Abstract
We investigated the relationship between positive surgical margin (PSM)-related factors and biochemical recurrence (BCR) and the ability of intraoperative frozen sections to predict significant PSM in patients with prostate cancer. The study included 271 patients who underwent robot-assisted laparoscopic prostatectomy with bilateral nerve sparing and maximal urethral preservation. Intraoperative frozen sections of the periurethra, dorsal vein, and bladder neck were analyzed. The ability of PSM-related factors to predict BCR and significant PSM was assessed by logistic regression. Of 271 patients, 108 (39.9%) had PSM and 163 (60.1%) had negative margins. Pathologic Gleason score ≥8 (18.9% vs 7.5%, P = 0.015) and T stage ≥T3a (51.9% vs 24.6%, P < 0.001) were significantly more frequent in the PSM group. Multivariate analysis showed that Gleason pattern ≥4 (vs <4; hazard ratio: 4.386; P = 0.0004) was the only significant predictor of BCR in the PSM cohort. Periurethral frozen sections had a sensitivity of 83.3% and a specificity of 84.2% in detecting PSM with Gleason pattern ≥4. Multivariate analysis showed that membranous urethra length (odds ratio [OR]: 0.79, P = 0.0376) and extracapsular extension of the apex (OR: 4.58, P = 0.0226) on magnetic resonance imaging (MRI) and positive periurethral tissue (OR: 17.85, P < 0.0001) were associated with PSM of the apex. PSM with Gleason pattern ≥4 is significantly predictive of BCR. Intraoperative frozen sections of periurethral tissue can independently predict PSM, whereas sections of the bladder neck and dorsal vein could not. Pathologic examination of these samples may help predict significant PSM in patients undergoing robot-assisted laparoscopic prostatectomy with preservation of functional outcomes.
Keywords: biochemical recurrence, frozen section, positive surgical margin, robotic-assisted prostatectomy
INTRODUCTION
Pathologic Gleason score and stage and initial prostate-specific antigen (PSA) concentration have been found to predict prognosis in patients with localized prostate cancer.1 Positive surgical margins (PSMs) can increase the risk of biochemical recurrence (BCR).2 PSM can be caused by extracapsular extension of the tumor, technical error, or an artifact related to handling in the operating room or histology laboratory.3 Efforts have been made to both measure and reduce PSM.3 Exact prediction, through risk classification or imaging analysis, may provide surgical guidelines to reduce PSM rate.4 The surgeon's experience, the aggressiveness of nerve sparing, and detailed pathologic handling may also have an impact on PSM rates.5
Intraoperative frozen section assessment may reduce PSM rates and improve prognosis. The primary purpose of intraoperative frozen section is to confirm complete resection and the absence of residual tumor. Intraoperative frozen sections taken from different locations in periprostatic tissue, however, have yielded mixed results in assessing PSM in patients undergoing radical prostatectomy.6 It is also unclear whether negative conversion after positive frozen section can improve oncologic outcome, although one report suggested that only frozen sections can reduce the risk of BCR.6,7 To date, however, there has been no consensus on whether intraoperative frozen sections are predictive or on the location from which samples should be obtained. However, intraoperative frozen sections around the posterolateral pedicle may help surgeons decide whether or not to save the neurovascular bundle and maintain potency.8,9 The ability of intraoperative frozen sections taken from other locations of the prostate to predict PSM in patients who strongly wish to preserve the neurovascular bundle is not clear. This study therefore sought to identify significant PSM-related factors predictive of BCR and to confirm that intraoperative frozen section can predict significant PSM, retrospectively.
PATIENTS AND METHODS
The study protocol was approved by the Ethical Review Board of Asan Medical Center (Seoul, Korea). The study population included 271 patients with prostate cancer who underwent robot-assisted laparoscopic prostatectomy with intraoperative frozen sections between October 2008 and April 2016, retrospectively. Patients who received neoadjuvant therapy were excluded. Clinical and pathologic data were reviewed retrospectively, including age at diagnosis, initial PSA concentrations, body mass index, Charlson comorbidity index, magnetic resonance imaging (MRI) findings, Gleason score, T stage, surgical margin status, and frozen section results. Serum PSA concentrations were measured every 3–6 months for the first 2 years after surgery and annually thereafter. BCR was defined as two consecutive >0.2 ng ml−1 increases in PSA. The median follow-up period was 30.2 (interquartile range: 15.4–39.8) months. Informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data.
