Abstract
While substantial evidence points towards obesity and associated cardiometabolic disorders being a major factor for poor outcomes in SARS-CoV2 infections (COVID-19), the complexity of the interplay between these two pandemics is becoming apparent. Indeed, as previously defined, this interaction between obesity and COVID-19 represents a ‘syndemic’ that requires both current and ongoing attention. At a mechanistic level the chronic inflammatory environment of obesity predisposes to life threatening events such as cytokine storm and enhanced coagulopathy. Obesity and its management are affected by diverse factors manifested at societal, educational, racial, and nutritional levels. A multidisciplinary approach is required to manage obese and type 2 diabetic patients, not only during the current COVID-19 crisis, but to decrease the growing burden of cardiometabolic disease and associated cardiovascular complications impacting future viral pandemics. Further, this syndemic has highlighted disparities in healthcare which need to be addressed to achieve equality in health outcomes in patients infected with COVID-19.
Highlights
-
•
Obesity and associated cardiometabolic disorders are major contributors to poor outcomes to SARS-CoV2 infection (COVID-19).
-
•
Excess deposition of visceral fat, in particular, is predictive of a poor outcome to SARS-CoV2 infection.
-
•
Obesity and COVID-19 interact creating a ‘syndemic’ with outcomes affected by societal, racial, nutritional and physical activity.
-
•
Preemptive measures are needed to address healthy weight, access to nutritional food, therapeutics and psychosocial issues.
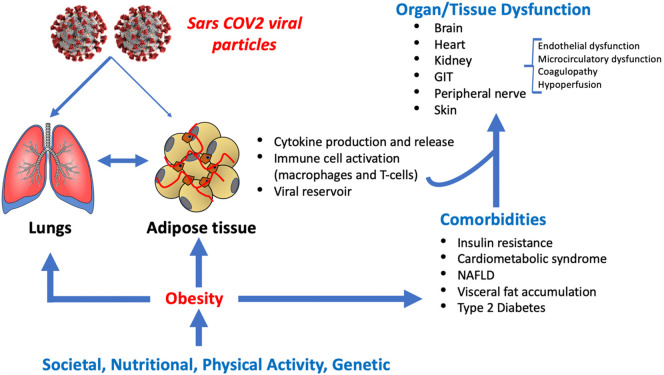
The current COVID-19 pandemic resulting from exposure to the RNA virus, SARS-CoV2, is a major cause of morbidity and mortality on a global scale. By the beginning of October 2020 there was more than 34,000,000 cases and over 1,000,000 deaths worldwide, with these numbers continuing to increase. Although initial emphasis was placed on its effects on the respiratory system it is now clear that COVID-19 is a systemic, multi-system disorder (Fig. 1 ). An apparent universal finding is that several chronic cardiometabolic disease states are associated with an increased predilection for adverse SARS-CoV2 infection outcomes. In particular, these conditions include diabetes, hypertension, cardiovascular disease (CVD) and chronic renal disease [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. All of these chronic diseases are more common in males and in aging populations which are also populations with poorer outcomes in patients contracting SARS-CoV2 infection [10]. These observations suggest that there may be a common underlying denominator that predisposes to poorer outcomes in these populations. An integral underlying condition that links these disease states is obesity. To this point, increased adipose tissue mass, especially when deposited centrally, is a major risk factor for cardiometabolic abnormalities including insulin resistance, dyslipidemia, hypertension and systemic inflammation, which ultimately lead to diabetes, exacerbated hypertension, cardiovascular disease, NASH and chronic kidney disease [[11], [12], [13], [14]].
Fig. 1.
Obesity is a contributor to poor outcomes in COVID-19. While obesity is a common factor in several comorbidities that have been implicated as risk factors for morbidity and mortality associated with COVID-19 infection, complex interactions exist not only at the cellular level but as relates to the multifactorial issues underlying fat distribution. This schematic has drawn on ideas presented in recent publications [35,38,67]. GIT – gastrointestinal tract; NAFLD – non alcohol fatty liver disease.
