Abstract
The present study aimed to evaluate the influence of serum vitamin D levels on semen quality and testosterone levels. This is a cross-sectional study conducted at Androscience, Science and Innovation Center in Andrology and High-Complex Clinical and Andrology Laboratory in Sao Paulo, Brazil, with 508 male patients, aged 18–60 years, from 2007 to 2017. Seminal parameters and serum sexual hormones were correlated with serum vitamin D concentrations in 260 men selected by strict selection criteria. Patients were divided into normozoospermic group (NZG, n = 124) and a group with seminal abnormalities (SAG, n = 136). Evaluation included complete physical examination, past medical history, habits and lifestyle factors, two complete seminal analysis with sperm functional tests, serum levels of 25-hydroxy-vitamin D3 (25(OH)VD3), total and free testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), sex hormone-binding globulin (SHBG), total cholesterol, homeostatic model assessment of insulin resistance (HOMA-IR) index, and karyotype. The mean concentration of 25(OH)VD3 was significantly lower in the SAG (P < 0.001) and positively correlated with all baseline seminal parameters and total testosterone levels. In addition, serum vitamin D3 concentration was found to be positively correlated with sperm concentration (β= 2.103; P < 0.001), total number of spermatozoa with progressive motility (β = 2.069; P = 0.003), total number of motile spermatozoa (β = 2.571; P = 0.015), and strict morphology (β = 0.056; P = 0.006), regardless of other variables. This is the first comparative study to address the issue of serum vitamin D3 content between normozoospermic patients and those with sperm abnormalities. It clearly demonstrates a direct and positive relationship between serum vitamin D level and overall semen quality, male reproductive potential, and testosterone levels.
Keywords: male infertility, semen quality, sexual hormones, sperm motility, testosterone, vitamin D deficiency
INTRODUCTION
Classically, vitamin D (VD3) is recognized as a key regulator of calcium, phosphorus, and bone health homeostasis.1 However, in addition to bones, VD3 targets a wider range of biological organs and processes, including fat metabolism,2 thyroid function,3 immune response,4 cardiovascular function,5 the central nervous system,6 and the reproductive system.7,8 Vitamin D deficiency (VDD) secondary to modern lifestyles, food habits (such as a strict vegan diet or poor nutrition), milk allergies, and, more recently, the excessive use of sunscreens for the adequate prevention of skin cancer and melanoma in particular, is considered a public health problem in both developed and developing countries.9 According to the Dietary Reference Intakes for Calcium and Vitamin D (Institute of Medicine, 2011),10 the prevalence of VDD (estimated by a serum concentration of 25-hydroxy-vitamin D3 [25(OH)VD3] ≤20 ng ml−1) has been estimated between 30% and 93%, even in tropical countries where sun exposure is not a limitation.9 In a recent meta-analysis, the prevalence of VDD in Brazil reached 28.2%, while VD3 insufficiency affected up to 45.3% of the general population, with an average serum level of 25(OH)VD3 at about 27 ng ml−1.11
Adequate testicular function requires a cascade of complex events, the detailed investigation of which has been the objective of many studies.12,13 It is generally accepted that male infertility is largely underestimated using only basic sperm analysis as recommended by the World Health Organization (WHO). When the etiology of male infertility is unclear and the cause for sperm dysfunction is unknown, idiophatic cases are often directly guided for the in vitro fertilization (IVF) lab, not allowing more relevant, better cost-effective and less risky andrological treatments.14,15 An ongoing debate on global VDD in the general population, and the recognition that VD3 is a pleiotropic signaling molecule, has encouraged studies of its action on nonconventional target organs and tissues, i.e., other than bone health and metabolism.1,16,17 The multiple localization of VD3 receptor (VDR) in human spermatozoa (including the sperm head, the postacrosomal region, the neck, and sperm midpiece) supports VD3 potential actions on sperm functions.18,19,20,21 In addition, the fact that the VDR has been detected in spermatogonia, spermatids, spermatozoa, Leydig, Sertoli, and maturing germ cells supports the idea that VD3 could be a potent actor in male reproductive performance.19,20,21 Finally, the fact that all the VD3 metabolic enzymes (including 25-hydroxylase, 1α-hydroxylase, and 24-hydroxylase) are found broadly expressed in the male reproductive tract and are present in spermatozoa18,22 also supports the idea that VD3 is likely to modulate male reproductive functions. In rodent VDD models, sperm motility, concentration, and normal morphology were reported to decrease and these effects were mainly attributed to VDD-induced hypocalcemia as they were increased by calcium and phosphorus supplementation.23 In human VDD, similar sperm phenotypical and functional impairments have been reported together with impacts on female reproductive performances and offspring; however, the link with hypocalcemia remains obscure anxsd controversial.24,25 In addition, the link between VDD and serum androgen levels is still poorly understood.26 Several studies suggesting that VDD may influence male reproductive function have been reported in both fertile and infertile men.24,25,27,28,29,30 However, the cohort size limits the conclusions and all the associations disappeared after adjustment for confounders using multivariate analysis.27 This prompted us to evaluate serum VD3 and testosterone concentrations in relation to semen parameters in normozoospermic patients compared with patients showing defective semen parameters.
