Abstract
Oxidative stress is prevalent among infertile men and is a significant cause of sperm DNA damage. Since sperm DNA damage may reduce embryo quality and increase miscarriage rates, it is possible that untreated sperm oxidative stress may impair in vitro fertilization (IVF) live birth rates. Given that the antioxidant Menevit is reported to reduce sperm DNA damage, it was hypothesized that men's consumption of this supplement may alter IVF outcomes. Therefore, a retrospective cohort study was conducted analyzing outcomes for couples undergoing their first fresh embryo transfer. Men were classified as controls if they were taking no supplements, health conscious controls if taking “general health” supplements, or Menevit users. Men with karyotype abnormalities, or cycles using donated, frozen and surgically extracted sperm were excluded. Among the final study cohort of 657 men, live birth rates were significantly higher in Menevit users than controls (multivariate adjusted odds ratio [OR]: 1.57, 95% confidence interval [CI]: 1.01–2.45, P = 0.046), but not between controls taking no supplements and those using general health supplements, thereby suggesting that potential health conscious behavior in supplement users is unlikely responsible for the superior outcomes in Menevit users. Interestingly, in a post hoc sensitivity analysis, live birth rates among Menevit users were statistically superior to controls for lean men (OR: 2.73, 95% CI: 1.18–6.28; P = 0.019), not their overweight/obese counterparts (OR: 1.29, 95% CI: 0.75–2.22, P = 0.37). The results of this large cohort study therefore support a positive association between men's use of the Menevit antioxidant during IVF treatment and live birth rates, especially in lean individuals.
Keywords: antioxidant, body mass index, in vitro fertilization, live birth, oxidative stress, sperm
INTRODUCTION
Male factor infertility is a significant indication for in vitro fertilization (IVF) throughout the world, with impaired sperm quality accounting for at least half of all IVF treatment cycles.1 IVF intracytoplasmic sperm injection (ICSI) appears to be an ideal treatment for male factor infertility as it facilitates fertilization even when sperm quality is extremely poor. However, it is recognized that if sperm has significant degrees of DNA fragmentation, this may result in suboptimal blastocyst formation, lower IVF pregnancy rates,2,3 and an increase in the chance of pregnancy loss4 – all reducing IVF efficiency. As such, optimization of sperm health prior to the onset of IVF treatment should be a key therapeutic target.
It is well recognized that oxidative stress is a key driver for sperm DNA damage.5,6 Oxidative stress (OS) occurs when the production of “free radicals” (reactive oxygen species or ROS) by sperm and activated leukocytes exceeds their neutralization by protective antioxidants naturally present within sperm, the male genital tract secretions, and seminal plasma.7 Importantly, it is estimated that up to 70% of men in an infertile relationship have biochemical evidence for sperm OS,7 with OS damage to sperm also being more commonly present in infertile men with totally normal routine semen analysis parameters.8
Recently, the first international position statement on the management of male oxidative stress infertility (MOSI) was published, highlighting the increasing importance of this condition.9 The escalating prevalence of MOSI is multifactorial,7 with obesity and poor diet likely being key drivers. Obesity is associated with a chronic state of low-grade systemic inflammation, increased leukocyte production of free radicals, and associated oxidative stress.10 Furthermore, obesity has also been reported to initiate activation of macrophage ROS production within the male reproductive tract, which results in sperm oxidative damage,11,12 plus an overall decline in sperm quality.13,14 Second, the increased consumption of “fast-food” meals, often high in pro-inflammatory fats and low in protective dietary antioxidants, is reported to trigger oxidative stress.15 With increasing rates of obesity,16 it is highly likely that the clinical problem of MOSI will escalate in the future, highlighting the urgent need for new therapeutic interventions to optimize men's fertility potential.
Lifestyle changes such as weight loss and increased intake of antioxidant-rich fresh fruit and vegetables, plus fish rich in anti-inflammatory omega-3 oils, are all likely to benefit sperm health.17,18 However, most men are unable to sustain these improvements in diet for a significant period of time, even when intensely supported.19 As an alternative, there has been a proliferation in antioxidant supplements being marketed to combat MOSI.20 A recent Cochrane systematic review21 has concluded that some antioxidant preparations can reduce sperm DNA damage (4 randomized controlled trials [RCTs], 254 men, P < 0.0001), confirming the biological plausibility that antioxidant treatment could benefit IVF outcomes. However, at present, there are only 2 small RCTs involving 90 men examining the impact of male antioxidant use on IVF outcome,22,23 with the Cochrane review21 concluding that there is only low-quality evidence supporting men's use of antioxidants to improve IVF live birth rates (odds ratio [OR]: 3.61, 95% confidence interval [CI]: 1.27–10.29, P = 0.02). This concern highlights the need for more research in this important area and was a trigger to conduct the current study.
Given the observations above linking infertile status, sperm oxidative stress, and DNA damage with reduced IVF success rates, we hypothesized that minimization of this damage through the use of male antioxidant therapy would be associated with improved IVF outcomes. Furthermore, as obese men are known to exhibit higher degrees of sperm oxidative stress, we hypothesized that male body mass index (BMI) may be an important effect modifier in this relationship, with obese men benefiting differently to nonobese men with antioxidant use. Therefore, the primary objective of this study was to analyze the impact of the men's use of the Menevit antioxidant, a nutraceutical reported to improve sperm DNA integrity,24 on pregnancy outcomes in a large group of IVF patients. Secondary outcomes were the impact of antioxidant use on IVF embryology outcomes, principally the number of high-quality embryos available for transfer or cryopreservation. Sperm quality was not a primary study outcome as sperm DNA fragmentation and oxidative stress were not assessed.
