Abstract
No specific drugs have been approved for coronavirus disease 2019 (COVID-19) to date as the development of antivirals usually requires time. Therefore, assessment and use of currently available antiviral drugs is critical for a timely response to the current pandemic. Here, we have reviewed anti-SARS-CoV-2 potencies of available antiviral drug groups such as fusion inhibitors, protease inhibitors, neuraminidase inhibitors, and M2 ion-channel protein blockers. Although clinical trials to assess the efficacy of these antivirals are ongoing, this review highlights important information including docking and modeling analyses, in vitro studies, as well as results from clinical uses of these antivirals against COVID-19 pandemic.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Antiviral
Abbreviations
- 3CLPro
3C-like cysteine protease
- AAK1
AP2-associated protein kinase 1
- ACE2
angiotensin-converting enzyme 2
- CMV
cytomegalovirus
- COVID-19
coronavirus disease 2019
- E
envelope protein
- ERGIC
endoplasmic reticulum-Golgi apparatus compartment
- HA
hemagglutinin envelope glycoprotein
- HAV
hepatitis A virus
- HCV
hepatitis C virus
- HE
hemagglutinin-esterase
- HSV
herpes simplex virus
- ICU
intensive care unit
- INF-β
interferon
- M
membrane protein
- MERS-CoV
Middle East respiratory syndrome coronavirus
- Mpro
main protease
- N
nucleocapsid
- NNRTI
non-nucleoside reverse-transcriptase inhibitors
- NRTI
nucleoside reverse-transcriptase inhibitor
- NRTTI
nucleoside reverse transcriptase translocation inhibitors
- Nsp
non-structural protein
- NtRTI
nucleotide reverse-transcriptase inhibitor
- Ppro
papain-like protease
- R0
reproductive number
- RdRp
RNA-dependent RNA polymerase
- S
glycoprotein spike
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TMPRSS2
transmembrane serine protease 2
1. Introduction
The current coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major threat to human civilization and leaves many challenges ahead.1 , 2 As of July 11, 2020, there were more than 12 million confirmed cases of COVID-19, with more than 500,000 deaths.3 The most common symptoms of COVID-19 include fever, dry cough, dyspnea, chest pain, fatigue and myalgia, whereas headache, dizziness, abdominal pain, diarrhea, nausea, and vomiting are less commonly observed.4 Although most of the SARS-CoV-2 infections are asymptomatic or have mild clinical symptoms, 20.3% of the hospitalized patients require intensive care unit (ICU) admission, resulting in a significant burden on healthcare facilities.5 The disease severity, in part, seems to be associated with dysregulation of the host immune response.6 The basic reproductive number (R0) of SARS-CoV-2 is higher than that of SARS Coronavirus (SARS-CoV)7 with a mortality rate of up to 6.2% as of April 13, 2020.8
SARS-CoV-2 belongs to the family Coronaviridae, subfamily Coronavirinae and genus Betacoronavirus, along with SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV).9 , 10 SARS-CoV-2 has a spherical enveloped particle-containing positive-stranded RNA that binds to the nucleocapsid (N) inside the membrane protein (M) and the envelope (E) comprises of glycoprotein spikes (S).11 S protein is a primary receptor-binding domain (RBD) and is critical for viral entry into the host cells through cellular receptor angiotensin-converting enzyme 2 (ACE2).12 , 13 Similar to other viruses, SARS-CoV-2 hijacks the host cell machinery and multiplies via viral attachment, fusion, penetration, uncoating, transcription, translation, and virion release.14, 15, 16, 17, 18
Specific effective drugs against SARS-CoV-2 have not yet been discovered and no specific drug has been approved for the treatment of COVID-19. Rapid assessment of the currently available antiviral drugs to be used for COVID-19 patients is therefore crucial in this time of crisis as well as discovering newer drugs.5 , 19 Since the virus hijacks the host system via attaching to and then penetrating the host cells, followed by further critical steps (uncoating, reverse transcription, transcription, translation, and releasing of the virion), the principal target of antiviral drugs is to block the viral replication cycle at any of these stages. Currently, there are more than eighty antiviral drugs available and approved for treating viral infections in humans.20 Over 50% of these drugs are used to treat HIV infection, with the rest being used against influenza A and B, Ebola virus, cytomegalovirus (CMV), hepatitis A and C virus (HAV and HCV), and herpes simplex virus (HSV). In the current pandemic, some available antivirals have been used to treat COVID-19 cases in some countries.21 , 22 Since clinical trials to assess the efficacy of available antivirals for COVID-19 are still ongoing, the types of antivirals being used globally vary widely. This review summarizes antiviral drugs that can be potentially used for SARS-CoV-2 infection including the rationales, docking and modeling analysis, in vivo and in vitro findings, as well as results from new investigational drug protocols and clinical trials during this emergency and crisis.
2. SARS-CoV-2 life cycle and potential targets: The rationales
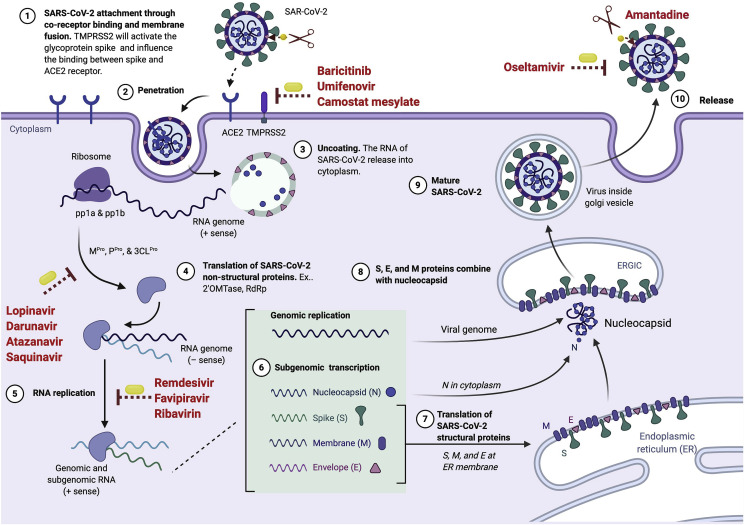
Major biochemical events and components in the replication cycle of coronavirus are considered as targets against which antiviral drugs are currently being developed. These include the spike protein, proteolytic enzymes, and RNA dependent RNA polymerase.23 SARS-CoV-2 is transmitted among humans mainly via respiratory droplets, although it may also follow an airborne transmission mode.24 , 25 The virus enters the host cells through two pathways, either via endosomes or plasma membrane fusion. In both mechanisms, the viral S protein mediates attachment to the membrane of the host cell and engages angiotensin-converting enzyme 2 (ACE2) as the entry receptor26, 27, 28, 29 (Fig. 1 ). A recent study showed the attachment between S protein and ACE2 is activated by a host protease called transmembrane serine protease 2 (TMPRSS2).26 , 30 The virus uses S protein to neutralize antibodies, making it easier to bind to the host receptors.31 Although the detailed fusion machinery of SARS-CoV-2 is not fully understood, Betacoronavirus mostly use hemagglutinin-esterase (HE) to link to sialic acid on the glycoprotein surface.32 , 33 These fusion steps could be inhibited using fusion inhibitors (Fig. 2 ).