PATIENTS AND METHODS
Study design and patients
This cross-sectional study collected data from the records of 508 male patients, aged 18–60 years, who attended Androscience, Science and Innovation Center in Andrology and High-Complex Clinical and Andrology Laboratory in Sao Paulo, Brazil, a reference center for male infertility, hypogonadism, and male health, from 2007 to 2017. The main reasons for consulting these patients were infertility, erectile dysfunction, loss of libido, premature ejaculation and other sexual problems, prevasectomy visit, or as part of a general health checkup. The data collected included sex hormone evaluation, semen analysis, and functional testing of semen (WHO standards).31 Serum 25(OH)VD3 (in ng ml−1) concentrations were included and each subject was classified according to the Endocrine Society guidelines.32 When 25(OH)VD3 serum concentration falls below 20 ng ml−1, the patient is in a state of VDD, from 20 ng ml−1 to 30 ng ml−1, and is qualified as VD3 insufficiency; when 25(OH)VD3 serum concentration is over 30 ng ml−1, the patient is considered normal. Data also included information on medications, physical exercise, tobacco, alcohol, and other drugs. The presence of clinical varicocele according to the criteria modified by Dubin and Amelar33 was verified as well as other testicular abnormalities. Overweight and obesity status of each patient were also assessed by defining the body mass index (BMI) with the following reference values (BMI: eutrophic <25 kg m−2, overweight ≥25 to <30 kg m−2, and obesity ≥30 kg m−2).34
Rigorous exclusion criteria included any previous history of medical, lifestyle, or serious unhealthy habits that could affect sperm quality or impair testicular function: genetics or infectious and inflammatory diseases, use of synthetic or illicit drugs, and cancer and related therapies, as described in Figure 1. In addition, data collection was strictly limited to a 6-month interval between the initial assessment and blood and semen testing. As a consequence, out of the 508 patients, final sample size was reduced to 260 subjects (Figure 1). These were subsequently divided into two groups according to the World Health Organization Laboratory Manual for the Examination and Processing of Human Semen (WHO, 2010).31 The seminal abnormalities group (SAG; n = 136) included patients with one or more seminal abnormalities, based on the 5th centile of recommendations: concentration <15 × 106 ml−1, progressive motility <32%, and semen morphology <4%. The normozoospermic group (NZG, n = 124) consisted of individuals with no identifiable differences from any of the semen's basic parameter reference values. This study was approved by the Research Ethics Committee of the School of Medicine of the University of Sao Paulo, Sao Paulo, Brazil (trial registration number: CAAE 48393715.9.0000.0065). Informed consent form was exempt by Ethics Committee because all data were extracted from medical records and there was no clinical intervention.
Figure 1.
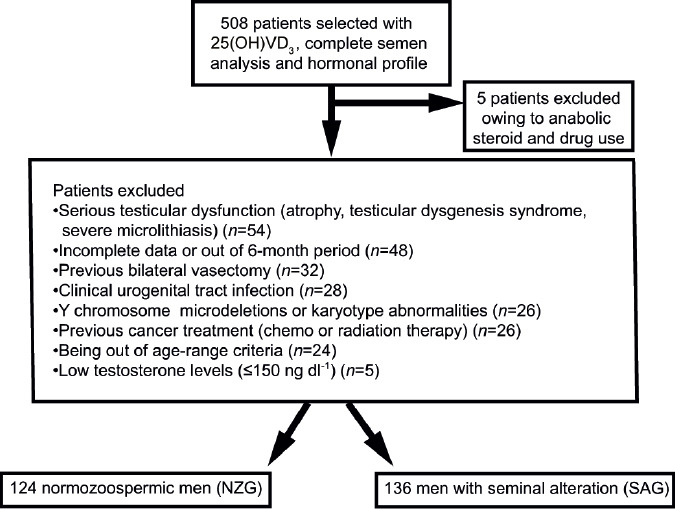
Selection diagram representing study design.
Semen and biochemical analyses
Blood tests come from a specific database and were carried out in high-quality certified laboratories. Serum concentrations of 25(OH)VD3 (in ng ml−1) were obtained by competitive chemiluminescent immunoassay (ECLIA binding assay, Diasorin, Stillwater, MN, USA) or by high-performance liquid chromatography (HPLC) with mass spectrometry. Serum luteinizing hormone (LH, in μU l−1), follicle-stimulating hormone (FSH, in μU l−1), sex hormone-binding globulin (SHBG, in nmol l−1), and free and total testosterone (in ng ml−1) were measured by an electrochemiluminescent immunoassay kit (ECLIA kit; Roche Diagnostics, Mannheim, Germany). Free testosterone levels were calculated from the Vermeulen formula.35 The homeostatic model assessment of insulin resistance (HOMA-IR) index and total cholesterol (in mg dl−1) were adopted as potential confounding variables. The HOMA-IR index was calculated from the formula: HOMA-IR = fasting glucose (in mg dl−1) × fasting insulin (in μUI ml−1)/22.5 as described by Bergman et al.36 in 2003. Fasting glucose was evaluated by calorimetric enzyme, whereas fasting insulin was monitored by ECLIA kit (Roche Diagnostics). All semen analyses were performed at Androscience, High Complexity Clinical and Research Andrology Laboratory. Samples were collected by masturbation after a period of 2–7 days of abstinence. Semen analysis was performed using a Makler counting chamber (Sefi-Medical Instruments, Haifa, Israel) and included the evaluation of macroscopic and microscopic semen parameters: volume (in ml), pH, concentration (×106 ml−1), progressive motility (%PR) and total motility (%TM), total number of spermatozoa (×106), total number of motile spermatozoa (×106), and total number of spermatozoa with progressive motility (×106) according to the WHO guidelines.31 The morphological analysis was carried out taking into account WHO31 and strict criteria by Kruger et al.37 The presence of antisperm antibodies was assessed using the Marscreen® commercial kit (Bioscreen, New York, NY, USA).
Statistical analyses
Patients were characterized by age and vitamin D3 deficiency as assessed according to the Endocrine Society guidelines.32 The presence of varicocele, smoking, alcohol consumption, physical activity level and other lifestyle habits, BMI, HOMA-IR index, basal serum hormone concentrations, and seminal parameters were recorded. Continuously changing data were described by mean and standard deviation (s.d.), whereas categorical data were described by absolute number, frequency, and proportion. Correlations between serum 25(OH)VD3 concentrations, semen parameters, sex hormones, HOMA-IR index, total cholesterol, BMI, and age were analyzed by Spearman's correlation coefficient. The Mann–Whitney U test was used to verify the association between 25(OH)VD3 and other clinical or epidemiological characteristics. This test was also used to identify an association between semen parameter values and sex hormones and all the variables described above. A multiple linear regression was performed to predict which variables (VD3, age, presence of varicocele, smoking, alcohol consumption, BMI, HOMA-IR index, and total testosterone concentration) could have a positive or negative influence on seminal parameters. The normality of semen parameters was confirmed and evaluated by partial regression graphs and residue tables in the predicted values. These data were also evaluated for residue independence by the Durbin–Watson test and homoscedasticity (variance homogeneity) by visual inspection of a residue graph against nonstandard predicted values. It was observed that both the collective linearity and the dependent variable with all independent variables were linear. The significance level was set at P < 0.05. All analyses were performed with SPSS 23.0 (IBM Corp, Armonk, NY, USA).
