Abstract
Androgen deprivation therapy (ADT) with gonadotropin-releasing hormone (GnRH) agonists and antagonists is the mainstay of advanced prostate cancer treatment. Both drug classes decrease levels of luteinizing hormone and follicle-stimulating hormones (FSH), thereby lowering testosterone to castrate levels. This is associated with adverse events (AEs), including cardiovascular (CV) disorders, bone fractures, metabolic dysfunction, and impaired cognitive function. This literature review discusses these AEs, with a focus on CV and bone-related events. A hypothesis-generating meta-analysis of six clinical trials showed a potentially increased risk for CV disorders with GnRH agonists versus the GnRH antagonist degarelix. While no study has directly compared GnRH agonists versus antagonists with a primary CV outcome, one hypothesis for this observation is that GnRH agonists lead to initial surges in FSH that may negatively impact CV health, whereas antagonists do not. GnRH agonists are associated with metabolic and cognitive AEs and while data are lacking for GnRH antagonists, no differences in risk are predicted. Other common AEs with ADT include injection site reactions, which are much more common with degarelix than with GnRH agonists, which may reflect differing administration and injection techniques. Future studies are needed to further evaluate and compare the safety profiles of GnRH agonists and antagonists, especially in patients with pre-existing CV disease and other co-morbidities. Physicians should carefully evaluate benefits and risks when prescribing ADT and ensure that side effects are well managed.
Keywords: androgen antagonists, metabolic syndrome, prostate cancer, safety
INTRODUCTION
Androgen deprivation therapy (ADT) is a first-line treatment for advanced prostate cancer with the goal of achieving castrate levels of testosterone (<0.5 ng ml−1).1 ADT using gonadotropin-releasing hormone (GnRH) agonists or antagonists works by blocking the hypothalamic-pituitary-testis feedback system, thereby lowering the levels of circulating androgens available to activate androgen receptors.2 Currently approved GnRH agonists, such as goserelin, leuprolide, buserelin, and triptorelin, are mainly available as intramuscular or subcutaneous depots. Degarelix, administered subcutaneously, is the only GnRH antagonist approved for the treatment of prostate cancer.3
GnRH agonists and antagonists represent the vast majority of ADT and, unlike bilateral orchiectomy, their effects are reversible. However, there are safety concerns including injection site reactions, hot flushes, cardiovascular disorders, metabolic dysfunction, and increased risk of fractures due to reduced bone mineral density (BMD).4,5,6
This literature review focuses on the safety profile of ADT using GnRH agonists and antagonists in patients with prostate cancer, with a special focus on cardiovascular and bone adverse events (AEs).
LITERATURE SEARCH AND EVIDENCE ACQUISITION
A PubMed search was conducted to identify publications from Phase III studies, meta-analyses, and retrospective population-based studies that evaluated the exposure of ADT in patients with prostate cancer. The inclusion criteria included use of either intermittent or continuous ADT (IADT or CADT, respectively), studies with adequate safety data on cardiovascular events, and effects on bone health. Phase II studies were excluded. The approved dosing regimen for both GnRH agonists and antagonists has been considered in the present review; exceptions are mentioned as applicable.
Safety data were tabulated for similarly captured events while it was descriptively presented when not in uniformity with rest of the data. The key events captured included cardiovascular events and fractures. The safety data for GnRH agonists and antagonists are presented to provide a comparative overview.
HORMONAL REGULATION
GnRH agonists and antagonists both suppress testosterone levels to castrate levels. GnRH agonists, after an initial overstimulation, down-regulate GnRH receptors in the pituitary leading to reduction in luteinizing hormone (LH), follicle-stimulating hormones (FSH), and testosterone. In contrast, GnRH antagonists directly block pituitary GnRH receptors providing rapid and sustained suppression of LH, FSH, and testosterone.1
The initial overstimulation of pituitary receptors by GnRH agonists leads to a surge of testosterone that, particularly in patients with metastatic disease, may very rarely exacerbate clinical symptoms (clinical flare) leading to bone pain, spinal cord compression, and even death; if the risk of flare is a concern, it can be managed with an anti-androgen.7 Microsurges of LH and testosterone can occur after each administration of these agents8 (this is sometimes known as the ‘acute-on-chronic’ phenomenon), but are generally not clinically appreciable. During treatment with GnRH agonists, FSH levels gradually increase and can cause an FSH “escape.”9 The benefit of the rapid and sustained suppression of FSH with GnRH antagonists is unclear, but given human prostate cancer cells have been shown to express FSH receptors, further analyses of these differences are warranted.10 Importantly, GnRH antagonists provide an alternative treatment approach without clinical flares for advanced or metastatic prostate cancer.8,11
IMPACT OF ADT ON CARDIOVASCULAR HEALTH
In 2010, the US Food and Drug Administration (FDA) issued a notification to add new safety warnings on GnRH agonists labels pertaining to the increased risk of diabetes, heart attack, sudden cardiac death, and stroke. Similar advisory statements were published by the American Heart Association, American Cancer Society, and American Urological Association;12 however, there is no such warning for the use of GnRH antagonists.
