Abstract
Background
Surgery is the cornerstone of gynecological cancer management, but inpatient treatment may expose both patients and healthcare staff to COVID-19 infections. Plans to mitigate the impact of the COVID-19 pandemic have been implemented widely, but few studies have evaluated the effectiveness of these plans in maintaining safe surgical care delivery.
Aim
To evaluate the effects of mitigating plans implemented on the delivery of gynecological cancer surgery during the COVID-19 pandemic.
Methods
A comparative cohort study of patients treated in a high-volume tertiary gyneoncological centre in the United Kingdom. Prospectively-recorded consecutive operations performed and early peri-operative outcomes during the same calendar periods (January–August) in 2019 and 2020 were compared.
Results
In total, 585 operations were performed (296 in 2019; 289 in 2020). There was no significant difference in patient demographics. Types of surgery performed were different (p = 0.034), with fewer cytoreductive surgeries for ovarian cancer and laparoscopic procedures (p = 0.002) in 2020. There was no difference in intra-operative complication rates, critical care admission rates or length of stay. One patient had confirmed COVID-19 infection (0.4%). The 30-day post-operative complication rates were significantly higher in 2020 than in 2019 (58 [20.1%] versus 32 [10.8%]; p = 0.002) for both minor and major complications. This increase, primarily from March 2020 onwards, coincided with the first peak of the COVID-19 pandemic in the UK.
Conclusions
Maintaining surgical throughput with meticulous and timely planning is feasible during the COVID-19 pandemic but this was associated with an increase in post-operative complications due to a multitude of reasons.
Keywords: COVID-19, Pandemic, cancer surgery, surgical morbidityvirus
1. Introduction
The pandemic of COVID-19 has put a significant strain on the healthcare system worldwide. Strategies to mitigate its impact have been proposed to prioritise finite resources in different healthcare settings. Consequently, elective surgery has been put on hold in many hospitals, even for patients with cancer [1]. Recently published evidence has shown that patients who received elective or emergency surgery and developed COVID-19 were vulnerable to pulmonary complications and more likely to die from their operations. This increased risk was particularly evident in the elderly (>70 year-old) who had undergone surgery for malignant disease [2]. As primary surgery is among an important treatment strategy for women with gynecological cancer, which are more prevalent in the elderly, delaying or relying solely on non-surgical treatment may potentially impact on their long-term outcomes.
On the other hand, the safety of staff and other patients are also paramount when planning surgical treatments. Aerosol transmission from general anesthetic airway management and, theoretically, in laparoscopic procedures have led to changes in intraoperative pathways [3,4]. Emerging evidence also suggests the importance of minimising the risks of hospital-acquired COVID-19 infection during the perioperative period. A recent UK-based study reported one in eight (12.5%) COVID-19 infections were acquired in hospital [5]. Moreover, a COVID-19-free surgical care pathway was associated with 47% fewer early (≤30 days) post-operative COVID-19 infections (HR 0.53; 95% CI 0.36–0.76) [6].
To balance the conundrum between the risks to operate during this pandemic and the negative impact of delaying treatment on oncological outcome, we undertook several radical changes in our cancer centre early during the pandemic with the aim to deliver safe and high-quality surgery to our patients. In this study, we aim to evaluate the effects of the implemented changes during the pandemic on service delivery and care outcomes. As few studies have evaluated the effectiveness of the implemented mitigation plans to maintain safe surgical care delivery; the aim of this study is to evaluate the impact of the COVID-19 pandemic and the influence of our mitigation plans on the care delivery and short-term perioperative outcomes for patients with gynecological cancers.
2. Methods
2.1. Patient selection
Consecutive surgical cases from two separate periods, 1 January 2019 to 12 August 2019 (referred as “2019” thereafter) and 1 January 2020 to 12 August 2020 (referred as “2020” thereafter), were captured prospectively from the Pan-Birmingham Gynecological Cancer Centre database. Our centre supports a population of 2.2 millions in the West Midlands region in the UK.