RESULTS
Sample characteristics
Table 1 presents the clinical and laboratory characteristics of the patients' seminal samples. The mean age was similar in the SAG and NZG groups. The mean serum 25(OH)VD3 concentration was significantly lower in the SAG group than the NZG group (Figure 2). The prevalence of VDD was significantly higher in patients with abnormal seminal parameters than in the normozoospermic group (33.1% in the SAG group and 25.0% in the NZG group, P < 0.001). The 25(OH)VD3 concentration was considered sufficient in 25.7% of SAG men and 41.9% of NZG men. The prevalence of overweight and obesity was significantly higher in patients with abnormal seminal characteristics than normozoospermic patients (77.1% for the SAG group and 54.6% for the NZG group). The same was true for serum insulin levels (P = 0.054). The incidence of alcohol consumption and smoking was similar in both groups (54.4% in the SAG group vs 61.2% in the NZG group, and 17.6% in the SAG group vs 16.5% in the NZG group, respectively). The frequency of varicocele was exactly the same in both groups (54.9%). No differences were observed in total cholesterol and HOMA-IR between the two groups (P = 0.734 and P = 0.071, respectively). Regarding hormonal profile, the SAG group had lower total and free testosterone levels (P = 0.019 and P = 0.018, respectively) and higher FSH (P = 0.018) than the NZG group.
Table 1.
Baseline clinical and laboratory features of the samples according to seminal analysis diagnosis group
| Clinical/laboratory characteristics | SAG (n=136) | NZG (n=124) | P |
|---|---|---|---|
| Age (year), mean±s.d. (range) | 38.73±8.45 (19.00–59.00) | 37.96±8.77 (18.00–60.00) | 0.615 |
| 25(OH)VD3 (ng dl−1), mean±s.d. (range) | 25.18±8.89 (7.00–68.00) | 30.06±12.10 (9.00–75.00) | <0.001* |
| Sufficient (>30.1 ng ml−1), n (%)a | 35 (25.7) | 52 (41.9) | 0.01* |
| Insufficient (20.1–30 ng ml−1), n (%)a | 56 (41.2) | 41 (33.1) | 0.01* |
| Deficient (≤20 ng ml−1), n (%)a | 45 (33.1) | 31 (25.0) | 0.01* |
| BMI (kg m−2), mean±s.d. (range)b,c | 27.72±4.16 (17.03–42.80) | 26.63±3.62 (20.86–36.90) | 0.013* |
| Underweight, n (%) | 1 (0.9) | - | |
| Normal BMI, n (%) | 23 (21.9) | 44 (45.4) | 0.01* |
| Overweight, n (%) | 54 (51.4) | 39 (40.2) | 0.01* |
| Obese, n (%) | 27 (25.8) | 14 (14.4) | 0.01* |
| Presence of varicocele (yes), n/total (%)c | 56/102 (54.9) | 56/102 (54.9) | 1.000 |
| Currently smoker (yes), n/total (%)c | 18/102 (17.6) | 16/97 (16.5) | 0.829 |
| Alcohol use (yes), n/total (%)c | 55/101 (54.4) | 60/98 (61.2) | 0.334 |
| Insulin (µU ml−1), mean±s.d. (range) | 10.12±6.42 (2.20–40.00) | 8.58±5.79 (2.00–29.00) | 0.054 |
| HOMA-IR, mean±s.d. (range) | 2.25±1.42 (0.46–8.39) | 1.98±1.50 (0.20–8.19) | 0.071 |
| Total cholesterol (mg dl−1), mean±s.d. (range) | 199.04±39.21 (103.00–317.00) | 197.33±40.74 (90.00–304.00) | 0.734 |
| Seminal parameters, mean (range)d | |||
| Semen volume (ml) | 3.00 (0.25–10.00) | 2.50 (0.50–8.50) | 0.244 |
| Total number of motile spermatozoa (106) | 23.25 (0–559.00) | 147.00 (11.0–1152.00) | <0.001* |
| Total number of spermatozoa (106) | 43.60 (0–866.70) | 205.75 (1.50–1440.00) | <0.001* |
| Sperm concentration (106 ml−1) | 16.00 (0–577.80) | 82.00 (15.00–800.00) | <0.001* |
| Total number of spermatozoa with progressive motility (106) | 5.41 (00–426.00) | 79.60 (6.93–748.00) | <0.001* |
| Total progressive motility (%) | 16.00 (0–65.00) | 50.00 (32.00–75.00) | <0.001* |
| Total motile sperm (%) | 45.00 (0–80.00) | 70.00 (15.00–90.00) | <0.001* |
| Hormonal parameters, median (range) | |||
| LH (µU l−1) | 4.50 (0.08–19.20) | 3.88 (0.06–10.10) | 0.203 |
| FSH (µU l−1) | 6.17 (0.22–41.50) | 4.31 (0.29–23.90) | 0.018* |
| Total testosterone (ng dl−1) | 463.84 (164.00–1123.00) | 522.99 (175.00–1231.00) | 0.019* |
| Free testosterone (ng dl−1) | 9.88 (3.56–32.00) | 14.22 (3.70–374.00) | 0.018* |
| SHBG (nmol l−1) | 37.57 (6.70–413.0) | 36.27 (8.00–415.00) | 0.641* |
aAccording to the Endocrine Society guidelines (2011);32 baccording to the WHO 2000;34 cthere were some missing data in the medical records; daccording to the WHO 2010.31 *P≤0.05 is considered statistically significant difference. SAG: seminal abnormalities group; NZG: normozoospermic group; 25(OH)VD3: 25-hydroxy-vitamin D3; BMI: body mass index; HOMA-IR: homeostatic model assessment insulin resistance; LH: luteinizing hormone; FSH: follicle-stimulating hormone; SHBG: sex hormone-binding globulin; s.d.: standard deviation
Figure 2.