Indeed, early reports from China and Italy indicated that obesity, even at relatively mild levels, predisposes to increased morbidity and mortality in patients with SARS-CoV2 infection [1,15], as was also reported in those with influenza A (H1N1) disease [16]. This relationship between obesity and poorer outcomes in COVID-19 infection has now been seen in a number of countries including the US, France, Spain and Mexico [15,17,18]. For example, increased risk for a poor Covid-19 outcome was seen at a BMI > 24.0 in a report from Shenzhen, China – a BMI that would be considered ‘normal’ in many societies, indicating that increasing risk starts with even high normal BMI and the risk increases progressively with increasing degrees of obesity. Subsequently, a number of early meta- analyses of studies from wide geographical regions have confirmed the impact of obesity on COVID-19 outcomes and have provided a more accurate effect estimate of the association. Further, the presence of obesity may shift the severity of COVID-19 infection and increased mortality to younger populations [[19], [20], [21]]. However, an important question is whether obesity is the root cause of the problem and/or whether its association with other cardiometabolic entities with the severity of COVID-19 infection and outcomes are independent.
A recent study reported in Metabolism [22] provided novel insights regarding the role of obesity and associated disorders in promoting poor outcomes in SARS-CoV2 infected patients. Obesity was identified as a critical underlying cause of poor outcome in a specific cohort of 200 infected Covid-19 patients in the Bronx borough in New York City. The patient population in the Bronx cohort, unlike prior reported homogeneous COVID-19 populations, was largely of African American and Hispanic (approximately 85%) makeup. This ethnic breakdown is considerably different from an earlier study of a wider New York City area where these groups approximated some 50% of COVID-19 patients [23]. Importantly, the report from the Bronx included patients representing underserved and economically disadvantaged minorities; thus, revealing the outcomes of COVID-19 in this vulnerable population where there is a paucity of information. Another strength of this study was the use of multivariable models to statistically dissect pathways of variables associated with the outcomes of interest and that severe obesity, assessed as a BMI of 35 kg/m2 or greater, was treated as a dichotomous, as well as a continuous variable in this analysis. Thus, the authors were able to show that obesity is an independent predictor of poorer outcome with COVID-19 infections in this vulnerable population. This approach adjusted for associated diseases such as diabetes, age, hypertension and cardiovascular disease and thus excluded them as significant confounders in this association indicating that obesity is at the root cause of the problem.
Several recent papers in Metabolism (one a meta-analysis of 16 studies including nearly 110,000 infected patients [24]; a similar meta-analysis of 45, 650 patients [25]; and the other a regional study) have now strengthened the observed relationship between obesity and adverse COVID-19 outcomes/severity. Further, these analyses suggest a relatively linear relationship between BMI and COVID-19 severity such that patients classified as overweight also show an increased risk for a poor outcome or death. In the study of Du this relationship was again shown to be independent of a number of other comorbidities [24].
An additional consideration relates to the contribution of body composition and specific fat depots vs. overall obesity. In this regard, two recent brief retrospective studies [26,27] have reported that increased visceral fat, determined using CT scans, is predictive of the severity of COVID-19 infection as defined by the need for admission to intensive care units and mechanical ventilation. Further, increased visceral fat was more predictive of a severe outcome than was increased subcutaneous fat. Importantly, visceral fat accumulation is more closely associated to inflammatory disorders including the cardiometabolic syndrome and type 2 diabetes [28]. Both studies further provided evidence for the utility of CT scans routinely taken in the context of care to assess lung involvement also provide abdominal fat information in hospitalized COVID-19 patients were superior to BMI, particularly in aging populations where body composition changes markedly. Another study reported that the presence of fatty liver (metabolic associated fatty liver disease), another condition associated with excess body and visceral fat as determined by CT scans, was associated with a more severe clinical course for Covid-19 infection [29]. In this study obesity was defined as BMI >25 kg/m2 perhaps indicating the importance of fat deposition in the liver as well as abdominal adiposity as opposed to BMI, per se. Also, important to consider, body fat distribution for a given BMI is also impacted by other factors including sex and race. Relevant to this, using data from the UK Biobank, a population-based cohort study, Zhu et al. [30] examined the relationship between a calculated polygenic risk score for obesity and COVID-19 severity. A higher risk score for BMI was associated with COVID-19 outcome perhaps indicating a role for genetic predisposition for obesity and poor outcome [30]. While future research will be required to identify specific genetic mechanisms, this could conceivably lead to a more personalized medicine approach for prevention or treatment.
It has further been suggested that SARS-CoV2 infection may impact adipose tissue specifically associated with cardiovascular structures, specifically epicardial and perivascular fat [[31], [32], [33]]. Increased adipose tissue in theses organs has been associated with inflammation, immune cell activation and cardiovascular disorders. Specific involvement of these fat depots in the course of COVID-19 infection requires further study.