All operations were performed by a single experienced surgeon (CSK) and included bilateral nerve sparing, bladder neck cauterization, and maximal urethra preservation (Figure 1a). After the removal of the prostate specimen, intraoperative frozen sections were obtained from the soft tissue of the periurethra, the remaining bladder neck, and the region of the dorsal vein (Figure 1b). The soft tissue of the periurethra was obtained circumferentially above the assumed muscular layer of the urethra. Bladder neck tissue was resected from the remaining bladder muscle circumferentially. The tissue from the dorsal vein was gained before dorsal vein suture for bleeding control. The surface of each prostate specimen was painted with India ink (Cancer Diagnostics, Inc., Durham, NC, USA), and PSM was defined as cancer cells in inked margins. Each specimen was cut into 3–5 mm sections. Divisions on MRI and pathology were defined in Figure 1c. Frozen section results were obtained after approximately 15 min, and additional resection was not performed.
Figure 1.
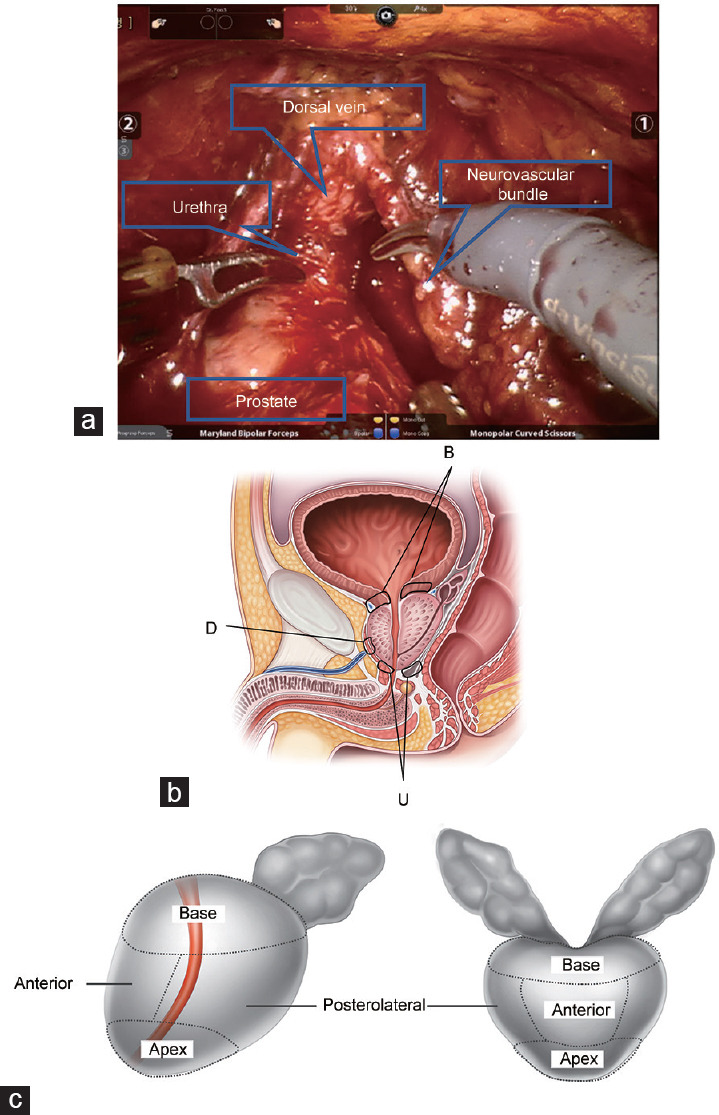
Illustration showing the locations from which intraoperative frozen sections were taken and surgical margins. (a) Intraoperative anatomy. (b) Locations for obtaining intraoperative frozen sections of periurethral tissue (U), bladder neck (B), and dorsal vein (D). (c) Prostate anatomy division by magnetic resonance imaging and pathology.
The baseline clinicopathological characteristics of the patients were expressed as mean ± standard deviation or numbers with percentages. Continuous variables in the PSM and negative surgical margin (NSM) groups were compared by Student's t-test, and categorical variables were compared by Chi-squared test. BCR-free survival was estimated using the Kaplan–Meier method, and survival in patients categorized by surgical margin status was compared by log-rank tests. Factors prognostic of BCR in the total population and the PSM group were analyzed by Cox proportional hazard regression. The results obtained from examination of intraoperative frozen sections at each site were used to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for PSM. Factors significant on univariate analyses (P < 0.50) were entered into multivariate logistic regression to determine factors independently predictive of significant PSM. All statistical analyses were performed with SPSS 12.0 software (SPSS Inc., Chicago, IL, USA) and R software (version 3.4.1; R Project for Statistical Computing, Vienna, Austria), with P < 0.05 considered statistically significant.