Fig. 1.
The life cycle of SARS-CoV-2 and possible inhibition targets of antiviral drugs. Fusion inhibitors inhibit the fusion process of viral entry, while protease inhibitors target some proteases. Transcription inhibitors target reverse transcription step by blocking RNA-dependent RNA polymerase and therefore prevent viral replication. Some of the transcriptase inhibitors are nucleoside reverse-transcriptases. Some antivirals target M2 channel protein.
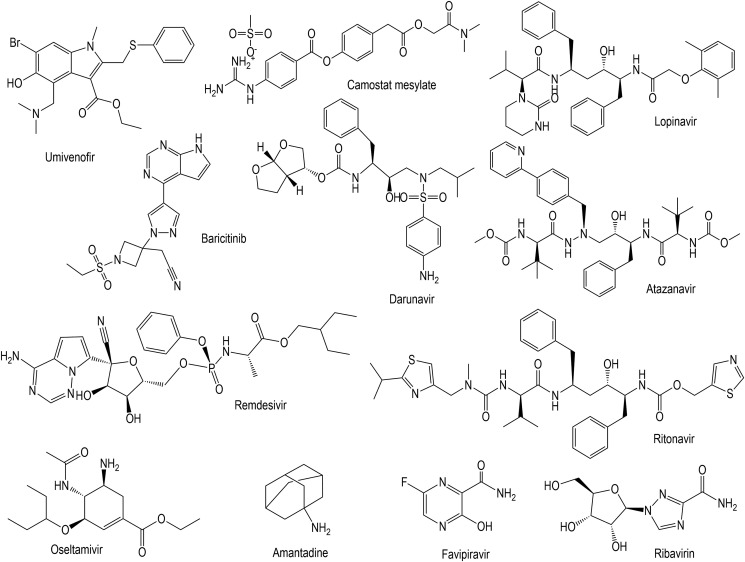
Fig. 2.
Structures of selected antiviral drugs that have therapeutic potential against SARS-CoV-2. Baricitinib, umifenovir and camostat mesylate are fusion inhibitors while lopinavir darunavir and atazanavir are protease inhibitors. Reverse transcription inhibitors such as remdesivir, favipiravir (Avigan) and ribavirin, neuraminidase inhibitors such as oseltamivir and M2 ion-channel protein blockers (amantadine) are potential against SARS-CoV-2.
After the completion of fusion, the envelope is peeled off, and the genome of SARS-CoV-2, along with its nucleocapsid, penetrates the host cell cytoplasm (Fig. 1).26 , 34 , 35 Its genome contains open reading frames 1a and 1b (ORF1a and ORF1b) genes that produce two polyproteins (pp), called pp1a and pp1b, that help in hijacking host ribosomes for viral translation process.36 , 37 These polyproteins are then cleaved by main protease (Mpro) and papain-like protease (Ppro) to produce several non-structural proteins.38 Beside Mpro and Ppro, 3C-like cysteine protease (3CLPro) has also been suggested to exist in SARS-CoV-2 based on 96% similarity with SARS-CoV using a three-dimensional analysis model.39 Theses proteases are essential for viral replication and transcription40 and protease inhibitors inhibiting these proteases (Fig. 2) are potential antivirals for SARS-CoV-2.
Subsequently, the replication process of the SARS-CoV-2 virus begins. Since the complete mechanisms of SARS-CoV-2 have not been thoroughly studied yet, the replication of SARS-CoV-2 can be explained based on SARS-CoV and MERS-CoV models. This is because the list of structural and non-structural proteins of SARS-CoV-2 is similar to those in these two viruses.14 A non-structural protein, called nsp12 forms a replication and transcription complex called RNA-dependent RNA polymerase (RdRp).41 In SARS-CoV, nsp12 associates with its cofactor (nsp7 and nsp8)42 , 43 and this protein complex produces a complementary negative-sense RNA using the original positive RNA as a template. The negative-strand RNA is then used by viral replicase to synthesize new positive RNA molecules to process another translation and replication step to form the genome of the newest viral particles.44 In SARS-CoV, topoisomerase III-beta mediates this process.45 These stages may be disrupted using reverse transcription inhibitors.
Post-translational modification is required for assembly and budding of the enveloped virus. The sub-genomic RNA forms a structural protein complex including S, E, M and N.46 S, E, and M then enter the endoplasmic reticulum.46 The positive-strand RNA and N form a nucleoprotein complex in the cytoplasm.47 Both complexes merge to complete the virus copy production in the endoplasmic reticulum-Golgi apparatus compartment (ERGIC). They are excreted to the extracellular region through the Golgi apparatus and vesicles as a mature virus and released from the cells to infect other cells.48
3. Antivirals for SARS-CoV-2 infection: results from labs, trials and patients
3.1. Fusion inhibitors
Fusion inhibitor is a group of antivirals that inhibit the fusion process during viral entry into the host cells (Fig. 1). Several drugs are available with umifenovir and camostat mesylate (Fig. 2) demonstrating antiviral activity against SARS-CoV-2.49, 50, 51, 52
Baricitinib
Similar to other viruses, SARS-CoV-2 enters the host cells through receptor-mediated endocytosis. The process of endocytosis is regulated by AP2-associated protein kinase 1 (AAK1).39 Therefore, the disruption of AAK1 will not only block the viral entry but also the intracellular viral assembly.39 Baricitinib is a Janus kinase (JAK) inhibitor with high potential to bind to and inhibit AAK1.53 Hence baricitinib can be used to inhibit both viral entry as well as the inflammatory response associated with SARS-CoV-2 infection (Fig. 2).53 JAK inhibitors such as ruxolitinib and fedratinib that are closely related to baricitinib inhibited clathrin-mediated endocytosis at higher doses and hence these may not be effective in reducing the viral infectivity at tolerable doses.54 Therapeutic use of baricitinib is associated with the occurrence of neutropenia, lymphocytopenia, and viral reactivation.55 Since SARS-CoV-2 infected patients have a lower absolute lymphocyte count, use of baricitinib may increase the incidence of co-infection.55 Further studies are required to analyze the risk-benefit ratio as well as the clinical utility of baricitinib therapy.