An important question regarding the mechanism(s) by which obesity predicts a poor outcome in heterogeneous COVID-19 populations including minority patients, as well as in more homogeneous populations from China, Italy and Spain. Suggested impacting factors have included an increased susceptibility to infection; mechanical factors impairing ventilation (obesity hypoventilation syndrome and obstructive sleep apnea), particularly when intubation is required [34,35]; obesity-related abnormalities in coagulation, fibrinolysis, inflammation, oxidative stress and disturbances in microcirculatory function [[36], [37], [38]]. In this regard, obesity represents a state of adipose tissue and systemic inflammation [39,40] (Fig. 1).
Metabolic changes in adipose tissue in the setting of obesity include expansion of inflammatory immune cell populations, increased secretion of inflammatory molecules and hormones including tumor necrosis factor α, interleukin 6, angiotensinogen, angiotensin II, aldosterone, leptin, resistin, and monocyte chemoattractant protein-1, and reduced secretion of the anti-inflammatory molecule adiponectin. This adipose tissue-induced inflammatory state is evidenced by increased levels of interleukin 6 and other inflammatory cytokines seen with coronavirus infections including SARS-CoV, MERS-CoV and SARS-CoV2 [41]. Elevated circulating levels of these inflammatory cytokines likely impairs vascular insulin metabolic signaling and associated nitric oxide mediated smooth muscle cell relaxation. There is increased recruitment and activation of pro-inflammatory immune cells into the vasculature, which then contribute to development of increased vascular permeability which enhances the ability of the virus to invade tissues such as the heart, vasculature, kidney and gut. Thus, the “inflammatory storm” seen with this disease may be coupled with an overactive immune system that exists in obesity.
Adipose tissue has also been suggested to act as reservoir for the SARS-CoV2 virus, contributing to viral shedding and associated amplification of cytokine production [35]. This, however, requires further investigation as visceral fat samples from a small number (n = 4) COVID-19 positive patients undergoing abdominal surgery were negative for viral RNA as detected by quantitative PCR [42]. Of additional importance to the relationship between obesity and COVID-19 infection is the involvement of the renin angiotensin II aldosterone system (RAAS). Obesity is associated with systemic and fat tissue RAAS activation with elevated aldosterone levels, which contribute to inflammation [43]. while ACE2 has been identified as the receptor for cellular uptake of SARS-CoV2 [44] and is expressed in adipose tissue [35]. While a preliminary study has reported increased ACE2 mRNA expression in visceral compared to subcutaneous adipose tissue (Favre, under review) further studies are required to determine whether ACE2 protein is upregulated with visceral adiposity, particularly in the context of SARS-CoV-2 infection. Related to this, a further study has reported that following weight loss, mRNA expression for ACE2 in subcutaneous adipose tissue decreased in a cohort of non-COVID patients (n = 143; BMI > 27 kg/m2) [45]. Interestingly, subjects showing the greatest decrease in ACE2 mRNA showed a higher degree of skeletal muscle insulin resistance, suggesting a relationship between ACE2 and insulin sensitivity [45]. The relevance of these finding to SARS-CoV2 infection, however, requires further study. Regardless of the exact cellular mechanisms, obesity and the associated systemic inflammatory state may be a common denominator underlying the poorer outcomes in patients with underlying diabetes, hypertension and CVD, especially in minorities with a high prevalence of these disorders. To this point, COVID-19 has been described as a modern day example of Virchow's Triad (damage to the vascular wall, stasis/impaired flow and hypercoagulation) exacerbated by obesity and associated metabolic abnormalities [38] (Fig. 1).
