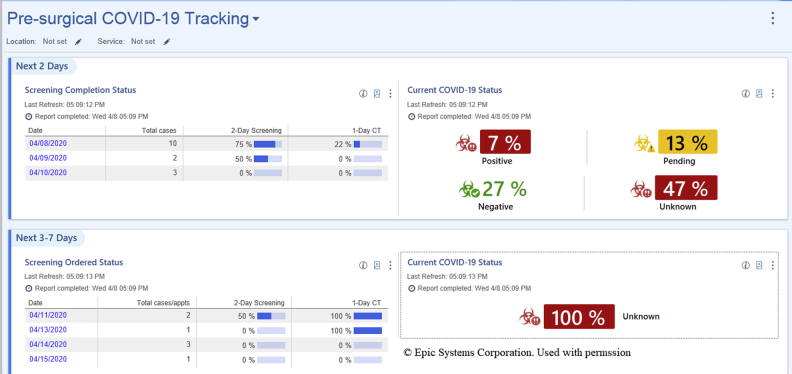
Another related example is the presurgical COVID-19 tracking dashboard (Figure 3) that allows users to run reports tracking screening completion status or displaying the ordered status of procedure-related tasks to determine the status of task completion rates for upcoming scheduled operations. The dashboard can also be configured using the parameters at the top to filter down to each location and service of interest.
Figure 3.
Presurgical coronavirus disease 2019 (COVID-19) tracking dashboard.
Inpatient services use a newly configured COVID-19 inpatient list that has SARS-CoV-2 infection status and risk score added as columns. The first column displays the patient’s COVID-19 risk factors score. The other 3 patient list columns are related to SARS-CoV-2 results during the last 14 days (pending, negative result, and positive result, respectively). The columns are found by searching the term “SARS” when adding a column to a list. The COVID-19 risk factors score identifies the number of risk factors a patient has for severe illness. The report is able to be personalized and can be viewed on personal schedules and patient lists.
There are 2 Epic dashboards available for leadership. Enterprise COVID-19 Leadership Dashboard, implemented using Tableau software, tracks real-time admissions, positive patients, and a number of laboratory tests and results for COVID-19. The dashboard captures data as of 23:59 the previous day and is set to refresh each morning at 07:00. The second leadership dashboard is the COVID-19 Population Explorer, which displays a distribution of COVID-19 confirmed cases by age, legal sex, and risk factors.
Several operational reports were also created using the COVID-19 registries data. These reports provide information that is used at an operational level to identify and to manage needs of patients. For example, a resource is made available for COVID-19 patients who tested positive or had pending test results and were being discharged from the hospital without a means of transportation. The registry data were used to arrange transportation proactively rather than reactively. Another example is the remote monitoring care team COVID-19 report used to notify, to monitor, and to advise patients with confirmed or probable COVID-19. Reports generated from these registries were used to manage patient care across various clinical care settings for multidisciplinary purposes including scheduling of appointments. For the inpatient practice, a COVID-19 report generated using the registry facilitates nursing leadership assessment of staffing and personal protective equipment required for hospitalized COVID-19 patients. A related subsequent report assists the care team responsible for monitoring the postdischarge status and home care.
A comprehensive report library in the EHR system serves as the repository for all the reports designed to provide information on COVID-19 patients. Providers use the report library to find COVID-19 status of their respective patient panel or inpatient list. The remote communication and bulk mailing tools within registries assist with prompt notification, monitoring, and linkage to the required medical and support services for patients. Targeted content is tailored for the various administrative and health care delivery teams. The registries are also the primary source used to identify patients eligible for relevant clinical trials, managed centrally through an institutional research COVID-19 task force. For all applications, users are instructed to use only the reports that are applicable to their role and business need and to observe Mayo Clinic policies for privacy and confidentiality.
