Abstract
Background
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection causes direct lung damage, overwhelming endothelial activation, and inflammatory reaction, leading to acute respiratory failure and multi-organ dysfunction. Ongoing clinical trials are evaluating targeted therapies to hinder this exaggerated inflammatory response. Critically ill coronavirus disease 2019 (COVID-19) patients have shown heterogeneous severity trajectories, suggesting that response to therapies is likely to vary across patients.
Research Question
Are critically ill COVID-19 patients biologically and immunologically dissociable based on profiling of currently evaluated therapeutic targets?
Study Design and Methods
We did a single-center, prospective study in an ICU department in France. Ninety-six critically ill adult patients admitted with a documented SARS-CoV-2 infection were enrolled. We conducted principal components analysis and hierarchical clustering on a vast array of immunologic variables measured on the day of ICU admission.
Results
We found that patients were distributed in three clusters bearing distinct immunologic features and associated with different ICU outcomes. Cluster 1 had a “humoral immunodeficiency” phenotype with predominant B-lymphocyte defect, relative hypogammaglobulinemia, and moderate inflammation. Cluster 2 had a “hyperinflammatory” phenotype, with high cytokine levels (IL-6, IL-1β, IL-8, tumor necrosis factor-alpha [TNF⍺]) associated with CD4+ and CD8+ T-lymphocyte defects. Cluster 3 had a “complement-dependent” phenotype with terminal complement activation markers (elevated C3 and sC5b-9).
Interpretation
Patients with severe COVID-19 exhibiting cytokine release marks, complement activation, or B-lymphocyte defects are distinct from each other. Such immunologic variability argues in favor of targeting different mediators in different groups of patients and could serve as a basis for patient identification and clinical trial eligibility.
Key Words: critical care, immunology, inflammation, respiratory failure
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; NK, natural killer; PCA, principal components analysis; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TNF⍺, tumor necrosis factor-alpha
FOR EDITORIAL COMMENT, SEE PAGE 1706
Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is an emerging pathogen, which originated in late 2019 in China and is responsible for a form of severe viral pneumonia or coronavirus disease 2019 (COVID-19).1, 2, 3, 4 In early 2020, this disease evolved in a worldwide pandemic, and it constitutes a universal challenge for health-care settings.5 , 6 From a clinical standpoint, distinct patients trajectories have emerged, with different disease progression courses as well as different disease stages.7 In this regard, several clinical parameters have been described as potential risk factors for severe forms of COVID-19, namely age, clinical frailty, or preexisting comorbidities such as immunosuppression.2 , 3 , 8 SARS-CoV-2-related lung injury results from the interplay between direct viral damage to the alveolar epithelial cell and excessive endothelial activation.9 Both insults lead to the exaggerated cytokine production that is responsible for the most severe respiratory cases. This inflammation is composed of many overlapping signaling pathways, mediated by multiple key players, chiefly interleukins and the complement system.9 , 10 Early evidence pointed to IL-6 and IL-1β as pivotal biomarkers of disease severity.11 , 12 The complement system proteins act as key mediators of the innate immune response and are present in the pulmonary alveolar epithelium,13 , 14 with some evidence of deposits of terminal complex components C5b-9 in the lung microvasculature in COVID-19 patients.15 Additionally, the anaphylatoxin C5a has been involved in pulmonary endothelium damage in ARDS, and murine models of induced Middle East respiratory syndrome-coronavirus
and SARS-CoV-1.13 , 16 Therefore, early trials have not only evaluated antiviral17 , 18 efficacy but rapidly sought to evaluate the impact of targeted antiinflammatory therapies,19 , 20 namely, C5a,21 IL-6,22 and IL-1 inhibitors, or other immunomodulatory therapies such as steroids, hydroxychloroquine, IV immunoglobulins, or convalescent plasma,23 to alleviate the inflammatory reaction, thereby avoiding the need for mechanical ventilation and case fatality.
However, one could suppose that eligibility for these specific therapies may not be identical for all patients. Therefore, we hypothesized that critically ill COVID-19 patients with established clinical severity could be biologically and immunologically dissociable, and we sought to characterize this heterogeneity at baseline (ICU admission), with a particular focus on therapeutic targets currently being evaluated in ongoing clinical trials.
Study Design and Methods
Study Design and Participants
This study was approved by the Ethics Committee of the French Intensive Care Society (FICS; CE SRLF n°20-32).