Suboptimal diet is a known cause of the cardiometabolic syndrome, diabetes and CVD with quality of diet being affected by race, ethnicity, and socioeconomic status [46]. Nutritional status further impacts immune function and levels of systemic inflammation [47]. As a result of such information, the issue of maintaining an appropriate nutritional diet during the COVID-19 pandemic has been raised and is of particular importance given the COVID-19 pandemic converges with the obesity pandemic to create a syndemic. In particular, the benefit of a Mediterranean Diet (MD) rich in fruits, vegetables, cereals, legumes, fish and monounsaturated/polyunsaturated fatty acids has been advocated. Importantly, consumption of a MD has been linked to having cardiovascular and general health benefits including through decreasing levels of obesity and exerting favorable effects on components of the cardiometabolic syndrome. Mechanistically the benefits of a MD may result from lower energy density, higher fiber intake, reduced intake of saturated fats and improved antioxidant defense mechanisms. Even in areas where it may be expensive to buy fruits and vegetables or other more expensive components of the MD, consumption of less costly dried fruits such as raisins/ which have anti-oxidative and inflammatory activities [48], could potentially benefit or prevent severe infections [49,50]. Relevant to the outcome severity for COVID-19 infection such diets have been reported to decrease systemic inflammation and blood coagulation [51]. Further, a MD has been shown to exert beneficial effects in those with the cardiometabolic syndrome [52] and diabetes [53,54]. In order to assess the benefits of a MD in those with COVID-19 studies have been initiated during lockdown periods in both Spain (COVIDiet Study [55]) and Italy (EHLC-COVID19) [56]. Importantly, the Italian study also assesses lifestyle habits in terms of smoking, sleep quality, physical activity and shopping choices. While both studies indicate there is good short-term adherence to the MD they further seek to describe long term adherence and impact on chronic disease including susceptibility and outcomes to COVID-19. As inexpensive diets high in refined carbohydrates, especially those containing high amounts of fructose corn syrup, promote obesity and type 2 diabetes [57,58] consideration should also be given to restricting their intake or possibly following vegetarian/plant-based diets which have been shown to have beneficial effects on obesity and CV health [59].
The possible role of dietary supplements in COVID-19 prevention and treatment has been raised in both the popular press and medical literature [60] and considerable interest has been directed towards a possible association between low vitamin D levels and poorer outcomes of COVID-19 infection [61]. Interestingly, and relevant to the above discussion, a recent studied showed that adherence to a MD both decreased obesity and enhanced vitamin D levels [62]. As vitamin D deficiency has been suggested to be common, particularly in people of African American and Hispanic descent, it has been proposed that efforts should be made to correct low vitamin D levels in communities at risk for COVID-19 infection [63] until randomized clinical trials confirm its protective role or alternatively show that the observed associations might be due to uncontrolled confounding by obesity or other factors. Collectively, these observations also support the need to consider the role played my micronutrients in healthy diets in reducing inflammation and minimizing risk of infection.
Given the contribution of obesity and cardiometabolic comorbidities to poor outcomes following COVID-19 infection, pharmacological treatments should be prioritized towards body weight loss and decreasing CVD risk factors. Consistent with this approach glucose lowering agents, incretins and SGLT2 inhibitors represent potential therapeutic classes meeting this aim [64,65]. Adequate preventative management of glycemia and lipids, along with monitoring of blood pressure and physical activity in patients with diabetes during the COVID-19 pandemic has recently been highlighted [66].
Also, increasingly recognized is that the impact of obesity needs to be considered beyond mechanistic interactions at a cellular and organ level. Indeed, psychological and societal factors including ethnic differences, health and nutritional disparities [[67], [68], [69], [70], [71], [72]] and weight stigma [73] should be taken into consideration The impact of obesity in specific ethnic/racial groups has been similarly observed in other Northern and Central American minority communities including Native Americans and people of Mexican/Hispanic descent. This represents a considerable health and resource concern to these communities as the prevalence of adult obesity in Mexico is approximately 40% in women and 27% in men. Similarly, the prevalence of obesity (BMI > 30 kg/m2) in Native Americans in the US is greater than 48% (https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=40). The widespread finding of an association between obesity, especially abdominal obesity, and COVID-19 severity strengthens the likelihood of a causative relationship for poor outcome in these minority populations and likely applies to other racial minorities showing poorer COVID-19 outcomes [72] (https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020#ethnic-breakdown-of-deaths-by-age-and-sex).
Recently, in the context of COVID-19 outcomes, the complex relationships between health disparities and obesity have been raised [67,69,70]. Access to healthy foods, medical services, public health initiatives and nutritional education contribute to poor nutrition and obesity, particularly in disadvantaged communities. Such factors are considered to favor the excess consumption of nutrient dense foods, high in refined carbohydrates, that lead to obesity [74]. This situation appears to be further complicated by socioeconomic factors, leading to denser living and work environments that does not favor social distancing or the ability to ‘work from home’. Related to this, Townshend et al. have provided a very interesting and important perspective on the relationship between obesity and the course of COVID-19 infection, emphasizing the impact of an obesity stigma in leading to health disparities [73]. Accordingly, this stigma may be reflected in both a patient's reluctance to seek healthcare, and poorer care as a result of a negative stereotype that healthcare providers may unwittingly apply to this population. Both of these situations add to the mechanistic and social interactions that occur between the obese state and poor COVID-19 infection outcomes (Fig. 1).