Discussion
Registries have demonstrated utility in the management of many health conditions.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 We rapidly developed, implemented, and used EHR patient registries to manage the daily operations of one of the largest multistate group practices in the United States during the ongoing COVID-19 pandemic. The primary function of the COVID-19 registries was to equip our health system to process real-time data and to classify patients into the appropriate diagnostic category. The registries also complemented other innovative contributions from our health system.2,3,10, 11, 12,25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 The COVID-19 registries are used extensively to manage the daily operations of the clinical practice. For example, surgical services use patient registry data and dashboards (Figure 1) to develop a new clinical workflow process implemented at the preoperative evaluation clinic to manage the testing, symptom screening, patient education, and preoperative optimization of surgical patients.39 In addition, artificial intelligence is applied to the registry data to enhance screening and diagnosis for high-risk patients using various risk scores. Registry data are also used to coordinate with state and federal health agencies to optimize supply chain management decisions and allocation of staff and resources across sites.37,40
COVID-19 dashboards have been used at the population health level.41,42 Many medical specialties have already invested into developing COVID-19 patient registries.43, 44, 45, 46 Although they are useful for research, patient registries outside the EHR system have limited capacity to receive real-time data of patients. A large regional public academic health center used rapid EHR configurations to manage COVID-19 that incorporated some of the features of our effort.47 That center developed a COVID-19 operational dashboard, tracked COVID-related infection in the EHR embedded database, and tracked persons under investigation. Another health system used the COVID-19 registry in the EHR to develop predictive models.48,49 A global COVID-19 patient registry was used to conduct case-fatality research.50 Similarly, another health system created an EHR dashboard to observe patients and developed a patient registry in the Epic for patients with potential or confirmed COVID-19 diagnosis. Whereas the authors used an EHR-based COVID-19 registry, the primary focus was to support telehealth during the pandemic.51 To that end, we built dashboards and analytical reports for various stakeholders in the health system according to their current as well as anticipated (eg, resource allocation) clinical practice needs. Broad access to the EHR self-service analytics tools also enables users to create ad hoc reports at their discretion. The remote monitoring type reports (using the registry data) allow clinicians and staff the ability to monitor their specific cohorts of interest. Examples of monitoring include the ability to track status of infection, testing dates, follow-up dates, and the like.
Compared with other EHR optimization efforts to manage COVID-19,41,51,52 our digital health surveillance strategies not only supported workflow optimization for tailored health services but also provided a basis for COVID-19 research to understand long-term effects that COVID-19 may have in patients. For example, our registry data can be used to generate cohorts to compare patients who ever tested positive vs patients who never tested positive vs patients who tested positive multiple times. Another research example involves the identification of patients eligible to consent to analysis of both leftover clinical samples and prospectively collected biobank samples. Mayo Clinic led the national Expanded Access Treatment Protocol for COVID-19 convalescent plasma,53 sponsored by the Food & Drug Administration, and the registries served as a key component of this effort for Mayo Clinic patients. In addition to these research applications, the registries also support the necessary reporting to the state health department and contact tracing to prevent community transmission.
An important limitation of any EHR registry is the diagnostic accuracy of testing.54, 55, 56 Thus, our registries also incorporated other rule criteria, such as clinical diagnosis from the problem list and ICD-10 codes used for the diagnosis and treatment of COVID-19. The latter was implemented off-cycle to allow immediate tracking of health care claims and statistics for COVID-19 cases diagnosed on or after April 1, 2020. The code could be used only for cases diagnosed on or after April 1, 2020. In general, COVID-19 cases diagnosed before April 1, 2020, were reported using ICD-10 codes that describe the patient’s signs, symptoms, or associated illnesses. ICD-10 codes and associated guidelines may continue to evolve as more is learned about the clinical manifestations, transmission, and long-term effects of the virus. Of note, the COVID-19 registry required frequent maintenance, as might be expected during a pandemic. For example, when the Centers for Disease Control and Prevention changed guidelines, registry rules were changed accordingly. Our methodology employed is generalizable to the extent that other health systems also using Epic can adopt our registry framework. However, the registry methodology and specific application have not been validated in other populations of patients.
Conclusion
We developed and implemented a systematic approach to the use of EHR patient registries to manage the COVID-19 pandemic that proved feasible and useful in a large multistate group clinical practice in the United States. The key to harnessing the potential of digital surveillance tools to promote patient-centered care during the COVID-19 pandemic was to use the registry data, reports, and dashboards as informatics tools to inform decision-making.