The European Association of Urology (EAU) states that there is no consistent evidence that ADT is associated with nonfatal cardiovascular diseases (CVD).13,14 Importantly, the randomized controlled trials (RCTs), showing no increased CVD risk from ADT,14 tended to exclude older patients, and patients with more comorbidities.15 There is also an uncertainty around the duration of ADT use that would precipitate CVD, with some studies suggesting the risk only arises after long-term treatment,16,17 while others suggest 4–6 months of ADT can increase the 5-year cumulative incidence of cardiovascular death by 5.5%.18,19
A Surveillance, Epidemiology, and End Results (SEER) analysis of 140 474 patients with nonmetastatic prostate cancer between 1995 and 200920 showed that GnRH agonists were associated with an increased risk of coronary artery disease (hazard ratio [HR]: 1.11, 95% confidence interval [CI]: 1.07–1.15), acute myocardial infarction (HR: 1.09, 95% CI: 1.04–1.15), and sudden cardiac death (HR: 1.18, 95% CI: 1.12–1.24) (all P < 0.0001) as compared with ADT-naïve patients (Table 1). Another population-based study of 182 757 patients with loco-regional prostate cancer,21 showed that GnRH agonists were associated with increased risk of peripheral arterial disease (HR: 1.15, 95% CI: 1.11–1.19) and venous thromboembolism (HR: 1.09, 95% CI: 1.04–1.15), see Table 1. In this study, 47.8% of patients received GnRH agonists, while only 2.2% of patients received orchiectomy.
Table 1.
Cardiovascular events with the use of androgen deprivation therapy
| GnRH antagonist (degarelix/abarelix) | GnRH agonists (goserelin/leuprolide) | Orchiectomy | Combined androgen blockade | ADT naïve/no ADT | |
|---|---|---|---|---|---|
| Keating et al.222006 | |||||
| Coronary heart disease | NA | 1.16 (1.10–1.21)a | 0.99 (0.91–1.07) | NA | NA |
| Myocardial infarction | NA | 1.11 (1.01–1.21)a | 0.94 (0.82–1.09) | NA | NA |
| Sudden cardiac death | NA | 1.16 (1.05–1.27)a | 1.01 (0.87–1.18) | NA | NA |
| Diabetes | NA | 1.44 (1.34–1.55)a | 1.34 (1.20–1.50)a | NA | NA |
| Keating et al.23 2010 | |||||
| Coronary heart disease | NA | 144 (135.7–152.2)b | 210.5 (150.9–270.0)b | 157.7 (129.4–186.0)b | NA |
| Myocardial infarction | NA | 12.8 (11.1–14.4)b | 24.3 (12.4–36.3) | 10.2 (5.2–15.2) | NA |
| Sudden cardiac death | NA | 21.6 (19.4–23.7)b | 23.3 (11.5–35.1) | 20.1 (13.0–27.2) | NA |
| Stroke | NA | 18.5 (16.5–20.5)b | 26.2 (13.8–38.7) | 14.8 (8.8–20.9) | NA |
| Diabetes | NA | 159.4 (150.6–168.3)b | 190.4 (137.6–243.2)b | 144.6 (117.2–172.0)b | |
| Smith et al.78 2010 | |||||
| Supraventricular arrhythmia (%) | 2 | 4 | NA | NA | NA |
| Ischemic heart disease (%) | 4 | 10 | NA | NA | NA |
| Cardiac failure (%) | <1 | 2 | NA | NA | NA |
| Peripheral vascular atherosclerosis (%) | 1 | ≤1 | NA | NA | NA |
| Stroke (%) | 2 | ≤1 | NA | NA | NA |
| Serious arrhythmia (%) | 2 | 5 | NA | NA | NA |
| Discontinuation due to cardiac disorders (%) | 1 | 2 | NA | NA | NA |
| Death due to cardiac arrest (n) | 2 | NA | NA | NA | NA |
| Death due to myocardial infarction (n) | 1 | 2 | NA | NA | NA |
| Death due to cardiac failure (n) | 1 | 1 | NA | NA | NA |
| Death due to cardiac disorder (n) | NA | 1 | NA | NA | NA |
| Hu et al.21 2012 | |||||
| Peripheral arterial diseasec | NA | 30.5 (29.6–31.4)d | 27.1 (24.4–29.7)d | NA | 21.0 (20.7–21.3) |
| Venous thromboembolismc | NA | 13.4 (12.8–14.0)d | 14.0 (12.2–15.9)d | NA | 10.4 (10.2–10.6) |
| Gandaglia et al.20 2014 | |||||
| Coronary artery disease (%) | NA | 26.9 (26.1–27.7)e | 23.2 (20.2–26.2) | NA | 25.1 (24.4–25.8) |
| Acute myocardial infarction (%) | NA | 16.6 (15.9–17.3)e | 14.8 (12.2–17.4) | NA | 14.8 (14.2–15.4) |
| Sudden cardiac death (%) | NA | 17.7 (17.1–18.4)e | 16.4 (13.7–19.2) | NA | 14.2 (13.7–14.8) |
| Albertsen et al.162014 – overall number of cardiovascular events and related deaths | |||||
| CV event | 42 | 37 | NA | NA | NA |
| CV related deaths | 20 | 22 | NA | NA | NA |
| Hazard ratio | 0.60 (95% CI: 0.41–0.87) | NA | NA | NA | |
| Albertsen et al.162014 – number of events in patients with pre-existing cardiovascular disease at baseline | |||||
| CV event | 21 | 23 | NA | NA | NA |
| CV related deaths | 9 | 13 | NA | NA | NA |
| Hazard ratio | 0.44 (95% CI: 0.26–0.74) | NA | NA | NA | |
aP<0.05 w.r.t. no treatment; values presented as hazard ratio (95% CI), bP<0.001; values presented as rate (95% CI); P values reflect if rate of each outcome with any of the treatments differed from that with no androgen deprivation therapy; events per 1000 person-years, cEvents per 1000 person-years, dP<0.001; values presented as rate (95% CI); P values based whether the rate for men during GnRH agonist treatment differed from the rate under no treatment and whether the rate for men treated with orchiectomy differed from the rate under no treatment, eP<0.001 w.r.t. no treatment; events per 1000 person-years; values presented as rate (95% CI). ADT: androgen deprivation therapy; CI: confidence interval; CV: cardiovascular; GnRH: gonadotropin-releasing hormone; NA: data not available from cited publication; w.r.t.: with reference to
SEER Medicare data from 1992 to 2001 in a cohort of 73 196 men aged 66 years and older,22 suggested that ADT usage for ≥4 months was associated with a 16% risk for both coronary heart disease (adjusted HR: 1.16, 95% CI: 1.10–1.21; P < 0.05) and sudden cardiac death (adjusted HR: 1.16, 95% CI: 1.05–1.27; P < 0.05). A further population-based study23 observed that GnRH agonists were associated with increased risk of coronary heart disease (Table 1). Also, combined androgen blockade with anti-androgens significantly increased the risk of incident coronary heart disease (HR: 1.27, 95% CI: 1.0–1.53). However, anti-androgen therapy alone did not show significant differences for any of the outcomes. While these studies collectively suggested increased cardiovascular risk with GnRH agonists, they included insufficient men treated with GnRH antagonists for conclusions to be drawn regarding the safety of GnRH antagonists.