2.2. Data collection
Patients' demographics (including American Society of Anesthesiologists Physical Status Classification system [ASA] grades and World Health Organization [WHO] performance status), comorbidities, surgical descriptions, FIGO stage, length of post-operative hospital stay and critical care admission, intra-operative complications, COVID-19 infection status (as measured by polymerase chase reactive [PCR] RNA tests from nasal and pharyngeal swabs, ROCHE), 30-day post-operative complications and mortality were collated from digital electronic health records retrospectively. Anonymous information relating to surgical team members COVID-19 infectivity status (PCR or serology tests) was also collected. The study was approved by our hospital's clinical governance department.
2.3. Mitigation plans
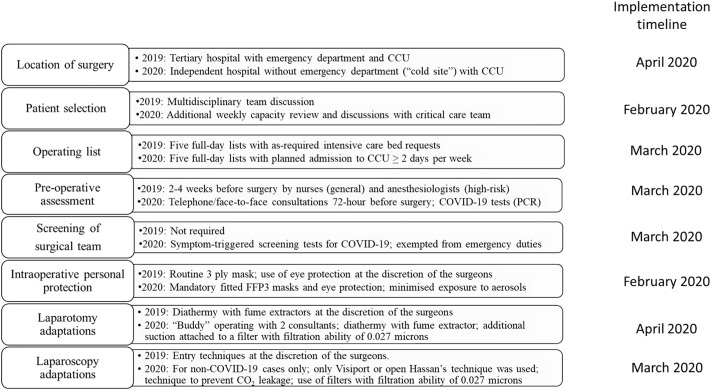
The formal mitigation plan with all relevant steps to minimise COVID-19 infection during open and laparoscopic surgery, along with the surgical modifications introduced, is summarised in Fig. 1 . These steps were formulated at a multidisciplinary consensus meeting with all relevant stakeholders with our organization, including surgeons, anesthetic and critical care teams, clinical nurse specialists, senior nurses and the senior management team. The mitigation plan was introduced in stages according to the severity of COVID-19 pandemic, where the incident peaked in the UK in March 2020 [7], through the National Health Service (NHS) England resilience initiative to overcome the COVID-19 pandemic. The mitigation plan was fully implemented from 7th April 2020.
Fig. 1.
Mitigation plans undertaken to deliver gynecological cancer surgery during the COVID-19 pandemic.
Between February 2020 to April 2020, we introduced the use of additional personal protection for our surgical team, and surgical staff was not involved in managing patients with COVID-19 patients. Segregation of wards and operating theatre into COVID-19-free areas began in early March and screening for patients with COVID-19 questionnaires took effect from mid-of March 2020. From 7th April 2020, our cancer service was relocated to a “private sector hospital” (a privately funded hospital that was contracted to undertake NHS work during the COVID-19 pandemic) with capacity to offer post-operative high-dependency care. Importantly, the hospital did not have an emergency department and did not admit or treat patients with suspected or proven COVID-19. The hospital provided supporting and nursing staff, and infrastructures for our surgical and anesthetic team to continue to deliver major surgery for our patients.
Patients were required to self-isolate 14 days prior to surgery and undertake a robust pre-operative healthcare questionnaire specific for COVID-19 symptoms. From 22nd April 2020, mandatory COVID-19 screening test using PCR was introduced to all pre-operative patients 3 days prior to their surgery. Surgical team members were fitted with FFP3 masks and eye shields (visors), and surgery was modified to minimise the potential of aerosol transmission during laparotomy or laparoscopic surgery (Fig. 1).
2.4. Statistical analysis
Data were analysed using STATA® Version 16 (StataCorp, USA) and Prism (v8.0, GraphPad, USA). Categorical data were compared using chi-squared tests and non-parametric comparisons of continuous variables were performed using Mann-Whitney U test. A p-value of <0.05 was considered significant.
3. Results
3.1. Patient demographics
During the study period, 585 operations were performed (296 in 2019 and 289 in 2020). The majority of patient were over 60 years old, overweight or obese, ASA grade 2 and WHO performance status of 0. Approximately a fifth and a third of patients had respiratory and cardiovascular co-morbidities, respectively (Table 1 ). There was no statistically significant difference in patient demographics (Table 1). In patients with ovarian cancer, the overall neoadjuvant chemotherapy (NACT) rates were not significantly different between the two cohorts.
Table 1.