RESULTS
The clinicopathological characteristics of the 271 included patients are presented in Table 1. PSM was confirmed in 108 (39.9%) patients and NSM in 163 (60.1%) patients. Of the 271 patients, 226 (83.4%) were at high risk according to the D'Amico classification. The PSM group was significantly younger (63.8 ± 7.3 vs 65.6 ± 6.7 years, P = 0.039) and had significantly higher PSA levels (9.2 ± 7.5 vs 7.1 ± 4.8 ng ml−1, P = 0.013) and maximal tumor percentage of positive biopsy core (51.3% ± 31.0% vs 39.1% ± 25.3%, P = 0.001), as well as having a higher percentage of patients with clinical Gleason scores ≥8 (27.8% vs 18.4%, P = 0.014), than the NSM group. Tumor volume (6.6 ± 7.7 vs 2.6 ± 2.6 ml, P < 0.001) and the percentages of patients with pathologic Gleason scores ≥8 (18.9% vs 7.5%, P = 0.015) and T stage ≥T3a (51.9% vs 24.6%, P < 0.001) were significantly higher in the PSM than those in the NSM group, as were the percentages of positive frozen sections from periurethral tissue (40.7% vs 11.7%, P < 0.001) and the dorsal vein site (13.9% vs 1.2%, P < 0.001).
Table 1.
Basic characteristics of patients and tumors
| Characteristics | PSM (n=108) | NSM (n=163) | Total (n=271) | P |
|---|---|---|---|---|
| Age (year), mean±s.d. | 63.8±7.3 | 65.6±6.7 | 64.9±7.0 | 0.039 |
| Initial PSA (ng ml−1), mean±s.d. | 9.2±7.5 | 7.1±4.8 | 7.9±6.0 | 0.013 |
| Body mass index (kg m−2), mean±s.d. | 24.3±3.3 | 24.7±2.6 | 24.5±2.9 | 0.315 |
| Hypertension, n (%) | 70 (64.8) | 89 (54.6) | 112 (41.3) | 0.122 |
| Diabetes mellitus, n (%) | 23 (21.3) | 29 (17.8) | 52 (19.2) | 0.576 |
| Charlson comorbidity index, n (%) | ||||
| 0 | 9 (8.3) | 18 (11.0) | 27 (10.0) | 0.720 |
| 1 | 62 (57.4) | 94 (57.7) | 156 (57.6) | |
| ≥2 | 37 (34.3) | 51 (31.3) | 88 (32.5) | |
| D’Amico risk, n (%) | ||||
| Low | 2 (1.9) | 15 (9.2) | 17 (6.3) | 0.032 |
| Intermediate | 14 (13.0) | 14 (8.6) | 28 (10.3) | |
| High | 92 (85.2) | 134 (82.2) | 226 (83.4) | |
| Clinical T2 stage, n (%) | 78 (72.2) | 133 (81.6) | 211 (77.9) | 0.095 |
| Extracapsular invasion on MRI, n (%) | 30 (27.8) | 24 (14.7) | 54 (19.9) | 0.144 |
| Seminal vesicle invasion on MRI, n (%) | 7 (6.5) | 12 (7.4) | 19 (7.0) | 0.454 |
| Clinical Gleason score, n (%) | ||||
| 6 | 30 (27.8) | 52 (31.9) | 82 (30.3) | 0.014 |
| 7 | 48 (44.4) | 81 (49.7) | 129 (47.6) | |
| 8 | 14 (13.0) | 25 (15.3) | 39 (14.4) | |
| ≥9 | 16 (14.8) | 5 (3.1) | 21 (7.7) | |
| Prostate volume on TRUS (ml), mean±s.d. | 34.1±10.1 | 34.9±14.5 | 34.6±12.9 | 0.675 |
| Membranous urethra length on MRI (mm), mean±s.d. | 12.0±4.0 | 12.1±2.8 | 12.1±3.4 | 0.919 |
| Percent of positive core (%), mean±s.d. | 57.8±33.5 | 51.4±37.0 | 53.9±35.7 | 0.155 |
| Maximal tumor percent of positive core (%), mean±s.d. | 51.3±31.0 | 39.1±25.3 | 44.0±28.3 | 0.001 |
| Prostate volume (ml), mean±s.d. | 29.4±9.7 | 31.0±13.1 | 30.4±11.8 | 0.264 |
| Tumor volume (ml), mean±s.d. | 6.6±7.7 | 2.6±2.6 | 4.2±5.6 | <0.001 |