Between March 1 and April 30, 2020, all consecutive adult patients referred for severe SARS-CoV-2 infection (defined as the need for oxygen > 9 L/min to achieve oxygen saturation levels of Spo 2 > 94%, or the need for high-flow nasal oxygen or mechanical ventilation on the first day of ICU stay) were prospectively included on admission to the medical ICU of the Saint-Louis Hospital, Paris, France. Laboratory confirmation for SARS-Cov-2 was defined as a positive result of real-time reverse transcriptase–polymerase chain reaction assay of nasopharyngeal or rectal swabs.
Data Collection
Data were collected by local investigators, using electronic case report forms, then centralized and anonymized. We collected epidemiologic, demographic, medical history, biologic, and immunologic data on the day of ICU admission.
We collected routine blood examinations at ICU admission, including blood count, coagulation profile, and serum biochemical tests. Blood samples at admission were also collected for each patient for subsequent biomarkers measurements. Serum IL-6, IL-1β, tumor necrosis factor alpha (TNF⍺), and IL-8 were analyzed according to the manufacturer’s instructions (Ella, ProteinSimple). Concomitant with clinical and biological data, circulating levels of C3 and soluble C5b-9 were determined according to the instructions of the manufacturer (Siemens and Quidel).
Statistical Analysis
Continuous variables are described as median and interquartile range (IQR) and compared using the Kruskal-Wallis test; categorical variables are summarized by counts (percentages) and compared using Fisher exact test, as appropriate.
We performed a hierarchical clustering in a principal component approach (namely, hierarchical clustering on principal components) to identify different phenotypes. First, we performed a principal component analysis (PCA), including a set of biological and immunological data, including D-dimers, a panel of cytokines (serum IL-6, IL-1β, TNF⍺), two complement biomarkers (C3 and soluble C5b-9), gamma-globulin level and lymphocyte counts (natural killer [NK] cells, CD8+, CD4+ T cells, and B cells). These covariables have been selected a priori because of their importance in severe SARS-CoV-2 infection and related potential therapeutic implications. Variables were standardized as they were measured in different units. Then, a hierarchical cluster analysis based on the first four dimensions of the PCA was used to determine subgroups of patients according to these characteristics. The clustering of patients was performed using Euclidean distance and the Ward agglomerative method. Missing data were imputed using iterative PCA. Briefly, we estimated the number of dimensions to use in the reconstruction formula, and then missing values were predicted using an iterative PCA algorithm.24 Details about the method used are available in the online data supplement (e-Figs 1-4, e-Table 1).
All tests were two-sided, and P < 5% was considered to indicate significant associations. Analyses were performed using R statistical platform, version 3.0.2 (https://cran.r-project.org/), using packages FactomineR and missMDA.
Results
Demographics and Characteristics
During the study period, 96 patients were admitted to the ICU, and all were included in the current study. Median age was 58 years (IQR [53-67]), and most patients were male (n = 69, 72%). Main comorbidities included hypertension (n = 52; 55%), diabetes mellitus (n = 28; 30%), and cardiovascular disease (n = 11; 12%). Most common past medications included antiplatelet therapy (n = 21; 22%), statins (n = 23; 24%), or antihypertensive drugs, mainly angiotensin-converting-enzyme inhibitors (n = 11; 12%) or angiotensin II receptor blockers medication (n = 16; 17%) (Table 1 ). Twenty-six patients had a history of cancer or solid organ transplantation. Among them, 16 patients were still receiving immunosuppressive therapy on admission (eg, chemotherapy, immunotherapy, glucocorticoids, or other immunosuppressive treatment; Table 2 ).
Table 1.
