Abstract
Population-level herd immunity is critical for long-term control of SARS-CoV-2. However, proposals to reach the herd immunity threshold through naturally acquired infection, rather than vaccination, have complicated public health efforts and popularized policies that will lead to widespread transmission and mortality. Vaccination is the only viable path to herd immunity.
Population-level herd immunity is critical for long-term control of SARS-CoV-2. However, proposals to reach the herd immunity threshold through naturally acquired infection, rather than vaccination, have complicated public health efforts and popularized policies that will lead to widespread transmission and mortality. Vaccination is the only viable path to herd immunity.
Main Text
Of the many strategies that have been proposed for controlling the global coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), herd immunity through natural infection may be the most deeply flawed and outright dangerous. In early October, three scientists issued a document called the Great Barrington Declaration, calling for “focused protection” of the vulnerable, while encouraging young, healthy people to otherwise resume their normal lives1. The justification given for this approach is that the whole community would benefit from the protection conferred upon the more vulnerable by others that have built up herd immunity. This has influenced public health policy in the United States, as the authors of the declaration met with members of the White House Coronavirus Task Force and compelled them to advocate for a focused protection strategy. In practice, this has resulted in minimal federal guidance to encourage essential interventions, such as face masks and distancing, intended to reduce transmission. These unfortunate developments in the pandemic response are based on a fundamental misunderstanding of herd immunity and how it is achieved. Herd immunity has never been achieved through naturally acquired infections and is only possible at global population scale through mass immunization.
Herd immunity is based on our understanding of viral pathogens as obligate intracellular parasites that require a host for replication. If enough people are immune to infection, then the virus cannot be transmitted to new susceptible hosts and will be eliminated from circulation within the population. When a sufficient proportion of the population are immune and thus thwart the pathogen’s ability to circulate, that population has reached the herd immunity threshold. Throughout history, consequential human pathogens that caused debilitating disease, such as smallpox and polio, have circulated throughout the population for centuries or millennia without ever reaching this threshold. They have only been vanquished through immunization campaigns that have required years of effort and investment.
Herd immunity is a relatively recent concept, and some have taken umbrage at the term as it equates human populations with animals. However, this reflects the origin of the term, which was originally coined by livestock veterinarians in the early 20th century referring to epidemics of “contagious abortion,” or pathogens that caused spontaneous miscarriages in herds of cattle and sheep. By the 1950s, the term was applied to newly developed vaccines and their potential for preventing widespread viral diseases such as polio at population scale2. As herd immunity as a concept became more broadly associated with immunization campaigns, it gained that specific meaning. Until recently, herd immunity generally referred to population immunity acquired through vaccination.
The recent reversion of the term to its original context—immunity acquired through infection or immunization—has created a host of misconceptions about how the herd immunity threshold might be reached for SARS-CoV-2. The prospect of reaching herd immunity through natural infection is not an expeditious process, in part because of the relationship between the herd immunity threshold and the basic reproduction number (R0). R0 measures the average number of secondary infections caused by one infected person in a population of completely susceptible individuals. In the most basic terms, the herd immunity threshold is defined mathematically as 1-1/R0 3. Given that estimates of R0 throughout the SARS-CoV-2 pandemic around the world have ranged from 2 to 3 in the absence of interventions to reduce transmission, the herd immunity threshold is estimated to be in the range of 50%–67%4.
However, R0 is not a static number, making the herd immunity threshold difficult to estimate. R0 is not solely determined by viral infectivity and virulence and rarely reflects the variables present in the real world. Interventions intended to reduce transmission can reduce R0 substantially, as can many variables that influence susceptibility, including genetic traits, receptor distribution, and immune status of the host. Furthermore, even in populations that are completely susceptible, they do not remain completely susceptible over time as a pathogen spreads through the population. For SARS-CoV-2, R0 has varied by country and region, depending on the intervention measures applied in those locations. Although substantial spread has occurred in heavily affected countries, even in the United States, which leads the world in COVID-19 cases, large seroprevalence studies have indicated that seroprevalence was generally less than 10% after the first surge in spring 20205. Current CDC estimates suggest that seroprevalence around the United States does not exceed 20%–25% in heavily affected states such as New York and is much lower throughout most of the rest of the country6. While seropositivity alone is not proof of protective immunity, the low overall seroprevalence suggests that the majority of people have not been exposed. Reaching herd immunity through naturally acquired infection will at minimum require doubling or tripling the number of cases and require at least 2–3 years, and possibly longer should community transmission decrease. As more than 270,000 people in the United States have died of COVID-19 and millions report persistent long-haul symptoms after recovery, attempting to reach herd immunity in this way would be catastrophic.