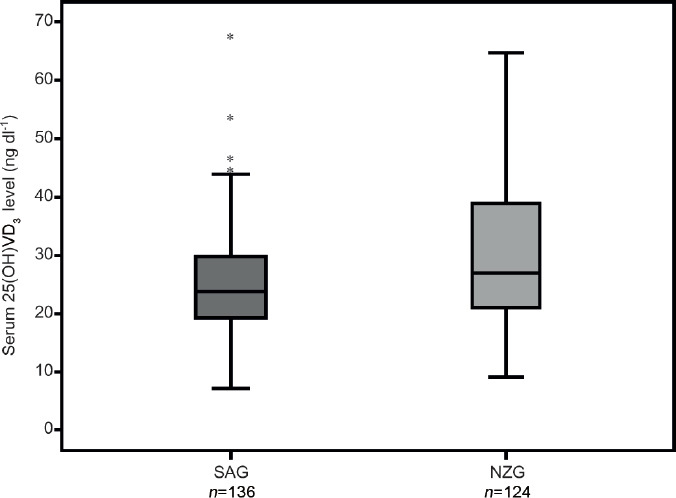
Box-plot graph of 25-hydroxy-vitamin D3 serum levels distribution in both groups. The box displays the first quartile, median, and third quartile value distribution, whereas the horizontal line within it represents the median. Error bars represents the maximum and minimum VD3 levels and the black dots represents few outliers. *SAG: seminal abnormalities group; NZG: normozoospermic group; 25(OH)VD3: 25-hydroxy-vitamin D3; VD3: vitamin D.
Vitamin D and semen quality
We identified a significant correlation between serum 25(OH)VD3 concentrations and all basic seminal parameter values, mainly with percentage of total sperm motility (P = 0.001; Supplementary Figure 1 (1.6MB, tif) ), even using multivariate analysis (Table 2). The best-correlated sperm parameters were associated with a 25(OH)VD3 concentration of about 30 to 40 ng ml−1 (Figure 2). Total serum testosterone concentration was positively correlated with values of three semen parameters: total sperm motility (β = 0.025; P < 0.004), total progressive motility (β = 0.020; P < 0.009), and sperm morphology according to the WHO criteria (β = 0.007; P = 0.021). In contrast, varicocele had a statistically significant impact only in the total number of spermatozoa (β = 62.930; P = 0.039). In particular, the 25(OH)VD3 levels positively and independently influenced four sperm quality parameters, including sperm concentration (β = 2.103; P < 0.001), total number of motile spermatozoa (β = 2.571; P = 0.015), total number of spermatozoa with progressive motility (β = 2.069; P = 0.003), and Kruger's strict criteria morphology (β = 0.056; P = 0.006).
Table 2.
Contribution of each variable to seminal quality parameters by β coefficient (significance value, P) from multivariate regression analysis
| Constant | Total number of spermatozoa | Sperm concentration | Total number of motile spermatozoa | Total number of spermatozoa with progressive motility | Total progressive motility | Total sperm motility | Normal morphology (WHO 2010) | Morphology (strict) |
|---|---|---|---|---|---|---|---|---|
| 25(OH)VD3 | 3.231 (0.033*) | 2.103 (<0.001*) | 2.571 (0.015*) | 2.069 (0.003*) | 0.366 (0.015*) | 0.426 (0.013*) | 0.117 (0.053*) | 0.056 (0.006*) |
| Age | −1.439 (0.480) | 0.394 (0.615) | −0.967 (0.499) | −0.536 (0.565) | −0.120 (0.554) | −0.331 (0.152) | 0.007 (0.930) | −0.011 (0.681) |
| BMI | −0.974 (0.522) | 0.195 (0.739) | −0.591 (0.580) | −0.519 (0.455) | −0.014 (0.925) | −0.124 (0.470) | 0.035 (0.569) | 0.015 (0.468) |
| Total testosterone | 0.081 (0.294) | 0.033 (0.262) | 0.069 (0.200) | 0.038 (0.281) | 0.020 (0.009*) | 0.025 (0.004*) | 0.007 (0.021*) | 0.002 (0.058) |
| Total cholesterol | 0.248 (0.439) | 0.139 (0.261) | 0.148 (0.510) | 0.034 (0.817) | −0.025 (0.436) | −0.008 (0.829) | 0.003 (0.840) | −0.002 (0.582) |
| HOMA-IR | −6.558 (0.509) | 3.177 (0.405) | −5.536 (0.427) | −2.170 (0.632) | 0.094 (0.924) | 1.070 (0.341) | 0.363 (0.363) | −0.057 (0.582) |
| Alcohol | −51.649 (0.069) | −20.007 (0.066) | −33.197 (0.095) | −13.701 (0.289) | −1.234 (0.661) | −5.481 (0.087) | 0.685 (0.547) | −0.052 (0.891) |
| Smoking | −0.886 (0.973) | −5.282 (0.605) | −2.210 (0.906) | −5.855 (0.630) | −1.332 (0.615) | −0.700 (0.816) | −0.698 (0.506) | 0.164 (0.638) |
| Varicocele | 62.930 (0.039*) | 16.758 (0.152) | 31.890 (0.135) | 20.889 (0.132) | −2.291 (0.448) | −4.348 (0.206) | −0.593 (0.626) | 0.332 (0.411) |
Predictors (constant): 25(OH)VD3, varicocele, HOMA-IR, age, alcohol, total testosterone, BMI, smoking. Dependent variable: total number of motile spermatozoa, total number of spermatozoa, total number of spermatozoa with progressive motility, total progressive motile, total motile, morphology by WHO, morphology by Kruger. *P≤0.05 is considered statistically significant difference. 25(OH)VD3: 25-hydroxy-vitamin D3; BMI: body mass index; HOMA-IR: homeostatic model assessment insulin resistance; WHO: World Health Organization
Vitamin D and clinical and laboratorial parameters
We found a negative correlation between serum 25(OH)VD3 concentrations and HOMA-IR concentrations (r = −0.21; P = 0.009). No correlation with BMI (P = 0.330), total serum cholesterol levels (P = 0.850), smoking (P = 0.500), or alcohol intake (P = 0.104) was established.