Overall Population and Cluster Characteristics
| Overall (N = 96) | Cluster 1 (n = 34) | Cluster 2 (n = 20) | Cluster 3 (n = 42) | P | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 58 [53-67] | 57 [50-67] | 61 [55-67] | 60 [53-66] | .638 |
| BMI | 28 [23-31] | 25 [23-31] | 27 [26-31] | 28 [25-31] | .268 |
| Male | 69 (72) | 25 (74) | 18 (90) | 26 (62) | .068 |
| Underlying conditions | |||||
| Hypertension | 52 (55) | 16 (49) | 12 (60) | 24 (57) | .693 |
| Diabetes mellitus | 28 (30) | 10 (30) | 5 (25) | 13 (31) | .916 |
| Cardiovascular disease | 11 (12) | 5 (15) | 3 (15) | 3 (7) | .499 |
| Solid organ transplant | 10 (10.4) | 6 (17.6) | 3 (15.0) | 1 (2.4) | .048 |
| Past history of malignancy | 16 (16.8) | 12 (35.3) | 2 (10.0) | 2 (4.9) | .002 |
| Active malignancya | 6 (6.2) | 6 (17.6) | 0 (0) | 0 (0) | <.005 |
| Clinical characteristics | |||||
| SAPS-II score | 28 [21-39] | 29 [22-37] | 36 [25-49] | 25 [18-34] | .04 |
| SOFA score | 4 [2-7] | 5 [2.25-8] | 5.5 [2.75-8.25] | 2 [2-6] | .019 |
| Respiratory | 2 [2-3] | 2 [2-3] | 2 [2-4] | 2 [1.25-3] | .221 |
| Hemodynamic | 0 [0-3] | 0 [0-3] | 3 [0-3] | 0 [0-3] | .131 |
| Renal | 0 [0-1] | 0 [0-1] | 0.5 [0-1.25] | 0 [0-0] | .042 |
| Liver | 0 [0-0] | 0 [0-0] | 0 [0-0] | 0 [0-0] | NS |
| Neurologic | 0 [0-0] | 0 [0-0] | 0 [0-0] | 0 [0-0] | NS |
| Coagulation | 0 [0-0] | 0 [0-1] | 0 [0, 0] | 0 [0, 0] | .009 |
| Time from symptom onset, days | 8 [6-12] | 6 [3-12] | 7 [4-8] | 9 [5-12] | .12 |
| Oxygen flow on arrival, L/min | 9 [6-12] | 9 [6-10] | 12 [6-15] | 9 [6-12] | .292 |
| RR, breaths/min | 28 [23-34] | 29 [20-30] | 30 [25-35] | 28 [24-34] | .483 |
| Oxygenation strategies on day 1 | |||||
| Standard oxygen alone | 51 (53.1) | 18 (52.9) | 8 (40.0) | 25 (59.5) | .36 |
| HFNC | 30 (31.2) | 12 (35.3) | 6 (30.0) | 12 (28.6) | .846 |
| Mechanical ventilation | 15 (15.6) | 4 (11.8) | 6 (30.0) | 5 (11.9) | .173 |
| Biological markers | |||||
| LDH, U/L | 809 [634-908] | 696 [558-842] | 870 [799-935] | 850 [710-903] | .036 |
| D-dimers, μg/L | 1,360 [780-2,840] | 1,230 [730-1,960] | 1,780 [955-3,155] | 1,360 [820-2,740] | .646 |
| CRP, mg/L | 181 [84-261] | 132 [77-226] | 263 [133-322] | 179 [94-238] | .075 |
| Ferritin, μg/L | 1,272 [636-2,234] | 1,238 [523-2,272] | 1,658 [1,183-2,099] | 1,045 [641-1,644] | .128 |
| Cytokine release | |||||
| TNF⍺, pg/mL | 22.7 [18.7-28.0] | 18.2 [14.4-23.8] | 29.7 [23.4-36.7] | 22.2 [19.2-26.5] | <.001 |
| IL-1β, pg/mL | 0.44 [0.32-0.86] | 0.44 [0.32-0.59] | 1.01 [0.75-1.27] | 0.36 [0.32-0.52] | <.001 |
| IL-6, pg/mL | 74 [41-137] | 89 [54-147] | 135 [79-220] | 46.7 [29.7-76.5] | <.001 |
| IL-8, pg/mL | 50 [31-78] | 44 [28-59] | 57 [47-90] | 42 [31-74] | .048 |
| Lymphocytes typing | |||||
| Total lymphocytes, cells/mm3 | 790 [580-1,170] | 710 [550-960] | 750 [340-1,240] | 960 [720-1,380] | .023 |
| T lymphocytes, cells/mm3 | 539 [343-764] | 567 [370-752] | 344 [244-525] | 637 [392-799] | .087 |
| CD8+ T lymphocytes, cells/mm3 | 182 [114-269] | 235 [170-356] | 101 [67-201] | 177 [134-238] | .027 |
| CD4+ T lymphocytes, cells/mm3 | 332 [184-464] | 322 [162-395] | 186 [168-375] | 413 [239-539] | .016 |
| NK cells, cells/mm3 | 106 [76-153] | 93 [57-117] | 139 [94-299] | 103 [73-156] | .011 |
| B lymphocytes, cells/mm3 | 104 [54-184] | 49 [14-82] | 100 [65-130] | 183 [143-283] | <.001 |