In a meta-analysis comprising six randomized controlled trials (n = 2328),16 a significantly lower risk of cardiovascular events or mortality with GnRH antagonists compared with GnRH agonists, was observed (HR: 0.60, 95% CI: 0.41–0.87; P = 0.008). Furthermore, in patients with pre-existing cardiovascular disorders (n = 708), the risk of cardiac events was lowered by 56% in patients treated with GnRH antagonists compared to agonists, within 1 year of initiating the therapy (HR: 0.44, 95% CI: 0.26–0.74; P = 0.002). Moreover, a 12-month randomized controlled trial24 showed that GnRH antagonist treatment halved the risk of cardiovascular events compared with GnRH agonists in patients with pre-existing cardiovascular disorders (n = 143).
Although the original scope of this review excluded Phase II studies, a recent small prospective comparative study of men with prostate cancer and pre-existing CVD found that 20% experienced a major CV and cerebrovascular event with GnRH agonists compared with 3% with GnRH antagonists (P = 0.013). The absolute risk reduction at 12 months of using GnRH antagonists was 18.1% (95% CI: 4.6%–31.2%; P = 0.032).25
Given no study has compared GnRH agonists versus antagonists with a primary outcome of CV health, further comparator studies are required to confirm whether the risk of cardiovascular events is indeed lower with GnRH antagonists over GnRH agonists, but some authors already recommend GnRH antagonists rather than GnRH agonists for ADT in patients with pre-existing CVDs.26,27
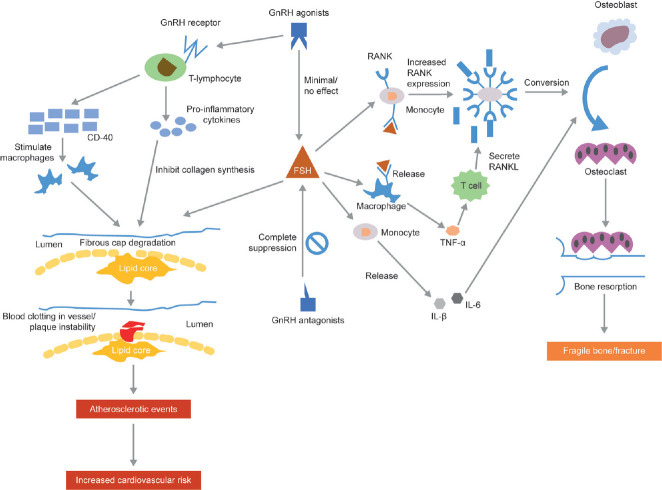
The increased cardiovascular risk observed with testosterone suppression could be attributed to the resultant metabolic effects, such as changes in body composition, obesity, insulin resistance, hyperglycemia, dyslipidemia, and hypertension along with endothelial dysfunction and arterial wall thickening.28,29,30 In addition, GnRH agonists stimulate the GnRH receptors on T-lymphocytes leading to plaque instability, and possible atherosclerotic events,30,31 further increasing CVD risk.
If confirmed in prospective studies, the increased propensity of GnRH agonists to cause CVD over GnRH antagonists could potentially be explained by their differential effect on FSH (Figure 1).32 GnRH antagonists rapidly reduce FSH levels (<90%), whereas GnRH agonists cause an initial increase followed by gradual decrease (≤50%).30,33 Since FSH is involved in augmenting fat and lipid storage, this may cause disruption of vascular integrity, unstable plaques, and other metabolic changes leading to CVD.2,32,34 FSH receptors have been found on the endothelial surface and FSH may therefore impact on endothelial cell function.16,35 However, the Phase II study referred to above did not find a difference in endothelial function between men treated with a GnRH agonist or antagonist (the primary endpoint of the study).25 Also, if FSH was the main driver of such differences, orchiectomy, which leads to increased FSH, should have the highest rates of CVD disease, yet meta-analyses suggest similar cardiovascular risks with orchiectomy as those with GnRH agonists.36,37 As such, the clinical importance of FSH differences is unclear at best.
Figure 1.
Mechanism of action for cardiovascular disease and fractures. FSH: follicle-stimulating hormone; GnRH: gonadotropin-releasing hormone; IL: interleukin; RANK: receptor activator of nuclear factor kappa-B; RANKL: receptor activator of nuclear factor kappa-B ligand; TNF: tumor necrosis factor.