PARTICIPANTS AND METHODS
Study population
The study cohort was a retrospective analysis of 945 consecutively screened couples undergoing their first IVF/ICSI cycle at a private assisted reproductive treatment (ART) unit (Repromed, Adelaide, Australia) between January 2013 and October 2016. While an earlier RCT had reported a doubling of clinical pregnancy rates in Menevit users compared to controls,23 for the purpose of determining the power for this study, we assumed a more conservative increase in live birth rate of 50% (30% live birth rate in the control vs 45% in the Menevit group). With 132 couples in the smaller Menevit group, and 466 couples in the non-Menevit group, we had more than 80% power at a Type 2 error rate of α = 0.05 to detect a difference in live birth rate of 30% versus 45%.
Repeat cycles of IVF for the same couple during the study period were not considered, as is standard practice for retrospective studies. Other exclusion criteria included utilization of donor eggs or sperm, surgical sperm retrieval, and couples using frozen sperm. The latter two exclusions were made as both surgical sperm retrieval25 and use of cryopreserved sperm26 are known to influence sperm DNA integrity. Cycles in which no mature egg was retrieved were excluded as sperm quality was not a contributor to IVF outcome. However, cycles with complete fertilization failure, or no embryo transfer due to embryo arrest, were included in the final analysis as these outcomes may have a paternal origin. IVF cycles in which no fresh embryo transfer occurred because of the clinician or patient's decision to freeze all embryos (preimplantation genetic screening, ovarian hyperstimulation syndrome, or other medical and social reasons) were excluded from the final analysis due to the potential to bias outcomes independent of sperm quality.
Both paternal and maternal height and weight measured by the clinic before the couple's treatment cycle were used to generate body mass index (BMI, in kg m−2). Couples where the female partner aged greater than 38 years, or had a BMI of ≥30 kg m−2, were excluded from the cohort as these maternal factors are known to negatively influence IVF outcomes, independent of sperm quality-the primary research question of interest. Men aged greater than 45 years were also excluded from the study as this group is known to be at increased risk of sperm DNA replication errors that may negatively impact on IVF outcomes,27 but is not amenable to antioxidant treatment. Similarly, men with a genetic cause for their infertility on karyotype testing (translocation) were excluded from the study.
Study outcomes
Patient prognostic details that were recorded included female serum anti-Müllerian hormone (AMH), number of prior pregnancies and IVF treatment cycles, underlying etiology of infertility (as assessed by the treating physician), and both female and male age plus BMI. The treatment cycle outcomes analyzed included the number of oocytes collected and fertilized, number of embryos transferred, embryo utilization rates (embryos of sufficient morphological quality to freeze), plus biochemical and clinical pregnancy rates, and live birth outcomes.
This study design was a retrospective cohort where the use of nutritional supplements was primarily decided by the patients in consultation with their community pharmacist, but sometimes with the encouragement of their treating family physician or IVF specialist, especially when male factor was identified on a routine semen analysis. Direct tests of sperm oxidative stress were not available during the study period, and therefore, no patient was placed on targeted antioxidant treatment because they had been identified as exhibiting sperm oxidative stress.
Use of supplements was recorded by patients on their semen analysis questionnaire completed on the day the IVF semen sample was produced. For the purposes of the cohort study, subjects were classified into three groups depending on their use of supplements from these clinical records. Those men taking no supplements were considered as the control group. Those men taking Menevit, but no other antioxidant or mineral supplement, were classified as the Menevit group. Men that were taking supplements other than Menevit were treated as a separate group of “health conscious controls (HCC)” and used as a comparison group, but were not included in the final cohort analysis. This was in recognition of the possibility that use of supplements may be associated with other healthy lifestyle behaviors that could potentially favorably influence IVF outcomes. Men consuming Menevit plus other antioxidant vitamin/mineral supplements or anti-inflammatory preparations (omega-3 fish oil) were excluded from the analysis as it would have been impossible to determine which supplement was potentially influencing IVF outcomes. The duration and compliance of supplement use was not recorded on the IVF semen sample questionnaire.
The Menevit nutraceutical therapy consists of a single daily capsule containing 6 mg lycopene, 400 IU vitamin E, 100 mg of vitamin C, 25 mg of zinc, 26 μg of selenium, 500 μg of folate, and 333 μg of garlic oil (Bayer Consumer Care, Sydney, Australia).23 This product is available in community pharmacies throughout Australia, New Zealand, and Asia without a physician's prescription.
IVF treatment protocol
Controlled ovarian stimulation was conducted using recombinant follicle-stimulating hormone (FSH; Puregon; MSD, Sydney, Australia or Gonal-F; Serono, Sydney, Australia) or highly purified urinary gonadotropin (Menopur, Ferring, Sydney, Australia) in a gonadotropin-releasing hormone (GnRH) antagonist cycle (ganirelix, MSD). Follicle development was monitored by ultrasound and serum estradiol, and a human chorionic gonadotropin (hCG) trigger (250 μg Ovidrel; Serono) was administered when two or more follicles ≥17 mm in diameter were present. Oocytes were collected by transvaginal oocyte retrieval performed 36 h after hCG administration under light sedation. Fertilization was achieved using either routine insemination or ICSI according to sperm quality and clinician judgment, with conformation of fertilization being assessed 16–18 h later by the presence of two pronuclei and two polar bodies. On the day of insemination, a Neubauer hemocytometer was used to determine sperm concentration in the IVF sample, and sperm motility was determined manually under ×40. However, sperm normal morphology was not formally quantified in the IVF insemination sample.
Embryo morphology was assessed on either day 4 or day 5 (assigned based on clinician availability for embryo transfer), based on previous published guidelines.28,29 Embryos were selected for transfer based on morphology with the highest graded embryos selected for fresh transfer.