Demographics of patients who underwent operations (n = 585).
| 2019 (n = 296) |
2020 (n = 289) |
p-Value | ||
|---|---|---|---|---|
| Age⁎ (Years) |
61.5 (16.3) | 61.2 (14.4) | 0.593 | |
| BMI⁎ (kg/m2) |
29.6 (7.7) | 29.8 (6.6) | 0.328 | |
| ASA grade | 1 | 46 (15.4%) | 32 (11.1%) | 0.176 |
| 2 | 155 (52.4%) | 150 (51.9%) | ||
| 3 | 87 (29.4%) | 103 (35.6%) | ||
| 4 | 1 (0.3%) | 3 (1.0%) | ||
| WHO performance status | 0 | 217 (73.3%) | 218 (75.4%) | 0.973 |
| 1 | 39 (13.2%) | 41 (14.2%) | ||
| 2 | 19 (6.4%) | 18 (6.2%) | ||
| 3 | 8 (2.7%) | 11 (3.8%) | ||
| 4 | 1 (0.3%) | 1 (0.4%) | ||
| Co-morbidities | Respiratory | 59 (19.9%) | 43 (14.9%) | 0.107 |
| Cardiovascular | 109 (36.8%) | 97 (33.6%) | 0.409 | |
| Diabetes | 46 (15.5%) | 46 (15.9%) | 0.900 | |
| Others | 91 (30.7%) | 78 (27.0%) | 0.317 |
Mean (SD); BMI = body mass index; ASA grade = American Society of Anesthesiologists Physical Status Classification system; WHO = World Health Organization; NACT = neoadjuvant chemotherapy. In the 2019 cohort, ASA grade and WHO performance status were not recorded in 7 (2.2%) and 14 (4.4%) patients, respectively.
3.2. COVID-19 status
Seventy-two hours before surgery, all patients underwent pre-operative screening for symptoms of COVID-19 using a standardised questionnaire. As none reported any COVID-19-associated symptoms, all were admitted for surgery. In addition, pre-operative testing for COVID-19 infection was introduced on 22 April 2020, with nasopharyngeal swabs sent for a polymerase chain reaction (PCR)-based COVID-19 tests 72 h before surgery. Only 11 (6.7%) patients were tested using the PCR-based screening test before 22nd April 2020, prior to mandatory pre-operative COVID-19 screening started. These patients were screened because they were at risk of being exposed to COVID-19 and none were tested positive. After PCR-based screening test was made mandatory, one patient was tested positive for COVID-19 but she was asymptomatic. Her repeat test after 14 days was negative and she did not develop any symptoms related to COVID-19, hence, she was admitted for surgery.
3.3. Surgical procedure performed
Details of the surgical procedures performed are summarised in Table 2 and Supplementary Fig. 1. There was no significant difference between the numbers of major operations performed between the cohorts (243 [82.1%] versus 241 [83.4%], p = 0.678), nor the type of gynecological cancers and stages of disease treated. Differences in the type of operations performed between the two cohorts were identified. Fewer primary cytoreductive surgery (12 [4.2%] versus 21 [7.1%]), secondary cytoreductive surgery (2 [0.7%] versus 6 [2.0%]) and laparoscopic hysterectomies (36 [12.5%] versus 66 [22.3%]) were performed in 2020, compared to the same period in 2019 (Table 2). During the same period, more radical hysterectomies (20 [6.9%] versus 13 [4.4%]) and open hysterectomies (38 [13.2%] versus 27 [9.1%]) were performed. Consistent with the observations in the differences of operation types, fewer laparoscopic procedures were performed in 2020, compared to 2019 (57 [19.7%] versus 91 [30.7%]). The total numbers of radical vulvectomies and related groin lymphadenectomies in 2019 and 2020, were 41 (13.9%) and 51 (17.6%), respectively.
Table 2.