In 2017 The Lancet expanded on the term Syndemics (as introduced by Singer [75]), as “a conceptual framework for understanding diseases or health conditions that arise in populations and that are exacerbated by the social, economic, environmental, and political milieu in which a population is immersed” [76]. It has become clear that the coexisting COVID-19 pandemic and the growing obesity epidemic combine to fit the definition of a syndemic and represent a global challenge requiring research, public health, nutrition and educational approaches. This dire situation is clearly exacerbated by reduced availability of affordable, high quality nutrition [77].
In summary, recently published studies indicate that obesity and the associated inflammatory state represent an independent risk factor for a more severe clinical course during SARS-CoV2 infection. As recently highlighted [78] assessment of COVID-19 patients in terms of anthropometric and metabolic data should be considered an important component of assessing patient risk for possible poor outcomes. Further, the increasing prevalence of obesity and related cardiometabolic disorders [79] will likely represent challenges to clinical management during the ongoing COVID-19 pandemic and future pandemic viral exposures. Preemptive approaches, at multiple levels, will be required to address the complex problem of obesity including maintenance of healthy weight, provision of access to affordable and nutritious foods, and attention to psychosocial issues in these patients (Fig. 1). Finally, vigilance will need to paid to possible long-term medical consequences of SARS-CoV2 infection in surviving obese patients.
Declaration of competing interest
No conflicts of interest.
References
- 1.Cai Q., Chen F., Wang T., Liu X., Wu Q., He Q. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 2.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas M.E., Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill M.A., Mantzoros C., Sowers J.R. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020;107:154217. doi: 10.1016/j.metabol.2020.154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7.Rebelos E., Moriconi D., Virdis A., Taddei S., Foschi D., Nannipieri M. Letter to the editor: importance of metabolic health in the era of COVID-19. Metabolism. 2020;108:154247. doi: 10.1016/j.metabol.2020.154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2(7):572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020;69(9):1857–1863. doi: 10.2337/dbi19-0023. Epub 2020 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan N., Schick F., Haring H.U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26(2):292–300. doi: 10.1016/j.cmet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Aroor A.R., McKarns S., Demarco V.G., Jia G., Sowers J.R. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62(11):1543–1552. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia G., Hill M.A., Sowers J.R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim S., Taskinen M.R., Boren J. Crosstalk between nonalcoholic fatty liver disease and cardiometabolic syndrome. Obes Rev. 2019;20(4):599–611. doi: 10.1111/obr.12820. [DOI] [PubMed] [Google Scholar]
- 15.Barrasa H., Rello J., Tejada S. SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39(5):553–561. doi: 10.1016/j.accpm.2020.04.001. Epub 2020 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan O.W., Bramley A., Fowlkes A., Freedman D.S., Taylor T.H., Gargiullo P. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denova-Gutierrez E., Lopez-Gatell H., Alomia-Zegarra J.L., Lopez-Ridaura R., Zaragoza-Jimenez C.A., Dyer-Leal D.D. The association between obesity, type 2 diabetes, and hypertension with severe COVID-19 on admission among Mexicans. Obesity (Silver Spring) 2020;28(10):1826–1832. doi: 10.1002/oby.22946. Epub 2020 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng M., Qi Y., Deng L., Wang H., Xu Y., Li Z. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (Silver Spring) 2020;28(10):1815–1825. doi: 10.1002/oby.22943. Epub 2020 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong S.W.X., Young B.E., Leo Y.S., Lye D.C. Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients. Clin Infect Dis. 2020;8 doi: 10.1093/cid/ciaa548. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Y., Lv Y., Zha W., Zhou N., Hong X. Association of Body mass index (BMI) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metabolism. 2020;154373 doi: 10.1016/j.metabol.2020.154373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y., Lu Y., Huang Y.M., Wang M., Ling W., Sui Y. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen A., Bressem K., Albrecht J., Albrecht J., Thiess H.M., Vahldiek J. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;154317 doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe M.C.D., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. Epub 2020 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 29.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Association of obesity and its genetic predisposition with the risk of severe COVID-19: analysis of population-based cohort data. Metabolism. 2020;112:154345. doi: 10.1016/j.metabol.2020.154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim I.C., Han S. Epicardial adipose tissue: fuel for COVID-19-induced cardiac injury? Eur Heart J. 2020;41(24):2334–2335. doi: 10.1093/eurheartj/ehaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity (Silver Spring) 2020;28(8):1367. doi: 10.1002/oby.22867. [DOI] [PubMed] [Google Scholar]