Umifenovir
Umifenovir, also called arbidol, is a nucleoside antiviral targeting the hemagglutinin envelope glycoprotein (HA) in the fusion machinery of influenza virus.56 A recent study reported that umifenovir monotherapy to COVID-19 patients in China resulted in negative viral conversion where the virus was not detected in 14 days.57 Randomized clinical trials are underway to assess the efficacy of umifenovir in China.58 , 59 Arbidol and arbidol mesylate compounds have shown inhibitory effects on SARS virus-replication under in vitro conditions and are currently under trial to ascertain their therapeutic potentials in treating pneumonia caused due to SARS-CoV-2 in COVID-19 patients.49 , 50
Camostat mesylate
Camostat mesylate – a serine protease inhibitor – is another candidate drug that targets the fusion step in viruses. SARS-CoV-2 gains entry within the target host cells either through ACE-2 receptor and/or TMPRSS2 receptors, and camostat mesylate acts as a TMPRSS2 inhibitor.60 It downregulates expression of SARS-CoV-2 spike (S) protein to prevent surface fusion and thereby blocks the cellular entry of the virus.51 , 52 A previous study found that camostat mesylate prevented SARS-CoV entry into human bronchial epithelial cells.61 Another in vitro study showed that camostat mesylate and E-64d (a cysteine protease inhibitor) could efficiently block TMPRSS2 binding of SARS-CoV-2.26 Clinical trials are ongoing to assess the effectiveness of a combination therapy of hydroxychloroquine and camostat mesylate vis-a-vis hydroxychloroquine alone in Denmark62 and Germany.63
Another serine protease inhibitor, nafamostat mesylate was found to possess 15-fold higher efficiency in inhibiting the entry of SARS-CoV-2 virus into host cells.64 Hence due to its more potent antiviral activity and favorable safety profile, nafamostat mesylate can be considered as a better alternative to camostat mesylate.64 Nafamostat mesylate is also used to treat disseminated intravascular coagulation (DIC). Hence, it will be further beneficial in managing the DIC with enhanced fibrinolysis seen in COVID-19 patients.65
3.2. Protease inhibitors
Some protease inhibitors such as lopinavir, darunavir, and atazanavir have the potential to be used against COVID-1957, 66, 67 (Fig. 2). Computer-aided drug design techniques can be used for identifying potential drug repurposing candidates against viral proteases. In a computational drug repurposing study, drugs such as carfilzomib, valrubicin, eravacycline, lopinavir, and elbasvir were found to inhibit the main protease in SARS-CoV-2.68 Further in vitro and in vivo studies are required to confirm the efficacy of these drugs.
Lopinavir
Currently, lopinavir is used in combination with ritonavir for treatment and prevention of HIV infection. It has been reported that lopinavir inhibited SARS-CoV-2 at a half-maximal effective concentration (EC50) - the level of a drug that induces a response halfway between the baseline and maximum after a specified exposure time - of 26.36 μM.69 Administration of lopinavir as an emergency drug in China increased the eosinophil count among COVID-19 patients.70 In an in silico study, a combination of lopinavir and ritonavir – both used as HIV protease inhibitors – inhibited the main protease (MPro) of SARS-CoV-2 71. A previous study showed that a specific combination of lopinavir-ritonavir, known as Kaletra® demonstrated antiviral effects against SARS-CoV both in vitro and in clinical trials.72 Therefore, the lopinavir-ritonavir combination is being used as an emergency treatment for COVID-19 patients in some countries73 , 74 (Table 1 ). Lopinavir-ritonavir alone or in combination with interferon (INF)-β – an inflammation regulator molecule – have been listed by WHO as options for “solidarity” clinical trial for COVID-19.75 Ritonavir-lopinavir commination could reduce the viral load and improve the clinical symptoms of COVID-19.57 Combination of ritonavir-lopinavir and umifenovir also substantially halted the progression of lung damage too.76 In one study, lopinavir-ritonavir treatment was associated with a better outcome but did not significantly accelerate the clinical improvement of severe COVID-19 infection.74 Although the efficacy of lopinavir has not been assessed for COVID-19, ritonavir-lopinavir combination has been used in treating COVID-19 cases in some countries such as the USA,77 Singapore,78 Japan79 and other countries that follow International Pulmonologists consensus80 as emergency response measures (Table 1). Clinical trials are ongoing to assess the efficacy of lopinavir-ritonavir for COVID-19 in China,81 Canada,82 Spain,83 France,84 Hong Kong,85 Thailand,86 and the US.87
Table 1.
Current use of existing antiviral drugs for COVID-19.
| Class of drug | Current application | US FDA approved for current application | Emergency use for COVID-19 |
|---|---|---|---|
| Fusion inhibitor | |||
| Umifenovir (Arbidol) | Influenza | No | Singapore, China |
| Protease Inhibitor | |||
| Lopinavir | HIV | Yes (September 2000) | USA, Japan, Singapore, Italy, China, IPC (Lopinavir-Ritonavir fix dose) |
| Darunavir | HIV-1 | Yes (July 2016) | Italy (Darunavir-Ritonavir fix dose) |
| Atazanavir | HIV-1 | Yes (July 2003) | Singapore |
| Saquinavir | HIV-1 | Yes (December 1995) | Singapore |
| Nucleoside reverse transcriptase inhibitor | |||
| Emtricitabine | HIV-1 | Yes (July 2003) | Singapore (Emtricitabine-Tenofovir fix dose) |
| Azvudine | HIV-1 | No | Singapore |
| Nucleotide reverse transcriptase inhibitor | |||
| Remdesivir | Ebola | Not yet | WHO, IPC, USA, Singapore, Italy |
| Favipiravir (Avigan) | Influenza | Not yet | Singapore, Japan, Indonesia |
| Ribavirin | HCV | Yes (April 2004) | Singapore, IPC |
| Sofosbuvir | HCV | Yes (December 2013) | Singapore |
| Neuraminidase inhibitor (Virus release inhibitor) | |||
| Oseltamivir (Tamiflu) | Influenza A & B | Yes (December 1999) | IPC, Singapore, Indonesia |
Darunavir
Darunavir, an anti-HIV drug, has been recommended for COVID-19 treatment in Italy.88 It is used in a combined regimen along with cytochrome P-450 inhibitors like ritonavir or cobicistat and in vitro studies have demonstrated their replication inhibitory effect against SARS-CoV-2.66 A clinical trial in Thailand is underway to assess the effectiveness of darunavir combination with other antivirals and hydroxychloroquine for COVID-19 patients.86 A combination of darunavir and cobicistat is also being tested in an ongoing clinical trial in China.89 A fixed-dose combination of darunavir and cobicistat, known as PREZCOBIX®, is also being used to treat COVID-19 (Table 1).90 Recently, HIV positive patients who were already under treatment with darunavir, were found to be infected with COVID-19, raising concerns over the efficacy of this HIV protease inhibitor.91 This suggests that darunavir might not be effective in preventing SARS-CoV-2 infection at the current adopted dosage of 800 mg.91
Atazanavir
An in silico study showed that atazanavir bound more strongly to the active site of SARS-CoV-2 MPro as compared to lopinavir67 and an in vitro study found that atazanavir inhibited SARS-CoV-2 replication.67 A previous study on HIV infected patients showed that a combination of atazanavir with ritonavir improved glucose uptake and lipid parameters and decreased fasting glucose more effectively as compared to lopinavir-ritonavir combination.92 , 93 This suggests that atazanavir might be an alternative for lopinavir when combined with ritonavir for COVID-19 treatment; however, further study is warranted. Currently, this antiviral drug is as an option for COVID-19 treatment (Table 1).