| Gamma globulins, g/L | 9.1 [7.4-11.5] | 7.7 [6.9-8.9] | 8.8 [7.1-12.3] | 10.9 [9.1-12.0] | <.001 |
| HLA-DR/monocyte, count | 8,631 [6,828-13,962] | 7,712 [5,939-11,668] | 7,852 [6,701-10,265] | 1,1073 [8,533-16,559] | .144 |
| Complement pathway | |||||
| C3, mg/L | 1,305 [1,173-1,550] | 1,260 [1,150-1,540] | 1,230 [1,160-1,340] | 1,445 [1,243-1,630] | .072 |
| sC5b-9, ng/mL | 373 [270-471] | 292 [217-449] | 368 [330-442] | 392 [357-492] | .034 |
| SC5b-9 > 360, ng/mL | 43 (56) | 10 (35) | 9 (56) | 24 (75) | .006 |
| ICU outcome | |||||
| Time of follow-up, days | 15 [7-20.25] | 17 [12-21] | 13 [7-19] | 15 [7-19] | .609 |
| Mechanical ventilation | 53 (55) | 18 (53) | 15 (75) | 20 (48) | .123 |
| Noninvasive ventilation (NIV) | 4 (4.2) | 0 (0.0) | 1 (5.0) | 3 (7.1) | .338 |
| ECMO | 4 (4.3) | 1 (2.9) | 1 (5.3) | 2 (4.9) | NS |
| AKI in ICU | 42 (44) | 12 (35) | 15 (75) | 15 (36) | .007 |
| Renal replacement therapy | 13 (13.5) | 3 (8.8) | 5 (25.0) | 5 (11.9) | .269 |
| Venous thromboembolic events | 13 (13.5) | 3 (8.8) | 2 (10.0) | 8 (19.5) | .4 |
| In-ICU mortality | 29 (31) | 11 (32.4) | 11 (55) | 7 (17.5) | .015 |
Values are given in No. (%) or median [interquartile range (IQR)]. Univariate analysis according to cluster status was done using Fisher exact test for categorical variables, and Kruskal-Wallis test for continuous nonnormal variables. AKI was defined using the Kidney Disease Improving Global Outcome (KDIGO) classification. Mechanical ventilation status was defined as any requirement for mechanical ventilation during ICU stay. AKI = acute kidney injury; CRP = C-reactive protein; ECMO = extracorporeal membrane oxygenation; HFNC = high-flow nasal canula; LDH = lactate dehydrogenase; NIV = noninvasive ventilation; NK = natural killer; RR = respiratory rate; RRT = renal replacement therapy; SAPS-II = simplified acute physiology score (SAPS II); sC5b-9 = soluble membrane attack complex; SOFA = Sequential Organ Failure Assessment; TNF-⍺ = tumor necrosis factor-alpha.
Chemotherapy during the last 6 months.
Table 2.
Demographic, Clinical, and Biological Characteristics of Patients According to Immunologic Status Before ICU Admission
| Overall (N = 96) | Immunocompetent (n = 80) | Immunocompromised (n = 16) | P | |
|---|---|---|---|---|
| Solid organ transplant | 10 (10.4) | … | 10 (10.4) | … |
| Kidney | 9 (9.4) | … | 9 (9.4) | … |
| Heart | 1 (1.0) | … | 1 (1.0) | … |
| None | 86 (89.6) | … | 86 (89.6) | … |
| Active malignancy | 6 (6.3) | … | 6 (6.3) | … |
| Lymphoproliferative | 3 (3.1) | … | 3 (3.1) | … |
| Myeloma | 3 (3.1) | … | 3 (3.1) | … |
| Immunomodulatory treatments | … | |||
| Corticosteroids | 12 (12.5) | … | 12 (12.5) | … |
| Daratumumab | 1 (1.1) | … | 1 (1.1) | … |
| Rituximab/obinituzumab | 3 (3.1) | … | 3(3.1) | … |
| Ixazomib | 1 (1.1) | … | 1 (1.1) | … |
| Belatacept | 2 (2.1) | … | 2 (2.1) | … |
| Cyclosporin | 6 (6.3) | … | 6 (6.3) | … |
| Tacrolimus | 1 (1.1) | … | 1 (1.1) | … |
| Biological markers | ||||
| D-dimers, μg/L | 1,360 [780-2,840] | 1,310 [770-2,790] | 1,500 [953-2,948] | .608 |
| Ferritin, μg/L | 1,272 [636-2,234] | 1,182 [626-1,971] | 1,645 [1,230-2,272] | .213 |
| CRP, mg/L | 181 [84-261] | 181 [84-251] | 201 [103-272] | .489 |
| Cytokine release | ||||
| TNF⍺, pg/mL | 22.7 [18.7-28] | 22.7 [18-29] | 22 [19.5-26] | .682 |
| IL-1β, pg/mL | 0.44 [0.32-0.86] | 0.44 [0.33-0.87] | 0.54 [0.32-0.84] | .746 |