Summary of operations performed and perioperative outcomes (n = 585).
| 2019 (n = 296) |
2020 (n = 289) |
p-Value | ||
|---|---|---|---|---|
| Disease site | Uterus | 99 (33.5%) | 84 (29.1%) | 0.341 |
| Ovary | 99 (33.5%) | 110 (38.1%) | ||
| Cervix | 39 (13.2%) | 26 (9.0%) | ||
| Vulva | 52 (17.6%) | 58 (20.1%) | ||
| Vagina | 5 (1.7%) | 7 (2.4%) | ||
| Other | 2 (0.7%) | 4 (1.4%) | ||
| FIGO stage | 1 | 113 (38.2%) | 100 (34.6%) | 0.890 |
| 2 | 30 (10.1%) | 27 (9.3%) | ||
| 3 | 67 (22.6%) | 68 (23.5%) | ||
| 4 | 31 (10.5%) | 36 (12.5%) | ||
| Recurrence | 6 (2.0%) | 9 (3.1%) | ||
| Not cancer | 36 (12.2%) | 34 (11.8%) | ||
| Not available | 13 (4.4%) | 14 (4.8%) | ||
| Major/minor | Major | 243 (82.1%) | 241 (83.4%) | 0.678 |
| Minor | 53 (17.9%) | 48 (16.6%) | ||
| NACT (ovarian cancer only)⁎ | No | 63 (63.6%) | 68 (61.8%) | 0.786 |
| ≤ 3 cycles | 16 (16.2%) | 15 (13.6%) | ||
| > 3 cycles | 20 (20.2%) | 27 (24.6%) | ||
| Type of operation | Exenterative surgery | 1 (0.3%) | 3 (1.0%) | 0.034 |
| Primary cytoreductive surgery | 21 (7.1%) | 12 (4.2%) | ||
| Delayed cytoreductive surgery | 26 (8.8%) | 31 (10.7%) | ||
| Secondary cytoreductive surgery | 6 (2.0%) | 2 (0.7%) | ||
| Radical hysterectomy (open/laparoscopic) | 13 (4.4%) | 20 (6.9%) | ||
| Staging surgery (open/laparoscopic) | 37 (12.5%) | 39 (13.5%) | ||
| TAH BSO+/− pelvic lymphadenectomy | 27 (9.1%) | 38 (13.2%) | ||
| TLH BSO +/− pelvic lymphadenectomy | 66 (22.3%) | 36 (12.5%) | ||
| Vulvectomy/vulval excision/vaginectomy | 28 (9.5%) | 28 (9.7%) | ||
| Groin lymphadenectomy⁎⁎ | 6 (2.0%) | 9 (3.1%) | ||
| Vulvectomy + groin lymphadenectomy⁎⁎ | 7 (2.4%) | 14 (4.8%) | ||
| Other | 58 (19.6%) | 57 (19.7%) | ||
| Laparoscopic | Yes | 91 (30.7%) | 57 (19.7%) | 0.002 |
| No | 205 (69.3%) | 232 (80.3%) | ||
| First surgeon | Attending surgeons | 172 (58.1%) | 243 (84.1%) | <0.001 |
| Not attending surgeons ⁎⁎⁎ | 124 (41.9%) | 46 (15.9%) | ||
| Intraoperative complications | 24 (8.1%) | 26 (9.0%) | 0.701 | |
| CCU admission | 56 (18.9%) | 64 (22.2%) | 0.712 | |
| Unplanned | 14 (4.7%) | 14 (4.8%) | ||
| Length of stay (days)⁎⁎⁎⁎ | 3 (1–4) | 3 (1–5) | 0.528 | |
| Post-operative complications | 32 (10.8%) | 58 (20.1%) | 0.002 | |
|
|
|
||
|
|
|
||
|
|
|
FIGO = International Federation of Gynecology and Obstetrics; TAH = total abdominal hysterectomy; TLH = total laparoscopic hysterectomy; TRLH = total radical laparoscopic hysterectomy; BSO = bilateral salpingoophorectomy; CCU=Critical Care Unit.
For patients with ovarian cancer only, 99 and 110 in the 2019 and 2020 cohorts, respectively.
Groin lymphadenectomy includes sentinel node sampling.
Including doctors-in-training and surgical care practitioners.
Median(IQR)
Further comparison was also made to evaluate if any changes in surgical throughput after our service was relocated to independent sector (Supplementary Fig. 1). There was no significant change in surgical case-load before (pre-April 2020) and after service relocation (April 2020 onwards), and the surgical throughput for 2020 and 2019 was largely similar between the two time period (April to August).