IMPACT OF ADT ON GLUCOSE METABOLISM
Data indicate that low levels of testosterone and steroid hormone-binding globulin could lead to insulin resistance or altered insulin sensitivity,38,39 central obesity and rise in intra-abdominal fat mass,40,41 all under the umbrella of metabolic syndrome.42 A significant increase from baseline in serum insulin levels (26%–63%) following 3 months of ADT has been observed,43 though the fasting glucose levels remained unchanged. The resulting insulin resistance may also stimulate the production of inflammatory adipocytokines as these are associated with both increased adiposity and insulin resistance.44 These findings were confirmed in long-term studies; there was also an increased prevalence of fasting hyperglycemia,45 with about 44% patients reaching fasting plasma glucose levels of >126 mg dl−1.22 A retrospective analysis showed that patients on ADT were 36% more likely to develop diabetes versus those not on ADT, particularly during the 1st year of ADT.46 Hyperinsulinemia is also shown to be independently associated with cardiovascular mortality.47
In a meta-analysis of eight studies including 157 588 patients,48 ADT was associated with an increased risk of developing diabetes. Of the 65 695 patients receiving GnRH agonists, 7136 developed diabetes compared with only 6987 of 91 893 non-ADT users (risk ratio: 1.39, 95% CI: 1.27–1.53; P < 0.001). Deterioration in glycemic control with ADT has also been reported in patients with pre-existing diabetes.43 The effects of GnRH antagonists on insulin sensitivity have not been studied, but there is every reason to believe the effects would be similar.
ADT AND OTHER METABOLIC CHANGES
Dyslipidemia, particularly hypercholesterolemia and hypertriglyceridemia, also occurs with ADT. A prospective 12-month study of 40 patients with prostate cancer receiving ADT49 showed increased serum total cholesterol, low-density lipoprotein cholesterol, and triglycerides. Interestingly, high-density lipoprotein levels were also increased,49,50 which is in contrast with typical metabolic syndrome criteria. Most of these effects appear within the first 3 months of treatment.43
Both short- and long-term studies of ADT have shown changes in body composition, especially reduction in skeletal muscle mass, and increase in body fat, within 3–6 months of starting ADT.49,51,52,53,54,55 These lead to obesity56 and are potential precursors of metabolic syndrome. The data show that metabolic syndrome is more common in men undergoing long-term ADT (>50%) than in patients receiving non-ADT treatment (22%) and eugonadal counterparts (20%).57 Again, given these metabolic syndrome-like effects of ADT are thought to be due to low testosterone, there is every reason to believe identical effects would be seen with GnRH antagonists.
IMPACT OF ADT ON BONE HEALTH
ADT accelerates the rate of bone loss in men by 5- to 10-fold the normal rate,58 which may increase the rate of fractures (Figure 1).59 The relative fracture risk in 50 613 elderly patients (≥66 years) was 1.45 (95% CI: 1.36–1.56) in patients receiving nine or more doses of GnRH agonist in the first 12 months after diagnosis.59 In a 10-year study of 80 844 elderly patients (>65 years) treated with GnRH agonists,60 18.4% experienced at least one fracture and 4.1% had a fracture requiring hospitalization. Importantly, a positive dose-response relationship was observed between fracture incidence and cumulative dose of GnRH agonists.
Testosterone suppression results in a concomitant drop in estrogen, which reduces bone density.61 The rate of bone loss, especially in the spine and hip, is greatest in the 1st year of treatment,58 but could continue even when ADT is discontinued. However, a study of 236 men scheduled for ADT found pre-existing osteoporosis in 11%, while 40% had osteopenia.62
The GnRH antagonist degarelix was directly compared with the GnRH agonist leuprolide in a randomized study. Reductions in serum alkaline phosphatase (S-ALP) were significantly greater with degarelix (P < 0.05).63 The effect of this on bone density, bone strength, or fracture risk remains to be determined.
FSH, including that of prostate cancer cell origin, also adversely impacts differentiation of monocyte osteoclast precursors into osteoclasts64 and seems to be a pro-inflammatory hormone inducing monocytes to release interleukin (IL)-1β and IL-6 which increase osteoclast development and bone resorption.65 Due to their complete suppression of FSH, there is a theoretical advantage that GnRH antagonists may cause less osteoporosis, though there are no data to support this. As such, prospective studies comparing fracture risk and bone loss between GnRH agonists and antagonists are required to test this.
OTHER FREQUENT ADT-RELATED ADVERSE EVENTS INJECTION-SITE REACTIONS
Injection-site reactions
Injection site reactions have been reported as one of the major adverse events with GnRH antagonists (Table 2).66,67,68,69 In a randomized clinical trial, a significantly higher rate of injection-site reactions was reported with subcutaneous degarelix (40%, P < 0.001) compared to intramuscular leuprolide injection (<1%).33 The difference in incidence of injection-site reactions might be attributed to the differing modes of administration and injection volumes.
Table 2.
