Abstract
Coronavirus disease 2019 (COVID-19) has become the greatest threat to human society in a century. To better devise control strategies, policymakers should adjust policies based on scientific evidence in hand. Several countries have limited the epidemics of COVID-19 by prioritizing containment strategies to mitigate the impacts on public health and healthcare systems. However, asymptomatic/pre-symptomatic transmission of COVID-19 complicated traditional symptom-based approaches for disease control. In addition, drastic population-based interventions usually have significant societal and economic impacts. Therefore, in Taiwan, the containment strategies consisted of the more extended case-based interventions (e.g., case detection with enhanced surveillance and contact tracing with active monitoring and quarantine of close contacts) and more targeted population-based interventions (e.g., face mask use in recommended settings and risk-oriented border control with corresponding quarantine requirement). The success of the blended approach emphasizes not only the importance of evidence-supported policymaking but also the coordinated efforts between the government and the people.
Keywords: Covid-19, Disease control, Transmission dynamics, Health policy, Quarantine, Isolation
1. Introduction
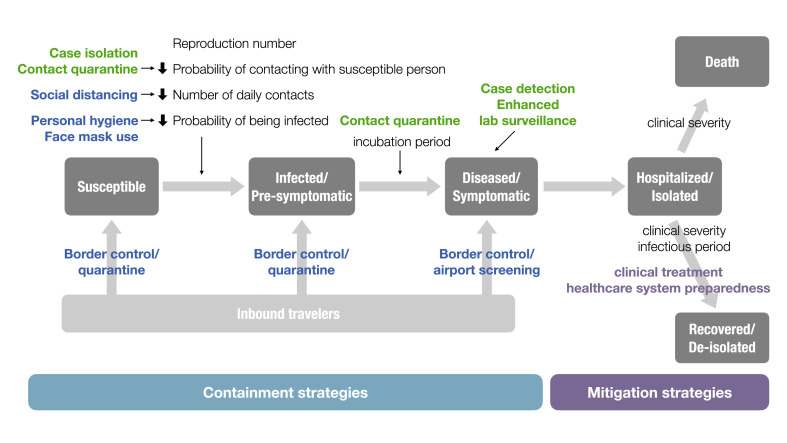
After the discovery of coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China, in December 2019, this novel disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become the most severe infectious disease threat in a century. Scientists and policymakers struggled to devise best practices, which we knew little about at the early stages of the pandemic, to stop the spread of the disease. Because effective pharmaceutical interventions were lacking, non-pharmaceutical interventions (NPIs), e.g., quarantine, social distancing, personal hygiene, and face mask use, become mainstays of COVID-19 control [[1], [2], [3], [4]]. Nevertheless, the widespread use of NPIs significantly disrupted normal life, especially when drastic interventions such as travel restrictions, city-wide lockdowns, shelter-in-place, or continued school closure, were implemented. These measures also had severe economic impacts, making sustainment of these control measures difficult. Modifying disease control strategies that strike a balance between COVID-19 control and extreme economic downturn requires understanding the key epidemiological parameters and transmission dynamics of COVID-19 (Fig. 1 ).
Fig. 1.
Conceptual diagram of key COVID-19 control measures adapting from the framework of Susceptible-Exposed (Infected)-Infectious-Recovered (SEIR) compartmental model.
Black texts indicate key epidemiological parameters. Colored texts indicate interventions targeting different epidemiological parameters or people at different stages of disease (green: case-based; blue: population-based; purple: interventions for mitigation). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2. R0 and case fatality rate: choosing between containment and mitigation strategies
While the estimated reproduction number (R0) in the initial outbreaks in Wuhan, China, and other countries was relatively high, ranging from 2.5 to 5.1, in outbreaks that started by disease introduction through the importation of cases, R0 was lower, from 2.1 to 3.2 [5]. The lowered R0 might have resulted from better awareness and knowledge about COVID-19 transmission, better preparedness, or corresponding interventions [6]. In settings where epidemics were under control, the effective reproduction number (Rt) could be reduced to < 1 [7,8]. Because COVID-19 and pandemic influenza have similar R0, clinical characteristics, and clinical severity [9], several countries attempted to develop control strategies based on their pandemic influenza preparedness plans and vacillated between containment (prevent community transmission) and mitigation (reduce disease burden) strategies (Fig. 1) [10].