Furthermore, relying on natural infection rather than vaccination to reach the herd immunity threshold assumes that infection and vaccination induce comparable immune responses with similar durability. There is growing evidence that this is not the case. Many pathogenic viruses, including SARS-CoV-2, inhibit the activity of type I interferons, which drive innate antiviral responses that are critical to both initial suppression of virus replication and subsequent induction of robust adaptive immunity. SARS-CoV-2 infection profoundly suppresses type I and type III interferons compared to influenza A virus in vivo 7. Systemic suppression of type I interferon is associated with severe COVID-198, which is also linked in multiple studies to lymphopenia9. As type I interferons drive Th1 polarization, which enhances both neutralizing antibody and CD8+ cytotoxic T cell responses, it is reasonable to hypothesize that the subversion of these responses could impact adaptive mechanisms of viral clearance and the development of immunological memory. Although most COVID-19 patients do develop detectable antibody responses, multiple studies have observed that serum antibody titers may rapidly decline within months, for SARS-CoV-2 as well as other coronaviruses10. While the significance to functional immune protection is unknown, it does suggest that SARS-CoV-2 infection may result in atypical long-term immune responses. A recent study showed substantial depletion of follicular CD4+ T cells and a loss of germinal centers in the lymph nodes and spleens of patients who died of COVID-19, and this was accompanied by severely reduced follicular B cells11. As follicular CD4+ T cells within germinal centers are necessary for differentiation of memory B cells, this finding suggests a possible mechanism by which SARS-CoV-2 infection may impair the development of long-lasting, durable immunity.
Immunity induced by vaccination is likely to produce very different responses. The current vaccine candidates in late-stage clinical trials do not cause SARS-CoV-2 infection, therefore will not interfere with or evade either innate or adaptive immune responses. Although several of the candidates are viral-vectored vaccines, these undergo an abortive, non-pathogenic replication cycle and do not suppress these immune responses. Therefore, they are less likely to result in subversion of memory immune responses. Data from pre-clinical and phase 1/2 trials support this finding: while infection results in a wide spectrum of antibody titers, immunization consistently produces neutralizing titers comparable to the highest titers seen in convalescent patients12 , 13. While there is not yet data on how this impacts durability, it suggests that immune responses elicited by vaccines are fundamentally distinct from those produced by naturally acquired infection. Thus, reaching herd immunity through immunization rather than infection will not only occur more quickly and with vastly less morbidity and mortality, it will likely result in greater functional immune protection for a longer duration of time (Figure 1 ).
Figure 1.
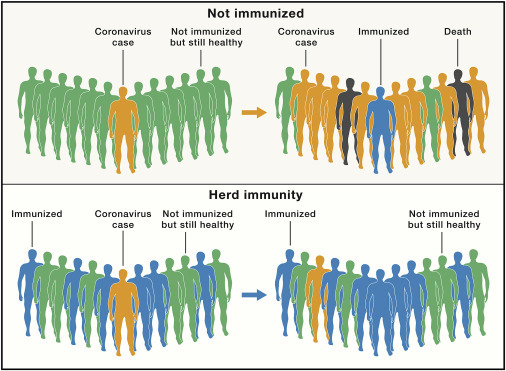
SARS-CoV-2 Spread in Populations with Different Susceptibility
SARS-CoV-2 spread in a susceptible population (top panel) and in a population that has reached the herd immunity threshold (lower panel).
Many questions remain about how herd immunity will contribute to the ultimate control of the SARS-CoV-2 pandemic and the long-term prospects for preventing future outbreaks. However, several facts are abundantly clear. Although vaccines, when available, will require months to distribute and tremendous efforts to overcome vaccine hesitancy, they still will reach the herd immunity threshold, whatever that may be, in far less time than natural infection would permit. They may produce more robust, longer-lasting, and more protective immune responses than infection. Most importantly, decades of reliable research demonstrate that vaccines are a safe and highly effective means of preventing widespread infectious diseases and are the only morally and scientifically acceptable approach for achieving herd immunity at national or global scale. Attempting to reach herd immunity through natural infection will result in devastating losses of both life and quality of life for those infected and are completely insupportable as a public health strategy for controlling a generational pandemic.
Acknowledgments
Declaration of Interest
The author declares no competing interests
References
- 1.Kulldorf M., Gupta S., Bhattacharya J. 2021. Great Barrington Declaration.https://gbdeclaration.org/ [Google Scholar]
- 2.Jones D., Helmreich S. A history of herd immunity. Lancet. 2020;396:810–811. doi: 10.1016/S0140-6736(20)31924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R.M., May R.M. Vaccination and herd immunity to infectious diseases. Nature. 1985;318:323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- 4.Omer S.B., Yildirim I., Forman H.P. Herd immunity and implications for SARS-CoV-2 control. JAMA - JAMA. 2020 doi: 10.1001/jama.2020.20892. [DOI] [PubMed] [Google Scholar]
- 5.Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., Fry A.M., Cannon D.L., Chiang C.F., Gibbons A. Seroprevalence of antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May12, 2020. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- 6.CDC COVID Data Tracker https://covid.cdc.gov/covid-data-tracker/#national-lab.
- 7.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Carbit B., Bondet V., Chenevier-Gobeaux C. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020 doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko N., Kuo H.H., Bocau J., Farmer J.R., Allard-Chamard H., Mahajan V.S., Piechocka-Trocha A., Lefteri K., Osborn M., Bals J. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020;183:143–157.e13. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020 doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y., Dai T., Wei Y., Zhang L., Zheng M., Zou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020 doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