List of common TEAEs
| Treatment arms | Crawford et al.66 2011 | Crawford et al.67 2014 | Garnick and Mottet11 2012 | Klotz et al.33 2008 | Mason et al.69 2013 | Geiges et al.68 2015 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Degarelix 240/80 mga | Leuprolide 7.5 mg switched to degarelix 240/80 or 160 mga | Degarelix, 240/80 mga | Degarelix, 240/160 mga | Leuprolide switched to degarelix, 240/80 or 160 mga | Abarelix + GnRH agonists | Degarelix (240/80 mg, 240/160 mg)a | Leuprolide 7.5 mg | Degarelix 240/80 mga | Goserelin (3.6 mg)/bicalutamide (50 mg) | Degarelix 240/80 mga | |
| Patients, n (%) | 125 (100) | 134 (100) | 125 (100) | 126 (100) | 134 (100) | NA | 409 (100) | 201 (100) | 181 (100) | 64 (100) | NA |
| Hot flushes, n (%) | NA | NA | 61 (29) | 61 (30) | 40 (30) | 124 (70) | 105 (26) | 43 (21) | 108 (60) | 40 (63) | NA |
| Fatigue, n (%) | NA | NA | 6 (3) | 13 (6) | 8 (6) | 46 (26) | 20 (5) | 13 (6) | 10 (6) | 6 (9) | NA |
| Diarrhoea, n (%) | NA | NA | NA | NA | NA | 15 (9) | NA | NA | NA | NA | NA |
| Dizziness, n (%) | NA | NA | NA | NA | NA | 15 (9) | NA | NA | NA | NA | NA |
| Insomnia, n (%) | NA | NA | NA | NA | NA | 14 (9) | NA | NA | NA | NA | NA |
| Increased ALT, n (%) | NA | NA | 13 (6) | 14 (7) | 6 (4) | NA | 37 (9) | 11 (5) | NA | NA | NA |
| Increased AST, n (%) | NA | NA | 11 (5) | 10 (5) | 5 (4) | NA | 21 (5) | 6 (3) | NA | NA | NA |
| Back pain, n (%) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 18 (1.8) |
| Pain, n (%) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 42 (4.2) |
| Headache, n (%) | NA | NA | NA | NA | NA | 13 (7) | NA | NA | NA | NA | NA |
| Chills, n (%) | NA | NA | 14 (7) | 7 (3) | 2 (2) | NA | 18 (4) | NA | NA | NA | NA |
| Pyrexia, n (%) | NA | NA | 13 (6) | 15 (7) | 9 (7) | NA | NA | NA | NA | NA | NA |
| Nausea, n (%) | NA | NA | NA | NA | NA | 13 (7) | NA | NA | 2 (1) | 3 (5) | NA |
| Urinary frequency, n (%) | NA | NA | NA | NA | NA | 12 (7) | NA | NA | NA | NA | NA |
| Decreased libido, n (%) | NA | NA | NA | NA | NA | 9 (5) | NA | NA | 12 (7) | 4 (6) | NA |
| Erectile dysfunction, n (%) | NA | NA | 2 (<1) | 4 (2) | 7 (5) | NA | NA | 14 (8) | 6 (9) | NA | |
| Weakness, n (%) | NA | NA | NA | NA | NA | 9 (5) | NA | NA | NA | NA | NA |
| Arthralgia, n (%) | NA | NA | NA | NA | NA | 9 (5) | 17 (4) | 18 (9)* | NA | NA | NA |
| Cough, n (%) | NA | NA | NA | NA | NA | 9 (5) | NA | NA | NA | NA | NA |
| Weight gain, n (%) | NA | NA | 21 (10) | 13 (6) | 16 (12) | 8 (5) | 40 (10) | 24 (12) | NA | NA | 20 (2.0) |
| Constipation, n (%) | NA | NA | NA | NA | NA | 8 (5) | 17 (4) | 10 (5) | NA | NA | NA |
| Dyspnoea, n (%) | NA | NA | NA | NA | NA | 8 (5) | NA | NA | NA | NA | NA |
| Injection site reaction, n (%) | NA | NA | Pain: 64 (31), erythema: 40 (19), swelling: 17 (8), nodule: 13 (6), induration: 9 (4) | Pain: 67 (33), erythema: 50 (25), swelling: 16 (8), nodule: 17 (8), induration: 12 (6) | Pain: 36 (27), erythema: 26 (19), swelling: 10 (7), nodule: 6 (4), induration: 4 (3) | NA | 162 (40) | 1 (<1) | Injection site pain: 60 (33), injection site erythema: 45 (25), injection site pruritus: 13 (7), injection site swelling: 11 (6), injection site induration: 9 (5) | Injection site pain: 1 (2), injection site erythema: 0 (0), injection site pruritus: 0 (0), injection site swelling: 0 (0), injection site induration: 0 (0) | Erythema: 86 (8.5) |
| Hypertension, n (%) | NA | NA | NA | NA | NA | NA | 26 (6) | 8 (4) | NA | NA | 6 (0.6) |
| Musculoskeletal, n (%) | 26 | 17 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypercholesterolemia, n (%) | NA | NA | NA | NA | NA | NA | 19 (5) | 5 (2) | NA | NA | NA |
| Asthenia, n (%) | NA | NA | NA | NA | NA | NA | NA | NA | 13 (7) | 6 (9) | NA |
| Urinary tract infections, n (%) | NA | NA | NA | NA | NA | NA | 13 (3) | 18 (9) | NA | NA | NA |
a‘/’ indicates induction on a higher dose (e.g., 240 mg) and subsequent maintenance on a lower dose (e.g., 80 mg). ALT: alanine transaminase; AST: aspartate transaminase; GnRH: gonadotropin-releasing hormone; NA: data not available from cited publication; TEAEs: treatment emergent adverse events
Cognitive impairment/dementia
Although not extensively studied, ADT appears to be associated with depression, cognitive impairment, and mood disorders. In a retrospective study, it was observed that the incidence of depressive, cognitive (Alzheimer's disease/dementia) and constitutional disorders increased with the use of ADT.59 A meta-analysis including 90 543 patients, 38 307 of whom were exposed to ADT,70 showed a statistically significant association between ADT use and Alzheimer's disease risk (HR: 1.88, 95% CI: 1.10–3.20; P = 0.021). Decline in cognitive functions, visual-spatial abilities, and executive functioning and worsened mood as measured from Profile of Mood States have also been reported.71 A possible mechanism of action could be the suppressed testosterone levels following ADT since testosterone is implicated in cognitive function, in which case the effects would be predicted to be equal for GnRH agonists and antagonists.