Saquinavir and other protease inhibitors
Saquinavir and other protease inhibitors such as indinavir, amprenavir, and nelfinavir might also display similar effects against COVID-19 like protease inhibitors mentioned earlier, due to a high degree of similarity between the structures (Fig. 1). An in silico study demonstrated that saquinavir and indinavir inhibited 3CLPro activity in SARS-CoV-2.94 Another study showed that saquinavir, indinavir, amprenavir and nelfinavir inhibit SARS-CoV-2 in vitro 95 with nelfinavir showing the best inhibition in comparison with others.95 Saquinavir has been used in treatment of COVID-19 patients in Singapore (Table 1). In another computational study, two more candidates were identified, raltegravir and paritaprevir that showed the potential to inhibit 3CLPro activity in SARS-CoV-2.96 Recently, potential anti-viral phytochemicals that have activity against the 3CLpro of SARS-CoV-2 were identified while screening medicinal plant library and the top antiviral candidates identified can be further evaluated using in vitro studies.97
3.3. Reverse transcription inhibitors
Another strategy to combat SARS-CoV-2 infection involves targeting the reverse transcription step by blocking RdRp and therefore preventing viral replication (Fig. 1). A few potential inhibitors are nucleoside reverse-transcriptase inhibitors (NRTIs), nucleotide reverse-transcriptase inhibitors (NtRTIs), non-nucleoside reverse-transcriptase inhibitors (NNRTIs) and nucleoside reverse transcriptase translocation inhibitors (NRTTIs).
Remdesivir
Remdesivir is a monophosphoramidate prodrug with a molecular mass of 602.6 g/mol and chemical formula C27H35N6O8P. Designated as GS-5734, remdesivir is a nucleotide analog possessing a wide spectrum of antiviral properties against majority of the single stranded RNA viruses like coronaviruses (including MERS-CoV and SARS-CoV-2) Lassa fever virus, Junin virus, Ebola-Marburg virus, respiratory syncytial virus, Nipah virus and Hendra virus.98 , 99 After entering the host cells, GS-5734 is metabolized into GS-441524 that is capable of reducing RNA replication of SARS-CoV, MERS-CoV, zoonotic and endemic human delta coronaviruses under in vitro conditions, while in vivo results have shown antiviral potential against bat and human coronaviruses in primary epithelial and lung cell culture systems.100 , 101
Remdesivir is an NtRTI drug that is worthy of a “solidarity” clinical trial for COVID-19, according to WHO.75 It acts as an RNA-dependent RNA polymerase (RdRp) inhibitor100 and its pharmacokinetics and characteristics have been studied in SARS-CoV and MERS-CoV infections.102 It alters functions of viral exonuclease and due to disturbed proof reading, viral genomic RNA replication and production declines.98 Since it can prevent viral replication and can be recommended for COVID-19 patients to prevent the severity of disease progression, randomized, double blind clinical trials with such patients are underway in phase-3 to confirm the therapeutic potential of remdesivir.66 , 103
Previous studies found that remdesivir was effective against MERS-CoV; it reduced the viral loads in the affected part in mice, therefore supported to regain the normal pulmonary functions104 , 105 and was also proposed as a therapeutic agent against SARS-CoV-2.106 A preliminary study found that the viral load in nasopharyngeal and oropharyngeal swabs reduced significantly after 12 days of remdesivir administration.107 An in vitro study reported that a combination of remdesivir and chloroquine, an anti-malarial drug, effectively inhibited SARS-CoV-2 growth in Vero E6 cells.108 Clinical trials are ongoing to assess the efficacy of remdesivir for COVID-19 in the US,109 Norway,110 and France.111 Remdesivir has been used to treat COVID-19 cases in the USA and Singapore. The first case of COVID-19 in the USA was recovered using intravenously administered remdesivir107 (Table 1).
Favipiravir (Avigan)
Favipiravir (T705), a purine (guanine) nucleotide analog is a derivative of pyrazine carboxamide (6‐fluoro‐3‐hydroxy‐2‐pyrazinecarboxamide) and an RdRp inhibitor.112 It was initially developed against influenza but attracted attention for COVID-19 treatment due to its large spectrum antiviral properties.113 Favipiravir is a prodrug and becomes an active molecule called favipiravir ibufuranosyl-5′-triphosphate (T‐705‐RTP) upon administration.114 It competes with guanine nucleosides during RNA viral replication by getting integrated with viral RNA, resulting in selectively blocking the RdRp to arrest the synthesis of viral RNA.115 Favipiravir is considered as a potential candidate drug for COVID-19, however while administering favipiravir, drug-drug interaction must be taken into consideration with already prescribed medication which may alter the pharmacokinetics and plasma concentration of favipiravir.116 , 117 This antiviral was associated with rapid clearance of Ebola virus in an animal model.118 Ongoing clinical trials in China119 report favipiravir treatment in patients was significantly correlated with a shorter viral clearance time as compared to untreated patients.120 Its efficacy has also been assessed in a randomized clinical trial.121 Favipiravir is currently being used for COVID-19 treatment in Japan79 and Indonesia122 (Table 1).