| Pathologic Gleason score, n (%) | ||||
| 6 | 12 (11.9) | 36 (22.5) | 48 (18.4) | 0.015 |
| 7 | 70 (69.3) | 112 (70.0) | 182 (69.7) | |
| 8 | 4 (4.0) | 3 (1.9) | 7 (2.7) | |
| ≥9 | 15 (14.9) | 9 (5.6) | 24 (9.2) | |
| Pathologic T stage, n (%) | ||||
| T2 | 52 (48.1) | 123 (75.5) | 175 (64.6) | <0.001 |
| T3a | 41 (38.0) | 28 (17.2) | 69 (25.5) | |
| T3b | 15 (13.9) | 12 (7.4) | 27 (10.0) | |
| Length of PSM (mm), mean±s.d. | 11.1±15.0 | 11.1±15.0 | ||
| Number of PSMs, n (%) | ||||
| 1 | 63 (58.3) | 63 (23.2) | ||
| ≥2 | 45 (41.7) | 45 (16.6) | ||
| Gleason pattern of PSM, n (%) | ||||
| <4 | 48 (44.4) | 48 (17.7) | ||
| ≥4 | 60 (55.6) | 60 (22.1) | ||
| Location of PSM, n (%) | ||||
| Apex | 62 (57.4) | 0 (0) | 62 (22.9) | |
| Base | 21 (19.4) | 0 (0) | 21 (7.7) | |
| Anterior | 37 (34.3) | 0 (0) | 37 (13.7) | |
| Posterolateral | 41 (38.0) | 0 (0) | 41 (15.1) | |
| Positive frozen section, n (%) | ||||
| Periurethra tissue | 44 (40.7) | 19 (11.7) | 63 (23.2) | <0.001 |
| Bladder neck | 6 (5.6) | 2 (1.2) | 8 (3.0) | 0.090 |
| Dorsal vein site | 15 (13.9) | 2 (1.2) | 17 (6.3) | <0.001 |
PSMs: positive surgical margins; NSM: negative surgical margin; s.d.: standard deviation; PSA: prostate-specific antigen; MRI: magnetic resonance imaging; TRUS: transrectal ultrasonography
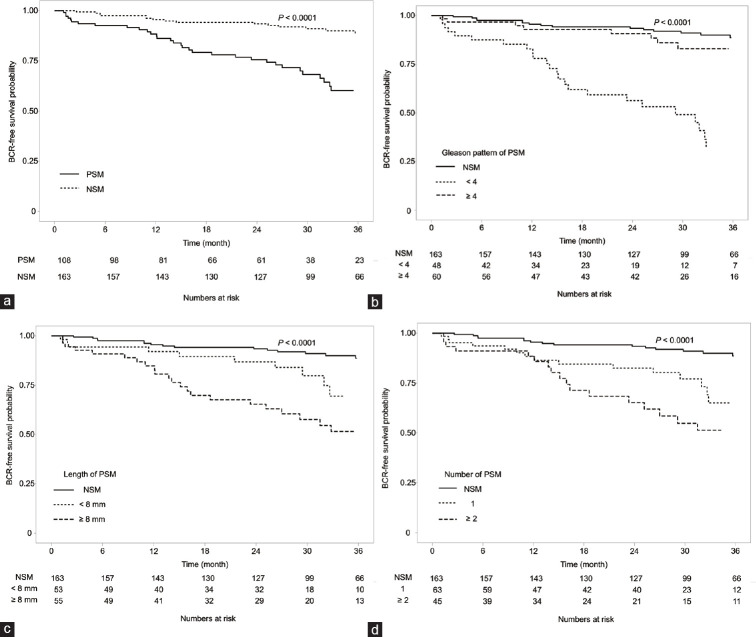
The 3-year BCR-free survival rate was 60.2% in the PSM group and 88.6% in the NSM group (P < 0.0001, Figure 2a). The estimated median BCR-free survival was 57.6 (95% confidence interval [CI]: 49.7–65.4) months in the PSM group, and 80.8 (95% CI: 75.2–86.4) months in the NSM group. Analysis of 3-year BCR-free survival rates according to Gleason pattern showed that these rates were significantly lower in patients with Gleason pattern ≥4 and PSM (31.8%) than those in patients with Gleason pattern <4 and PSM (82.9%) and those in patients with NSM (88.6%, P < 0.0001; Figure 2b). The estimated median BCR-free survival was 35.9 (95% CI: 26.6–45.2) months in patients with Gleason pattern ≥4 and 71.4 (95% CI: 62.3–80.4) months in patients with Gleason pattern <4 (P < 0.001). The median 3-year BCR-free survival rates were 51.4% and 69.5% (P = 0.062) in patients with PSM ≥8 mm and <8 mm, respectively. Compared with patients in the NSM group, the median BCR-free survival durations were significantly shorter in patients with Gleason pattern ≥4 (52.2 months; 95% CI: 41.8–62.7 months, P < 0.001) and Gleason pattern <4 (45.9 months; 95% CI: 39.1–52.8 months, P = 0.009) (Figure 2c). The 3-year BCR-free survival rates were 65.2% in patients with PSM at one site and 51.3% in patients with PSM at ≥2 sites (P = 0.152). Compared with the NSM group, the median BCR-free duration was also significantly shorter in patients with PSM at one site (52.9 months; 95% CI: 44.4–61.3 months, P = 0.001) and at ≥2 sites (52.3 months; 95% CI: 60.5–64.1 months, P < 0.0001) (Figure 2d).