Supplementary Figure 2 (175.2KB, tif) shows the positive Spearman's correlation between 25(OH)VD3 concentrations and total testosterone levels (r = 0.161; P = 0.014). In addition, 25(OH)VD3 concentration and BMI concentration were predictors of total testosterone concentration (β = 2.751, P = 0.019, and β = −9.621, P = 0.002, respectively; Table 3). Smoking, alcohol consumption, and the presence of varicocele had no influence on sex hormone outcomes in either group and were not independent predictors of any controlled variables.
Table 3.
Contribution of each individual variable to sexual hormone parameters by standardized β coefficient from multivariate regression analysis
| Constant | Total testosterone | FSH | SHBG |
|---|---|---|---|
| 25(OH)VD3 | 2.751 (0.019*) | 3.306 (0.112) | 0.133 (0.177) |
| Age | −1.927 (0.180) | 0.039 (0.111) | 0.401 (0.001*) |
| BMI | −9.621 (0.002) | 0.069 (0.175) | −0.935 (<0.001*) |
| Total cholesterol | 0.017 (0.956) | −0.012 (0.022*) | −0.008 (0.754) |
Data are expressed as β coefficient (P value). Predictors (constant): 25(OH)VD3, age, total cholesterol, BMI, smoking, varicocele. Dependent variable: total testosterone, free testosterone, LH, FSH, SHBG. *P≤0.05 is considered statistically significant difference. 25(OH)VD3: 25-hydroxy-vitamin D3; BMI: body mass index; LH: luteinizing hormone; FSH: follicle-stimulating hormone; SHBG: sex hormone-binding globulin
DISCUSSION
An ongoing debate regarding global VDD deficiency in the general population has encouraged intensive investigation of its action upon nonclassical target organs and tissues, other than bone health and metabolism.1,16,17 Vitamin D has been recognized as an all-around signaling molecule and male reproductive organs are among its target tissues.38
In this cross-sectional study, with strict inclusion criteria, we found that 66.5% of patients (from a cohort of 260 men in a tropical country where sun exposure is not a limitation) consulting for sexual and reproductive health problems were in a situation of vitamin D insufficiency. Considering their functional and structural sperm parameters, we clearly demonstrated here that serum 25(OH)VD3 levels were positively correlated with values of all seminal parameters that are part of the standardized WHO-recommended semen analysis. The strongest correlation was with sperm motility, as it has already been reported elsewhere.25 Nevertheless, these results further indicate that regardless of a wide number of confounding variables, serum VD3 concentration had a positive influence on the total number of spermatozoa with progressive motility. An easily understandable and useful equation formulated from our findings demonstrates that every “one unit” (ng ml−1) increase in 25(OH)VD3 serum concentrations correlates with a 2.1% increase in progressive motile spermatozoa in the ejaculate. Other factors that may affect sperm quality and reduce fertility status, such as exposure to tobacco and alcohol, had no influence and were not significant as confounders for seminal parameters in our multivariate analysis. Progressive motility is one of the most important indicators of sperm quality provided by basic semen analysis and has a positive predictive value for natural conception and even intrauterine insemination (IUI), as demonstrated in a large retrospective review of 1728 IUI cycles, demonstrated that among 38 clinical, hormonal, metabolic and semen variables analyzed, only 3 of them were associated with successful IUI outcome: female age <37.7 years at the time of treatment, absence of pelvic surgery, and the strongest correlation with postwash sperm motility higher than 40.0% (P = 0.006).39 Therefore, theoretically, VD3 supplementation could, in selected cases, decrease the invasiveness of proposed assisted reproductive technologies and turn previous IVF or intracytoplasmic sperm injection (ICSI) cases into a promising less invasive with minor risks and more cost-effective scenario of simple IUIs.
The results of observational studies concerning serum 25(OH)D3 concentrations and seminal parameters are divergent. Some studies have reported a positive correlation with sperm motility and morphology,24,25,40,41 while others have shown a negative27 or even no effect on seminal parameters.28 Our data concur with Hammoud et al.24 who also suggested that low serum VD3 concentrations may be associated with poor seminal parameter values. These authors reported an inverted U-shaped relationship between serum VD3 concentration and five seminal parameters, including sperm concentration, percentage of progressively motile spermatozoa, total number of spermatozoa with progressive motility, total number of spermatozoa, and percentage of normal sperm heads.24 In a cross-sectional study of 300 Danish men, serum VD3 concentrations were also found to be correlated with total and progressive sperm motility as well as with normal sperm morphology.30 In addition, in another Danish cross-sectional study involving 307 men, a high level of VD3 (37.0–90.0 ng ml−1) was associated with a lower median total number of spermatozoa and a lower percentage of sperm of normal morphology.27 This unexpected finding in this latest study probably reflects a low number of men in the low-VD3 group insufficient to detect an effect on sperm quality, rather than indicating that low VD3 levels are not associated with poor seminal parameters. In vitro, serum 25(OH)VD3 concentrations are correlated with increased intracellular calcium concentration in human spermatozoa.40 This hypothesis was recently strengthened by the observation that in a randomized and placebo-controlled trial, higher live-birth rates were seen in oligozoospermic men with low serum VD3 basal levels after they received a combined VD3 and calcium supplementation.41 The same group reported that serum calcium levels, VD3 levels, and progressive sperm motility are correlated and that 25(OH)VD3 induces a rapid increase in intracellular calcium concentration through a VDR-mediated action.40 In addition, progressive sperm motility also has a positive correlation with a higher probability of obtaining hyperactivated sperm motility in the female environment.42 With these elements at hand, we propose a potential mechanism of action in which sperm motility is modulated by VDR-regulated calcium flows in the male genital tract (Figure 3). This model could explain the improvement in sperm motility and the stimulation of the acrosomal response in men with higher serum VD3 levels. It could also explain the broad role of VD3 that will act at both the mature spermatozoon and testicular levels, since VDR has been detected in mature spermatozoa and also in Leydig, Sertoli, and differentiating germ cells from spermatogonia and spermatids to spermatozoa.19,20,21,22 The multiple locations of VDR in human spermatozoa (including the sperm head, the post-acrosomal region, the neck, and sperm midpiece) also support its various actions on functional sperm characteristics.18,19,20,21
Figure 3.