Ribavirin
Ribavirin is a guanine derivative analog that has antiviral activity against HCV, with an in vitro study report that it has antiviral activity against SARS-CoV-2.123 FDA advocated the therapeutic efficiency of ribavirin, remdesivir, penciclovir, favipiravir, nitazoxanide, nafamostat, and chloroquine against this strain based on in vitro trials.124
Ribavirin antiviral mechanism works to hamper the function of polymerases, hinders the RNA capping to destabilize the viral RNA and finally obstruct replication. Along with this, ribavirin inhibits the function of inosine monophosphate dehydrogenase enzyme to prevent the production of guanosine and hence promotes degradation of viral RNA.125 If at all any replication would with ribavirin intake, probability of arbitrary mutation in the RNA is immense, leading to loss of virulence in progeny viruses.124
Its efficacy in treating SARS-CoV-2 patients is being tested in clinical trials in Hong Kong.126 According to the Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced pneumonia in China for temporary treatment of COVID-19, ribavirin is one of recommended drugs that is administered with a combination with either IFN alpha or lopinavir-ritonavir.127 Docking and modeling analysis using ribavirin together with sofosbuvir and remdesivir indicated that ribavirin is a promising candidate drug for COVID-19 treatment and can be administered either by intra-venous route or orally.128 Ribavirin and sofosbuvir are currently part of the therapeutic regimen to treat COVID-19 in some countries (Table 1).
Other transcription inhibitors
Other FDA approved NtRTIs such as adefovir, tenofovir alafenamide, tenofovir disoproxil, abacavir, ganciclovir, and didanosine have similar structural characteristics either with remdesivir or ribavirin, and therefore, probably have antiviral activity against SARS-CoV-2. Other transcriptase inhibitor drugs such as NRTIs (lamivudine, stavudine, zidovudine, emtricitabine, zalcitabine, and azvudine) and NNRTIs (efavirenz, nevirapine, delavirdine, and rilpivirine) might also have antiviral properties against SARS-CoV-2. Though some of them have been evaluated in silico through molecular docking studies,129 further studies are warranted to ascertain their clinical efficiency.
3.4. Neuraminidase inhibitors
Oseltamivir – a neuraminidase inhibitor – is effective in preventing influenza130 and was successful in treating influenza in children.131 Neuraminidase inhibitor drugs such as oseltamivir, zanamivir, and peramivir, are not expected to be effective against COVID-19, mainly because neuraminidase has not been found in SARS-CoV-2. However, studies have reported the use of a combination of oseltamivir with ganciclovir and lopinavir/ritonavir to treat COVID-19 patients in Wuhan.132 , 133 A computational study also supported synergistic effects of oseltamivir-lopinavir-ritonavir combination against SARS-CoV-2.134 Oseltamivir administered with ceftriaxone and terbutaline has been used to treat COVID-19 cases in Afghanistan.135 A case report also found that the CT-scan of the lungs of a COVID-19 patient showed significant improvement after a three day course of oseltamivir.136 Oseltamivir is currently being used as a recommended option for COVID-19 treatment in Indonesia and Singapore (Table 1).
3.5. M2 ion-channel protein blockers
The M2 channel protein on the viral envelope is essential in maintaining pH across the viral envelope that is critical during cell entry and movement across the trans-Golgi membrane of host cells during viral maturation. This M2 ion-channel protein is one of the targets to combat influenza viruses.137 The structures of some M2 ion-channel protein inhibitors such as amantadine, adamantane, and rimantadine are represented in Fig. 2. A previous study showed that amantadine could block the p7 protein of HCV that is crucial in forming ion channels in host cell membranes.138 A report in 1973 showed that amantadine had a potent effect against Coronavirus 229E in vitro,139 and later a report also showed that amantadine was able to block protein-membrane channel activity of SARS-CoV.140 Despite increasing evidence suggesting that amantadine has antiviral potency suitable for COVID-19 therapy,141 , 142 more studies are warranted to assess its efficacy.
4. Non-antiviral drugs against SARS-CoV-2 infection: Old dog new tricks
4.1. Importin α/β1-mediated nuclear import inhibitors
An FDA-approved broad-spectrum antiparasitic drug called ivermectin has recently exhibited potent in vitro antiviral activity against SARS-CoV-2. A single dose of the drug induced ~5000-fold reduction in viral RNA content.143 The broad-spectrum antiviral activity of ivermectin against several RNA viruses is mediated by the inhibition of importin α/β1-mediated nuclear import.143 SARS-CoV-2 being an RNA virus, a similar mechanism is expected to facilitate the inhibitory activity of ivermectin.143 The drug combination of ivermectin and hydroxychloroquine was proposed as a combination therapy for the prophylaxis or treatment of COVID-19. This combination may produce a synergistic effect due to the inhibition of both, viral entry as well as viral replication.144 However pharmacokinetic analysis indicates that higher dosage is required for achieving the antiviral activity. Therefore, the recommended inhibitory concentration is very difficult to administer in human beings.145 In a recent observational case-controlled study, ivermectin therapy at a dose of 150 mcg/Kg was reported to lower the mortality rate as well as the duration of hospital stay.146 Further randomized clinical controlled studies are required before concluding the efficacy of ivermectin in SARS-CoV-2 infected patients.
4.2. Chloroquine and hydroxychloroquine: repurposed drug, FDA approved
Chloroquine (9‐aminoquinoline) is a proven and reliable anti-malarial drug, which has been found useful against SARS-CoV-2 infections and hence is now proposed and approved to be used for the treatment of COVID-19 patients by clinicians, with its new insights being explored fully.33 , 147 , 148 It blocks the entry of the virus by either altering the structural configuration of cell receptors or by competitively binding to the cellular receptors.149 It can amend the glycosylation of ACE-2 cellular receptors needed for SARS-CoV-2 entry.149 On the other hand, this drug can also reduce synthesis of sialic acid receptors to prevent the attachment of SARS-CoV-2 to the host cells. Chloroquine and hydroxychloroquine possess better binding affinity to host cell receptors as compared to the S protein of SARS-CoV-2 and therefore due to competitive binding to sialic acid and gangliosides present on the surface of the target cell, it prevents attachment and entry of the virus.150 In addition to the antiviral activity, chloroquine possesses anti-inflammatory activity that might contribute to its efficacy in treating COVID-19 patients. Studies conducted on animal models of melioidosis suggest that the anti-inflammatory property of chloroquine is mediated by the inhibition of glycogen synthase kinase-3β.151 Before the large-scale recommendation of off-label use, the potential of chloroquine to cause detrimental cardiac effects must be considered.152 Currently a randomized controlled trial has been registered to evaluate the prophylactic potential of hydroxychloroquine in preventing secondary infections and severe clinical symptoms among individuals who came into contact with SARS-CoV-2 infected individuals.153
5. Conclusion and further perspectives
Even though specific antiviral drugs for COVID-19 have not been discovered or approved by the FDA, the use of some available antiviral drugs that target specific steps within the life cycle of SARS-CoV-2 could be an alternative therapeutic strategy for dealing with this pandemic. Fusion inhibitors, protease inhibitors and transcription inhibitors are some of the promising groups of antivirals to be considered for the same. Apart from antiviral drugs, several promising approaches are also being used to treat COVID-19 such as convalescent plasma, the use of which has shown a reduction in viral load and morbidity of patients.154 , 155 IFN-⍺/β156 , 157 and IL-6R inhibitor158 , 159 have also showed promising effects and are currently being assessed in several clinical trials.