All embryo transfers were conducted under ultrasound guidance by appropriately trained physicians. Luteal support was provided using vaginal progesterone (Crinone, Merck) and oral estrogen (estradiol valerate 2 mg b.d. Bayer) for a minimum of 2 weeks, or alternatively hCG (Pregnyl, Merck) support (1500 IU subcutaneous day 4 and day 7 postoocyte retrieval).
Treatment outcomes
A pregnancy was classified as a positive biochemical pregnancy when the serum β-hCG level exceeded 20 IU on day 16 after embryo transfer. Clinical viable pregnancy was classified as the presence of a viable fetal heartbeat on ultrasound examination between 7- and 8-week gestation. Live birth rates were determined by the delivery of a live born infant(s) per embryo transfer procedure.
Ethical statement
Institutional review board approval to retrospectively access existing patient records was obtained from the institutional Scientific Review Board (Repromed Scientific Advisory Committee, approval number: 0219), as per the Australian guidelines. Participants had previously given written consent for this type of retrospective data extraction and analysis.
Statistical analyses
Subject characteristics were described using median and interquartile range for continuous variables and number (percentage) for categorical variables. The characteristics of the 3 groups of subjects were compared using the Kruskal–Wallis test for continuous variables and the Chi-squared test of independence for categorical variables. For final comparison of pregnancy outcomes, we analyzed only the Menevit group and the control group of men that did not use any supplements. Fertility outcomes were assessed using univariate and multivariate negative binomial regression with the estimated association between use of Menevit versus no supplements reported as a rate ratio (RR) with 95% CIs and associated P-value from a Wald test. Pregnancy outcomes were assessed using univariate and multivariate binary logistic regression with the estimated effect of Menevit reported as an OR and 95% CI and associated P-value from a Wald test. Maternal age, paternal age, maternal BMI, paternal BMI, the etiology of infertility (male, tubular, ovulatory dysfunction, endometriosis, unexplained, combined male and female, or other), and the number of eggs collected were included as covariates in the multivariate analysis for these outcomes. As a sensitivity analysis, we also assessed whether the effect of Menevit on pregnancy outcomes varied according to the presence of being overweight (BMI >25 kg m−2) by stratifying the analysis according to men with a BMI above or below 25 kg m−2. Since maternal age is a key determinate of IVF live birth, we also calculated and plotted the marginal predicted probabilities of a live birth according to maternal age, stratified according to paternal BMI (above or below 25 kg m−2) and the use of Menevit. Model predictors included treatment group, maternal age, paternal age, maternal BMI, etiology, the number of eggs collected, a binary indicator variable for BMI <25 kg m−2 versus ≥25 kg m−2, and an interaction between Menevit use and the BMI <25 kg m−2 indicator variable. A 2-sided Type 1 error rate of α = 0.05 was used for hypothesis testing. All analysis was performed using Stata version 16.0 (StataCorp, College Station, TX, USA).
RESULTS
Subject characteristics
From a starting cohort of 945 consecutive cycles undergoing case-note review and data extraction, a total of 288 cycles were excluded from the final analysis as they did not meet the stated inclusion/exclusion criteria, leaving a total of 657 cycles for analysis. The baseline characteristics of the three male supplement classification groups are outlined in Table 1. The majority of men in the final cohort were taking no supplements (n = 466, 70.9%), with 132 men (20.1%) using the Menevit nutraceutical. The remaining 59 men in the health conscious control group (9.0% total cohort) were taking various “general health” multivitamin and mineral supplements (n = 47), fish oil (n = 8), followed by herbal products (garlic extract, echinacea, gingko, or St. John's wort; n = 4), protein supplements (n = 2), probiotics (n = 1), or a combination of the above, none specifically designed to augment sperm health.
Table 1.
Description of study participants
| Study group | Controls (no supplements, n=466 | Anti-oxidant nutraceutical (Menevit, n=132) | Health-conscious controls (general health supplements, n=59) | P |
|---|---|---|---|---|
| Female age (year) | 32.7 (29.8–35.0) | 32.3 (29.7–34.4) | 33.9 (30.5–35.2) | 0.09 |
| Female BMI (kg m−2) | 23.3 (21.2–26.2) | 23.1 (21.0–26.0) | 24.5 (21.3–26.8) | 0.52 |
| Prior IVF cycles (n) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.92 |
| Gravidity | 0 (0–1) | 0 (0–1) | 1 (0–1) | 0.34 |
| Parity | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0.10 |
| Serum AMH (pmol) | 26 (13.3–42.3) | 25.4 (14.0–44.1) | 19.3 (10.5–44.7) | 0.55 |
| Male age (year) | 34.1 (31.3–37.4) | 33.9 (30.5–37.0) | 35.6 (32.3–37.8) | 0.04 |
| Male BMI (kg m−2) | 27.1 (24.5–30.2) | 26.6 (24.2–30.3) | 27.9 (24.5–30.1) | 0.95 |
| Etiology of infertility, n (%) | ||||
| Male | 100 (21.5) | 50 (37.9) | 13 (22.0) | <0.001 |
| Tubal | 37 (7.9) | 8 (6.1) | 4 (6.8) | |
| Ovulatory | 66 (14.1) | 10 (7.6) | 8 (13.6) | |
| Endometriosis | 42 (9.0) | 9 (6.8) | 6 (10.2) | |
| Unexplained | 96 (20.6) | 15 (11.4) | 18 (30.5) | |
| Other | 59 (12.7) | 7 (5.3) | 7 (11.9) | |
| Combined male and female | 66 (14.2) | 33 (25.0) | 3 (5.1) |
All data are expressed as median (IQR), aside from etiology which is expressed as numbers of cases (%). AMH: anti-Müllerian hormone; IVF: in vitro fertilization; BMI: body mass index; IQR: interquartile range
There were no significant differences between the three groups for maternal age, maternal BMI, the number of prior IVF cycles, gravidity, parity, serum AMH, and paternal BMI (Table 1). However, health conscious controls were slightly older than subjects in the Menevit group (median age: 35.6 vs 34.1 years; P = 0.04). In addition, there was also a difference in the etiology of the couple's infertility, with the Menevit group having a higher frequency of male factor infertility (prior abnormal semen analysis result; 37.9% vs 22.0% and 21.6% in health controls and supplemented controls, respectively; P < 0.001). Very few men or women in the study cohort smoked, with almost identical rates in the two primary comparison groups (male smoker: 6.6% controls vs 6.8% Menevit; female smoker: 2.3% controls vs 3.8% Menevit group).