Footnotes
Grant Support: This work was supported by internal funding from Mayo Clinic.
Potential Competing Interests: The authors report no competing interests.
References
- 1.Shah A., Kashyap R., Tosh P., Sampathkumar P., O’Horo J.C. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. 2020;95(4):646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tosh P.K., Bucks C.M., O’Horo J.C., DeMartino E.S., Johnson J.M., Callies B.I. Elements of an effective incident command center. Mayo Clin Proc. 2020;95(9S):S3–S7. doi: 10.1016/j.mayocp.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A., Challener D., Tande A.J., et al. Drive-through testing: a unique, efficient method of collecting large volume of specimens during the SARS-CoV-2 (COVID-19) pandemic. Mayo Clin Proc. 2020;95(7):1420–1425. doi: 10.1016/j.mayocp.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razonable R.R., Pennington K.M., Meehan A.M., et al. A collaborative multidisciplinary approach to the management of coronavirus disease-19 in the hospital setting. Mayo Clin Proc. 2020;95(7):1467–1481. doi: 10.1016/j.mayocp.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohta N.S., Sampathkumar P. Learnings from Mayo Clinic’s methods for scaling a coordinated and comprehensive plan for Covid-19. NEJM Catal Innov Care Deliv. 2020 March 19. [Google Scholar]
- 6.Alwashmi M.F. The use of digital health in the detection and management of COVID-19. Int J Environ Res Public Health. 2020;17(8):2906. doi: 10.3390/ijerph17082906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golinelli D., Boetto E., Carullo G., Nuzzolese A.G., Landini M.P., Fantini M.P. Adoption of digital technologies in health care during the COVID-19 pandemic: systematic review of early scientific literature. J Med Internet Res. 2020;22(11):e22280. doi: 10.2196/22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judson T.J., Odisho A.Y., Neinstein A.B., et al. Rapid design and implementation of an integrated patient self-triage and self-scheduling tool for COVID-19. J Am Med Inform Assoc. 2020;27(6):860–866. doi: 10.1093/jamia/ocaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gliklich R.E., Dreyer N.A., Leavy M.B. 3rd ed. Agency for Healthcare Research and Quality; Rockville, MD: 2014. Registries for Evaluating Patient Outcomes: A User’s Guide. Report No. 13(14)-EHC111. [PubMed] [Google Scholar]
- 10.Burger C.D., Mikhail A.E., Orenstein R., Ebbert J.O., Vergidis P., Badley A.D. Research response to SARS-CoV-2/COVID-19. Mayo Clin Proc. 2020;95(9S):S52–S55. doi: 10.1016/j.mayocp.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A.S., Tande A.J., Challener D.W., O’Horo J.C., Binnicker M.J., Berbari E.F. Diagnostic stewardship: an essential element in a rapidly evolving COVID-19 pandemic. Mayo Clin Proc. 2020;95(9S):S17–S19. doi: 10.1016/j.mayocp.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tande A.J., Challener D.W., Shah A.S., O’Horo J.C., Beam E. Community-based drive-through and walk-through testing centers. Mayo Clin Proc. 2020;95(9S):S20–S22. doi: 10.1016/j.mayocp.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmittdiel J., Bodenheimer T., Solomon N.A., Gillies R.R., Shortell S.M. Brief report: the prevalence and use of chronic disease registries in physician organizations. J Gen Intern Med. 2005;20(9):855–858. doi: 10.1111/j.1525-1497.2005.0171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hser Y.I., Mooney L.J., Saxon A.J., Miotto K., Bell D.S., Huang D. Chronic pain among patients with opioid use disorder: results from electronic health records data. J Subst Abuse Treat. 2017;77:26–30. doi: 10.1016/j.jsat.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant R.W., Cagliero E., Sullivan C.M., et al. A controlled trial of population management: diabetes mellitus: putting evidence into practice (DM-PEP) Diabetes Care. 2004;27(10):2299–2305. doi: 10.2337/diacare.27.10.2299. [DOI] [PubMed] [Google Scholar]