3.4. Intraoperative and early post-operative outcomes
All our patients were routinely followed up two to three weeks post-operatively and then referred onwards for adjuvant treatment if relevant. All patients have direct open access to our post-operative hospital service within 8 weeks of being discharged where 30 days post-operative complications were captured. There was no difference in unplanned post-operative admission (4.7% and 4.8% in 2019 and 2020, respectively) into level 2 or 3 Critical Care Unit (CCU) and the length of post-operative stay (3 days; Table 2). No patient was admitted with symptoms suggestive of COVID-19 to CCU.
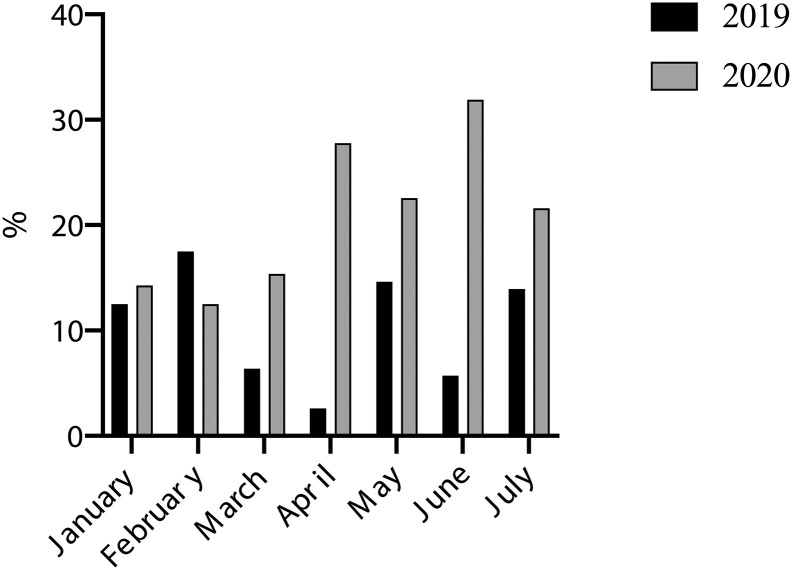
The Clavien-Dindo classification was used to classify post-operative surgical complication [8]. Although there was no difference in intra-operative complication rates, the 30-day post-operative complication rates were significantly higher in 2020 than in 2019 (58 [20.1%] versus 32 [10.8%]; p = 0.002). The increase was observed for both minor (Clavien-Dindo Grade I-II) and major (Clavien-Dindo Grade III-V) complications (Table 2). Most of this difference was attributed by increasing rates of infections, wound issues and post-operative ileus (Fig. 2, Supplementary Table 1a and b). The increase in post-operative complication rates were observed primarily from March 2020 onwards, coincided with the first wave of the COVID-19 pandemic reaches its peak in the UK (Fig. 3) [7]. One patient had a confirmed positive PCR test for COVID-19 infection, who was asymptomatic and made an uneventful post-operative recovery. The patient was screened for COVID-19 as post-operative blood test showed lymphopenia, a potential indicator of COVID-19 infection [9]. Further analysis revealed that there was a significantly higher number minor post-operative complications in the 2020 cohort for cervical and vulva cancer patients but not with other disease types (Supplementary Table 2).
Fig. 2.
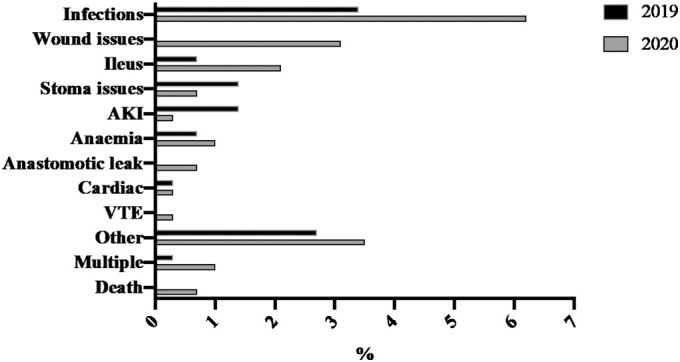
Thirty-day post-operative complication rates in 2019 (n = 32) and 2020 (n = 58) by the type of complications.
Fig. 3.
Thirty-day post-operative complication rates in 2019 (n = 32) and 2020 (n = 58) by calendar month. Only complete calendar months were included in this analysis.
