Abstract
Objectives
To develop an international consensus on managing penile cancer patients during the COVID-19 acute waves. A major concern for patients with penile cancer during the coronavirus disease 2019 (COVID-19) pandemic is how the enforced safety measures will affect their disease management. Delays in diagnosis and treatment initiation may have an impact on the extent of the primary lesion as well as the cancer-specific survival because of the development and progression of inguinal lymph node metastases.
Materials and methods
A review of the COVID-19 literature was conducted in conjunction with analysis of current international guidelines on the management of penile cancer. Results were presented to an international panel of experts on penile cancer and infection control by a virtual accelerated Delphi process using 4 survey rounds. Consensus opinion was defined as an agreement of ≥80%, which was used to reconfigure management pathways for penile cancer.
Results
Limited evidence is available for delaying penile cancer management. The consensus rate of agreement was 100% that penile cancer pathways should be reconfigured, and measures should be developed to prevent perioperative nosocomial transmission of COVID-19. The panel also reached a consensus on several statements aimed at reconfiguring the management of penile cancer patients during the COVID-19 pandemic.
Conclusions
The international consensus panel proposed a framework for the diagnostic and invasive therapeutic procedures for penile cancer within a low-risk environment for COVID-19.
Keywords: COVID-19, Delphi study, Pandemic, Penile cancer
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a major impact on healthcare pathways and delivery, including the onset of unavoidable delays in managing patients with aggressive cancers [1]. This population includes rare cancers such as penile cancer, for which patients typically present late in the course of the disease due to patient embarrassment or misdiagnosis [2,3].
Delaying penile cancer treatment can lead to disease progression of the primary tumor [4,5] such that organ-sparing surgery may no longer be feasible [6]; similarly, the development of metastatic disease in the inguinal lymph nodes has an impact on cancer specific survival [7], [8], [9], [10]. To mitigate the health risks to patients because of the reconfiguration of services during the COVID-19 pandemic, prioritization of patients with time-critical cancers has been proposed [11]. An additional challenge to delivery is the need to protect both patients and healthcare workers from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [12]. Given the current focus on more common time-critical malignancies, rare cancers such as penile cancer are at risk of being deemed low priority because of their low incidence [7,13], in a manner that would exacerbate the current disease.
Healthcare provisions in the European Union aim to provide high-quality cost-effective care. The European Reference Networks (ERNs) [14] were developed to facilitate cross-border cooperation between healthcare providers to improve care for patients with rare diseases. The ERN Urogenital-Diseases section (eUROGEN) is one of 24 ERNs (https://eurogen-ern.eu/) and aims to improve the diagnosis and facilitate more equitable access to high-quality treatment and care of rare urogenital diseases.
Although guidance on prioritization of urologic cancers has been available through individual organizations, a more detailed adaptation of pathways for rare tumors such as penile cancer is lacking [15,16]. The Delphi technique is a structured communication process, originally created as a systematic, interactive decision method that incorporates expert opinions [17] to develop a mutual agreement by using questionnaires and the resulting feedback to further the discussion in each subsequent round. The primary aims of this study were to develop guidance on reconfiguring the management pathways for patients with penile cancer, during the acute Covid-19 waves, as it is deemed a time-critical cancer.
2. Material and methods
The study consisted of 3 phases, whereby each phase informed the subsequent phase. First, a review of the COVID-19 literature indexed in the PubMed and Scopus databases was conducted. During this step, an analysis of the current published National Comprehensive Cancer Network (NCCN) [18] and the European Association of Urology (EAU) [19] guidelines on penile cancer was also completed and although the European Society of Medical Oncology (ESMO) guidelines were reviewed it was felt that the other 2 guidelines covered the same points [20]. Second, this analysis was used to develop the questions presented to the expert consensus group. Third, the consensus statements created were used to develop recommendations aimed at reconfiguring management pathways for penile cancer during the COVID-19 acute waves.
The first teleconference was conducted on May 15, 2020, and the second on June 5, 2020. The available evidence was used to develop questions for incorporation into the Delphi process. Separately, penile cancer guidelines and recommendations published by the EAU and NCCN [18,19] were reviewed by 5 of the authors (AM, OOC, FC, HA, EP), and the information summarized in these documents was used to establish the standard of care for penile cancer patients.