Figure 2.
Kaplan–Meier analysis of biochemical recurrence-free survival according to characteristics of surgical margins. (a) Presence of surgical margins; (b) Gleason pattern of positive surgical margins; (c) length of positive surgical margins; and (d) number of positive surgical margins. PSM: positive surgical margin; NSM: negative surgical margin; BCR: biochemical recurrence.
Cox proportional hazard model 1, in which each variable was adjusted by initial PSA concentration, pathologic Gleason score, and pathologic T stage, showed that PSM (hazard ratio [HR]: 2.681, 95% CI: 1.474–4.878, P = 0.0012) was a significant risk factor for BCR (Table 2). In this model, each PSM-related variable, including Gleason pattern ≥4 (HR: 4.434, 95% CI: 2.347–8.380, P < 0.0001), tumor lengths (<8 mm, HR: 2.497, 95% CI: 1.156–5.394, P = 0.0198; and ≥8 mm, HR: 2.804, 95% CI: 1.441–5.456, P = 0.0024), number of tumors (one, HR: 2.448, 95% CI: 1.240–4.828, P = 0.0098; and ≥2, HR: 3.056, 95% CI: 1.471–6.347, P = 0.0027), and tumor location (apex, HR: 3.861, 95% CI: 1.919–7.768, P = 0.0002; anterior, HR: 2.727, 95% CI: 1.162–6.400, P = 0.0212; and posterolateral, HR: 2.691, 95% CI: 1.300–5.570, P = 0.0076), was significantly associated with BCR. In multivariate model 2, which included all variables associated with PSM, only Gleason pattern ≥4 (vs< 4; HR: 4.386, 95% CI: 1.938–9.928, P = 0.0004) was significantly associated with BCR.
Table 2.
Cox hazard analysis of factors associated with biochemical recurrence-free survival
| Models | Total cohort (univariate model) | Total cohort (multivariate model 1)a | PSM cohort (multivariate model 2)b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| PSM versus NSM | 3.310 | 1.900–5.740 | <0.0001 | 2.681 | 1.474–4.878 | 0.0012 | |||
| Gleason pattern of PSM (vs NSM) | |||||||||
| Gleason pattern <4 | 1.440 | 0.660–3.140 | 0.3640 | 1.212 | 0.522–2.813 | 0.6551 | 1.000 | Reference | |
| Gleason pattern ≥4 | 6.670 | 3.670–12.100 | <0.0001 | 4.434 | 2.347–8.380 | <0.0001 | 4.386 | 1.938–9.928 | 0.0004 |
| Length of PSM (vs NSM) | |||||||||
| <8 mm | 2.310 | 1.110–4.840 | 0.0260 | 2.497 | 1.156–5.394 | 0.0198 | 1.000 | Reference | |
| ≥8 mm | 4.160 | 2.230–8.090 | <0.0001 | 2.804 | 1.441–5.456 | 0.0024 | 1.236 | 0.536–2.854 | 0.6191 |
| Number of PSMs (vs NSM) | |||||||||
| 1 | 2.670 | 1.390–5.140 | 0.0030 | 2.448 | 1.240–4.828 | 0.0098 | 1.000 | Reference | |
| ≥2 | 4.250 | 2.230–8.090 | <0.0001 | 3.056 | 1.471–6.347 | 0.0027 | 1.147 | 0.445–2.955 | 0.7767 |
| Location of PSM (vs NSM) | |||||||||
| Apex | 3.660 | 1.990–6.730 | <0.0001 | 3.861 | 1.919–7.768 | 0.0002 | 1.000 | Reference | |
| Base | 5.210 | 2.370–11.420 | <0.0001 | 1.826 | 0.742–4.491 | 0.1898 | 1.294 | 0.272–6.160 | 0.7464 |
| Anterior | 3.120 | 1.500–6.480 | 0.0020 | 2.727 | 1.162–6.400 | 0.0212 | 0.765 | 0.131–4.467 | 0.7663 |
| Posterolateral | 3.560 | 1.800–7.040 | <0.0001 | 2.691 | 1.300–5.570 | 0.0076 | 0.908 | 0.158–5.219 | 0.9143 |
aIn multivariate model 1, each variable was adjusted for initial PSA, pathologic Gleason score, and pathologic T stage; bIn multivariate model 2, all variables related to PSM were entered into the PSM cohort. CI: confidence interval; HR: hazard ratio; PSM: positive surgical margin; NSM: negative surgical margin; PSA: prostate-specific antigen
Table 3 shows the sensitivity, specificity, PPV, and NPV for positive intraoperative frozen sections at each site. Positive periurethral tissue had a sensitivity of 64.5% and a specificity of 89.0% for all PSMs, as well as a sensitivity of 83.3% and a specificity of 84.2% for PSMs with a Gleason pattern ≥4. Multivariate logistic analysis showed that membranous urethra length on MRI (odds ratio [OR]: 0.79, 95% CI: 0.63–0.99, P = 0.0376), extracapsular extension of apex on MRI (OR: 4.58, 95% CI: 1.24–17.32, P = 0.0226), and positive frozen periurethra sections (OR: 17.85, 95% CI: 5.40–73.25, P < 0.0001) were associated with PSM of the apex (Table 4). Maximal tumor percentage of positive core was associated with PSM of the base (OR: 1.05, 95% CI: 1.02–1.10, P = 0.0023), and anterior extracapsular extension on MRI was associated with anterior PSM (OR: 6.26, 95% CI: 1.42–25.33, P = 0.0106).