There were two early post-operative death (≤30 days) in 2020 (versus none in 2019). An 83-year-old patient with advanced lower genital tract melanoma died of respiratory symptoms one day after her palliative hysterectomy, with suspected COVID-19 exposure, although her nasopharyngeal swab for COVID-19 infection was negative by PCR test. Another patient died from massive bilaterally pulmonary embolism 10 days after her operation.
3.5. Impact on staff wellbeing and training
At the time of the COVID-19 pandemic, our surgical team comprised of six surgical oncologists for gynecological cancers, 4 senior doctors-in-training, and 2 surgical care practitioners. Between January to August 2020, one of our surgeon reported flu-like illness and was self-isolated for 14 days. The surgeon then undertook COVID-19 serology test when it was made available in May 2020 and the test was negative for past and recent COVID-19. Serology screening test for COVID-19 was made available to staff by our hospital and participation was voluntarily. Of the remaining 11 members of surgical staff, 9 undertook COVID-19 serology testing between May to June 2020, with all testing negative for COVID-19 antibodies, indicating that none had previous or current infection. One staff member had 2 consecutive COVID-19 nasopharyngeal PCR swab tests instead of a serology test and the results were negative. One staff member did not have serology testing and did not report any COVID-19 symptoms throughout the study period.
As a gynecological oncology sub-speciality surgical training centre, we evaluated the impact of this pandemic on surgical training by assessing the roles of the primary surgeons who performed the operations. The proportion of operations performed by doctors-in-training decreased from 41.9% to 15.9% when comparing between 2019 and 2020 (p < 0.001; Table 2).
4. Discussions
There has been concerted effort globally to determine strategies for delivering safe cancer surgery during the COVID-19 pandemic by reorganization of services and developing COVID-19-free surgical care pathways. This study aimed to evaluate the impact of the pandemic by comparing the surgery performed during the same period in 2020 with a contemporary cohort in 2019 at an established high-volume tertiary gyneoncology centre. Our mitigation plan was introduced in stages as the pandemic progressed and full implementation of change took place in April 2020, when COVID-19 pandemic was at its height in the UK [8].
With the measures aim to mitigate the effects of clinical service disruption (Fig. 1), we did not observe significant differences in patient demographics (Table 1) or disease stages, and have maintained the number and complexity of operations performed (Table 2). However, some patient pathways were inevitably altered. We identified a significant reduction of laparoscopic operations performed during the pandemic (19.7% in 2020 versus 30.7% in 2019; p = 0.002). We therefore attributed this observed difference in potential deferral of surgery in selected patients with early low-grade endometrial cancers and significant medical co-morbidities who were offered hormonal therapies. Delivery of complex ovarian cancer was considered particularly challenging during this pandemic [10]. Nevertheless, with our mitigation plan, we were still able to maintain our surgical throughput to undertake complex cytoreductive surgery for patients with ovarian cancer. We observed that fewer primary and secondary cytoreductive operations were performed for ovarian cancers in 2020 compared to 2019 (Supplementary Fig. 1), consistent with our pragmatic solution to prioritise the limited access to CCU facilities available during the COVID-19 pandemic. We also observed an increase in the number of patients undergoing radical vulvectomy, with or without groin lymphadenectomies, during the pandemic. In addition to approaches described in Fig. 1, surgery for vulvar cancer, which often affects the elderly population, was made possible by undertaking these procedures under regional anesthesia. However, patients who required major reconstructive surgery underwent general anesthesia instead.
The one patient who was diagnosed with COVID-19 post-operatively and the other who died of suspected COVID-19, albeit her nasopharyngeal swab test was negative, occurred before our service were relocated to the independent section. Neither patients had pre-operative COVID-19 nasopharyngeal swab test as it was not routine at that time but both had underwent stringent screening with COVID-19 symptom questionnaires. The surgical team who operated on the patients were already routinely wearing protective clothing (see Fig. 1). No intra-operative or operative COVID-19 case or mortality was reported after our service was relocated from April 2020.
The UK was among the worst affected country by the COVID-19 pandemic with the West Midlands having an incidence rate of 2906 per 100,000, among one of the most critically affected regions in the country [7]. Hence, our data suggest that pre-operative COVID-19 screening (based on a questionnaire and/or nasopharyngeal PCR swab approach), in combination with a period of self-isolation, is efficient to prevent perioperative COVID-19 infection. Ensuring the staff involved are stationed in ‘COVID-free’ care areas and isolated from high-risk areas, has enabled patient care to continue as normal for many.