3. Expert panel
A total of 21 experts from across Europe were convened in tandem with expertise from the eUROGEN workstream, the EAU guidelines panel, the EAU Section of Infections in Urology and the EAU Research Foundation to discuss and develop an international standard for managing penile cancer patients during the COVID-19 pandemic. The experts were selected by the senior author of this paper (AM) considering the scientific and clinical competence in the field. Of the 21 panel members, 16 were qualified as urologists, 2 were experts in infectious diseases control as well as virology, 1 was a medical oncologist, 1 was a clinical oncologist, and 1 was a specialist penile cancer nurse (Supplement-1).
4. Internet survey and Delphi process
The Delphi process consisted of 4 rounds. An internet survey using Google Forms (Google) was generated and sent to the panel members, with a request for responses to be returned within 24 hours. Consensus achievement was defined as attaining ≥80% agreement on each question based on previous studies [17]. The survey was subdivided into 4 sections with 82 questions overall relevant to a particular theme: General questions (4 questions), environment [6], patients (63), and healthcare workforce [11].
An accelerated electronic consensus-reaching exercise using the Delphi methodology was then applied and completed over 3 consecutive days. Questions that achieved ≥80% consensus among the answers were removed from the next survey round. Repeated iterations of anonymous voting continued over 3 rounds, in which an individual's vote in the next round was informed by knowledge of the entire group's results from the previous round. For inclusion in the final compiled recommendations, each survey item needed to reach a group consensus (i.e., show ≥80% agreement) by the end of the first 3 survey rounds. However, the panel decided to conduct a final survey round during the second meeting to reach a consensus on 4 statements that failed to reach the consensus agreement rate of ≥80%. Levels of consensus are reported in Supplement-2.
5. Results
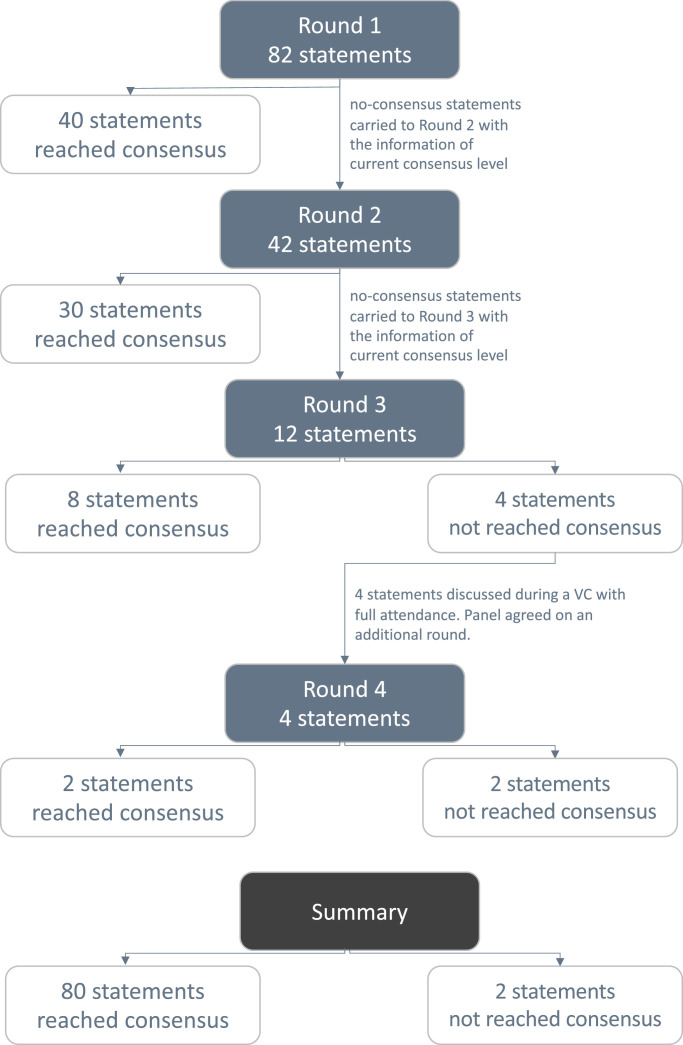
All 21 experts participated in each of the survey rounds. Throughout the process, a consensus was reached on almost all items (80/82, 97.56%). Fig. 1 summarizes the outcomes of each of the 4 rounds, and Table 1 presents the statements obtained.