Table 3.
Sensitivity, specificity, and predictive values of intraoperative frozen sections showing positive surgical margins at each site
| Accuracy | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| All PSMs | ||||
| Periurethra tissue | 64.5 | 89.0 | 63.5 | 89.4 |
| Bladder neck | 19.0 | 98.4 | 50.0 | 93.5 |
| Dorsal vein site | 24.3 | 96.6 | 52.9 | 89.0 |
| PSM with Gleason pattern ≥4 | ||||
| Periurethra tissue | 83.3 | 84.2 | 39.7 | 97.6 |
| Bladder neck | 9.1 | 97.3 | 12.5 | 96.2 |
| Dorsal vein site | 31.3 | 95.3 | 29.4 | 95.7 |
PPV: positive predictive value; NPV: negative predictive value; PSMs: positive surgical margins
Table 4.
Multivariate logistic regression of factors predicting positive surgical margins including Gleason pattern ≥4 at each location
| Apex | Base | Anterior | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Membranous urethra length on MRI (continuous) | 0.79 | 0.63–0.99 | 0.0376 | NA | 0.84 | 0.65–1.05 | 0.1516 | ||
| Maximal tumor percent of positive core (continuous) | 1.02 | 1.00–1.04 | 0.0834 | 1.05 | 1.02–1.10 | 0.0023 | |||
| Extracapsular extension of each site on MRI (yes vs no) | 4.58 | 1.24–17.32 | 0.0226 | 6.26 | 1.42–25.33 | 0.0106 | |||
| Positive frozen section of periurethra tissue (yes vs no) | 17.85 | 5.40–73.25 | <0.0001 | NA | NA | ||||
| Positive frozen section of bladder neck (yes vs no) | NA | NA | |||||||
| Positive frozen section of dorsal vein site (yes vs no) | NA | NA | |||||||
PSA: prostate-specific antigen; MRI: magnetic resonance imaging; NA: not analyzed; CI: confidence interval; OR: odds ratio
DISCUSSION
This study confirmed that PSM was associated with an increased risk of BCR after robot-assisted laparoscopic prostatectomy. The most important PSM-related predictive factor was the Gleason pattern of PSM. Intraoperative frozen sections of the bladder neck and dorsal vein site showed poor sensitivity, whereas frozen sections of periurethral tissue showed acceptable sensitivity and specificity for detecting PSM with Gleason pattern ≥4. Consequently, positivity of frozen sections of periurethral tissue was significantly associated with significant PSM in the apex of the prostate.
Although tumor-related factors, including tumor, node, metastasis (TNM) stage, Gleason score, and preoperative PSA concentration, are closely related to BCR, they cannot be controlled. By contrast, PSM at the time of surgery is partially controllable. Pathologists at our institution routinely observe higher Gleason patterns on PSM, determine the length of each PSM, and mark the location of each on a painted prostate shape using transparent paper. Although PSMs can be subclassified by, for example, length, location, and number,10 few studies have reported that Gleason score of PSM was independently associated with BCR.11 Adjuvant radiation may reduce the rate of poorer outcomes in patients with PSM with high Gleason score. Only one study to date has included the length, location, and numbers of PSM with Gleason pattern in multivariate analysis, with this study reporting that patients with Gleason pattern ≥4 on PSM had a 2-fold higher risk of BCR.12 In the present study, PSM with Gleason pattern ≥4 was positively correlated with pathologic Gleason score (data not shown) and independently associated with a 4.4-fold increased risk of BCR despite adjustment for pathologic Gleason score. Tumor progression in these patients may be associated with the aggressive characteristics of Gleason pattern ≥4.13
Apical PSM is the most common location in patients undergoing radical prostatectomy.14 Dissection of the apex may be especially challenging, because of the lack of an obvious border between the apex and the urethral sphincter. Maximal urethral preservation could result in unavoidable apical PSM.15 In the present study, short membranous urethral length was associated with a high risk of apical PSM. In addition, the fibromuscular band denoting the capsule and periprostatic tissue is faint in the apex.16 Surgical handling of the specimen could result in a faint capsule, causing false positive results. Apical PSM could therefore be associated not only with tumor-related factors but also with other factors, emphasizing the importance of finding significant PSM on the apex. Intraoperative frozen sections of periurethral tissue can independently predict PSM, whereas PSM of base and anterior did not. Anterior fibromuscular stroma and benign adenoma constructs from anterior aspect of the prostate to the bladder neck, and this can be the barrier against the extension by carcinoma.17 Therefore, the frozen sections of bladder neck and dorsal vein might show the low accuracy for predicting PSM.