| IL-6, pg/mL | 74 [41-137] | 76 [41-143] | 51 [33-81] | .225 |
| IL-8, pg/mL | 50 [31-78] | 49 [30-76] | 52 [41-57] | .866 |
| Lymphocytes typing | ||||
| Total lymphocytes, cells/mm3 | 790 [580-1,170] | 890 [690-1,340] | 560 [320-690] | <.001 |
| T lymphocytes, cells/mm3 | 539 [343-764] | 593 [357-816] | 410 [271-468] | .012 |
| CD8+ T lymphocytes, cells/mm3 | 182 [114-269] | 180 [113-262] | 203 [118-280] | .804 |
| CD4+ T lymphocytes, cells/mm3 | 332 [184-464] | 375 [196-491] | 168 [69-257] | .001 |
| NK cells, cells/mm3 | 106 [76-153] | 111 [88-170] | 67 [37-116] | .024 |
| B lymphocytes, cells/mm3 | 104 [54-184] | 125 [71-197] | 14 [11-33] | <.001 |
| Gamma globulins, g/L | 9.1 [7.4-11.5] | 10 [8.4-11.7] | 7.2 [4.5-7.5] | <.001 |
| HLA-DR/monocyte, count | 8,631 [6,828-13,962] | 8,631 [7,224-13,116] | 8,327 [4,558-13,608] | .662 |
| Complement pathway | ||||
| C3, mg/L | 1,305 [1,173-1,550] | 1,340 [1,180-1,565] | 1,240 [1,148-1,370] | .151 |
| sC5b-9, ng/mL | 373 [270-471] | 381 [286-491] | 318 [213-443] | .175 |
Patients with immunocompromised status included patients with solid organ transplant and active malignancy, with immunomodulatory treatments. AKI = acute kidney injury; ECMO = extracorporeal membrane oxygenation; HFNC = high-flow nasal canula; IQR = interquartile range; NK = natural killer; RR = respiratory rate; RRT = renal replacement therapy; SAPS-II: simplified acute physiology score (SAPS II); sC5b-9 = soluble membrane attack complex;
SOFA = sequential organ failure assessment; TNF-⍺ = tumor necrosis factor-alpha
Time from symptom onset to ICU admission was 8 (6-12) days. On admission, mean respiratory rate was 28 (23-34) breaths/min, and median oxygen flow requirement to achieve Sao 2 > 94% was 9 (6-12) liters per minute. Sequential Organ Failure Assessment score at admission was 4 (2-7). Most common symptoms were shortness of breath (n = 86; 90%), fever (n = 80; 83%), cough (n = 75; 78%), and fatigue (n = 68; 71%), and myalgias (n = 40; 42%), headaches (n = 13; 14%), and diarrhea (n = 16; 17%) were less frequent. Chest radiographs showed bilateral interstitial pneumonia (median number of quadrants involved on chest radiograph: 4 [3-4]).
Biological Findings
Laboratory findings on the day of ICU admission are summarized in Table 1. At baseline, the most common abnormalities were elevated inflammation markers, characterized by increased levels of C-reactive protein (179 [83-256] mg/L), and fibrinogen (6.79 [5.76-7.75] g/L). Eighty-two (92%) patients had elevated D-dimers (>500 μg/L). Lymphopenia (<1,500 cells/mm3) was found in 78 (85%) patients, with a median value of 790 (580-1170) cells/mm3, affecting CD4+ T cells (332 [184-464] cells/mm3), CD8+ T cells (182 [114-269] cells/mm3), and B lymphocytes (104 [54-184] cells/mm3). We found that the level of C3 was elevated (>1,250 mg/L) in 45 (56%) patients with concomitant elevated levels of the soluble membrane attack complex sC5b-9 in 43 (53%) consistent with an activation of the terminal complement pathway.
Increased IL-6 was found in 82 patients (>95%) (74 pg/mL [41, 137]), together with IL-1β in 59 (75%) (0.44 pg/mL [0.32, 0.86]), IL-8 in 76 (>95%) (50 pg/mL [31, 78]), and TNF⍺ in 74 (94%) (22.7 pg/mL [18.7, 28.0]).
Cluster Analysis
Analysis in clusters characterized three distinct immunophenotypes (Table 1, Fig 1 , e-Fig 5).