Fig. 1.
Outcomes following each survey round.
Table 1.
Consensus statements
| Impact of COVID-19 pandemic on penile cancer patients | |
| |
| COVID-19 considerations for the healthcare workforce that will deliver penile cancer management in COVID-19 cold sites | |
| |
| Rationalizing the general management of penile cancer patients during the COVID-19 pandemic | |
| |
| Rationalizing of penile cancer diagnosis during the COVID-19 pandemic | |
| |
| Rationalizing the primary treatment of penile cancer patients during the COVID-19 pandemic | |
| General statements | |
| |
| Tis/Ta penile cancer | |
| Involving only the foreskin |
|
| Involving only the glans |
|
| T1(G1/G2) penile cancer | |
| Involving only the foreskin |
|
| Involving only the glans |
|
| T1(G3/G4) penile cancer | |
| Involving less than 50% of the glans |
|
| Involving more than 50% of the glans |
|
| T2 penile cancer | |
| Involving less than 50% of the glans |
|
| Involving more than 50% of the glans |
|
| T3–T4 penile cancer | |
| |
| Patients should self-isolate following the surgical treatment of penile cancer until their catheter is removed. | |
| cN0 patients | |
| Risk stratification for cN0 penile cancer patients in relation to lymph node metastasis should be deemed necessary to make treatment decisions. | |
| Low-risk cN0 patients |
|
| Intermediate-risk cN0 patients |
|
| High-risk cN0 patients |
|
| cN1 patients | |
| |
| cN2 patients | |
| |
| cN3 patients | |
| |
| Metastatic disease | |
| |
COVID-19 = coronavirus disease 2019; DSNB = dynamic sentinel lymph node biopsy; EAU = European Association of Urology; NCCN = National Comprehensive Cancer Network; PPE = personal protective equipment; 5-FU = 5-fluorouracil.
5.1. COVID-19 and the environment
The panel unanimously agreed to deliver penile cancer diagnostic or invasive therapeutic procedures only in hospitals with appropriate COVID-19 screening and prevention protocols and/or with newly reconfigured COVID-19–free zones that separate patient screening and treatment zones. The panel agreed that all patients should be tested before any hospital admission for invasive procedures, ideally by double-swab testing at 1 week and at 48 hours before hospital admission.
5.2. Reconfiguration of diagnostic pathways for new penile lesions
With the intent to reduce the number of hospital visits, the panel agreed that patients with penile lesions that are clinically obvious cancers, according to penile cancer expert evaluation, should not be biopsied in order to confirm the diagnosis before pursuing definitive treatment. More importantly, patients who have penile lesions with a low index of suspicion, according to penile cancer expert evaluation, should not undergo biopsy but instead be maintained under surveillance. The panel also agreed that all patients with a new penile cancer diagnosis should only undergo a physical examination that avoids stimulated penile magnetic resonance imaging (MRI, requiring alprostadil injection for stimulation) to evaluate preoperative disease extent.
5.3. Tis/Ta/T1 G1–G3 penile cancer
Several surgical options are available for treating penile cancer. The panel agreed that during the COVID-19 pandemic, these invasive procedures should be performed, whenever possible, under local anesthesia (LA).
Patients who have Tis/Ta/T1 grade (G) 1–G3 penile cancer on biopsy involving only the foreskin should undergo a radical circumcision under LA. For this group, the panel voted against the use of other topical treatments. If surveillance is chosen, the first face-to-face appointment for follow-up should be scheduled 3 months from the last evaluation.
Patients who have pTis/Ta penile cancer involving <50% of the glans should undergo topical treatment with 5-fluorouracil or imiquimod. If topical treatment cannot be administered or if the lesion also involves the foreskin, wide local excision with circumcision under LA is preferable.