Although countries with better preparedness and outbreak response may have a smaller R0, indicating the possibility to use mitigation strategies for disease control, the choice between containment and mitigation strategies is not dichotomous. Because of the lack of immunity to SARS-CoV-2 in the general population, public health and the healthcare system may be quickly overwhelmed from the exponential growth of patients when the disease is introduced into the community [11]. Early implementation of containment policies may spare public health and healthcare systems from being inundated, allowing communities to continue mitigation plans. However, mitigation of disease relies heavily on pharmaceutical interventions such as effective vaccines to alleviate disease burden. The lack of effective pharmaceutical treatments and vaccines for COVID-19 further decreases the possibility of implementing mitigation strategies alone to lessen the disease burden.
Therefore, several countries, such as South Korea, Australia, and New Zealand, chose to use “containment as mitigation” approach or “elimination strategy.” By prioritizing containment strategies, they successfully suppressed epidemics and preserved public health and healthcare capacity for mitigation [10,12].
3. Pre-symptomatic/asymptomatic transmission and its implication on policy
Because the most infectious period of Severe Acute Respiratory Syndrome (SARS) starts after symptom onset, SARS outbreak in 2003 was contained based on symptom-based approaches, including fever screening and isolation of symptomatic patients. However, COVID-19 is a different story. Both the virological and epidemiological studies suggested that the transmission of COVID-19 may start at least 2 days before symptom onset, peak soon after patients become symptomatic, and decrease to low levels after 10 days [[13], [14], [15], [16], [17], [18]]. In addition to disease transmission before symptom onset, there are also patients who have asymptomatic infections [[17], [18], [19], [20]]. Although there is little evidence of confirmed transmission caused by asymptomatic patients, most researchers still consider asymptomatic patients to have some degree of transmissibility, especially when patients were pauci-symptomatic, instead of being true asymptomatic [20,21].
Pre-symptomatic and asymptomatic disease transmission have policy implications:
First, the effectiveness of symptom- or test-based measures are reduced. Symptom- or test-based approach to identify COVID-19 patients usually cause longer delays for diagnosis. By the time patients have been diagnosed, onward transmission is likely to have occurred. This delay is further exacerbated in patients with severe clinical manifestations, which usually occur in the second week after disease onset [22,23]. Therefore, when patients present with fever, dyspnea, or other symptoms/signs of pneumonia at the clinic, they had already spent their most infectious period in the community. The opportunity of stopping onward transmission would have been missed. The first wave of COVID-19 in Europe and the Americas followed this pattern.
Second, pre-symptomatic/asymptomatic transmission points to the importance of more extensive approaches to disease control, including social distancing measures like movement restriction, school closures, restriction on mass gathering, or shelter-in-place. Pre-symptomatic and asymptomatic disease transmission also suggests the need to extend case-based interventions, such as the use of mass testing or quarantine requirement, especially for asymptomatic individuals at higher risk for infection.
3.1. Proactive response and blended approach in Taiwan
Because of the aforementioned epidemiologic characteristics, most countries that had delayed response failed to implement containment strategies during the tiny window of opportunity to reduce community transmission, and struggled to strike a balance between easing the social distancing restrictions and fighting against the re-surging epidemics.
Having learned the importance of surveillance from the SARS outbreak in 2003, Taiwan established comprehensive surveillance systems [24] and was one of the few countries that could initiate response at a very early stage of the COVID-19 outbreak [25]. The Taiwanese government decided to initiate more proactive responses, aiming for containment and thus mitigating the burden on public health and healthcare systems, right after the detection of COVID-19 outbreak in Wuhan [25]. Therefore, Taiwan was able to adopt containment measures and preserve the capacity for mitigation. In Taiwan, only around 500 cases were confirmed by the end of September 2020 with 7 deaths. No locally-acquired cases hav been identified since mid-April [51].
Critical measures for containment in Taiwan included two parts: 1) case-based interventions, including case detection via active and passive surveillance systems, contact tracing, and outbreak management; and 2) population-based interventions, including travel restriction, border control, social distancing, personal hygiene, and face mask use (Fig. 1) [26].