- 16.Navaneethan S.D., Jolly S.E., Schold J.D., et al. Development and validation of an electronic health record–based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6(1):40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendu M.L., Ahmed S., Maron J.K., et al. Development of an electronic health record–based chronic kidney disease registry to promote population health management. BMC Nephrol. 2019;20(1):72. doi: 10.1186/s12882-019-1260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Classen D., Li M., Miller S., Ladner D. An electronic health record–based real-time analytics program for patient safety surveillance and improvement. Health Aff (Millwood) 2018;37(11):1805–1812. doi: 10.1377/hlthaff.2018.0728. [DOI] [PubMed] [Google Scholar]
- 19.Hersh W. Electronic health records facilitate development of disease registries and more. Clin J Am Soc Nephrol. 2011;6(1):5–6. doi: 10.2215/CJN.09901110. [DOI] [PubMed] [Google Scholar]
- 20.Cronin C.E., Franz B., Schuller K.A. Expanding the population health workforce: strategic priorities of hospital organizations in the United States. Popul Health Manag. 2020 Mar 5 doi: 10.1089/pop.2019.0138. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Foster M., Albanese C., Chen Q., et al. Heart failure dashboard design and validation to improve care of veterans. Appl Clin Inform. 2020;11(1):153–159. doi: 10.1055/s-0040-1701257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe M.G., Lee G.A., Young J.D., Sidney S., Go A.S. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner E.H., Austin B.T., Davis C., Hindmarsh M., Schaefer J., Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 24.Hswen Y., Brownstein J.S. Real-time digital surveillance of vaping-induced pulmonary disease. N Engl J Med. 2019;381(18):1778–1780. doi: 10.1056/NEJMc1912818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wieland M.L., Doubeni C.A., Sia I.G. Community engagement with vulnerable populations. Mayo Clin Proc. 2020;95(9S):S60–S62. doi: 10.1016/j.mayocp.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storino C.B., Watson J.C., Sanchez W., Brown M.J., Tande A.J., Loftus C.G. Revamping outpatient care for patients without COVID-19. Mayo Clin Proc. 2020;95(9S):S44–S46. doi: 10.1016/j.mayocp.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sher T., Burger C.D., DeMartino E.S., de Moraes A.G., Sharp R.R. Resuscitation and COVID-19: recalibrating patient and family expectations during a pandemic. Mayo Clin Proc. 2020;95(9):1848–1851. doi: 10.1016/j.mayocp.2020.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampathkumar P., Beam E., Breeher L.E., O’Horo J.C. Precautions, utilization of personal protective equipment, and conservation strategies during the COVID-19 pandemic. Mayo Clin Proc. 2020;95(9S):S11–S13. doi: 10.1016/j.mayocp.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razonable R.R., Carmona E.M., Vergidis P., Wilson J.W., Marshall W.F., 3rd Clinical guidance and the delivery of care for patients with coronavirus disease 2019. Mayo Clin Proc. 2020;95(9S):S23–S25. doi: 10.1016/j.mayocp.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razonable R.R., Marshall W.F., 3rd, Stevens R.W., Kumar S., Murad M.H., Wilson W.R. Rapid appraisal system for COVID-19 medical information. Mayo Clin Proc. 2020;95(9S):S26–S28. doi: 10.1016/j.mayocp.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucchinetti C.F., von Bormann A.G., Nagel J.J., et al. Engaging and empowering the front lines during the COVID-19 outpatient practice reactivation. Mayo Clin Proc. 2020;95(9S):S47–S51. doi: 10.1016/j.mayocp.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J.F., Greenway M.R., Nasr D.M., et al. Telestroke in the time of COVID-19: the Mayo Clinic experience. Mayo Clin Proc. 2020;95(8):1704–1708. doi: 10.1016/j.mayocp.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrugia G., Plutowski R.W. Innovation lessons from the COVID-19 pandemic. Mayo Clin Proc. 2020;95(8):1574–1577. doi: 10.1016/j.mayocp.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Didehban R., Caine N.A., Glenn S.W., Hasse C.H. Role of the administrative partner and the physician-administrator partnership. Mayo Clin Proc. 2020;95(9S):S38–S40. doi: 10.1016/j.mayocp.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniels C.E., Caine N.A., Brown M.J., Berbari E.F., Williams A.W. The silver lining for health care during and after the pandemic. Mayo Clin Proc. 2020;95(9S):S69–S71. doi: 10.1016/j.mayocp.