Wide local excision with circumcision under LA should be considered as the first option in patients with pT1 (G1–G4) penile cancer involving <50% of the glans. Glansectomy with circumcision without reconstruction under LA should be adopted as a second option only for T1 G3–G4 patients with a lesion involving <50% of the glans for which wide local excision is not feasible.
Glansectomy with circumcision without reconstruction (graft) under a LA should instead be considered as the first option for pT1 G1–G4 patients with a lesion involving >50% of the glans and in all patients with T2 penile cancer. For pTa disease involving >50% of the glans, glansectomy with circumcision without reconstruction (graft) under a LA should be considered if wide local excision is not feasible. Partial penectomy or total penectomy and perineal urethrostomy should be adopted for treating T3/T4 penile cancer patients.
5.4. Reconfiguration of the therapeutic pathways for cN0 patients
The entire panel agreed that the EAU risk stratification for patients with cN0 penile cancer with lymph node metastasis is necessary to inform treatment decisions.
During the COVID-19 pandemic, low-risk patients with cN0 penile cancer (Table 2 ) should undergo surveillance without any other imaging and should have a first follow-up appointment at 3 months after the last evaluation. Intermediate- and high-risk patients with cN0 penile cancer (Table 2) should undergo dynamic sentinel lymph node biopsy to detect the presence of micrometastatic disease. Among intermediate-risk patients, ultrasound surveillance of the inguinal nodes is an option if nuclear medicine facilities are unavailable. Conversely, in high-risk patients, inguinal lymphadenectomy should be considered.
Table 2.
European Association of Urology (EAU) risk groups for penile cancer patients with no palpable inguinal lymph nodes for lymph node metastasis
| Low | Intermediate | High |
|---|---|---|
| Tis Ta T1a |
T1b G2 |
≥T2 or G3/G4 |
5.5. Reconfiguration of the therapeutic pathways for cN1/pN1 patients
During the COVID-19 pandemic, immediate inguinal lymphadenectomy can be delayed, no more than 3 months, for cN1 patients until a definitive pathologic diagnosis of nodal disease is obtained. However, cN1 patients (with lymph-node size less than 2 cm) should undergo computed tomography of the chest, abdomen, and pelvis to assess the inguino-pelvic lymph nodes and to detect any distant metastatic disease. A percutaneous lymph node biopsy should be performed to confirm the diagnosis. More importantly, if the percutaneous lymph node biopsy is negative, then an excisional biopsy should be performed. Conversely, for cN1 patients with lymph-node size more than 2 cm, immediate inguinal lymphadenectomy (ILND) should be considered.
According to the panel, pN1 patients should undergo unilateral ILND to reduce the chance of comorbidity and decrease the length of hospital stay. ILND, when required, should not be delayed more than 3 months [10].
Both cN1patients with node size more than 2 cm and pN1 patients should undergo contralateral dynamic sentinel lymph node biopsy or modified inguinal lymph node dissection to detect the presence of micrometastatic disease.
5.6. Reconfiguration of the therapeutic pathways for cN2–N3 and metastatic patients
According to the panel, during the COVID-19 pandemic, the management of cN2–3 and metastatic patients should follow current guidelines [18,19]. The panel agreed to consider giving adjuvant or palliative therapy only to patients with a good performance status. Also, 80% of the expert panel reported not offering neoadjuvant chemotherapy for cN2 disease in their routine practice before the pandemic. Conversely, neoadjuvant therapy may be reserved for cN3 patients with a good performance status and who have non-resectable disease.
5.7. Environment
Regarding the environment, 3 key concepts were suggested to largely shape the remaining statements of the consensus. The first concept was the need to simultaneously ensure the safety of the patients and healthcare staff against the risk of COVID-19. The second concept was the suggestion to assume that, at any given time point, a patient or healthcare professional can still be contagious. Finally, the third concept is that the working schedule of penile cancer surgical teams can be adapted to ensure that treatment delivery continues when appropriate during the pandemic. Both patients and healthcare professionals should be tested for COVID-19 unless proven to be immune (i.e., by serology analysis). More importantly, all healthcare professionals should receive high-level universal personal protective equipment for use during surgical procedures.