IVF outcomes
Table 2 shows the results of the univariate and multivariate analysis of the fertility outcomes comparing men taking Menevit and the control group taking no supplements. There were no significant differences between the two groups in either univariate or multivariate analysis for the number of eggs collected, embryos collected, embryos transferred, embryos cryopreserved, and sperm motility. After adjustment for confounding, sperm concentration was 25% lower in men taking Menevit compared to the control group (RR = 0.75, 95% CI: 0.62–0.92, P = 0.005), consistent with the higher rate of male factor infertility in this group. The proportion of cycles using ICSI to achieve fertilization was 91.1% in the Menevit group and 83.0% in the controls, with their respective fertilization rates being 60.5% and 58.6%. Failed fertilization or arrested embryo development was a rare event, occurring at a rate of between 5% and 6% in all three groups (P = 0.73).
Table 2.
Univariate and multivariate analysis of embryology outcomes for Menevit users versus controls (no supplements)
| Statistical analysis | Univariate | Multivariatea | |||
|---|---|---|---|---|---|
| Median (IQR) | Rate ratio (95% CI) | P | Rate ratio (95% CI) | P | |
| Oocytes collected | |||||
| Control (n=466)b | 9 (6–15) | 1.00 | 1.00 | ||
| Menevit (n=132)b | 9.5 (5.5–14.0) | 1.08 (0.90–1.13) | 0.89 | 0.98 (0.87–1.09) | 0.67 |
| Embryo’s produced | |||||
| Control | 5 (3–8) | 1.00 | 1.00 | ||
| Menevit | 5.5 (3.0–9.0) | 1.04 (0.91–1.18) | 0.56 | 1.01 (0.88–1.15) | 0.93 |
| Embryo’s transferred | |||||
| Control | 1 (1–1) | 1.00 | 1.00 | ||
| Menevit | 1 (1–1) | 0.99 (0.81–1.20) | 0.91 | 0.99 (0.81–1.21) | 0.91 |
| Embryo’s cryopreserved | |||||
| Control | 1.5 (0–4) | 1.00 | 1.00 | ||
| Menevit | 1.5 (0–4) | 1.08 (0.86–1.36) | 0.51 | 1.03 (0.81–1.32) | 0.79 |
| Sperm concentration (106 ml−1) | |||||
| Control | 50 (24–83) | 1.00 | 1.00 | ||
| Menevit | 39 (13–66) | 0.78 (0.64–0.94) | 0.009 | 0.75 (0.62–0.92) | 0.005 |
| Sperm motility (%) | |||||
| Control | 56 (45–67) | 1.00 | 1.00 | ||
| Menevit | 54 (41–64.5) | 0.94 (0.88–1.01) | 0.109 | 0.94 (0.87–1.02) | 0.13 |
Estimates obtained using a negative binomial regression model for count outcomes. aAdjusted for maternal age, paternal age, maternal BMI and paternal BMI; bnumber of IVF cycles reaching oocyte retrieval with at least one oocyte collected. IQR: interquartile range; CI: confidence interval; BMI: body mass index; IVF: in vitro fertilization
Pregnancy outcomes
Table 3 shows the results of the univariate and multivariate analysis of the pregnancy outcomes comparing men taking Menevit and the control group of men taking no supplements. There a significantly higher odds of a clinical viable pregnancy for the Menevit group of men compared to controls (adjusted OR: 1.62, 95% CI: 1.06–2.47, P = 0.026), and a significantly higher odds of a live born per embryo transfer procedure for the Menevit group of men compared to controls (adjusted OR: 1.57, 95% CI: 1.01–2.45, P = 0.046). There was also a trend toward a higher chance of a biochemical pregnancy for men in the Menevit group compared to controls (adjusted OR: 1.52, 95% CI: 0.99–2.34, P = 0.053).
Table 3.
Pregnancy outcomes for Menevit users versus controls (no supplements)
| Statistical analysis | Subjects, % (n/total) | Univariate | Multivariatea | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Biochemical pregnancy rateb | |||||
| Control | 49.4 (230/466) | 1.00 | 1.00 | ||
| Menevit | 58.3 (77/132) | 1.43 (0.97–2.12) | 0.069 | 1.52 (0.99–2.34) | 0.053 |
| Clinical viable pregnancy rateb | |||||
| Control | 38.8 (181/466) | 1.00 | 1.00 | ||
| Menevit | 50.0 (66/132) | 1.57 (1.07–2.32) | 0.022 | 1.62 (1.06–2.47) | 0.026 |
| Live birth delivery ratec | |||||
| Control | 38.0 (166/437) | 1.00 | 1.00 | ||
| Menevit | 48.0 (59/123) | 1.50 (1.01–2.25) | 0.047 | 1.57 (1.01–2.45) | 0.046 |
Estimates obtained using a binary logistic regression model. aAdjusted for maternal age, paternal age, maternal BMI, paternal BMI and the number of oocytes collected; bbiochemical and clinical pregnancy rates are expressed per cycle reaching retrieval with at least 1 oocyte collected; clive birth delivery rate is expressed per cycle reaching embryo transfer, excluding cycles with total fertilization failure or arrested embryo development. CI: confidence interval; BMI: body mass index; OR: odds ratio
In sensitivity analysis that stratified the analysis by male BMI, there was a significantly higher odds of a live birth for lean men taking Menevit (BMI below 25 kg m−2) compared to controls (OR: 2.73, 95% CI: 1.18–6.28, P = 0.019), but there was no difference in the odds of a live birth for overweight and obese men taking Menevit compared to controls (OR = 1.29, 95% CI: 0.75–2.22, P = 0.37).