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Challener D.W., Shah A., O’Horo J.C., Berbari E., Binnicker M.J., Tande A.J. Low utility of repeat real-time PCR testing for SARS-CoV-2 in clinical specimens. Mayo Clin Proc. 2020;95(9):1942–1945. doi: 10.1016/j.mayocp.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloch E.M., Shoham S., Casadevall A., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clinic Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berbari E.F., Williams A.W., Williamson M.J., Caine N.A., Nath K.A., Farrugia G. Introduction. Mayo Clin Proc. 2020;95(9S):S1–S2. doi: 10.1016/j.mayocp.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pai S.L., Irizarry-Alvarado J.M., Pitruzzello N.E., Bosch W., Aniskevich S., III Responding to the COVID-19 pandemic: a new surgical patient flow utilizing the preoperative evaluation clinic. Am J Med Qual. 2020;35(6):444–449. doi: 10.1177/1062860620946741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joyner M.J., Bruno K.A., Klassen S.A., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wissel B.D., Van Camp P., Kouril M., et al. An interactive online dashboard for tracking COVID-19 in US counties, cities, and states in real time. J Am Med Inform Assoc. 2020;27(7):1121–1125. doi: 10.1093/jamia/ocaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman E.E., McMahon D.E., Fitzgerald M.E., et al. The American Academy of Dermatology COVID-19 registry: crowdsourcing dermatology in the age of COVID-19. J Am Acad Dermatol. 2020;83(2):509–510. doi: 10.1016/j.jaad.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson P.C., Yazdany J. The COVID-19 Global Rheumatology Alliance: collecting data in a pandemic. Nat Rev Rheumatol. 2020;16(6):293–294. doi: 10.1038/s41584-020-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ntaios G., Michel P., Georgiopoulos G., et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 2020;51(9):e254–e258. doi: 10.1161/STROKEAHA.120.031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naik H., Alhusayen R., Frew J., et al. Global Hidradenitis Suppurativa COVID-19 Registry: a registry to inform data-driven management practices. Br J Dermatol. 2020;183(4):780–781. doi: 10.1111/bjd.19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeves J.J., Hollandsworth H.M., Torriani F.J., et al. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J Am Med Inform Assoc. 2020;27(6):853–859. doi: 10.1093/jamia/ocaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jehi L., Ji X., Milinovich A., et al. Individualizing risk prediction for positive coronavirus disease 2019 testing. Chest. 2020;158(4):1364–1375. doi: 10.1016/j.chest.2020.05.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jehi L., Ji X., Milinovich A., et al. Development and validation of a model for individualized prediction of hospitalization risk in 4,536 patients with COVID-19. PLoS One. 2020;15(8):e0237419. doi: 10.1371/journal.pone.0237419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohamad Alkhouli A., Annie F., Bates M.C., Bhatt D.L. Sex differences in COVID-19 case fatality rate: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613–1620. doi: 10.1016/j.mayocp.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford D., Harvey J.B., McElligott J., et al. Leveraging health system telehealth and informatics infrastructure to create a continuum of services for COVID-19 screening, testing, and treatment. J Am Med Inform Assoc. 2020;27(12):1871–1877. doi: 10.1093/jamia/ocaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jehi L., Ji X., Milinovich A., et al. Individualizing risk prediction for positive COVID-19 testing: results from 11,672 patients. Chest. 2020;158(4):1364–1375. doi: 10.1016/j.chest.2020.05.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyner M.J., Wright R.S., Fairweather D., et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 2020;58(6):e00512–e00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kontou P.I., Braliou G.G., Dimou N.L., Nikolopoulos G., Bagos P.G. Antibody tests in detecting SARS-CoV-2 infection: a meta-analysis. Diagnostics (Basel) 2020;10(5):319. doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West C.P., Montori V.M., Sampathkumar P. COVID-19 testing: the threat of false-negative results. Mayo Clin Proc. 2020;95(6):1127–1129. doi: 10.1016/j.mayocp.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]