5.8. Statements that did not reach the consensus
The panel could not reach a consensus on the question regarding the administration of neo-adjuvant chemotherapy to patients with cN2 penile cancer disease during the COVID 19 pandemic. In all, 72.4% of the panel agreed on administering neo-adjuvant chemotherapy to patients with cN2 patients during the pandemic. In addition to this, during the final consensus meeting, the majority of the panel members declared that neo-adjuvant chemotherapy is not in their routine clinical practice for the treatment of cN2 patients even if there is no pandemic. The panel also did not reach a consensus on the question: Owing to the possible risk of COVID infection for the aerosol-generating procedure, should the inguinal lymphadenectomy be performed with an open-access instead of using a laparoscopic/robotic approach on patients? 54.5% of the panel agreed on either open or laparoscopic/robotic surgery due to the experience of the surgical team.
6. Discussion
In this study, an international expert panel has developed a series of statements to reconfigure the management for penile cancer patients during the COVID-19 acute waves. This study primarily aims to modify recommendations of the current NCCN and EAU penile cancer guidelines to make them applicable during the COVID-19 pandemic. The adaptation was guided mainly by 2 necessities: firstly to minimize the number of hospital visits and hospitalization and secondly to prevent COVID-19-related complications attributed to cancer treatment. A consensus was reached regarding multiple items related to the diagnosis and management of penile cancer throughout the pandemic. During the COVID-19 acute waves non-urgent procedures should be postponed and non-invasive interventions should be encouraged so that the requirement for general anesthesia is restricted due to the morbidity associated with perioperative SARS-CoV-2 infection [21].
The panel fully agreed that all invasive diagnostic and therapeutic procedures must be performed in COVID-19–free zones. This recommendation is in line with those from the Societa Italiana di Chirurgia Oncologica, which stressed the need for the implementation of COVID-19–free hospitals/units to minimize the risk of COVID-19 infection among cancer patients during hospitalization [21], [22], [23]. Creating clinical areas exclusively committed to cancer care, as already established in parts of Italy and the United Kingdom, may allow safer, COVID-19–free pathways for these patients and theoretically minimizes the risk of exposure for healthcare professionals to COVID-19 patients [21]. Furthermore, these COVID-19–free zones/hospitals should adopt COVID-19 screening and prevention protocols. This recommendation is in line with those reported in the current literature [24]. Granata et al. recently compared the COVID-19 infection risk between patients who underwent an invasive urological procedure in a referral COVID-19 hospital and a COVID-19–free hospital, respectively, and reported that early implementation of extraordinary measures to restrict the spread of the virus could offer good protection in both hospital types [25].
The panel agreed that all patients with a new penile cancer diagnosis should only undergo a physical examination that avoids stimulated penile MRI. The main risk with not performing a penile MRI before surgery is to underestimate or overestimate the volume and the T stage of the disease. However, based on the panel, the preoperative physical examination and the intraoperative findings provide enough information to evaluate the extent of the disease correctly.
Regarding the surgical management of the disease, the panel agreed that surgeons should pursue, whenever possible, performing the surgical procedure under LA. Although penile-preserving surgery (i.e., glansectomy with skin-grafting) increases patients’ quality of life based on preservation of penile length, it requires a longer operating time and the need for general anesthesia. Furthermore, procedures requiring skin grafting have an inherent risk for complications such as graft loss or infection, which can increase the length of hospitalization and readmission rates [6]. Thus, the panel proposed that glansectomy and partial penectomy should be performed under LA, whenever possible, for T2 and T3 lesions, and to avoid reconstruction with skin grafting.