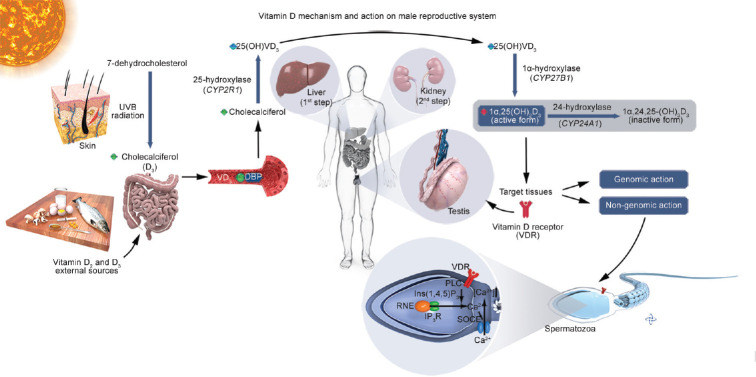
Vitamin D (VD3) mechanism and action on male reproductive system. After sun exposure, the first step of VD3 synthesis occurs in the skin, where ultraviolet B radiation converts 7-dehydrocholesterol into cholecalciferol (vitamin D3). Vitamin D2 and D3 coming from food sources or external administration, are absorbed in the gut and transported to the bloodstream, where cholecalciferol couples with VD3 binding protein (DBP) and is carried to the liver. Cholecalciferol (vitamin D3) is biologically inactive, and must undergo two hydroxylation steps to form the active 1α,25-dihydroxy-vitamin D3 (1α,25(OH)2D3). The first step is a reaction mediated by the hepatic CYP2R1 gene that codifies the 25-hydroxylase enzyme. The second step is conducted by renal CYP27B1 that codifies the enzyme 1α-hydroxylase, leading to the formation of the biological active form, 1α,25(OH)2D3, responsible for a multitude of genomic and nongenomic actions through binding and activating VD3 receptor (VDR) in many target tissues, including Leydig and Sertoli cells in testis, their precursor cells and spermatozoa. 1α,25(OH)2D3 can be inactivated by the 24-hydroxylase enzyme (codified by CYP24A1 gene), transforming into the inactive form 1α,24,25-trihydroxy-vitamin D3 (1α,24,25-(OH)3D3). The nongenomic effect of VDR in human spermatozoa occurs when the active form, 1α,25(OH)2D3 activates VDR in the neck region inducing PLC activation leading to IP3 production which subsequently opens IP3R-gated calcium channels in the RNE increasing intracellular Ca2+ concentration. Subsequently, the initial Ca2+ release from RNE might be supported by SOCE.38 VD3: vitamin D; CYP: cytochrome P450 gene superfamily; CYP2R1 gene: cytochrome P450 family 2 subfamily R member 1; CYP27B1 gene: cytochrome P450 family 27 subfamily B member 1; CYP24A1 gene: cytochrome P450 family 24 subfamily A member 1; VDR: vitamin D receptor; PLC: phospholipase C; IP3: Inositol 1,4,5-trisphosphate; IP3R: inositol 3-phosphate receptor; RNE: redundant nuclear envelope; SOCE: store-operated calcium entry. Illustration was developed by Androscience®.
In our cohort, we found a negative correlation between serum VD3 concentration and HOMA-IR, suggesting the possible influence of serum VD3 on glucose metabolism and sperm function. Recently, Ding et al.43 looked at the impact of VD3 supplementation on spermatogenesis in diabetic rats. They concluded that high blood sugar levels could be harmful to germ cells and affect the quantity and quality of spermatozoa. However, when animals received supplements with high doses of VD3, they had significantly fewer abnormalities (mainly in sperm concentration and motility) than the control group. The authors concluded that VD3 could play a protective role in overall testicular function, including increased reproductive capacity to withstand harmful effects of diabetes. It has been suggested that this effect was caused by the attenuation of inflammation, inactivating the caspase-dependent cascades that regulate apoptosis. In addition, the authors suggested that VD3 could be seen as a promising form of treatment for testicular dysfunction in diabetic patients. Furthermore, serum VD3 and BMI were determined to be positive and negative predictors of total testosterone levels, respectively. BMI also has a negative influence on SHBG, which leads to a better understanding of the lack of correlation between VD3 and free testosterone levels. Nevertheless, the overall influence of VD3 action on the increase in testosterone levels observed in our study is probably less important than the negative effects represented by the increase in BMI (Table 3). These variables appear to be an important factor influencing seminal quality.12 However, the multicollinearity analysis excluded this influence in our cohort.