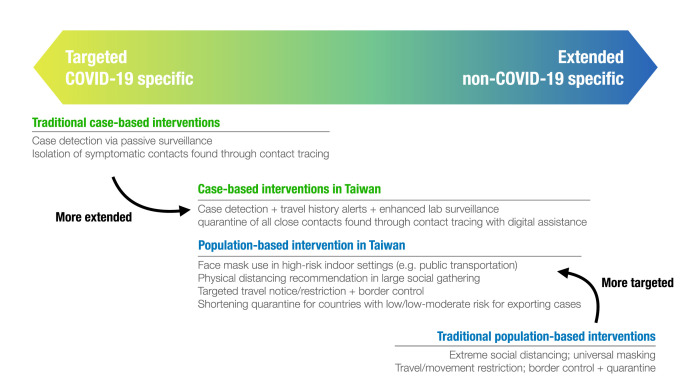
Because pre-symptomatic transmissions exist, traditional case-based interventions, such as contact tracing with health monitoring, would not be sufficient for COVID-19 control [[27], [28], [29]]. Wide-ranged population-based interventions, which indiscriminately prevent transmission at the pre-symptomatic and symptomatic phase (Fig. 1), might have greater effects but also cause significant societal and economic impacts [30]. Therefore, Taiwan’s blended approach aims to make the targeted case-based interventions more extended to cover pre-symptomatic/asymptomatic patients, and make the extended population-based approach more targeted to preserve public health and healthcare capacities (Fig. 2 ).
Fig. 2.
Key extended case-based and targeted population-based interventions for COVID-19 control in Taiwan.
3.2. Case-based interventions
3.2.1. Case detection through active travel history alert and enhanced laboratory surveillance
In Taiwan, case detection’s sensitivity and timeliness were improved by adapting and enhancing existing surveillance systems [24,[25], [51]] . COVID-19 was listed as a notifiable disease on January 15. Every patient who met the case definition, including clinical, epidemiological, and laboratory criteria, would be required to be reported to the National Notifiable Disease Surveillance System. A critical component of the epidemiological criterion was travel history. The first imported COVID-19 case had lived in Wuhan, China, and was detected in Taiwan on January 21, 2020 [31]. Travel history listed in the case definition expanded as the disease spread from Wuhan to other parts of China, Europe, the Americas, and the entire world by mid-March [25,26,32]. Immigration records were linked to the electronic medical records using the existing information infrastructure of the National Health Insurance System, and provided travel history alerts for physicians seeing patients in clinics [26]. The alerts significantly increased doctors’ awareness of patients’ travel history and the sensitivity of case detection.
As the number of locally-acquired cases increased, several enhanced laboratory surveillance measures were implemented to detect possible cases without relevant history [26]. Respiratory samples from patients with suspected novel influenza A infections, patients reported to have influenza infections with severe complications, and selected patients who presented with influenza-like illnesses at sentinel clinics, were tested for COVID-19. The first patient without clear source of infection was reported on February 16 through enhanced surveillance.
When the number of patients with unknown COVID-19 infection sources increased, the criteria for lab testing were extended to high-risk groups (e.g., healthcare workers) who had no relevant travel or exposure history. The time from patients’ disease onset to notification was kept at <3 days, indicating good efficiency and sensitivity of Taiwan’s surveillance system, and contributed to timely control of early outbreaks [33,[34], [51]].
3.2.2. Contact tracing with corresponding quarantine measures
In Taiwan, contact tracing was performed by case investigation teams that consisted of trained field epidemiologists and public health workers in central and local health departments. When a COVID-19 patient was confirmed, the case investigation team would investigate every possible contact. Close contact was a person who had face-to-face contact with a confirmed case for more than 15 min [15]. Contact tracing is usually completed within 48 h of case confirmation [35]. Contacts at higher risk for being infected (close contacts) were required to undergo 14-day quarantine with health monitoring. Contacts exposed in high transmission risk settings, e.g., households, hospitals, or in a cluster, may be tested for COVID-19, regardless of their symptoms, to detect cases earlier [15]. An electronic tracking platform was implemented to facilitate close contacts’ follow-up and reduced the workload of contact tracers [35].