Funding
This compilation is a review article written by its authors and required no substantial funding to be stated.
Declaration of competing interest
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
References
- 1.Malik Y.S., Kumar N., Sircar S., et al. 2020. Pandemic Coronavirus Disease (COVID-19): Challenges and A Global Perspective; p. 2020040469. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee P., Nagi N., Agarwal A., et al. The 2019 novel coronavirus disease (COVID-19) pandemic: a review of the current evidence. Indian J Med Res. 2020;151(2):147–159. doi: 10.4103/ijmr.IJMR_519_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harapan H., Itoh N., Yufika A., et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav Med Infect Dis. 2020:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keam S., Megawati D., Patel S., et al. Immunopathology and immunotherapeutic strategies in SARS-CoV-2 infection. Rev Med Virol. 2020 doi: 10.1002/rmv.2123. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Gayle A., Wilder-Smith A., et al. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Trav Med. 2020 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehr A.R., Perlman S. Springer; Coronaviruses: 2015. Coronaviruses: An Overview of Their Replication and Pathogenesis; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrell C., Howard C., Murphy F. fifth ed. Academic Press; United States: 2016. Fenner and White's Medical Virology. [Google Scholar]
- 11.Chatterjee S. Understanding the nature of variations in structural sequences coding for coronavirus spike, envelope, membrane and nucleocapsid proteins of SARS-CoV-2. 2020. https://ssrncom/abstract=3562504 Available at: SSRN: [DOI]
- 12.Zhu X., Liu Q., Du L., et al. Receptor-binding domain as a target for developing SARS vaccines. J Thorac Dis. 2013;5:S142. doi: 10.3978/j.issn.2072-1439.2013.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual M.R. 2020. Coronavirus SARS-CoV-2: Analysis of Subgenomic mRNA Transcription, 3CLpro and PL2pro Protease Cleavage Sites and Protein Synthesis. arXiv:200400746. [Google Scholar]
- 15.Yufika A., Wagner A.L., Nawawi Y., et al. Parents' hesitancy towards vaccination in Indonesia: a cross-sectional study in Indonesia. Vaccine. 2020;38:2592–2599. doi: 10.1016/j.vaccine.2020.01.072. [DOI] [PubMed] [Google Scholar]
- 16.Yan C., Cui J., Huang L., et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020;26(6):773–779. doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia S., Liu M., Wang C., et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walls A.C., Park Y.J., Tortorici M.A., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–293.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabaan A.A., Al-Ahmed S.H., Sah R., et al. Preprints; 2020. SARS-CoV-2/COVID-19 and advances in developing potential therapeutics and vaccines to counter This emerging pandemic virus–a review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young B.E., Ong S.W.X., Kalimuddin S., et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A.K., Brindisi M., Shahabi D., et al. Drug development and medicinal chemistry efforts toward SARS-coronavirus and covid-19 therapeutics. ChemMedChem. 2020;15(11):907–932. doi: 10.1002/cmdc.202000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Wang Y., Zhu J., et al. Emerging well-tailored nanoparticulate delivery system based on in situ regulation of the protein corona. J Contr Release. 2020;320:1–18. doi: 10.1016/j.jconrel.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Nandakumar A., Xing Y., Aranha R.R., et al. Human plasma protein corona of abeta amyloid and its impact on islet amyloid polypeptide cross-seeding. Biomacromolecules. 2020;21(2):988–998. doi: 10.1021/acs.biomac.9b01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan R., Zhang Y., Li Y., et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z., Xiao X., Wei X., et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020;92:595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walls A.C., Park Y.-J., Tortorici M.A., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y.-R., Cao Q.-D., Hong Z.-S., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreakΓÇôan update on the status. Military Med Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng Q., Langereis M.A., van Vliet A.L., et al. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci Unit States Am. 2008;105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaux C.A., Rolain J.-M., Colson P., et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed T., Noman M., Almatroudi A., et al. 2020. A Novel Coronavirus 2019 Linked with Pneumonia in China: Current Status and Future Prospects. [Google Scholar]
- 35.Patiyal S., Kaur D., Kaur H., et al. 2020. A Web-Based Platform on COVID-19 to Maintain Predicted Diagnostic, Drug and Vaccine Candidates. [DOI] [PubMed] [Google Scholar]
- 36.Sah R., Rodriguez-Morales A., Jha R., et al. Complete genome sequence of a 2019 novel coronavirus (SARS-CoV-2) strain isolated in Nepal. Microbiol Resour Announc. 2020;9 doi: 10.1128/MRA.00169-20. e00169-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30(7):1346–1351.e2. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Xue S., Yang H., et al. Recent progress in the discovery of inhibitors targeting coronavirus proteases. Virol Sin. 2016;31:24–30. doi: 10.1007/s12250-015-3711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shannon A., Le N.T.T., Selisko B., et al. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antivir Res. 2020:104793. doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subissi L., Posthuma C.C., Collet A., et al. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc Natl Acad Sci Unit States Am. 2014;111:E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fo Ferron, Subissi L., De Morais A.T.S., et al. Structural and molecular basis of mismatch correction and ribavirin excision from coronavirus RNA. Proc Natl Acad Sci Unit States Am. 2018;115:E162–E171. doi: 10.1073/pnas.1718806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy P.D., Pogany J. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol. 2012;10:137–149. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasanth K.R., Hirano M., Fagg W.S., et al. bioRxiv; 2020. Topoisomerase III-Beta Is Required for Efficient Replication of Positive-Sense RNA Viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nal B., Chan C., Kien F., et al. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J Gen Virol. 2005;86:1423–1434. doi: 10.1099/vir.0.80671-0. [DOI] [PubMed] [Google Scholar]