Although intraoperative frozen sections have been used to eliminate residual tumor, few studies have shown the benefits of conversion from positive to negative margins on oncologic outcomes. Negative conversion of margins around the neurovascular bundle resulted in a BCR similar to that in patients with NSM.9 About 30% of frozen sections of the urethra and bladder neck in patients with low- and intermediate-risk prostate cancer were found to have positive margins, with negative conversion of these positive sections by further resection found to be helpful in reducing the risk of BCR.7 However, one study reported that frozen sections had limited effect on BCR, but increased the risk of incontinence.18 Other opposite opinions for frozen sections include their low sensitivity, the high risk of PSM at other sites, the higher false positive rate due to difficulties in assessing tumors on frozen sections, and low productivity relative to cost.19 Our results indicated that periurethral intraoperative frozen sections were significantly predictive of PSM, whereas frozen sections of the bladder neck and dorsal vein site were not. In a previous study, the incidence of positivity on frozen sections of the bladder neck was lower (0.8%) than that at other sites because of the relatively wide range of the former or sufficient removal close to the ureteral orifice.20 Moreover, MRI has advantages confirming significant tumor at the apex.21 MRI findings in the present study were also significantly predictive of PSM on the apex and in anterior locations. Meanwhile, the frozen section in the posterolateral aspect of prostate which was in contact with the neurovascular bundle could help reduce PSM and functional outcomes.22 A randomized controlled study about frozen section of the posterolateral prostate will suggest more evidences.23
This study had several limitations. First, selection bias could not be ruled out, because the operator chose the performance of the dorsal vein frozen section when he was suspicious about the involvement of tumor. Second, the locations of intraoperative frozen sections and PSM may not match exactly. We used the same surgical technique to obtain frozen sections from all included patients, but these tumor specimens were resected and analyzed in a pathology laboratory after surgery. Third, this study could not determine whether additional resection is necessary, because we did not perform additional resection. Fourth, the ability of PSM of the bladder neck to predict BCR may be limited, because we cauterized the entire cross-section of the bladder neck to control bleeding and eliminate residual tumor. Despite these limitations, however, this is the first study to confirm the ability of frozen sections at various locations to predict significant PSM.
PSM with Gleason pattern ≥4 was significantly predictive of BCR in patients who underwent robot-assisted laparoscopic prostatectomy with bilateral nerve sparing and maximal urethra preservation. Positive frozen sections of periurethral tissue were an independent predictor of significant PSM with high sensitivity and specificity, whereas positive frozen sections of the bladder neck and dorsal vein site were not significantly predictive. Intraoperative frozen sections of periurethral tissue may help predict significant PSM and preserve functional outcomes.
AUTHOR CONTRIBUTIONS
SYC and CSK conceived and designed the study, and obtained funding; WL and BL acquired data; DY and BHC analyzed data; THK interpreted findings; SYC drafted the manuscript; and CSK supervised the study. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2019R1C1C1005170) and Korea Health Technology R&D Project, the Korea Health Industry Development Institute (H16C2193).
