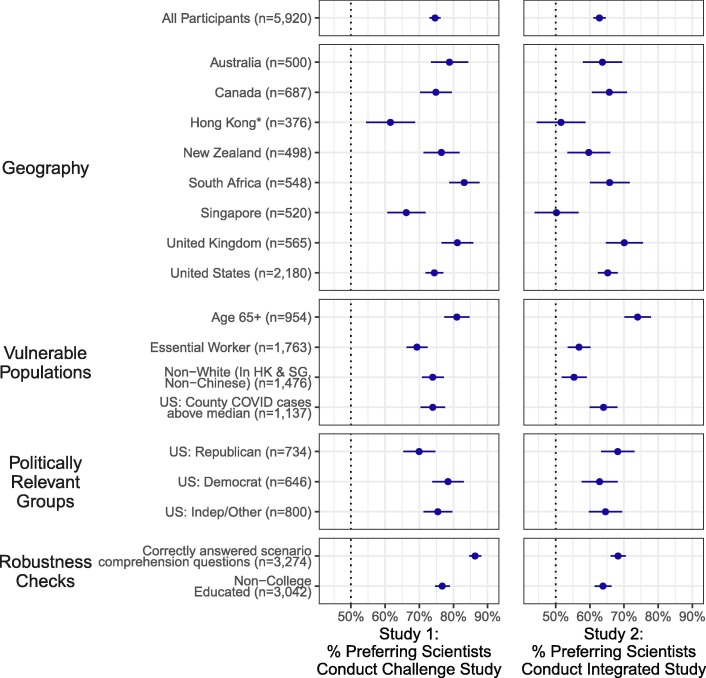
Fig. 2.
Broad Support for Challenge Trials and Integrated Trials That Accelerate COVID-19 Vaccine Development. Notes: The Figure shows the percent of respondents who preferred that scientists conduct each accelerated trial instead of a standard trial. Study 1 considered the use of a challenge trial in which participants are intentionally exposed to the virus instead of, in a standard trial, waiting for participants to be exposed to it in their daily lives. Study 2 considered the integration of smaller Phase II safety and immunogenicity trials into larger Phase III efficacy trials instead of, in a standard trial, waiting for the completion of a Phase II safety and immunogenicity trial before commencing a Phase III efficacy trial. The Figure shows the mean proportion of respondents who say they would prefer scientists to conduct each accelerated design for each study overall, by geography, and across various demographic subgroups. 95% confidence intervals surround the point estimates. Sample sizes shown are totals across both studies; respondents are approximately evenly split across the two studies. See Tables S3, S4, S7, and S8 for numerical values. * This Figure presents non-white respondents from Hong Kong only. There were an unanticipatedly large number of participants in Hong Kong who indicated they were white. The 2016 Hong Kong Census estimates that only 0.8% of the Hong Kong population identifies as white; we discuss this issue in further detail in the Supplementary Materials.