Although there was no difference in intraoperative complication rates, the 30-day post-operative complication rate was significantly higher in 2020 than in 2019 (20.1% vs 10.8%, p = 0.002; Table 2), regardless of severity. Infections (6.2% in 2020 versus 3.4% in 2019), wound issues (e.g. wound dehiscence without infection; 3.1% in 2020 versus 0% in 2019) and ileus (2.1% in 2020 versus 0.7% in 2019) were the commonest complications in 2020 (Fig. 2). The rise in post-operative complication rates coincident with the evolution of the pandemic (Fig. 3). There are multiple possible reasons behind this observed increase in post-operative complication rates. Firstly, as we transferred care from our specialist centre to the private sector, our gynaoncology nursing and ward staff were not transferred. Patients under the care of different surgical specialties were cared for on the same wards. The expertise developed over the years by our gyneoncology nursing staff was difficult to achieve within a short period. Secondly, when primary care was stretched during the pandemic, we had lower threshold to ask our patients to be reviewed by us in the tertiary care setting post-operatively, which could have led to detection bias. Nevertheless, it was also possible that we may have under estimated our post-operative complication rate, given that patients were less likely to attend hospital for minor complications during the height of the pandemic. Thirdly, it is possible that we prioritise the cases that were symptomatic, already deferred previously and/or received neoadjuvant chemotherapy in 2020. The results suggest the importance of considering team factors and additional attention on post-operative care for future pandemics or service re-configurations. Lastly, changes in surgical priority during the pandemic may have in part contributed to the rise in minor post-operative complications observed in 2020 (Supplementary Table 2a). For instance, there were significantly higher number of patients who underwent radical vulvectomy and groin lymphadenectomies. Both procedures are often associated with high post-operative morbidity. However, higher minor post-operative complications were also seen in patients undergoing surgery for cervical cancer despite cases of cervical cancer were lower in the 2020 than 2019 cohort. This could be explained by the higher number of minor cases (e.g. loop excision or biopsy of cervix) being undertaken in 2019 when compared to 2020 (Supplementary Table 2b).
An important reminder about the impact of this pandemic is not only on our patients, but also on our doctors-in-training. Appropriately, surgery performed by attending surgeons was recommended during the initial periods of this pandemic based on safety concerns. This has no doubt impacted on surgical training. Individualised strategies to ensure recuperation of lost training opportunities are paramount to ensure high-quality surgical services in the long run.
The main strength of our study lies in the fact that our surgical data were captured consecutively for the two time periods, with all patients comprehensively followed up for at least 30 days post-operatively. However, information on surgical morbidity and treatment outcomes were collected retrospectively, all information was available for the digital electronic records. Although our mitigation plan was not fully implemented until April 2020, we included data from January to March 2020 to show if there was any changes in our surgical practice and surgical morbidities as the pandemic progressed. Furthermore, the COVID-19 pandemic coincided with the onset of our annual seasonal winter flu which can be as disruptive as COVID-19 to our cancer service. Including data from January to March allows us to evaluate whether the seasonal winter flu in 2020 was likely to have independently influenced our surgical performance. Our study shows that the annual seasonal winter flu did not impact on our surgical performance in 2020. We acknowledged that this study focused on the clinical outcomes of patients who have overcome the challenges during the pandemic and underwent their surgical treatment, and further follow-up studies of those whose care pathways were altered and delayed are planned. Although historic comparator (the 2019 cohort) was used in this study, our internal clinical effectiveness audits had previously demonstrated minimal variations in perioperative outcomes over time (i.e. the significant increased in complication rates are unlikely to be due to normal year-on-year variations). Multi-centre studies comparing the approaches taken to mitigate the effects of this pandemic on surgical cancer care in different cancer types are awaited.