Does selection of patient populations impact the safety risk?
Managing ADT-related increased risk of cardiovascular, metabolic disorders and bone thinning metabolism is compounded by the fact that most patients with prostate cancer are aged over 50 years and affected by age-related changes in metabolic activities, glycemic control, bone density, and cardiovascular status.
Particularly for CVD, most of the studies evaluated the impact of ADT on death, rather than specific cardiovascular outcomes. In order to observe the true effect, patients with pre-existing CVD, those with comorbidities, and older patients should be evaluated. However, most of the prospective clinical trials excluded these patients.15 This is pertinent as there is a possibility that ADT is associated with cardiovascular mortality only in patients with a history of CVD.16,72,73 A recent meta-analysis showed that use of ADT was associated with a 38% greater risk of any type of nonfatal CVD compared with no ADT. Use of ADT seemed to increase the risk of nonfatal or fatal MI or stroke by 57% and 51%, respectively.42
RECOMMENDATIONS
Keeping these factors in mind, it is of utmost importance that the potential benefit and risks associated with ADT be evaluated and weighed judiciously, especially when the survival benefits are doubtful, such as in men with biochemical recurrence without metastases. Furthermore, considering that cancer itself has a deteriorating impact on the quality of life (QoL), it is worthwhile to minimize and adequately manage the treatment side effects.
In order to delay disease progression and alleviate the side effects attributed to ADT, some treating physicians are now offering IADT, which seems to be an alternative regimen without compromising the clinical outcomes, particularly in patients with nonmetastatic cancer and poorly differentiated cancer.74,75 However, there are studies that indicate there are few differences between IADT and CADT when it comes to overall safety and cardiovascular events.5,76
SUMMARY
This review highlights the major adverse events that are known to occur with ADT using GnRH agonists and antagonists. Arguably, CVDs are one of the most serious complications that have been linked with the use of ADT. These complications could add to the existing morbidity and mortality of prostate cancer, especially in patients with pre-existing disorders.77 Such concerns were severe enough for the FDA to add a warning against the use of GnRH agonists in patients with pre-existing cardiovascular disease.12 Similarly, it is important to manage other adverse events associated with ADT, such as bone and metabolic disorders, and cognitive impairment.
Currently, guidelines suggest GnRH agonists as the first-line therapy in patients with prostate cancer. The apparently preferable CVD safety profile of GnRH antagonists over GnRH agonists, which has yet to be prospectively validated in a study focused on CVD outcomes, suggests that GnRH antagonists offer an alternative first-line hormonal ADT treatment. These potential benefits may be offset by the inconvenience of monthly dosing with GnRH antagonists and increased injection site reactions.
A major limitation of this review was the unavailability of large randomized clinical trials comparing the safety profile of GnRH agonists and antagonists. Data discussed in the present review was mainly taken from retrospective or population-based studies and a few randomized trials that included major events like cardiovascular disease and fractures. Furthermore, only one of these studies provided a direct comparison of safety profiles.
It is hoped that the ongoing prospective randomized trial PRONOUNCE (NCT02663908) in a prostate cancer population with pre-existing CVD will increase the evidence base regarding whether or not GnRH antagonists reduce cardiovascular events in this cohort of patients relative to GnRH agonists.
AUTHOR CONTRIBUTIONS
SJF conceptualized the review, helped draft it, and provided critical feedback. PAA initiated and drafted the manuscript. In addition, he participated in its design and coordination. Both authors have read and approved the final version of the manuscript.
COMPETING INTERESTS
PAA has no conflict of interest related to the content of the manuscript. SJF is a consultant to Ferring Pharmaceuticals.