Comparison of pregnancy outcomes between controls taking no supplements and “health conscious” controls taking nonfertility-related supplements revealed no statistical difference in either biochemical pregnancy rates (49.4% vs 42.4%, P = 0.31), clinical viable pregnancy (38.8% vs 33.9%, P = 0.46), and live birth deliveries (38.0 % vs 33.9%, P = 0.55).
Predicted probability of a liveborn by male BMI status, maternal age, and the use of Menevit
Figure 1 shows the adjusted predicted probability of a live birth according to the use or not of Menevit, male BMI status (above or below BMI = 25 kg m−2), and maternal age. For men with a BMI <25 kg m−2, there was an overall 20.6% (95% CI: 2.4%–38.8%, P = 0.027) higher probability of a liveborn for those taking Menevit compared to controls taking no supplements (59.5% vs 38.9%). However, among men with a BMI ≥25 kg m−2, there was only a nonsignificant higher probability of 5.9% (95% CI: 0.0%–18.6%, P = 0.37) between those taking Menevit and controls (43.4% vs 37.5%).
Figure 1.
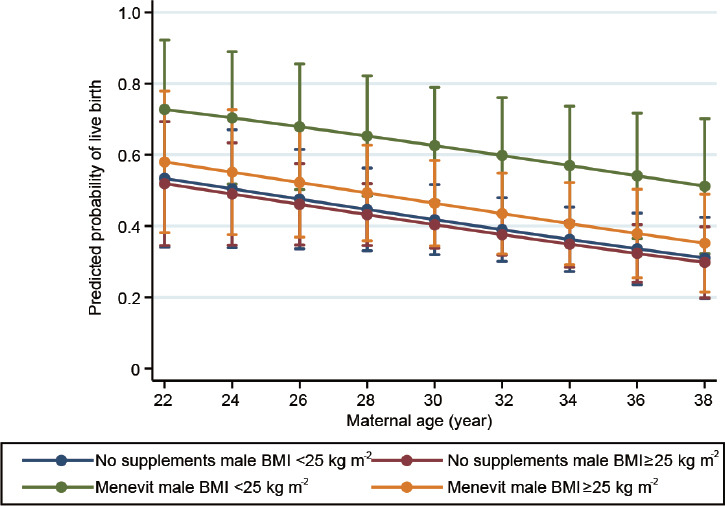
Predicted probability of a live birth delivery according to male BMI status, supplement use and maternal age. BMI: body mass index.
DISCUSSION
In this retrospective study, the largest of its kind to date, the use of the male fertility nutraceutical Menevit in lean men was associated with a 20.6% improvement in IVF live birth rates. While the overall improvement in live birth rate between controls and all Menevit users (irrespective of BMI) was only 10.7%, much less than the doubling in clinical pregnancy rate observed in an earlier RCT,23 the difference was still statistically significant and clinically meaningful. The observed difference in treatment efficiency likely reflects the fact that the original Menevit RCT targeted men with known oxidative stress and significant DNA damage, whereas in the current cohort study men self-selected for Menevit therapy. It is quite likely that some of the men taking Menevit in the current study did not have underlying oxidative stress, thereby reducing the potential benefit from antioxidant therapy. Furthermore, treatment compliance in the previous Menevit RCT was excellent, with 96% of participants missing <1 dose per week.23 In the current retrospective study, where participants were possibly less motivated and treatment compliance not monitored, it is highly likely that many men missed a significant number of Menevit doses. This may also have reduced the therapeutic effect, but our results probably best reflect the “real world” benefit from taking antioxidants in a nontrial clinical population.