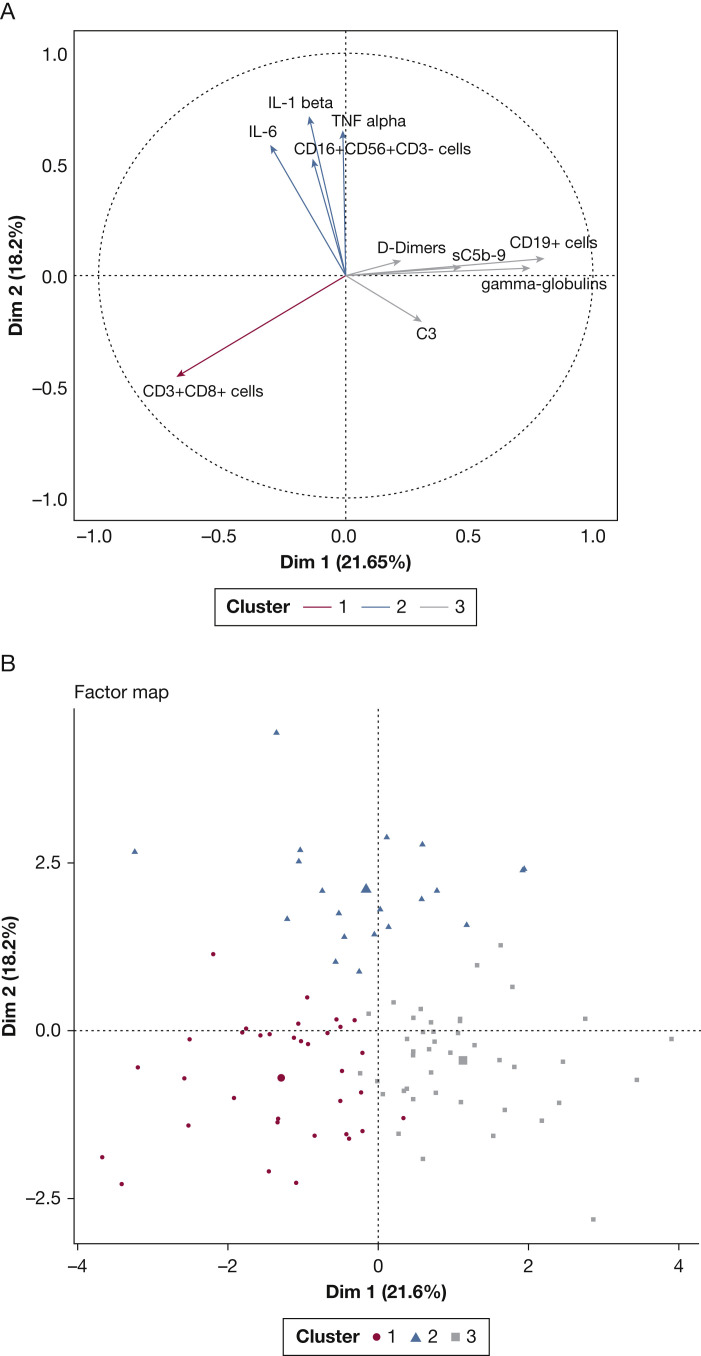
Figure 1.
Unsupervised analysis of immunologic data successfully discriminates critically ill patients with COVID-19 in three distinct clusters. Principal components analysis (PCA) on selected biologic and immunologic variables. A, Plotting of the two first principal components explaining 39.8% of the variance set; B, Factor map displaying the distribution of each patient after ascending hierarchical classification. C3 = fraction C3 of the complement; IL-6 = interleukin-6; IL-1β = interleukin 1 beta; NK = natural killer; sC5b9 = soluble membrane attack complex (MAC).
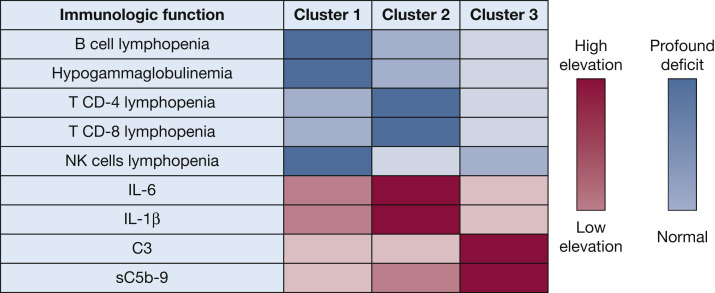
Thirty-four patients (35%) could be considered as a “humoral response deficiency” phenotype (cluster 1). These patients exhibited profound lymphopenia (710 [550, 960] cells/mm3), mainly on B cells (49 [14, 82] cells/mm3), and NK cells (93 [57, 117] cells/mm3) associated with hypogammaglobulinemia (7.7 g/L [6.9, 8.9]), which contrasted with relatively preserved T-cell count (567 [370, 752]) and moderate cytokine release (IL-1β [0.44 pg/mL (0.32; 0.59)], IL-6 [89 pg/mL (54-147)]) (Table 1). Most immunocompromised patients belonged to this cluster.