3.3. Population-based interventions
Population-based interventions implemented in Taiwan included border control, travel restriction, social distancing, personal hygiene, and face mask use. These measures, especially travel restrictions, border control, and social distancing, were not explicitly targeted to COVID-19 cases. But, they usually have more extensive impacts on the society and the economy, and were not traditionally recommended as critical measures for epidemic control. Although some population-based interventions such as face mask use and travel restrictions did not generate attention at the beginning of the pandemic, recent studies have shown promising impacts [[36], [37], [38]].
3.3.1. Travel restrictions and border control
From the early stage of the pandemic, targeted border control and travel restrictions were implemented by rapid risk assessment for specific regions or countries, which aligned with the travel history listed in the case definition for surveillance in Taiwan [51]. The targeted approach gave public health professionals and laboratories time to scale up their capacity without being overwhelmed. Using this framework, travel restriction and border control extended from the city of Wuhan to the whole world in accordance with global disease spread in two months. A 14-day quarantine for all inbound travelers has been required since mid-March [26].
Because more than 90% of patients’ incubation periods fell within seven days after exposure and the disease have a short infectious period [[14], [15], [16]], the risk of being infectious beyond the 14-day quarantine periods was considered low [39]. After COVID-19 in Taiwan seemed to have been ameliorated, the Central Epidemic Command Center (CECC), which oversees COVID-19 response for Taiwan, devised a strategy of shortening the quarantine periods to five or seven days for inbound travelers arriving from countries with low or low-moderate risk of exporting COVID-19 cases from specific countries [40].
3.3.2. Face mask use and supply
Although no recommendation for universal masking was pronounced by CECC, Taiwanese already developed the culture of wearing face masks, especially when they have respiratory symptoms or fever, after the SARS outbreak in 2003. This time, CECC took several important measures to secure the supply of face masks and reduced the barrier to using face masks in daily life [26]. The impact of increased face mask use is evident by the decrease of influenza and other respiratory diseases, another unexpected effect of COVID-19 control in Taiwan [34,41].
3.3.3. Physical and social distancing
By proactively implementing border control with quarantine and case-based interventions, no large-scale social distancing measures, e.g., movement restriction, lockdown, school closures, or shelter-in-place was initiated in Taiwan. The start of the spring semester was once delayed by two weeks in February, but no other delays of school opening were required afterward.
The general public was constantly reminded to practice a physical distance of 1.5 m indoors and 1 m outdoors and avoided crowded places. When physical distancing could not be practiced, such as on public transportations, face mask use is obligatory. Restaurants have remained open, though some have added partitions or took out seats to help keep patrons in practicing physical distancing. No restriction on people’s movements such as lockdown has been implemented, but face mask use on public transportation and certain enclosed indoor settings were recommended when the pandemic seemed to be prolonged [26].
4. Discussion
Real-time surveillance and risk assessment, early proactive intervention, extended case-based interventions, and targeted population-based interventions, all contributed to Taiwan’s success in controlling COVID-19. These efforts could only be made by better understanding the transmission dynamics of this novel pathogen and the refinement of existing public health systems and resources.
As we contemplate strategies in wait for the next wave of COVID-19, we must first realize that Taiwan’s current case-based intervention success comes with a high price tag on human resources, especially for contact tracing, which is labor-intensive, despite the assistance of electronic management system. The laboratory testing program, which is vital in identifying cases for additional investigation, also has limited capacity [42]. The design of testing strategy affects the sensitivity and comprehensiveness of case detection, especially when pre-symptomatic and asymptomatic transmissions increase, because the sensitivity of molecular testing in identifying SARS-CoV-2 infection is time-dependent [42]. If and when cases increase, accurately defining and devising testing strategies for case identification followed by case investigation and contact tracing will help prioritize the use of limited human and laboratory resources. For example, a periodic testing program in the high-risk population, or applying the test for specific groups whose time of exposure could be assumed (e.g., contacts of confirmed cases or inbound travelers), might be considered when we would like to expand the testing program to identify more asymptomatic patients before onward transmission occur [43]. Even though the contact tracing program was already robust in Taiwan, scaling up during large epidemic would still be of concern. In addition to digital assistance, such as electronic management system or contact tracing app, to reduce the workloads for humans, more accurate risk stratification based on the infectious period and high-risk exposure setting could ameliorate the program [15,44]. For example, because the most infectious period of COVID-19 falls within the first week after symptom onset, modifying case isolation and contact quarantine policies could free up the public health and healthcare capacities [15,16]. Japan’s cluster-based approach might be an alternative as well when surge capacity is anticipated [45].