We also observed a positive correlation between total and free serum testosterone levels and serum 25(OH)VD3 concentrations, in the NZG cohort. This is in agreement with the observation that in VDR-null mice, the testosterone/LH ratio is low, suggesting that testosterone levels are somehow regulated by VD3.22 However, a recent contradictory report demonstrated that a 6-month VD3+ calcium supplementation did not have any significant effect on serum testosterone levels in young men.44 It is thus clear that more investigations will be needed to elucidate the relationship existing between serum VD3 and serum testosterone concentrations; however, owing to the high prevalence of VDD worldwide, this is an issue that cannot be ignored any longer. Our findings in testosterone levels are not limited to the reproductive age group, but could be extrapolated to benefit the growing population of older men, who universally demand for better health standards, including improved quality of overall sexual satisfaction, in order that these results could stimulate new therapeutic developments.45 The elegant data reported here, in combination with nouvelle insights into the mechanisms of action of VD3 and its receptor (Figure 3), serve like an orientational compass pointing to the direction of maintaining serum VD3 concentration higher than 30 ng ml−1 for a better male reproductive performance. Whether higher VD3 levels could result in further improvement in sperm quality and/or testosterone levels, as well as, mitigate the harmful effects of oxidative stress, lipid peroxidation and DNA damage, remains a subject for future research.15,45
Together, the known VDR-mediated positive endocrine action of serum VD3 on the testis, as well as on spermatozoa and our present data showing that serum 25(OH)VD3 concentrations are positively correlated with sperm motility, nuclear integrity, and total testosterone levels, suggests that VD3 supplementation could be part of a therapeutic strategy designed to improve male fertility.46,47 Toward this goal, further investigation, including randomized, controlled, and double-blinded clinical trials as well as in vitro and in vivo studies, is needed to explore fully the therapeutic potential of VD3 supplementation in cases of male infertility. Given growing concerns over the widespread and uncontrolled use of assisted reproductive technologies, in particular, ICSI beyond medical and ethical boundaries, any simple and cheap clinical treatment measure to improve testicular function and sperm quality in vivo, such as VD3 supplementation, should be encouraged owing to its simplicity, low cost, and strong biological actions.47
AUTHOR CONTRIBUTIONS
JH, EMFC, PG, and RJA conceived the idea. IMC, JH, TAT, and JRP designed the study. IMC and JRP collected data. Data analysis was carried out by IMC, JRP, TAT, and PG. The first draft was written by IMC and EMFC and revised by TAT, JRP, EMFC, JRD, and JH. All authors read and approved the final version of the manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
Correlation between 25(OH)D3 and seminal parameters in 260 male subjects. Each blue point represents 25(OH)D3 concentration of one individual (each unity of measurement). Red line represents the correlation direct. 25(OH)D3: 25-hydroxy-vitamin D3; VD3 vitamin D.
Correlation between total testosterone levels and 25(OH)D3 in 260 male subjects. Each blue point represents 25(OH)D3 concentration for testosterone level in each patient. Red line represents the correlation direction tendency. r = Spearman's correlation coefficient. 25(OH)D3: 25-hydroxy-vitamin D3
ACKNOWLEDGMENTS
Androscience, Science and Innovation Center in Andrology and High-Complexity Clinical and Research Andrology Laboratory, provided technical and financial support. This study did not receive any financial support from external sources.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Anderson PH, Turner AG, Morris HA. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin Biochem. 2012;45:880–6. doi: 10.1016/j.clinbiochem.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Savastano S, Barrea L, Savanelli MC, Nappi F, Di Somma C, et al. Low vitamin D status and obesity: role of nutritionist. Rev Endocr Metab Disord. 2017;18:215–25. doi: 10.1007/s11154-017-9410-7. [DOI] [PubMed] [Google Scholar]
- 3.Nettore IC, Albano L, Ungaro P, Colao A, Macchia PE. Sunshine vitamin and thyroid. Rev Endocr Metab Disord. 2017;18:347–54. doi: 10.1007/s11154-017-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altieri B, Grant WB. Vitamin D and pancreas: the role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. 2017;57:3472–88. doi: 10.1080/10408398.2015.1136922. [DOI] [PubMed] [Google Scholar]
- 5.Muscogiuri G, Altieri B, de Angelis C, Palomba S, Pivonello R, et al. Shedding new light on female fertility: the role of vitamin D. Rev Endocr Metab Disord. 2017;18:273–83. doi: 10.1007/s11154-017-9407-2. [DOI] [PubMed] [Google Scholar]
- 6.Focker M, Antel J, Ring S, Hahn D, Kanal O, et al. Vitamin D and mental health in children and adolescents. Eur Child Adolesc Psychiatry. 2017;26:1043–66. doi: 10.1007/s00787-017-0949-3. [DOI] [PubMed] [Google Scholar]
- 7.Muscogiuri G, Annweiler C, Duval G, Karras S, Tirabassi G, et al. Vitamin D and cardiovascular disease: from atherosclerosis to myocardial infarction and stroke. Int J Cardiol. 2017;230:577–84. doi: 10.1016/j.ijcard.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 8.Tirabassi G, Cutini M, Muscogiuri G, Delli Muti N, Corona G, et al. Association between vitamin D and sperm parameters: clinical evidence. Endocrine. 2017;58:194–8. doi: 10.1007/s12020-016-1198-9. [DOI] [PubMed] [Google Scholar]
- 9.Fields J, Trivedi NJ, Horton E, Mechanick JI. Vitamin D in the persian gulf: integrative physiology and socioeconomic factors. Curr Osteoporos Rep. 2011;9:243–50. doi: 10.1007/s11914-011-0071-2. [DOI] [PubMed] [Google Scholar]
- 10.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington: National Academies Press (US); 2011. p. 636. [PubMed] [Google Scholar]
- 11.Pereira-Santos M, Santos JY, Carvalho GQ, Santos DB, Oliveira AM. Epidemiology of vitamin D insufficiency and deficiency in a population in a sunny country: geospatial meta-analysis in Brazil. Crit Rev Food Sci Nutr. 2018;8:1–8. doi: 10.1080/10408398.2018.1437711. [DOI] [PubMed] [Google Scholar]
- 12.Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7:153–61. doi: 10.1038/nrurol.2010.6. [DOI] [PubMed] [Google Scholar]
- 13.Mortimer D, Barratt CL, Bjorndahl L, de Jager C, Jequier AM, et al. What should it take to describe a substance or product as 'sperm-safe'. Hum Reprod Update. 2013;19(Suppl):i1–45. doi: 10.1093/humupd/dmt008. [DOI] [PubMed] [Google Scholar]
- 14.Pizzol D, Bertoldo A, Foresta C. Male infertility: biomolecular aspects. Biomol Conc. 2014;5:449–56. doi: 10.1515/bmc-2014-0031. [DOI] [PubMed] [Google Scholar]