It is interesting to note that in this study, and the previous Menevit RCT,23 no difference in embryo quality was observed between the Menevit and control groups, despite their being a significant live birth advantage with the consumption of the Menevit. This result probably reflects that embryo morphology is an insensitive marker of sperm oxidative DNA damage, with failure in embryo development due to high degrees of oxidative damage occurring well after day 5 of culture. It has even been suggested by some groups that oxidative stress tends to produce single-strand DNA breaks in the sperm genome, resulting in a lack of clinical pregnancy rather than impaired early embryo development.30 Conversely, double-strand DNA breaks are suggested to be due to a lack of DNA repair in meiosis and do present with low embryo quality and higher risk of miscarriage and implantation failure in IVF cycles.30
From a safety perspective, this study also provides support for the view that antioxidants are unlikely to be harmful to IVF outcomes. This is an important finding as one group has suggested that use of antioxidants could impair reproductive outcomes by producing a mild decondensation of sperm DNA,31 as ROS assist the formation of disulfide bridges between protamines, which may in turn impair IVF outcomes.32 However, reassuringly we have previously reported that consumption of the Menevit nutraceutical actually enhances sperm DNA condensation and integrity.24 We acknowledge that it is possible that sperm DNA decondensation may occur with other antioxidant preparations, especially those containing high doses of vitamin C known to interfere with disulfide bridge formation.31 In addition, the indiscriminate use of fertility antioxidants may produce “reductive stress” in all cells, which may impair normal cellular physiology and actually produce nonreproductive disease if taken long term.33
The retrospective cohort design of this study precludes any conclusion on whether the Menevit antioxidant improved sperm concentration or motility. Men with documented impaired sperm quality on routine semen analysis (WHO 2010)34 were twice as likely to take the Menevit antioxidant, explaining the significantly lower sperm concentration seen in that cohort's IVF sample. However, it is currently recognized that there is significant uncertainty on whether antioxidants improve traditional sperm parameters and chances of natural conception.21 The recently published Males, Antioxidants, and Infertility (MOXI) RCT35 is the largest trial to date examining the impact of one particular antioxidant preparation on sperm quality and non-IVF conception (natural conception, with the option of later intra uterine insemination [IUI]). This trial reported that 3 months of antioxidant treatment produced no benefit in terms of sperm concentration, motility, morphology, DNA integrity, or live birth rates. This lack of a fertility benefit is obviously a disappointing outcome, but not surprising given that sperm quality did not improve on the MOXI antioxidant formulation. However, these results differ to those of the current study where the context was the use of antioxidant supplements as an adjunct treatment to IVF, not natural or IUI-assisted conception. Furthermore, the Cochrane systematic review reported that several other antioxidant formulations have been shown to reduced sperm DNA damage in an RCT setting,21 thereby supporting a biologically plausible mechanism for enhancing fertility potential in IVF treatment. Finally, the Cochrane review21 also supports the use of antioxidants in the setting of IVF to boost live birth rates, although it is acknowledged that the strength of this conclusion is low due to the availability of only two small RCTs.22,23 We believe that the present large cohort study provides further support for the use of male antioxidants in the context of IVF treatment, at least for the Menevit formulation.
Our observation that lean men benefited more from Menevit antioxidant treatment than their overweight counterparts might appear counter-intuitive, given that obesity has been associated with both elevated levels of systemic oxidative stress36 and sperm oxidative DNA damage.11,37 However, it is possible that factors beyond oxidative stress could be confounding these results. Given that we did not directly measure oxidative stress or sperm DNA damage, this question will need to be answered by future prospective studies.
One important limitation of this study is that we are unable to answer the important question of how long men must take antioxidants in order to maximize IVF outcomes, since this information was not recorded. The previous Menevit RCT23 mandated 3 months of antioxidant pretreatment before the IVF treatment, based on the observation that this period would cover the entire 72-day duration of sperm production. Given that this earlier RCT confirmed a pregnancy benefit, and a later trial confirmed that 3 months of Menevit treatment reduced sperm DNA damage,24 a longer duration of pretreatment before IVF is unlikely to be of an additional benefit. Indeed, given that the majority of sperm DNA damage occurs during epididymal transit/storage phase of development,38,39 it is quite possible that only a short 1–2-week course of antioxidants may be adequate to optimize sperm health for IVF-related conception. One study in mice using a very similar antioxidant formulation to Menevit (with garlic oil substituted for green tea extract, but the remaining components remaining identical between the two formulations), has shown that only 12 days of antioxidant treatment is needed to reduce sperm DNA damage and improve reproductive outcomes.40 However, future studies will be needed to confirm the benefit of such short-term antioxidant therapy for men.
In addition, we acknowledge that our study was retrospective and observational and that the associations observed are not necessarily causal. However, our study had a number of strengths that helped reduce these concerns. First, we reduced the possibility of selection bias by including all consecutive couples over a long duration of time, excluding only those couples with clear female related poorer prognosis (age >38 years, BMI ≥30 kg m−2, no oocytes collected following egg retrieval), sperm donation cases, plus those cycles using surgical or cryopreserved sperm, both known to alter sperm DNA quality and IVF outcomes. The exclusion of “freeze all” cycles, primarily patients undergoing embryo preimplantation genetic screening, was felt necessary as antioxidant therapy may increase blastocyst formation rates, thereby increasing the number of embryos available for genetic screening. Since it has been reported that the transfer of a euploid embryo is more likely to result in clinical pregnancy than an untested embryo,41 we felt that inclusion of these preimplantation genetic testing -aneuploidy (PGT-A) cycles may unfairly bias results.
Second, we attempted to control for the possibility that men who consume supplements may be more health conscious (better diet, lower BMI) than nonsupplement users, indirectly resulting in superior IVF outcomes, by comparing IVF outcomes in men taking general health supplements unlikely to have sperm health promoting properties with controls taking no supplements. The lack of any difference suggests that a health-conscious bias is unlikely to account for the observed pregnancy benefit of Menevit treatment. However, we do acknowledge the relatively small sample size and clinical heterogeneity in this health-conscious group, with some participants taking supplements aimed to “augment” health in already healthy individuals (e.g., multi-vitamins, protein supplements), where others may be consuming supplements for mild ailments (e.g., St. John's wort for mild depression). Given these potential weaknesses, we did not include this health-conscious cohort in the final multivariate analysis (Table 2 and 3 and Figure 1).