5. Conclusion
Consistent with recent reports [6], COVID-19-free care pathways minimizes COVID-19 transmission perioperatively. In addition, for further peaks of COVID-19 outbreaks and future pandemics, additional plans and training are required for the post-operative periods to enhance recovery and minimise post-operative complications. Additional training for nursing staff who were inexperienced or new to caring for post-operative gynecological cancer patients may potentially reduce post-operative complications. However, despite the challenges faced during the COVID-19 pandemic, this study has demonstrated the feasibility of maintaining surgical throughput with meticulous planning by a cohesive multidisciplinary team in a timely manner. We believe that appropriating shielding and screening protocol in place for our patients coupled with a robust mitigation plan to protect surgical and theatre staffs have ensured the delivery of a safe cancer surgery service. Through a robust mitigation plan, we were able ring fence our cancer service to deliver surgical care with minimal disruption from the pandemic. However, in country with severely stretch health resources, the use of the Modified Elective Surgery Acuity Scale (ESAS) may help to prioritise surgical treatment and ensure continuity in cancer treatment during the pandemic [11].
Total numbers of operations performed, ovarian primary cytoreduction surgery (CRS), interval CRS and secondary CRS.
a: 30-day post-operative complications according to cancer sites for 2019 cohort. b: 30-day post-operative complications according to cancer sites for 2020 cohort
a: Number of cases of 30-day post-operative complications reported according disease site. b: Number of cases of 30-day post-operative complications reported according disease site vs. major or minor surgical procedures.
Author contribution
JY, EL and KS conceived the idea for the study, participated in its design and coordination, and provided final approval of the version to be published. EL, ZP, JL-Z, SC, AK, NM, FC, AU and BS performed data collection independently and then cross checked to ensure data accuracy. AE, SS, SK, KS, JB, JY are lead clinicians who provided the database and clinical details. EL performed statistical analysis. JY and EL wrote the manuscript with revisions and corrections provided by JB, KS, SK, SS, AE. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
- 1.Stahel P. How to risk-stratify elective surgery during the COVID-19 pandemic? Patient Saf. Surg. 2020;31(14):8. doi: 10.1186/s13037-020-00235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nepogodiev D., Bhangu A., Glasbey J.C., Li E., Omar O.M., Simoes J.F., et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odor P.M., Neun M., Bampoe S., Clark S., Heaton D., Hoogenboom E.M., et al. Anaesthesia and COVID-19: infection control. Br. J. Anaesth. 2020;125(1):16–24. doi: 10.1016/j.bja.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boghdady M.E., Ewalds-Kvist B.M. Laparoscopic surgery and the debate on its safety during COVID-19 pandemic: a systematic review of recommendations. Surgeon. 2020;11 doi: 10.1016/j.surge.2020.07.005. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter B., Collins J.T., Barlow-Pay F., Rickard, Bruce E., Verduri A., et al. Nosocomial COVID-19 infection: examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople) J. Hosp. Infect. 2020;106(2):376–384. doi: 10.1016/j.jhin.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasbey J.C., Bhangu A., COVIDSurg Collaborative Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J. Clin. Oncol. 2020;6 doi: 10.1200/JCO.20.01933. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The UK National COVID-19 surveillance reports. 2020. https://coronavirus.data.gov.uk/details/caseshttps://www.gov.uk/government/publications/national-covid-19-surveillance-reports Last updated on 5th December 2020.
- 8.Dindo D., Demartines N., Clavien P. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Deng Y., et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int. J. Infect. Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama J., El-Nashar S.A., Waggoner S., Traughber B., Kestersond J. Adjusting to the new reality: evaluation of early practice pattern adaptations to the COVID-19 pandemic. Gynecol. Oncol. 2020;158(2):256–261. doi: 10.1016/j.ygyno.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fader A.N., Huh W.K., Kesterson J., Pothuri B., Wethington S., Wright J.D., et al. When to operate, hesitate and reintegrate: society of gynecologic oncology surgical considerations during the COVID-19 pandemic. Gynecol. Oncol. 2020;158(2):236–243. doi: 10.1016/j.ygyno.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total numbers of operations performed, ovarian primary cytoreduction surgery (CRS), interval CRS and secondary CRS.
a: 30-day post-operative complications according to cancer sites for 2019 cohort. b: 30-day post-operative complications according to cancer sites for 2020 cohort
a: Number of cases of 30-day post-operative complications reported according disease site. b: Number of cases of 30-day post-operative complications reported according disease site vs. major or minor surgical procedures.