- 47.Chang C-k, Hou M.-H., Chang C.-F., et al. The SARS coronavirus nucleocapsid proteinΓÇôforms and functions. Antivir Res. 2014;103:39–50. doi: 10.1016/j.antiviral.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klumperman J., Locker J.K., Meijer A., et al. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J Virol. 1994;68:6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khamitov R.A., Loginova S., Shchukina V.N., et al. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 50.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasmi A., Noor S., Tippairote T., et al. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020:108409. doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuyama S., Nao N., Shirato K., et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson P., Griffin I., Tucker C., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020;395:e30. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stebbing J., Phelan A., Griffin I., et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Praveen D., Chowdary P.R., Aanandhi M.V. Janus kinase inhibitor-not an ideal option for management OF covid 19. Int J Antimicrob Agents. 2020;55(5):105967. doi: 10.1016/j.ijantimicag.2020.105967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci Unit States Am. 2017;114:206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhua Z., Luc Z., Xud T., et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jieming Q.U. 2020. Clinical Study of Arbidol Hydrochloride Tablets in the Treatment of Pneumonia Caused by Novel Coronavirus. NCT04260594. [Google Scholar]
- 59.Li L., Li Y. 2020. The Efficacy of Lopinavir Plus Ritonavir and Arbidol against Novel Coronavirus Infection (ELACOI) NCT04252885. [Google Scholar]
- 60.Uno Y. Camostat mesilate therapy for COVID-19. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02345-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawase M., Shirato K., van der Hoek L., et al. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Østergaard L. 2020. The Impact of Camostat Mesilate on COVID-19 Infection (CamoCO-19) NCT04321096. [Google Scholar]
- 63.Heinrich-Heine University . 2020. Combination Therapy with Camostat Mesilate + Hydroxychloroquine for COVID-19 (CLOCC) NCT04338906. [Google Scholar]
- 64.Hoffmann M., Schroeder S., Kleine-Weber H., et al. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64(6) doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asakura H., Ogawa H. Potential of heparin and nafamostat combination therapy for COVID-19. J Thromb Haemostasis. 2020;18(6):1521–1522. doi: 10.1111/jth.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 67.Fintelman-Rodrigues N., Sacramento C.Q., Lima C.R., et al. bioRxiv; 2020. Atazanavir Inhibits SARS-CoV-2 Replication and Pro-inflammatory Cytokine Production. [Google Scholar]
- 68.Wang J. Fast identification of possible drug treatment of coronavirus disease -19 (COVID-19) through computational drug repurposing study. J Chem Inf Model. 2020 doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu F., Xu A., Zhang Y., et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. pii: S1201-9712(20)30132-30136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Gene Genom. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chu C., Cheng V., Hung I., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim J., Jeon S., Shin H.-Y., et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.WHO . 2020. “Solidarity” Clinical Trial for COVID-19 Treatments. [Google Scholar]
- 76.Deng L., Li C., Zeng Q., et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.CDC CfDCaP . 2020. Information for Clinicians on Therapeutic Options for Patients with COVID-19. [Google Scholar]
- 78.SSH-SPH NSSHSoPH . 2020. COVID-19 Science Report: Therapeutics. [Google Scholar]
- 79.Ministry of Health LaW . first ed. 2020. Concept of Antiviral Drug Treatment for COVID-19. [Google Scholar]
- 80.IP IP . 2020. International Pulmonologist's Consensus on COVID-19. [Google Scholar]
- 81.Qiu Y. 2020. Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection. NCT04261907. [Google Scholar]
- 82.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 83.Bernal D. 2020. Clinical Trial to Evaluate Efficacy of 3 Types of Treatment in Patients with Pneumonia by COVID-19 (Covid-19HUF) NCT04346147. [Google Scholar]
- 84.Ader F., Espérou H. 2020. Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) NCT04315948. [Google Scholar]
- 85.Hung I. 2020. Lopinavir/Ritonavir, Ribavirin and IFN-Beta Combination for nCoV Treatment. NCT04276688. [Google Scholar]
- 86.Kongsaengdao S. 2020. Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID-19 : A Randomized Control Trial (THDMS-COVID-19) NCT04303299. [Google Scholar]
- 87.Freilich D. 2020. COVID MED Trial - Comparison of Therapeutics for Hospitalized Patients Infected with SARS-CoV-2 (COVIDMED) NCT04328012. [Google Scholar]
- 88.Nicastri E., Petrosillo N., Bartoli T.A., et al. National institute for the infectious diseases ΓÇ£L. SpallanzaniΓÇ¥, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020:12. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu H. 2020. Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID-19 (DC-COVID-19) NCT04252274. [Google Scholar]
- 90.Kumar S., Buckely K. Johnson & johnson launches multi-pronged response to coronavirus global public health threat. 2020. https://wwwjnjcom/johnson-johnson-launches-multi-pronged-response-to-coronavirus-global-public-health-threat
- 91.Riva A., Conti F., Bernacchia D., et al. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol Res. 2020:104826. doi: 10.1016/j.phrs.2020.104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stanley T.L., Joy T., Hadigan C.M., et al. Effects of switching from lopinavir/ritonavir to atazanavir/ritonavir on muscle glucose uptake and visceral fat in HIV infected patients. AIDS (London, England) 2009;23:1349. doi: 10.1097/QAD.0b013e32832ba904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noor M.A., Flint O.P., Maa J.-F., et al. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS. 2006;20:1813–1821. doi: 10.1097/01.aids.0000244200.11006.55. [DOI] [PubMed] [Google Scholar]
- 94.Hall D.C., Jr., Ji H.F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Trav Med Infect Dis. 2020:101646. doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamamoto N., Matsuyama S., Hoshino T., et al. bioRxiv; 2020. Nelfinavir Inhibits Replication of Severe Acute Respiratory Syndrome Coronavirus 2 in Vitro. [Google Scholar]
- 96.Khan R.J., Jha R.K., Amera G.M., et al. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2'-O-ribose methyltransferase. J Biomol Struct Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., et al. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Tawfiq J.A., Al-Homoud A.H., Memish Z.A. 2020. Remdesivir as a Possible Therapeutic Option for the COVID-19. Travel Medicine and Infectious Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ko W.C., Rolain J.M., Lee N.Y., et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55:105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gordon C.J., Tchesnokov E.P., Feng J.Y., et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020 doi: 10.1074/jbc.AC120.013056. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brown A.J., Won J.J., Graham R.L., et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agostini M.L., Andres E.L., Sims A.C., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheahan T.P., Sims A.C., Graham R.L., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Munster V.J., Koopmans M., van Doremalen N., et al. A novel coronavirus emerging in China - key questions for impact assessment. N Engl J Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 106.Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother. 2020;64(5) doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gilead Sciences . 2020. Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants with Severe Coronavirus Disease (COVID-19) NCT04292899. [Google Scholar]