Given that the impacts of population-based intervention could be reflected by their collateral effects on influenza or other respiratory diseases, the epidemics in countries with low influenza activity seemed not to be fully relieved [4,[46], [47], [48]]. This observation suggests the combined case-based and population-based interventions might still be necessary to minimize community transmission. On the other hand, drastic population-based interventions such as travel restrictions, border control, extreme social distancing, and universal use of face masks have generated significant economic, societal, and ecologic impacts. Travel restrictions and border control still led to 0.6% of economic contraction in Taiwan [49]. When thrown away, disposable face masks become wastes, polluting the environment, if not disposed of properly. Although these measures’ effectiveness in easing the epidemic might be evident, the cost-effectiveness has not been well-documented, especially when costs other than medical care are factored in.
To prepare for the next wave, sustainable strategies should be devised. For countries that stopped the first wave of the COVID-19 outbreak through city-wide or even country-wide lockdowns, developing a strategy for re-opening and preparing for the next wave using modified case-based and population-based interventions, relied on more refined public health policy planning and resource allocation. Compiling previous experience, learning from other countries, and adapting infectious disease modeling could lead to more creative and flexible strategies [10]. One modeling study in Taiwan showed that case-based interventions complement population-based interventions, especially when R0 is still high [34]. Establishing a more robust case-based intervention program to reduce disease burden could also reduce the need for drastic population-based interventions, thus lessening the negative effects these policies cause. For Taiwan, the aforementioned approaches are mainly containment strategies, aimed at reducing disease transmission. However, other countries’ experiences have shown that widespread community transmission might be inevitable, especially when drastic NPIs such as border control or social distancing could not be sustained. Therefore, scaling up preparedness and response plans is an important task, especially in the coming influenza season [50]. Although the experience in southern hemisphere countries showed that one collateral effect of COVID-19 response was a skipped influenza season [48], scaling-up testing capacity, using appropriate laboratory tests to distinguish SARS-CoV-2 from other respiratory viruses efficiently, and triaging patients with respiratory infections, would be major challenges in the winter months.
Ultimately, the goal of case-based and population-based intervention policies is to protect our healthcare capacity, which sick patients would all require eventually. For patients to have quality healthcare, we first need to ensure that our clinics and hospitals have adequate human resources, personal protective equipment, and laboratory testing capacity. Optimizing the strategies, including identifying community cases via targeted testing, triaging patients of varying disease severities, and adjusting isolation and quarantine practices, require better and more accurate understanding of the transmission dynamics of COVID-19. By accumulating more empirical intervention data from different settings worldwide and utilizing the information in modeling studies, we can better inform policymaking.
5. Conclusion
Through a blended approach that combined extended case-based and targeted population-based interventions, Taiwan had successfully controlled the first wave of COVID-19. The experience in implementing evidence-supported disease control measures and allocating limited resources may lead us to soon find a new normal for the next phase.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank I-Chun Huang for providing the idea to construct the conceptual diagram of COVID-19 control strategies.