Another important recommendation by the panel concerned the management of pN2–pN3 disease. The management options for these patients remain controversial, and there are discrepancies between the various guidelines. The EAU guidelines recommend adjuvant chemotherapy for pN2–pN3 M0 patients, whereas the NCCN guidelines do not include definitive statements regarding the administration of adjuvant chemotherapy, except in the setting of positive surgical margins, multiple lymph node involvement, and extranodal extension [26]. In this context, the panel agreed that adjuvant therapy should be performed only in patients with pN2–pN3 disease who have a good performance status. This recommendation is based on the evidence that patients without a good performance status are more vulnerable to severe COVID-19 [27]. Neoadjuvant chemotherapy can be an option as suggested by the EAU guidelines in the case of unresectable or recurrent lymph node metastases, whereas the NCCN guidelines recommend its use in selected patients with >4 cm (fixed or mobile), histologically proven, nodal metastases in pN2–pN3 M0 [26]. This lack of consensus was also apparent among the international expert panel during the Delphi process. Although the experts recommended considering neoadjuvant therapy for pN3 patients with a good performance status, no consensus was reached for pN2 patients. This recommendation is given to protect patients with a poor performance status, who are more vulnerable to severe COVID-19 [27]. Moreover, 80% of the panel declared that they did not routinely offer neoadjuvant treatment for cN2 disease, even before the pandemic.
This study is not without limitations. Urological surgeons were overrepresented on the panel, which reflects the fact that the current management of penile cancer is primarily surgical. We also appreciate that most participants on the panel were from the United Kingdom compared to other European countries. Although the panel composition was developed to reflect the high-volume penile cancer centers in Europe, it is inevitable that the UK will be overrepresented because of the earlier adoption of a centralized service in the UK. The concept of COVID-19 free hospitals also needs to be realistic. Although it is near impossible to ensure that there is no possibility of COVID-19 within the majority of facilities unless patients and staff are self-isolating and routinely swabbed on a daily basis, near COVID-19 free zones or COVID-19 protected areas which minimize the transmission of the virus are models that are likely to be employed.
7. Conclusions
To our knowledge, this work is the first unifying, international expert consensus study providing guidelines for reconfiguring the management of penile cancer patients during the COVID-19 acute waves. The study panel reached a consensus in 2 main domains. First, if invasive procedures for the management of penile cancer are necessary, then surgery should be carried out at a hospital where the likelihood of COVID-19–related hazards and consequences remain low. Second, an agreement was reached regarding reconfiguring the management pathways for penile cancer.
Conflict of interest
All authors of this manuscript have directly participated in planning, execution, and/or analysis of this study.
The contents of this manuscript have not been copyrighted or published previously.
The contents of this manuscript are not now under consideration for publication anywhere.
The contents of this manuscript will not be copyrighted, submitted, or published elsewhere while acceptance by Urologic Oncology: Seminars and Original Investigations in under consideration.
There are no directly related manuscript or abstracts, published or unpublished, by any authors of this manuscript.
No financial support or incentive has been provided for this manuscript.
Acknowledgments
This project is endorsed by the European Association of Urology Research Foundation.
Fabio Castiglione, MD is supported by The Margaret Spittle Research Fellowship, University College London Hospital, London UK. Asif Muneer is supported by the NIHR Biomedical Research Centre University College London Hospital.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2020.12.005.
Appendix. Supplementary materials
References
- 1.Naspro R, Da Pozzo LF. Urology in the time of corona. Nat Rev Urol. 2020;17:251–253. doi: 10.1038/s41585-020-0312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaux A, Netto GJ, Rodríguez IM. Epidemiologic profile, sexual history, pathologic features, and human papillomavirus status of 103 patients with penile carcinoma. World J Urol. 2013;31:861–867. doi: 10.1007/s00345-011-0802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arya M, Kalsi J, Kelly J, Muneer A. Malignant and premalignant lesions of the penis. BMJ. 2013;346:f1149. doi: 10.1136/bmj.f1149. [DOI] [PubMed] [Google Scholar]