REFERENCES
- 1.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Wu B, Zha Z, Zhao H, Jiang Y, et al. Positive surgical margin is associated with biochemical recurrence risk following radical prostatectomy: a meta-analysis from high-quality retrospective cohort studies. World J Surg Oncol. 2018;16:124. doi: 10.1186/s12957-018-1433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srigley JR. Key issues in handling and reporting radical prostatectomy specimens. Arch Pathol Lab Med. 2006;130:303–17. doi: 10.5858/2006-130-303-KIIHAR. [DOI] [PubMed] [Google Scholar]
- 4.Cormier L, Bastide C, Beuzeboc P, Fromont G, Hennequin C, et al. Prostate cancer surgical margin: review by the CCAFU (oncology committee of the French association of urology) Prog Urol. 2014;24:334–45. doi: 10.1016/j.purol.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Gettman MT, Blute ML. Radical prostatectomy: does surgical technique influence margin control? Urol Oncol. 2010;28:219–25. doi: 10.1016/j.urolonc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto H. Clinical benefits of frozen section assessment during urological surgery: does it contribute to improving surgical margin status and patient outcomes as previously thought? Int J Urol. 2017;24:25–31. doi: 10.1111/iju.13247. [DOI] [PubMed] [Google Scholar]
- 7.Pak S, Park S, Kim M, Go H, Cho YM, et al. The impact on oncological outcomes after radical prostatectomy for prostate cancer of converting soft tissue margins at the apex and bladder neck from tumour-positive to -negative. BJU Int. 2019;123:811–7. doi: 10.1111/bju.14480. [DOI] [PubMed] [Google Scholar]
- 8.Beyer B, Schlomm T, Tennstedt P, Boehm K, Adam M, et al. A feasible and time-efficient adaptation of NeuroSAFE for da Vinci robot-assisted radical prostatectomy. Eur Urol. 2014;66:138–44. doi: 10.1016/j.eururo.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Schlomm T, Tennstedt P, Huxhold C, Steuber T, Salomon G, et al. Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol. 2012;62:333–40. doi: 10.1016/j.eururo.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson AJ, Wood DP, Kattan MW, Klein EA, Scardino PT, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182:1357–63. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Kates M, Sopko NA, Han M, Partin AW, Epstein JI. Importance of reporting the Gleason score at the positive surgical margin site: analysis of 4,082 consecutive radical prostatectomy cases. J Urol. 2016;195:337–42. doi: 10.1016/j.juro.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Savdie R, Horvath LG, Benito RP, Rasiah KK, Haynes AM, et al. High Gleason grade carcinoma at a positive surgical margin predicts biochemical failure after radical prostatectomy and may guide adjuvant radiotherapy. BJU Int. 2012;109:1794–800. doi: 10.1111/j.1464-410X.2011.10572.x. [DOI] [PubMed] [Google Scholar]
- 13.Tollefson MK, Leibovich BC, Slezak JM, Zincke H, Blute ML. Long-term prognostic significance of primary Gleason pattern in patients with Gleason score 7 prostate cancer: impact on prostate cancer specific survival. J Urol. 2006;175:547–51. doi: 10.1016/S0022-5347(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 14.Smith JA, Jr, Chan RC, Chang SS, Herrell SD, Clark PE, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385–9. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Borin JF, Skarecky DW, Narula N, Ahlering TE. Impact of urethral stump length on continence and positive surgical margins in robot-assisted laparoscopic prostatectomy. Urology. 2007;70:173–7. doi: 10.1016/j.urology.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 16.Ayala AG, Ro JY, Babaian R, Troncoso P, Grignon DJ. The prostatic capsule: does it exist.Its importance in the staging and treatment of prostatic carcinoma? Am J Surg Pathol. 1989;13:21–7. [PubMed] [Google Scholar]
- 17.Brooks JD, Chao WM, Kerr J. Male pelvic anatomy reconstructed from the visible human data set. J Urol. 1998;159:868–72. [PubMed] [Google Scholar]
- 18.Ramirez-Backhaus M, Rabenalt R, Jain S, Do M, Liatsikos E, et al. Value of frozen section biopsies during radical prostatectomy: significance of the histological results. World J Urol. 2009;27:227–34. doi: 10.1007/s00345-008-0360-2. [DOI] [PubMed] [Google Scholar]
- 19.Ye H, Kong X, He TW, Jolis T, Choi K, et al. Intraoperative frozen section analysis of urethral margin biopsies during radical prostatectomy. Urology. 2011;78:399–404. doi: 10.1016/j.urology.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Lepor H, Kaci L. Role of intraoperative biopsies during radical retropubic prostatectomy. Urology. 2004;63:499–502. doi: 10.1016/j.urology.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Kenigsberg AP, Tamada T, Rosenkrantz AB, Llukani E, Deng FM, et al. Multiparametric magnetic resonance imaging identifies significant apical prostate cancers. BJU Int. 2018;121:239–43. doi: 10.1111/bju.13987. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi R, Cozzi G, Petralia G, Alessi S, Renne G, et al. Multiparametric magnetic resonance imaging and frozen-section analysis efficiently predict upgrading, upstaging, and extraprostatic extension in patients undergoing nerve-sparing robotic-assisted radical prostatectomy. Medicine (Baltimore) 2016;95:e4519. doi: 10.1097/MD.0000000000004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinneen E, Haider A, Allen C, Freeman A, Briggs T, et al. NeuroSAFE robot-assisted laparoscopic prostatectomy versus standard robot-assisted laparoscopic prostatectomy for men with localised prostate cancer (NeuroSAFE PROOF): protocol for a randomised controlled feasibility study. BMJ Open. 2019;9:e028132. doi: 10.1136/bmjopen-2018-028132. [DOI] [PMC free article] [PubMed] [Google Scholar]