- 33.Malavazos A.E., Goldberger J.J., Iacobellis G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur Heart J. 2020;41(24):2333. doi: 10.1093/eurheartj/ehaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J.F., Wang X.B., Zheng K.I., Liu W.Y., Chen J.J., George J. Letter to the editor: obesity hypoventilation syndrome and severe COVID-19. Metabolism. 2020;108:154249. doi: 10.1016/j.metabol.2020.154249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020;28(7):1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colantuoni A., Martini R., Caprari P., Ballestri M., Capecchi P.L., Gnasso A. COVID-19 Sepsis and microcirculation dysfunction. Front Physiol. 2020;11:747. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi W., Lv J., Lin L. Coagulopathy in COVID-19: focus on vascular thrombotic events. J Mol Cell Cardiol. 2020;146:32–40. doi: 10.1016/j.yjmcc.2020.07.003. Epub 2020 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaughan C.J., Cronin H., Ryan P.M., Caplice N.M. Obesity and COVID-19: a Virchow’s triad for the 21st century. Thromb Haemost. 2020 doi: 10.1055/s-0040-1714216. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 40.Tanti J.F., Ceppo F., Jager J., Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne) 2012;3:181. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 42.Safari S., Keyvani H., Alamdari N.M., Dehghanian A., Hashemi M.R., Honar B.N. Abdominal surgery in patients with COVID-19: detection of SARS-CoV-2 in abdominal and adipose tissues. Ann Surg. 2020;272(3):e253–e256. doi: 10.1097/SLA.0000000000004165. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lastra G., Sowers J.R. Obesity and cardiovascular disease: role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm Mol Biol Clin Investig. 2013;15(2):49–57. doi: 10.1515/hmbci-2013-0025. [DOI] [PubMed] [Google Scholar]
- 44.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L., Spranger L., Dominik S., Beer F., Brachs M., Spranger J. Metabolic impact of weight loss induced reduction of adipose ACE-2 – potential implication in COVID-19 infections? Metabolism. 2020;113:154401. doi: 10.1016/j.metabol.2020.154401. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kris-Etherton P.M., Petersen K.S., Velarde G., Barnard N.D., Miller M., Ros E. Barriers, opportunities, and challenges in addressing disparities in diet-related cardiovascular disease in the United States. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.119.014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silverio R., Goncalves D.C., Andrade M.F., Seelaender M. Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link? Adv Nutr. 2020 doi: 10.1093/advances/nmaa125. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanellos P.T., Kaliora A.C., Protogerou A.D., Tentolouris N., Perrea D.N., Karathanos V.T. The effect of raisins on biomarkers of endothelial function and oxidant damage; an open-label and randomized controlled intervention. Food Res Int. 2017;102:674–680. doi: 10.1016/j.foodres.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 49.Urpi-Sarda M., Casas R., Chiva-Blanch G., Romero-Mamani E.S., Valderas-Martinez P., Arranz S. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol Res. 2012;65(6):577–583. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Urpi-Sarda M., Casas R., Chiva-Blanch G., Romero-Mamani E.S., VValderas-Martinez P.S., Salas-Salvado J. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J Nutr. 2012;142(6):1019–1025. doi: 10.3945/jn.111.148726. [DOI] [PubMed] [Google Scholar]
- 51.Ostan R., Bene M.C., Spazzafumo L., Pinto A., Donini L.M., Pryen F. Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED study, an open-label randomized control trial. Clin Nutr. 2016;35(4):812–818. doi: 10.1016/j.clnu.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Kastorini C.M., Milionis H.J., Esposito K., Giugliano D., Goudevenos J.A., Panagiotakos D.B. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 53.Koloverou E., Panagiotakos D.B., Pitsavos C., Chrysohoou C., Georgousopoulou E.N., Grekas A. Adherence to Mediterranean diet and 10-year incidence (2002−2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev. 2016;32(1):73–81. doi: 10.1002/dmrr.2672. [DOI] [PubMed] [Google Scholar]