References
- 1.Davies N.G., Kucharski A.J., Eggo R.M., et al. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Heal. 2020;5:e375–e385. doi: 10.1016/s2468-2667(20)30133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai S., Ruktanonchai N.W., Zhou L., Prosper O., et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020:1–7. doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaxman S., Mishra S., Gandy A., et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020:1–8. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 4.Cowling B.J., Ali S.T., Ng T.W.Y., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. The Lancet Public Health. 2020 doi: 10.1016/s2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggerstaff M., Cowling B.J., Cucunubá Z.M., et al. Early insights from statistical and mathematical modeling of key epidemiologic parameters of COVID-19. Emerg. Infect. Dis. 2020;26(11):e1–e14. doi: 10.3201/eid2611.201074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali S.T., Wang L., Lau E.H.Y., et al. Serial interval of SARS-CoV-2 was shortened over time by nonpharmaceutical interventions. Science. 2020;369(6507):1106–1109. doi: 10.1126/science.abc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim E., Tariq A., Choi W., Lee Y., Chowell G. 2020. Transmission Potential and Severity of COVID-19 in South Korea.https://www.ijidonline.com/article/S1201-9712(20)30150-8/fulltext accessed October 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tariq A., Lee Y., Roosa K., et al. Real-time monitoring the transmission potential of COVID-19 in Singapore, March 2020. BMC Med. 2020;18:166. doi: 10.1186/s12916-020-01615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen E., Koopmans M., Go U., et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20(9):e238–e244. doi: 10.1016/s1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han E., Tan M.M.J., Turk E., et al. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396(10261):1525–1534. doi: 10.1016/s0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson N.M., Laydon D., Gilani G.N., et al. 2020. Impact of Non-pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand.https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf accessed October 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker M.G., Wilson N., Anglemyer A. Successful elimination of covid-19 transmission in New Zealand. N. Engl. J. Med. 2020;383(8):e56. doi: 10.1056/nejmc2025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wölfel R., Corman V.M., Guggemos W., et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature. 2020:1–10. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 14.He X., Lau E.H.Y., Wu P., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H.-Y., Jian S.-W., Liu D.-P., et al. Contact tracing assessment of COVID-19 transmission dynamics in taiwan and risk at different exposure periods before and after symptom onset. JAMA Internal Medicine. 2020;180 doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bullard J., Dust K., Funk D., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. 2020:ciaa638. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arons M.M., Hatfield K.M., Reddy S.C., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382(22):2081–2090. doi: 10.1056/nejmoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa N.W., Brooks J.T., Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann. Intern. Med. 2020;173:362–367. doi: 10.7326/m20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo L., Liu D., Liao X., et al. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in guangzhou, China: a prospective cohort study. Ann. Intern. Med. 2020:M20–2671. doi: 10.7326/m20-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J.-Y., Yun J.-G., Noh J.-Y., et al. Covid-19 in South Korea — challenges of subclinical manifestations. New Engl J Med. 2020 doi: 10.1056/nejmc2001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. Jama. 2020;323 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jian S.-W., Chen C.-M., Lee C.-Y., Liu D.-P. Real-time surveillance of infectious diseases: Taiwan’s experience. Health Security. 2017;15:144–153. doi: 10.1089/hs.2016.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng H.-Y., Li S.-Y., Yang C.-H. Initial rapid and proactive response for the COVID-19 outbreak — Taiwan’s experience. J. Formos. Med. Assoc. 2020;119:771–773. doi: 10.1016/j.jfma.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summers J., Cheng H.-Y., Lin H.-H., et al. Lancet Regional Heal - West Pac; 2020. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucharski A.J., Klepac P., Conlan A.J.K., et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect. Dis. 2020;20(10):1151–1160. doi: 10.1016/s1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellewell J., Abbott S., Gimma A., et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Global Heal. 2020;8(4):e488–e496. doi: 10.1016/s2214-109x(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peak C.M., Kahn R., Grad Y.H., et al. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study. Lancet Infect. Dis. 2020;20(9):1025–1033. doi: 10.1016/s1473-3099(20)30361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu I.Y., Alam P., Larson H., Lin L. Social consequences of mass quarantine during epidemics: a systematic review with implications for the COVID-19 response. J. Trav. Med. 2020;27(7):taaa192. doi: 10.1093/jtm/taaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng S.-C., Chang Y.-C., Chiang Y.-L.F., et al. First case of coronavirus disease 2019 (COVID-19) pneumonia in taiwan. J Formos Medical Assoc Taiwan Yi Zhi. 2020;119:747–751. doi: 10.1016/j.jfma.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C., Ng C., Brook R. Response to COVID-19 in taiwan. JAMA. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 33.Ng Y., Li Z., Chua Y.X., Chaw W.L., et al. Evaluation of the effectiveness of surveillance and containment measures for the first 100 patients with COVID-19 in Singapore — january 2–february 29, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:307–311. doi: 10.15585/mmwr.mm6911e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T.-C.V. Ng, H.-Y. Cheng, H.-H. Chang, , et al., Effects of Case- and Population-Based COVID-19 Interventions in Taiwan, MedRxiv 2020.08.17.20176255. 10.1101/2020.08.17.20176255. [DOI]
- 35.Jian S.-W., Cheng H.-Y., Huang X.-T., Liu D.-P. Contact tracing with digital assistance in Taiwan’s COVID-19 outbreak response. Int. J. Infect. Dis. 2020;101:348–352. doi: 10.1016/j.ijid.2020.09.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitze T., Kosfeld R., Rode J., Wälde K. 2020. IZA DP No 13319: Face masks considerably reduce COVID-19 cases in Germany: a synthetic control method approach.https://www.iza.org/publications/dp/13319/face-masksconsiderably-reduce-covid-19-cases-in-germany-a-synthetic-controlmethod-approach accessed October 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu D.K., Akl E.A., Duda S., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–19877. doi: 10.1016/s0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinazzi M., Davis J.T., Ajelli M., et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368 doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.S. Clifford, B.J. Quilty, T.W. Russell, et al., Strategies to reduce the risk of SARS-CoV-2 re-introduction from international travellers, MedRxiv 2020.07.24.20161281. 10.1101/2020.07.24.20161281. [DOI]
- 40.Taiwan CDC . 2020. Regulations Concerning Short-Term Business Travelers’ Applications for Shortened Quarantine Periods in Taiwan.https://www.cdc.gov.tw/En/File/Get/jb7gs02V5GixG0F7yhSigA accessed October 1, 2020. [Google Scholar]
- 41.Kuo S.-C., Shih S.-M., Chien L.-H., Hsiung C.A. Collateral benefit of COVID-19 control measures on influenza activity, taiwan. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2608.201192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications. N. Engl. J. Med. 2020;383 doi: 10.1056/nejmp2015897. [DOI] [PubMed] [Google Scholar]
- 43.Grassly N.C., Pons-Salort M., Parker E.P.K., et al. Comparison of molecular testing strategies for COVID-19 control: a mathematical modelling study. Lancet Infect. Dis. 2020 doi: 10.1016/s1473-3099(20)30630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cevik M., Marcus J.L., Buckee C., Smith T.C. SARS-CoV-2 transmission dynamics should inform policy. Clin. Infect. Dis. 2020:ciaa1442. doi: 10.1093/cid/ciaa1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshitani H. Cluster-based approach to coronavirus disease 2019 (COVID-19) response in Japan—February–April 2020. Jpn. J. Infect. Dis. 2020 doi: 10.7883/yoken.jjid.2020.363. [DOI] [PubMed] [Google Scholar]
- 46.Lee H., Lee H., Song K.-H., et al. Impact of public health interventions on seasonal influenza activity during the SARS-CoV-2 outbreak in Korea. Clin. Infect. Dis. 2020:ciaa672. doi: 10.1093/cid/ciaa672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soo R.J.J., Chiew C.J., Ma S., Pung R., Lee V. Decreased influenza incidence under COVID-19 control measures, Singapore - volume 26, number 8—august 2020 - emerging infectious diseases journal - CDC. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2608.201229. 1933–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen S.J., Azziz-Baumgartner E., Budd A.P., et al. Decreased influenza activity during the COVID-19 pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasell J. 2020. Which Countries Have Protected Both Health and the Economy in the Pandemic?https://ourworldindata.org/covid-health-economy?fbclid=IwAR0oC1xh3vJYctg1oQfVHYmWRRHr_zC0znn8MgtGvf4D2bitSfKGk_yhSLs accessed October 11, 2020. [Google Scholar]
- 50.Rubin R. What happens when COVID-19 collides with flu season? JAMA. 2020;324 doi: 10.1001/jama.2020.15260. [DOI] [PubMed] [Google Scholar]
- 51.Cheng H.-Y., Chueh Y.-N., Chen C.-M., et al. Taiwan’s COVID-19 Response: Timely Case Detection and Quarantine, January to June 2020. J Formos Med Assoc. 2020 doi: 10.1016/j.jfma.2020.10.023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]