- 4.Derakhshani P, Neubauer S, Braun M, Bargmann H, Heidenreich A, Engelmann U. Results and 10-year follow-up in patients with squamous cell carcinoma of the penis. Urol Int. 1999;62:238–244. doi: 10.1159/000030405. [DOI] [PubMed] [Google Scholar]
- 5.Pow-Sang MR, Ferreira U, Pow-Sang JM, Nardi AC, Destefano V. Epidemiology and natural history of penile cancer. Urology. 2010;76:S2–S6. doi: 10.1016/j.urology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Albersen M, Parnham A, Joniau S. Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol Oncol-Semin Ori. 2018;36:141–146. doi: 10.1016/j.urolonc.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulidou M, Sahdev V, Houssein S, Muneer A. Epidemiology of penile cancer. Curr Probl Cancer. 2015;39:126–136. doi: 10.1016/j.currproblcancer.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Parnham AS, Albersen M, Sahdev V. Glansectomy and split-thickness skin graft for penile cancer. Eur Urol. 2018;73:284–289. doi: 10.1016/j.eururo.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Alnajjar HM, MacAskill F, Christodoulidou M. Long-term outcomes for penile cancer patients presenting with advanced N3 disease requiring a myocutaneous flap reconstruction or primary closure-a retrospective single centre study. Transl Androl Urol. 2019;8:S13–S21. doi: 10.21037/tau.2019.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chipollini J, Tang DH, Gilbert SM. Delay to inguinal lymph node dissection greater than 3 months predicts poorer recurrence-free survival for patients with penile cancer. J Urol. 2017;198(6):1346–1352. doi: 10.1016/j.juro.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 11.Sud A, Jones M, Broggio J. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lurie N, Saville M, Hatchett R, Halton J. Developing covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 13.Castiglione F, Alnajjar HM, Christodoulidou M. Primary squamous cell carcinoma of the male proximal urethra: outcomes from a single centre. Eur Urol Focus. 2019 doi: 10.1016/j.euf.2019.02.016. S2405-4569(19)30051-3. [DOI] [PubMed] [Google Scholar]
- 14.Hollak CEM, Biegstraaten M, Baumgartner MR. Position statement on the role of healthcare professionals, patient organizations and industry in European reference networks. Orphanet J Rare Dis. 2016;11:7. doi: 10.1186/s13023-016-0383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diorio GJ, Leone AR, Spiess PE. Management of penile cancer. Urology. 2016;96:15–21. doi: 10.1016/j.urology.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Wallis CJD, Novara G, Marandino L. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur Urol. 2020;78(1):29–42. doi: 10.1016/j.eururo.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond IR, Grant RC, Feldman BM. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67:401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 18.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Penile Cancer. Version 2.2019. 2019.
- 19.Hakenberg OW, Minhas S, Necchi A, Protzel C, Watkin N., Compérat E. European Association of Urology Guidelines Office; 2020. European association of urology guidelines 2020 edition. [Google Scholar]
- 20.Van Poppel H, Watkin NA, Osanto S. Penile carcinoma: ESMO clinical practice guidelines. Ann Oncol. 2013;24(Suppl 6):vi115–vi124. doi: 10.1093/annonc/mdt286. 10.1093/annonc/mdt286 [DOI] [PubMed] [Google Scholar]
- 21.Restivo A, De Luca R, Spolverato G. The need of COVID19 free hospitals to maintain cancer care. Eur J Surg Oncol. 2020;46:1186–1187. doi: 10.1016/j.ejso.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campi R, Amparore D, Capitanio U. Assessing the burden of nondeferrable major uro-oncologic surgery to guide prioritisation strategies during the COVID-19 pandemic: insights from three Italian high-volume referral centres. Eur Urol. 2020;78(1):11–15. doi: 10.1016/j.eururo.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coccolini F, Perrone G, Chiarugi M. Surgery in COVID-19 patients: operational directives. World J Emerg Surg. 2020;15(1):25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVIDSurg Collaborative Global guidance for surgical care during the COVID-19 pandemic. Br J Surg. 2020;107(9):1097–1103. doi: 10.1002/bjs.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonio Maria G, Vasileios P, Giacomo Piero I. Urologic surgery and invasive procedures during coronavirus pandemic: Retrospective comparison of risk infection in a referral COVID hospital and in a free-COVID hospital. Urologia. 2020;87(4) doi: 10.1177/0391560320927106. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mistretta FA, Cyr S-J, Palumbo C. Adherence to guideline recommendations for perioperative chemotherapy in patients with pN2-3 M0 squamous cell carcinoma of the penis: temporal trends and survival outcomes. Clin Oncol. 2020;32:e93–101. doi: 10.1016/j.clon.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Bhatraju PK, Ghassemieh BJ, Nichols M. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.