ACKNOWLEDGMENTS
Medical writing support and technical editing of the manuscript, funded by Ferring Pharmaceuticals Ltd (UK), was provided by Bioscript Science, Macclesfield, UK. The funder played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Crawford ED. Hormonal therapy in prostate cancer: historical approaches. Rev Urol. 2004;6(Suppl 7):S3–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford ED, Rove KO, Schally AV, Rick FG, Block NL, et al. The role of the FSH system in the development and progression of prostate cancer. Am J Hematol Oncol. 2014;10:5–9. [Google Scholar]
- 3.Miyazawa Y, Kato H, Arai S, Furuya Y, Sekine Y, et al. Clinical endocrinological evaluation of the gonadal axis (testosterone, LH and FSH) in prostate cancer patients switched from a GnRH antagonist to a LHRH agonist. Basic Clin Androl. 2015;25:7. doi: 10.1186/s12610-015-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nead KT, Sinha S, Nguyen PL. Androgen deprivation therapy for prostate cancer and dementia risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20:259–64. doi: 10.1038/pcan.2017.10. [DOI] [PubMed] [Google Scholar]
- 5.Jin C, Fan Y, Meng Y, Shen C, Wang Y, et al. A meta-analysis of cardiovascular events in intermittent androgen-deprivation therapy versus continuous androgen-deprivation therapy for prostate cancer patients. Prostate Cancer Prostatic Dis. 2016;19:333–9. doi: 10.1038/pcan.2016.35. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuzuka K, Kyan A, Sato T, Orikasa K, Miyazato M, et al. Influence of 1 year of androgen deprivation therapy on lipid and glucose metabolism and fat accumulation in Japanese patients with prostate cancer. Prostate Cancer Prostatic Dis. 2016;19:57–62. doi: 10.1038/pcan.2015.50. [DOI] [PubMed] [Google Scholar]
- 7.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–6. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 8.Iversen P, Damber JE, Malmberg A, Persson BE, Klotz L. Degarelix monotherapy compared with luteinizing hormone-releasing hormone (LHRH) agonists plus anti-androgen flare protection in advanced prostate cancer: an analysis of two randomized controlled trials. Ther Adv Urol. 2016;8:75–82. doi: 10.1177/1756287215621471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shore ND, Abrahamsson PA, Anderson J, Crawford ED, Lange P. New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists. Prostate Cancer Prostatic Dis. 2013;16:7–15. doi: 10.1038/pcan.2012.25. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Josef E, Yang SY, Ji TH, Bidart JM, Garde SV, et al. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR) J Urol. 1999;161:970–6. [PubMed] [Google Scholar]
- 11.Garnick MB, Mottet N. New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. BJU Int. 2012;110:499–504. doi: 10.1111/j.1464-410X.2011.10708.x. [DOI] [PubMed] [Google Scholar]
- 12.Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–40. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, et al. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68:386–96. doi: 10.1016/j.eururo.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306:2359–66. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 15.Kelly WK, Gomella LG. Androgen deprivation therapy and competing risks. JAMA. 2011;306:2382–3. doi: 10.1001/jama.2011.1791. [DOI] [PubMed] [Google Scholar]
- 16.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–73. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Karantanos T, Karanika S. Assessing the cardiovascular risk of hormonal therapy in patients with prostate cancer. Ann Transl Med. 2016;4:99. doi: 10.21037/atm.2016.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 19.Roach M. Current trends for the use of androgen deprivation therapy in conjunction with radiotherapy for patients with unfavorable intermediate-risk, high-risk, localized, and locally advanced prostate cancer. Cancer. 2014;120:1620–9. doi: 10.1002/cncr.28594. [DOI] [PubMed] [Google Scholar]
- 20.Gandaglia G, Sun M, Popa I, Schiffmann J, Abdollah F, et al. The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: a population-based study. BJU Int. 2014;114:E82–9. doi: 10.1111/bju.12732. [DOI] [PubMed] [Google Scholar]
- 21.Hu JC, Williams SB, O'Malley AJ, Smith MR, Nguyen PL, et al. Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur Urol. 2012;61:1119–28. doi: 10.1016/j.eururo.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 23.Keating NL, O'Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higano CS, Crawford ED, Shore ND, Bosnyak Z, Malmberg A, et al. Risk of cardiovascular events with degarelix versus leuprolide after biochemical relapse of prostate cancer: exploratory analysis of a randomized controlled trial. J Clin Oncol. 2015;33:151. [Google Scholar]
- 25.Margel D, Peer A, Ber Y, Shavit-Grievink L, Tabachnik T, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199–208. doi: 10.1097/JU.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 26.Greiman AK, Keane TE. Approach to androgen deprivation in the prostate cancer patient with pre-existing cardiovascular disease. Curr Urol Rep. 2017;18:41. doi: 10.1007/s11934-017-0688-5. [DOI] [PubMed] [Google Scholar]
- 27.Clinton TN, Woldu SL, Raj GV. Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer. Expert Opin Pharmacother. 2017;18:825–32. doi: 10.1080/14656566.2017.1328056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SM, Kam SC. Metabolic effects of androgen deprivation therapy. Korean J Urol. 2015;56:12–8. doi: 10.4111/kju.2015.56.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 30.Rosario DJ, Davey P, Green J, Greene D, Turner B, et al. The role of gonadotrophin-releasing hormone antagonists in the treatment of patients with advanced hormone-dependent prostate cancer in the UK. World J Urol. 2016;34:1601–9. doi: 10.1007/s00345-016-1818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conteduca V, Di Lorenzo G, Tartarone A, Aieta M. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: an unresolved controversy. Crit Rev Oncol Hematol. 2013;86:42–51. doi: 10.1016/j.critrevonc.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Crawford ED, Schally AV, Pinthus JH, Block NL, Rick FG, et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol. 2017;35:183–91. doi: 10.1016/j.urolonc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu XM, Chan HC, Ding GL, Cai J, Song Y, et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell. 2015;14:409–20. doi: 10.1111/acel.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kittai AS, Blank J, Graff JN. Gonadotropin-releasing hormone antagonists in prostate cancer. Oncology (Williston Park) 2018;32:599–602. 604–6. [PubMed] [Google Scholar]