- 110.Aukrust P., Barratt-Due A., Kåsine T., et al. 2020. The Efficacy of Different Anti-viral Drugs in COVID 19 Infected Patients. NCT04321616. [Google Scholar]
- 111.Ader F. 2020. Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) NCT04315948. [Google Scholar]
- 112.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calling all coronavirus researchers: keep sharing, stay open. Nature. 2020;578:7. doi: 10.1038/d41586-020-00307-x. [DOI] [PubMed] [Google Scholar]
- 114.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhai P., Ding Y., Wu X., et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oestereich L., Lüdtke A., Wurr S., et al. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir Res. 2014;105:17–21. doi: 10.1016/j.antiviral.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Xuejiao L., Yingxia L. 2020. Clinical Study for Safety and Efficacy of Favipiravir in the Treatment of Novel Coronavirus Pneumonia (COVID-19) ChiCTR2000029600. [Google Scholar]
- 120.Cai Q., Yang M., Liu D., et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen C., Huang J., Cheng Z., et al. medRxiv; 2020. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. [Google Scholar]
- 122.ISR ISfR. 2020. Tata Laksana Pasien Covid-19. [Google Scholar]
- 123.Wang M., Cao R., Zhang L., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Khalili J.S., Zhu H., Mak N.S.A., et al. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Graci J.D., Cameron C.E. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.The University of Hong Kong . 2020. Lopinavir/Ritonavir, Ribavirin and IFN-Beta Combination for nCoV Treatment. NCT04276688. [Google Scholar]
- 127.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Therapeut. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 128.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang Y.-C., Tung Y.-A., Lee K.-H., et al. 2020. Potential Therapeutic Agents for COVID-19 Based on the Analysis of Protease and RNA Polymerase Docking. [Google Scholar]
- 130.Welliver R., Monto A.S., Carewicz O., et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. Jama. 2001;285:748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 131.Whitley R.J., Hayden F.G., Reisinger K.S., et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 132.Chu D.K.W., Pan Y., Cheng S.M.S., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muralidharan N., Sakthivel R., Velmurugan D., et al. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J Biomol Struct Dyn. 2020:1–7. doi: 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- 135.Mousavi S.H., Shah J., Giang H.T., et al. The first COVID-19 case in Afghanistan acquired from Iran. Lancet Infect Dis. 2020;20(6):657–658. doi: 10.1016/S1473-3099(20)30231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu Y., Xie Y-l, Wang X. Longitudinal CT findings in COVID-19 pneumonia: case presenting organizing pneumonia pattern. Radiology: Cardiothorac Imag. 2020;2 doi: 10.1148/ryct.2020200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skehel J.J., Hay A.J., Armstrong J.A. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978;38:97–110. doi: 10.1099/0022-1317-38-1-97. [DOI] [PubMed] [Google Scholar]
- 138.Griffin S.D., Beales L.P., Clarke D.S., et al. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535:34–38. doi: 10.1016/s0014-5793(02)03851-6. [DOI] [PubMed] [Google Scholar]
- 139.Mathur A., Beare A., Reed S.E. In vitro antiviral activity and preliminary clinical trials of a new adamantane compound. Antimicrob Agents Chemother. 1973;4:421–426. doi: 10.1128/aac.4.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Torres J., Maheswari U., Parthasarathy K., et al. Conductance and amantadine binding of a pore formed by a lysineΓÇÉflanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007;16:2065–2071. doi: 10.1110/ps.062730007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smieszek S., Przychodzen B., Polymeropoulos M.H. bioRxiv; 2020. Amantadine Disrupts Lysosomal Gene Expression; Potential Therapy for COVID19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cimolai N. Potentially repurposing adamantanes for COVID-19. J Med Virol. 2020;92(6):531–532. doi: 10.1002/jmv.25752. [DOI] [PubMed] [Google Scholar]
- 143.Caly L., Wagstaff K.M., Jans D.A. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antivir Res. 2012;95:202–206. doi: 10.1016/j.antiviral.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 144.Patri A., Fabbrocini G. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol. 2020;82(6) doi: 10.1016/j.jaad.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Momekov G., Momekova D. medRxiv; 2020. Ivermectin as a Potential COVID-19 Treatment from the Pharmacokinetic Point of View. [Google Scholar]
- 146.Patel A. 2020. Usefulness of ivermectin in COVID-19 illness. Available at: SSRN 3580524. [Google Scholar]
- 147.Duan Y.J., Liu Q., Zhao S.Q., et al. The trial of chloroquine in the treatment of corona virus disease 2019 COVID-19 and its research progress in forensic toxicology. Fa Yi Xue Za Zhi. 2020;36 doi: 10.12116/j.issn.1004-5619.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 148.Gao J., Hu S. Update on use of chloroquine/hydroxychloroquine to treat coronavirus disease 2019 (COVID-19) BioSci Trends. 2020;14(2):156–158. doi: 10.5582/bst.2020.03072. [DOI] [PubMed] [Google Scholar]
- 149.Quiros Roldan E., Biasiotto G., Magro P., et al. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol Res. 2020;158:104904. doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fantini J., Di Scala C., Chahinian H., et al. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Embi M.N., Ganesan N., Sidek H.M. Is GSK3β a molecular target of chloroquine treatment against COVID-19? Drug Discov Therapeut. 2020;14(2):107–108. doi: 10.5582/ddt.2020.03010. [DOI] [PubMed] [Google Scholar]
- 152.Lentini G., Cavalluzzi M.M., Habtemariam S. COVID-19, chloroquine repurposing, and cardiac safety concern: chirality might help. Molecules. 2020;25(8) doi: 10.3390/molecules25081834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mitjà O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Global Health. 2020;8(5):e639–e640. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J Am Med Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Casadevall A., Pirofski L-a. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Investigational F.D.A. Food and Drug Administration; Silver Spring, MD: 2020. COVID-19 Convalescent Plasma - Emergency INDs. [Google Scholar]
- 157.Ning Q. 2020. A Prospective/retrospective, Randomized Controlled Clinical Study of Interferon Atomization in the 2019-nCoV Pneumonia. NCT04254874. [Google Scholar]
- 158.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perrone F., Tumori I., Pascale F. 2020. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) (TOCIVID-19) NCT04317092. [Google Scholar]