- 54.Schroder H. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. J Nutr Biochem. 2007;18(3):149–160. doi: 10.1016/j.jnutbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Perez C., Molina-Montes E., Verardo V., Artacho R., Garcia-Villanova B., Guerra-Hernandez E.J. Changes in dietary behaviours during the COVID-19 outbreak confinement in the Spanish COVIDiet study. Nutrients. 2020:12(6). doi: 10.3390/nu12061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attina A., Cinelli G. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18(1):229. doi: 10.1186/s12967-020-02399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forshee R.A., Storey M.L., Allison D.B., Glinsmann W.H., Hein G.L., Lineback D.R. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr. 2007;47(6):561–582. doi: 10.1080/10408390600846457. [DOI] [PubMed] [Google Scholar]
- 58.Malik V.S., Schulze M.B., Hu F.B. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahleova H., Katz D.L. Editorial: vegetarian dietary patterns in the prevention and treatment of disease. Front Nutr. 2020;7:92. doi: 10.3389/fnut.2020.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adams K.K., Baker W.L., Sobieraj D.M. Myth busters: dietary supplements and COVID-19. Ann Pharmacother. 2020;54(8):820–826. doi: 10.1177/1060028020928052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Avolio A., Avataneo V., Manca A., Cusato J., De Nicolo A., Lucchini R. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5) doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrea L., Muscogiuri G., Laudisio D., Pugliese G., de Alteriis G., Colao A. Influence of the Mediterranean diet on 25- hydroxyvitamin D levels in adults. Nutrients. 2020;12(5) doi: 10.3390/nu12051439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manson J.E., Bassuk S.S. Commentary. Eliminating vitamin D deficiency during the COVID-19 pandemic: a call to action. Metabolism. 2020;112:154322. doi: 10.1016/j.metabol.2020.154322. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams D.M., Nawaz A., Evans M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther. 2020;11(6):1199–1216. doi: 10.1007/s13300-020-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh-Swaby O.R., Goodman S.G., Leiter L.A., Cheng A., Connelly K.A., Fitchett D. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2020;8(5):418–435. doi: 10.1016/S2213-8587(20)30038-3. [DOI] [PubMed] [Google Scholar]
- 66.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belanger M.J., Hill M.A., Angelidi A.M., Dalamaga M., Sowers J.R., Mantzoros C.S. Covid-19 and disparities in nutrition and obesity. N Engl J Med. 2020;383(11):e69. doi: 10.1056/NEJMp2021264. Epub 2020 Jul 15. [DOI] [PubMed] [Google Scholar]
- 68.Lassale C., Gaye B., Hamer M., Gale C.R., Batty G.D. Ethnic disparities in hospitalisation for COVID-19 in England: the role of socioeconomic factors, mental health, and inflammatory and pro-inflammatory factors in a community-based cohort study. Brain Behav Immun. 2020;88:44–49. doi: 10.1016/j.bbi.2020.05.074. Epub 2020 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thakur N., Lovinsky-Desir S., Bime C., Wisnivesky J.P., Celedon J.C., Health, Equality Diversity Committee of the American Thoracic, Society The structural and social determinants of the racial/ethnic disparities in the U.S. COVID-19 pandemic: what’s our role? Am J Respir Crit Care Med. 2020;202(7):943–949. doi: 10.1164/rccm.202005-1523PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7(3):398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vepa A., Bae J.P., Ahmed F., Pareek M., Khunti K. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab Syndr. 2020;14(5):1043–1051. doi: 10.1016/j.dsx.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Townsend M.J., Kyle T.K., Stanford F.C. Commentary: COVID-19 and obesity: exploring biologic vulnerabilities, structural disparities, and weight stigma. Metabolism. 2020;154316 doi: 10.1016/j.metabol.2020.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77. doi: 10.1016/j.cmet.2019.05.008. [e3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singer M., Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17(4):423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 76.The L. Syndemics: health in context. Lancet. 2017;389(10072):881. doi: 10.1016/S0140-6736(17)30640-2. [DOI] [PubMed] [Google Scholar]
- 77.Huizar M.I., Arena R., Laddu D.R. The global food syndemic: the impact of food insecurity, malnutrition and obesity on the healthspan amid the COVID-19 pandemic. Prog Cardiovasc Dis. 2020:572–585. doi: 10.1016/j.pcad.2020.07.002. S0033-0620(20)30139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ward Z.J., Bleich S.N., Cradock A.L., Barrett J.L., Giles C.M., Flax C. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]