We acknowledge some differences in patient characteristics between the two primary comparator groups, with significantly more male factor infertility in the Menevit group, while endometriosis and ovulatory dysfunction were slightly more common in the controls, although the latter did not reach statistical significance. It is improbable that these etiological differences biased the primary outcome of our study, since the North American (SART) database report examining nearly a quarter of a million IVF cycles has concluded that IVF live birth rates are not significantly different for couples with male factor, ovulatory dysfunction, or endometriosis related infertility, with success rates only being inferior in those women with diminished ovarian reserve.42 As the Menevit and control groups had comparable ovarian reserve status (serum AMH and number of oocytes retrieved; Table 1 and 2, the differences in etiology between these two comparator groups are very unlikely to account for the observed differences in live birth outcomes. In addition, while ICSI was more commonly used in the Menevit group than the controls, reflecting the higher incidence of male factor infertility, this difference in fertilization technique is also unlikely to positively bias pregnancy outcomes in the Menevit cohort as live birth rates tend to be marginally inferior in ICSI cycles than IVF, even in nonmale factor infertility patients.43 Furthermore, our performance of multivariate statistical analysis has allowed us to adjust for these etiological differences and other important confounders known to influence IVF outcomes. As such, we believe that the superior pregnancy outcomes observed in the Menevit group are more likely to reflect a true positive therapeutic benefit, rather than reflect a patient characteristic bias.
Finally, the study cohort was taken from a single IVF unit during a period of stable clinical and laboratory practices with minimal changes in medical and laboratory staff. As such, it is unlikely that practice or laboratory conditions have significantly biased the outcomes.
Only a large prospective RCT can provide level 1 evidence supporting the use of Menevit, or other male antioxidant compounds, to augment IVF outcomes. However, such placebo-controlled RCTs are extremely difficult to conduct. Specifically, we previously observed that once patients were informed that antioxidants may benefit sperm health or IVF outcomes, they generally refused to join our own RCT because of fear of being randomized to placebo, instead purchasing antioxidants readily available without prescription.23 As more evidence now exists for the beneficial effects of male antioxidants in IVF,21 placebo-controlled trials are likely to become even harder to recruit for in the future. Furthermore, we would like to highlight that medical guidance currently given to men seeking to “naturally” improve their fertility potential has generally been to consume more fresh fruit and vegetables, both rich in natural antioxidants, with this advice being based solely on the findings of observational studies linking fruit and vegetable consumption with better sperm quality,17,18 not prospective RCT's. Therefore, we would contend that it is similarly reasonable to advocate for the use of synthetic antioxidant supplements such as Menevit, even in the absence of large RCTs, provided that the antioxidant supplement is safe and low cost and has some evidence supporting its use.
Despite these concerns, we hope that the result of this study, together with earlier supporting studies23,24 will trigger interest in conducting a large multicenter RCT of male antioxidant use in the context of IVF treatment. In order to maximize the chances of a positive outcome, the trial should consider several important design aspects. First, it should use an antioxidant preparation and dose with documented ability to reduce sperm DNA damage, the only biologically plausible mechanism of benefit in an IVF setting.2–4 Second, it is imperative that the inclusion criteria for any RCT be documented evidence of sperm oxidative stress, possibly using the new inexpensive bench top assays for MOSI such as MiOXSYS system.9,44 Previous studies that failed to select for the presence of oxidative stress have most likely underestimated the potential therapeutic benefit of antioxidants, given that men without oxidative stress are unlikely to benefit.
CONCLUSION
The results of this large retrospective study show that the use of the male fertility nutraceutical Menevit is associated with superior live birth rates during IVF treatment, primarily in lean men. While acknowledging that large RCTs are still required to absolutely prove therapeutic benefit, the positive association between Menevit antioxidant use and live birth outcomes, combined with its low cost (<1 USD per day) and minimal side effects support this type of adjunct treatment. Further studies examining the minimal effective duration of use before IVF and the impact of men's antioxidants consumption on the long-term health of children born are still warranted.
AUTHOR CONTRIBUTIONS
AH, KT and HS performed the primary data extraction. RW and KT conducted the statistical analysis. ML and KT conceived the study, with all authors involved in the drafting of the manuscript. All authors aside from ML (deceased 2020) read and approved the final manuscript.
COMPETING INTERESTS
KT and ML both hold stock in Monash IVF (the owner of Repromed) and have a financial interest in Menevit fertility nutraceutical. Bayer, the manufacturer of Menevit, had no involvement (financial support, study design, and interpretation) in this study. AH, HS, and DZF are employees of Repromed, but declare no other conflicts of interest. RW has no conflict of interest.
ACKNOWLEDGMENTS
Professor Michelle Lane died just before the submission of this manuscript. We acknowledge her as an author given her significant intellectual input into the study and manuscript preparation. We wish to dedicate this manuscript to the memory of Michelle in recognition of the very significant contribution she made to reproductive science.