Twenty patients (21%) had a “hyperinflammatory” phenotype (cluster 2). These patients had very important hallmarks of cytokine release syndrome, with the highest pro-inflammatory cytokine values compared with other clusters (P < .001): IL-1β (1.01 [0.75-1.27] pg/mL), IL-6 (135 [79-220] pg/mL), and TNF⍺ (29.7 [23.4; 36.7] pg/mL) (Table 1). Another main feature seemed to be a T cell lymphocytopenia of both CD4+ (186 [168-374] cells/mm3), and CD8+ (101 [67-201] cells/mm3) lymphocytes. To note, sC5b-9 was discretely elevated in cluster 2 (368 [330; 442] ng/mL).
Finally, 42 patients (44%) exhibited a pattern of dependency on the terminal complement pathway with elevated median C3 concentrations (1,445 [1,243-1,630] mg/L), and a significant elevation of the soluble membrane attack complex sC5b-9 (392 ng/mL [357-492]) (P = .034) (Table 1). This cluster 3 could be named the “complement-dependent” phenotype. Figure 2 reports the respective importance of each of them in the partition process.
Figure 2.
Patients with COVID-19 have distinct immunologic dependencies: Colored representation of distinct immunologic patterns among critically ill patients with COVID-19. Quantitative levels of immunologic markers (cytokines, complement markers) are illustrated with proportional colored gradients. C3 = fraction C3 of the complement; IL-6 = interleukin-6; IL-1β = interleukin 1 beta; NK = natural killers; sC5b9 = soluble membrane attack complex (MAC).
Clinical characteristics and outcomes in the overall population and in each specific cluster are summarized in Table 1. As shown, clusters had similar clinical characteristics, and mortality rates varied from 55% (n = 11) in cluster 2 to 17.5% (n = 7) in cluster 3 (P = .015; Table 1). These results persisted after exclusion of immunocompromised patients (Table 2, e-Fig 6).
Discussion
Using a clustering approach on a vast array of immunologic and biologic variables on the day of ICU admission, our study provides new insights into the immunologic basis of heterogeneity in critically ill COVID-19 patients. This method without any a priori criteria allowed us to characterize, in a cohort of 96 patients, three distinct immunophenotypes, namely the “humoral response deficiency” phenotype (cluster 1), the “hyper-inflammatory” phenotype (cluster 2), and the “complement-dependent” phenotype (cluster 3).
Cluster 1 showed a high dependency on B-cell defects, associated hypogammaglobulinemia, and inflammation. Cluster 2 was characterized by the highest cytokine release (increased IL-6, IL-1β, TNF⍺, IL-8) and CD4 and CD8 T-cell defects, and carried the most severe mortality outcome. Finally, cluster 3 showed more discrete inflammation characteristics while having a high dependency on terminal complement activation (suggested by an increase in sC5b-9). Few studies have focused on the immunologic subtypes critically ill COVID-19 patients might display. A recent study25 has found a CD4 and CD8 lymphopenia in most patients, with a small subset of patients showing decreased NK cell levels, and normal or higher B-lymphocyte count. These defects appeared to be markedly more profound in critically ill patients as compared with patients with less severe disease. The complement pathway is also believed to play a pivotal role in the pulmonary lesion resulting from endothelial activation, and to date, data on complement activation in COVID-19 are scarce. A recent report10 suggested that, given the interplay between complement and inflammation mediators in the endothelial lesion, and the high dependency of the IL-6 cytokine release on C3 in SARS-CoV-1, both interventions targeting the IL-6 receptor and complement might act synergically on SARS-CoV-2.
The COVID-19 pandemic has affected millions of individuals, and no current specific treatment has been approved. The two previous outbreaks (SARS-CoV-1, and H1N1) did not provide conclusive data on the use of specific antiviral agents or antiinflammatory agents. Acute respiratory failure results in part from overwhelming inflammation causing extensive pulmonary and multiorgan endothelial lesions, largely described as a hallmark of severe forms. Therefore, the current pandemic has led to large-scale evaluations of many therapeutic antiviral and antiinflammatory agents in ongoing randomized control trials. Recent data provided evaluation of antiviral,17 , 18 targeted antiinflammatory,19 , 20 , 22 or immunomodulatory therapies.26 However, the use of such drugs might lead to different expected outcomes in critically ill patients, as compared with patients with less severe disease. Because severity and associated organ dysfunctions are the main drivers of mortality, any treatment that should be evaluated would need to be given early in the course of the disease to be beneficial. Moreover, this disease has shown a remarkable underlying heterogeneity in terms of patients’ profiles and severity, suggesting that a response to specific targeted therapies is likely to vary across patients. The three clusters identified in this study argue for targeting cytokines, complement system, or humoral response in different groups of patients with seemingly identical clinical severity. Taken together, our findings highlight that not all patients with severe COVID-19 who bear similar clinical characteristics have the same immunologic profile; they may not benefit from targeted therapies in the same way and therefore would not be eligible for the same targeted interventions.