- 15.Hallak J. Utility of sperm DNA fragmentation testing in different clinical scenarios of male reproductive abnormalities and its influence in natural and assisted reproduction. Transl Androl Urol. 2017;6:S509–12. doi: 10.21037/tau.2017.06.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, et al. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab. 2011;96:E646–52. doi: 10.1210/jc.2010-1628. [DOI] [PubMed] [Google Scholar]
- 17.Hlaing TT, Compston JE. Biochemical markers of bone turnover – uses and limitations. Ann Clin Biochem. 2014;51:189–202. doi: 10.1177/0004563213515190. [DOI] [PubMed] [Google Scholar]
- 18.Blomberg Jensen M, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25:1303–11. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 19.Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006;68:1345–9. doi: 10.1016/j.urology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Aquila S, Guido C, Perrotta I, Tripepi S, Nastro A, et al. Human sperm anatomy: ultrastructural localization of 1alpha,25-dihydroxyvitamin D receptor and its possible role in the human male gamete. J Anat. 2008;213:555–64. doi: 10.1111/j.1469-7580.2008.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aquila S, Guido C, Middea E, Perrotta I, Bruno R, et al. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009;7:140. doi: 10.1186/1477-7827-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Angelis C, Galdiero M, Pivonello C, Garifalos F, Menafra D, et al. The role of vitamin D in male fertility: a focus on the testis. Rev Endocr Metab Disord. 2017;18:285–305. doi: 10.1007/s11154-017-9425-0. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Chen L, Zhang W, Wang R, Goltzman D, et al. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015;308:E51–62. doi: 10.1152/ajpendo.00076.2014. [DOI] [PubMed] [Google Scholar]
- 24.Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, et al. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14:855–9. doi: 10.1038/aja.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbasihormozi S, Kouhkan A, Alizadeh AR, Shahverdi AH, Nasr-Esfahani MH, et al. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology. 2017;5:113–8. doi: 10.1111/andr.12280. [DOI] [PubMed] [Google Scholar]
- 26.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol. 2010;73:243–8. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramlau-Hansen CH, Moeller UK, Bonde JP, Olsen J, Thulstrup AM. Are serum levels of vitamin D associated with semen quality.Results from a cross-sectional study in young healthy men? Fertil Steril. 2011;95:1000–4. doi: 10.1016/j.fertnstert.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Jueraitetibaike K, Ding Z, Wang DD, Peng LP, Jing J, et al. The effect of vitamin D on sperm motility and the underlying mechanism. Asian J Androl. 2019;21:400–7. doi: 10.4103/aja.aja_105_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang B, Sun H, Wan Y, Wang H, Qin W, et al. Associations between testosterone, bone mineral density, vitamin D and semen quality in fertile and infertile Chinese men. Int J Androl. 2012;35:783–92. doi: 10.1111/j.1365-2605.2012.01287.x. [DOI] [PubMed] [Google Scholar]
- 30.Blomberg Jensen M, Gerner Lawaetz J, Andersson AM, Petersen JH, Nordkap L, et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum Reprod. 2016;31:1875–85. doi: 10.1093/humrep/dew152. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010. [Google Scholar]
- 32.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 33.Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril. 1970;8:606–9. doi: 10.1016/s0015-0282(16)37684-1. [DOI] [PubMed] [Google Scholar]
- 34.WHO. Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO; 2000. [PubMed] [Google Scholar]
- 35.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 36.Bergman RN, Zaccaro DJ, Watanabe RM, Haffner SM, Saad MF, et al. Minimal model-based insulin sensitivity has greater heritability and a different genetic basis than homeostasis model assessment or fasting insulin. Diabetes. 2003;8:2168–74. doi: 10.2337/diabetes.52.8.2168. [DOI] [PubMed] [Google Scholar]
- 37.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, et al. Sperm morphologic features as a prognostic factor inin vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 38.Blomberg Jensen M. Vitamin D and male reproduction. Nat RevEndocrinol. 2014;10:175–86. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- 39.Hendin BJ, Falcone T, Hallak J, Nelson DR, Vemullapalli S, et al. The effect of patient and semen characteristics on live birth rates following intrauterine insemination: a retrospective study. J Assist Reprod Genet. 2000;17:245–52. doi: 10.1023/A:1009402214820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26:1307–17. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 41.Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jørgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones, and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103:870–81. doi: 10.1210/jc.2017-01656. [DOI] [PubMed] [Google Scholar]
- 42.Freitas MJ, Vijayaraghavan S, Fardilha M. Signaling mechanisms in mammalian sperm motility. Biol Reprod. 2017;96:2–12. doi: 10.1095/biolreprod.116.144337. [DOI] [PubMed] [Google Scholar]
- 43.Ding C, Wang Q, Hao Y, Ma X, Wu L, et al. Vitamin D supplement improved testicular function in diabetic rats. Biochem Biophys Res Commun. 2016;473:161–7. doi: 10.1016/j.bbrc.2016.03.072. [DOI] [PubMed] [Google Scholar]
- 44.Saha S, Goswami R. Vitamin D and calcium supplementation, skeletal muscle strength and serum testosterone in young healthy adult males: randomized control trial. Clin Endocrinol. 2018;88:217–26. doi: 10.1111/cen.13507. [DOI] [PubMed] [Google Scholar]
- 45.Gharagozloo P, Gutierrez-Adan A, Champroux A, Noblanc A, Kocer A, et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum Reprod. 2016;31:252–62. doi: 10.1093/humrep/dev302. [DOI] [PubMed] [Google Scholar]
- 46.Pagani R, Ghayda RA, Hallak J. General Endocrine Therapy. In: Skinner MK, editor. Encyclopedia of Reproduction. Amsterdam: Academic Press; 2018. pp. 314–7. [Google Scholar]
- 47.Hallak J. A call for more responsible use of assisted reproductive technologies (ARTs) in male infertility: the hidden consequences of abuse, lack of andrological investigation and inaction. Transl Androl Urol. 2017;6:997–1004. doi: 10.21037/tau.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between 25(OH)D3 and seminal parameters in 260 male subjects. Each blue point represents 25(OH)D3 concentration of one individual (each unity of measurement). Red line represents the correlation direct. 25(OH)D3: 25-hydroxy-vitamin D3; VD3 vitamin D.
Correlation between total testosterone levels and 25(OH)D3 in 260 male subjects. Each blue point represents 25(OH)D3 concentration for testosterone level in each patient. Red line represents the correlation direction tendency. r = Spearman's correlation coefficient. 25(OH)D3: 25-hydroxy-vitamin D3