REFERENCES
- 1.Adamson GD, de Mouzon J, Chambers G, Zegers-Hochschild F, Mansour R, et al. International committee for monitoring assisted reproductive technology: world report on assisted reproductive technology, 2011. Fertil Steril. 2018;110:1067–80. doi: 10.1016/j.fertnstert.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 2.Borges E, Jr, Zanetti BF, Setti AS, Braga DP, Provenza RR, et al. Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil Steril. 2019;112:483–90. doi: 10.1016/j.fertnstert.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Deng C, Li T, Xie Y, Guo Y, Yang QY, et al. Sperm DNA fragmentation index influences assisted reproductive technology outcome: a systematic review and meta-analysis combined with a retrospective cohort study. Andrologia. 2019;51:e13263. doi: 10.1111/and.13263. [DOI] [PubMed] [Google Scholar]
- 4.McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2019;112:54–60. doi: 10.1016/j.fertnstert.2019.03.003. e3. [DOI] [PubMed] [Google Scholar]
- 5.De Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodelling and the formation of 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress. Biol Reprod. 2009;81:517–24. doi: 10.1095/biolreprod.109.076836. [DOI] [PubMed] [Google Scholar]
- 6.Santiso R, Tamayo M, Gosálvez J, Meseguer M, Garrido N, et al. Simultaneous determination in situ of DNA fragmentation and 8-oxoguanine in human sperm. Fertil Steril. 2010;93:314–8. doi: 10.1016/j.fertnstert.2009.07.969. [DOI] [PubMed] [Google Scholar]
- 7.Tremellen K. Oxidative stress and male infertility – a clinical perspective. Hum ReprodUpdate. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 8.Pasqualotto FF, Sharma RK, Kobayashi H, Nelson DR, Thomas AJ, Jr, et al. Oxidative stress in normospermic men undergoing infertility evaluation. J Androl. 2001;22:316–22. [PubMed] [Google Scholar]
- 9.Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, et al. Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37:296–312. doi: 10.5534/wjmh.190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13:423–44. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–8. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 12.Pearce KL, Hill A, Tremellen KP. Obesity related metabolic endotoxemia is associated with oxidative stress and impaired sperm DNA integrity. Basic Clin Androl. 2019;29:6. doi: 10.1186/s12610-019-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont C, Faure C, Sermondade N, Boubaya M, Eustache F, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013;15:622–5. doi: 10.1038/aja.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha EA, Sayed SK, Gaber HD, Abdel Hafez HK, Ghandour N, et al. Does being overweight affect seminal variables in fertile men? Reprod Biomed Online. 2016;33:703–8. doi: 10.1016/j.rbmo.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–72. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- 16.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–89. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 18.Nassan FL, Chavarro JE, Tanrikut C. Diet and men's fertility: does diet affect sperm quality? Fertil Steril. 2018;110:570–7. doi: 10.1016/j.fertnstert.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Crichton GE, Howe PR, Buckley JD, Coates AM, Murphy KJ, et al. Long-term dietary intervention trials: critical issues and challenges. Trials. 2012;13:111. doi: 10.1186/1745-6215-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins da Silva SJ. Male infertility and antioxidants: one small step for man, no giant leap for andrology? Reprod Biomed Online. 2019;39:879–83. doi: 10.1016/j.rbmo.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–31. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 23.Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007;47:216–21. doi: 10.1111/j.1479-828X.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 24.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–8. doi: 10.1016/s1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 25.Esteves SC, Roque M, Bradley CK, Garrido N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis. Fertil Steril. 2017;108:456–67e1. doi: 10.1016/j.fertnstert.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Amor H, Zeyad A, Alkhaled Y, Laqqan M, Saad A, et al. Relationship between nuclear DNA fragmentation, mitochondrial DNA damage and standard sperm parameters in spermatozoa of fertile and sub-fertile men before and after freeze-thawing procedure. Andrologia. 2018;50:e12998. doi: 10.1111/and.12998. [DOI] [PubMed] [Google Scholar]
- 27.Herati AS, Zhelyazkova BH, Butler PR, Lamb DJ. Age-related alterations in the genetics and genomics of the male germ line. Fertil Steril. 2017;107:319–23. doi: 10.1016/j.fertnstert.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Feil D, Henshaw RC, Lane M. Day 4 embryo selection is equal to Day 5 using a new embryo scoring system validated in single embryo transfers. Hum Reprod. 2008;23:1505–10. doi: 10.1093/humrep/dem419. [DOI] [PubMed] [Google Scholar]
- 29.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–11. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Ribas-Maynou J, Benet J. Single and double strand sperm DNA damage: different reproductive effects on male fertility. Genes (Basel) 2019;10:105. doi: 10.3390/genes10020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14:418–21. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- 32.Irez T, Sahmay S, Ocal P, Goymen A, Senol H, et al. Investigation of the association between the outcomes of sperm chromatin condensation and decondensation tests, and assisted reproduction techniques. Andrologia. 2015;47:438–47. doi: 10.1111/and.12286. [DOI] [PubMed] [Google Scholar]
- 33.Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: a possible cause of male infertility? Andrologia. 2019;51:e13162. doi: 10.1111/and.13162. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization; 2010. [Google Scholar]
- 35.Steiner AZ, Hansen KR, Barnhart KT, Cedars MI, Legro RS, et al. The effect of antioxidants on male factor infertility: the males, antioxidants, and infertility (MOXI) randomized clinical trial. Fertil Steril. 2020;113:552–60. doi: 10.1016/j.fertnstert.2019.11.008. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond) 2006;30:400–18. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 37.Vorilhon S, Brugnon F, Kocer A, Dollet S, Bourgne C, et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum Reprod. 2018;33:553–62. doi: 10.1093/humrep/dey038. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, et al. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–10. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 39.O'Flaherty C. Orchestrating the antioxidant defenses in the epididymis. Andrology. 2019;7:662–8. doi: 10.1111/andr.12630. [DOI] [PubMed] [Google Scholar]
- 40.McPherson NO, Shehadeh H, Fullston T, Zander-Fox DL, Lane M. Dietary micronutrient supplementation for 12 days in obese male mice restores sperm oxidative stress. Nutrients. 2019;11:2196. doi: 10.3390/nu11092196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30:473–83. doi: 10.1093/humrep/deu303. [DOI] [PubMed] [Google Scholar]
- 42.Stern JE, Brown MB, Wantman E, Kalra SK, Luke B. Live birth rates and birth outcomes by diagnosis using linked cycles from the SART CORS database. J Assist Reprod Genet. 2013;30:1445–50. doi: 10.1007/s10815-013-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimstad FW, Nangia AK, Luke B, Stern JE, Mak W. Use of ICSI in IVF cycles in women with tubal ligation does not improve pregnancy or live birth rates. Hum Reprod. 2016;31:2750–5. doi: 10.1093/humrep/dew247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016;106:566–73. doi: 10.1016/j.fertnstert.2016.05.013. e10. [DOI] [PubMed] [Google Scholar]