Our study suffers from limitations. In the current study, we focused only on data available on the day of ICU admission, regardless of the temporal evolution during the subsequent hospital stay. Also, we cannot exclude that a patient's profile may change over time. However, we chose to define immunophenotypes at baseline, because it is a timely window for deciding on specific treatment eligibility. We also included time from symptoms onset to ICU admission in the current analysis, to take into account a possible difference in the course of the disease. Our results generate hypotheses that need to be validated on larger cohorts. Whether the immunologic phenotypes described in this study can be expanded to other patients’ registries or can explain inconsistent results from clinical trials needs to be determined. Although this could be the biological translation of heterogeneity, this might be of use when selecting a targeted therapy. How these immunological clusters might be associated with morbidity and mortality is uncertain. Although the precise cause of death could not be identified, there are differences between the clusters of mortality and extra-respiratory damage (acute renal failure, thromboembolic complications) that should be clarified. Furthermore, sixteen patients of our cohort were still receiving immunosuppressive therapy for cancer or solid organ transplantation (Table 2). Such conditions could be associated with immunological parameters variation. However, we performed a sensitivity analysis after removing these patients (e-Table 2, e-Fig 6), which led to the same results. Then, our study was observational, and we cannot rule out that some heterogeneity was introduced in studied populations or procedures. However, general management and data collection were protocolized without great disparities. Finally, we focused on critically ill patients at an advanced stage of disease progression, and our results would need to be validated in less severe cases. Similarly, this study was conducted in a single center and needs to be confirmed in larger cohorts.
Interpretation
This study raises the hypothesis that, besides clinical overlap, critically ill patients with COVID-19 have heterogeneous immunological profiles. Our findings highlight that clinical trials might be analyzed based on this biological heterogeneity before concluding on clinical futility. For instance, trials that are being conducted for IL-6, IL-1β, or complement blockade might benefit from post hoc analysis stratifying primary or secondary endpoints by these immunophenotypes.
Take-home Points.
Study Question: Are critically-ill COVID-19 patients biologically and immunologically dissociable based on profiling of currently evaluated therapeutic targets?
Results: We found that patients were distributed in three clusters bearing distinct immunologic features and associated with different ICU outcomes.
Interpretation: Severe COVID-19 patients exhibiting cytokine release marks, complement activation, or B-lymphocyte defects are distinct from each other.
Acknowledgments
Author contributions: G. D. is the guarantor of the content of the manuscript, including the data and analysis. T. D., G. D., and E. A. designed and performed research; T. D. and G. D. analyzed the data; T. D., G. D., and E. A. wrote the manuscript; T. D., S. C. Z., V. F. B., F. M., E. L., M. D., R. P. L., L. Z., E. A., and G. D. collected the data; T. D., S. C. Z., V. F. B., F. M., E. L., M. D., R. P. L., L. Z., E. A., and G. D. approved the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: V. F. B.: consultancy or lecture fees or travel support from Alexion Pharmaceuticals, Inc., Apellis, and Roche. None declared (T. D., S. C.-Z., F. M., E. L., M. D., R. P. d. L., L. Z., E. A., G. D.).
Other contributions: This study was approved by the Ethics Committee of the French Intensive Care Society (FICS; CE SRLF n°20-32). The datasets used or analyzed during the current study are available from the corresponding author on reasonable request. The authors declare that they have no competing interests for the present work.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin K.M., Karas M.G., Ivascu N.S., Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020;201(11):1337–1344. doi: 10.1164/rccm.202004-1037CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risitano A.M., Mastellos D.C., Huber-Lang M. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40(6):511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R., Xiao H., Guo R., Li Y., Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4(1):1–7. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo R.-F., Ward P.A. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23(1):821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 15.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia C.C., Weston-Davies W., Russo R.C. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19-induced cytokine release syndrome. Chest. 2020;158(1):e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sciascia S., Aprà F., Baffa A. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38(3):529–532. [PubMed] [Google Scholar]
- 21.Diurno F., Numis F.G., Porta G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 22.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Q.-L., Yu Z.-J., Gou J.-J. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josse J., Pagès J., Husson F. Multiple imputation in principal component analysis. Adv Data Anal Classif. 2011;5(3):231–246. [Google Scholar]
- 25.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. J Zhejiang Univ Med Sci. 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.