- 36.Meng F, Zhu S, Zhao J, Vados L, Wang L, et al. Stroke related to androgen deprivation therapy for prostate cancer: a meta-analysis and systematic review. BMC Cancer. 2016;16:180. doi: 10.1186/s12885-016-2221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Z, Huang Y, Gong L, Gan S, Chan FL, et al. Association of androgen deprivation therapy with thromboembolic events in patients with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2018;21:451–60. doi: 10.1038/s41391-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 38.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–9. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 39.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–7. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 40.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90:2618–23. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 41.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 42.Bosco C, Crawley D, Adolfsson J, Rudman S, Van Hemelrijck M. Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta-analysis. PLoS One. 2015;10:e0117344. doi: 10.1371/journal.pone.0117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–8. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 44.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–8. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 46.Lage MJ, Barber BL, Markus RA. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology. 2007;70:1104–8. doi: 10.1016/j.urology.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–7. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Sun X, Zhao L, Chen X, Zhao J. Androgen deprivation therapy is associated with diabetes: Evidence from meta-analysis. J Diabetes Investig. 2016;7:629–36. doi: 10.1111/jdi.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 50.Behre HM, Bockers A, Schlingheider A, Nieschlag E. Sustained suppression of serum LH, FSH and testosterone and increase of high-density lipoprotein cholesterol by daily injections of the GnRH antagonist cetrorelix over 8 days in normal men. Clin Endocrinol (Oxf) 1994;40:241–8. doi: 10.1111/j.1365-2265.1994.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 51.Basaria S, Lieb J, 2nd, Tang AM, DeWeese T, Carducci M, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–86. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 52.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–9. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 53.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–5. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, McGovern K, Finkelstein JS, Smith MR. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer. 2005;104:1633–7. doi: 10.1002/cncr.21381. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, et al. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95:2136–44. doi: 10.1002/cncr.10967. [DOI] [PubMed] [Google Scholar]
- 56.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–32. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 57.Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–83. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 58.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–7. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 59.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 60.Beebe-Dimmer JL, Cetin K, Shahinian V, Morgenstern H, Yee C, et al. Timing of androgen deprivation therapy use and fracture risk among elderly men with prostate cancer in the United States. Pharmacoepidemiol Drug Saf. 2012;21:70–8. doi: 10.1002/pds.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen HT, von Schoultz B, Nguyen TV, Thang TX, Chau TT, et al. Sex hormone levels as determinants of bone mineral density and osteoporosis in Vietnamese women and men. J Bone Miner Metab. 2015;33:658–65. doi: 10.1007/s00774-014-0629-z. [DOI] [PubMed] [Google Scholar]
- 62.Cheung AS, Pattison D, Bretherton I, Hoermann R, Lim Joon D, et al. Cardiovascular risk and bone loss in men undergoing androgen deprivation therapy for non-metastatic prostate cancer: implementation of standardized management guidelines. Andrology. 2013;1:583–9. doi: 10.1111/j.2047-2927.2013.00093.x. [DOI] [PubMed] [Google Scholar]
- 63.Schroder FH, Tombal B, Miller K, Boccon-Gibod L, Shore ND, et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12-month, comparative, phase III study. BJU Int. 2010;106:182–7. doi: 10.1111/j.1464-410X.2009.08981.x. [DOI] [PubMed] [Google Scholar]
- 64.Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. 2011;53:141–4. doi: 10.1016/j.cyto.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cannon JG, Cortez-Cooper M, Meaders E, Stallings J, Haddow S, et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am J Physiol Regul Integr Comp Physiol. 2010;298:R790–8. doi: 10.1152/ajpregu.00728.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schroder F, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol. 2011;186:889–97. doi: 10.1016/j.juro.2011.04.083. [DOI] [PubMed] [Google Scholar]
- 67.Crawford ED, Shore ND, Moul JW, Tombal B, Schröder FH, et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology. 2014;83:1122–8. doi: 10.1016/j.urology.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Geiges G, Harms T, Rodemer G, Eckert R, Konig F, et al. Degarelix therapy for prostate cancer in a real-world setting: experience from the German IQUO (Association for uro-oncological quality assurance) Firmagon® registry. BMC Urol. 2015;15:122. doi: 10.1186/s12894-015-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mason M, Maldonado Pijoan X, Steidle C, Guerif S, Wiegel T, et al. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clin Oncol (R Coll Radiol) 2013;25:190–6. doi: 10.1016/j.clon.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, et al. Androgen deprivation therapy and future Alzheimer's disease risk. J Clin Oncol. 2016;34:566–71. doi: 10.1200/JCO.2015.63.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Dam D, Wassersug RJ, Hamilton LD. Androgen deprivation therapy's impact on the mood of prostate cancer patients as perceived by patients and the partners of patients. Psychooncology. 2016;25:848–56. doi: 10.1002/pon.3932. [DOI] [PubMed] [Google Scholar]
- 72.Nanda A, Chen MH, Braccioforte MH, Moran BJ, D'Amico AV. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–73. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 73.Ziehr DR, Chen MH, Zhang D, Braccioforte MH, Moran BJ, et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2015;116:358–65. doi: 10.1111/bju.12905. [DOI] [PubMed] [Google Scholar]
- 74.Parikh RA, Pascal LE, Davies BJ, Wang Z. Improving intermittent androgen deprivation therapy: lessons learned from basic and translational research. Asian J Androl. 2014;16:505–10. doi: 10.4103/1008-682X.125410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liede A, Hallett DC, Hope K, Graham A, Arellano J, et al. International survey of androgen deprivation therapy (ADT) for non-metastatic prostate cancer in 19 countries. ESMO Open. 2016;1:e000040. doi: 10.1136/esmoopen-2016-000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dason S, Allard CB, Wang JG, Hoogenes J, Shayegan B. Intermittent androgen deprivation therapy for prostate cancer: translating randomized controlled trials into clinical practice. Can J Urol. 2014;21:28–36. [PubMed] [Google Scholar]
- 77.Zareba P, Duivenvoorden W, Leong DP, Pinthus JH. Androgen deprivation therapy and cardiovascular disease: what is the linking mechanism? Ther Adv Urol. 2016;8:118–29. doi: 10.1177/1756287215617872. [DOI] [PMC free article] [PubMed] [Google Scholar]