Abstract
Background
Tocilizumab, an IL-6 receptor antagonist, can be used to treat cytokine release syndrome (CRS), with observed improvements in a coronavirus disease 2019 (COVID-19) case series.
Research Question
The goal of this study was to determine if tocilizumab benefits patients hospitalized with COVID-19.
Study Design and Methods
This observational study of consecutive COVID-19 patients hospitalized between March 10, 2020, and March 31, 2020, and followed up through April 21, 2020, was conducted by chart review. Patients were treated with tocilizumab using an algorithm that targeted CRS. Survival and mechanical ventilation (MV) outcomes were reported for 14 days and stratified according to disease severity designated at admission (severe, ≥ 3 L supplemental oxygen to maintain oxygen saturation > 93%). For tocilizumab-treated patients, pre/post analyses of clinical response, biomarkers, and safety outcomes were assessed. Post hoc survival analyses were conducted for race/ethnicity.
Results
Among the 239 patients, median age was 64 years; 36% and 19% were black and Hispanic, respectively. Hospital census increased exponentially, yet MV census did not. Severe disease was associated with lower survival (78% vs 93%; P < .001), greater proportion requiring MV (44% vs 5%; P < .001), and longer median MV days (5.5 vs 1.0; P = .003). Tocilizumab-treated patients (n = 153 [64%]) comprised 90% of those with severe disease; 44% of patients with nonsevere disease received tocilizumab for evolving CRS. Tocilizumab-treated patients with severe disease had higher admission levels of high-sensitivity C-reactive protein (120 vs 71 mg/L; P < .001) and received tocilizumab sooner (2 vs 3 days; P < .001), but their survival was similar to that of patients with nonsevere disease (83% vs 91%; P = .11). For tocilizumab-treated patients requiring MV, survival was 75% (95% CI, 64-89). Following tocilizumab treatment, few adverse events occurred, and oxygenation and inflammatory biomarkers (eg, high-sensitivity C-reactive protein, IL-6) improved; however, D-dimer and soluble IL-2 receptor (also termed CD25) levels increased significantly. Survival in black and Hispanic patients, after controlling for age, was significantly higher than in white patients (log-rank test, P = .002).
Interpretation
A treatment algorithm that included tocilizumab to target CRS may influence MV and survival outcomes. In tocilizumab-treated patients, oxygenation and inflammatory biomarkers improved, with higher than expected survival. Randomized trials must confirm these findings.
Key Words: COVID-19, cytokine release syndrome, disease severity, mechanical ventilation, survival, tocilizumab
Abbreviations: COVID-19, coronavirus disease 2019; CRS, cytokine release syndrome; hs-CRP, high-sensitivity C-reactive protein; IL-6R, IL-6 receptor; MV, mechanical ventilation; sIL2R, soluble IL-2 receptor; Spo2, oxygen saturation
In the absence of evidence-based treatments in the midst of a volatile coronavirus disease 2019 (COVID-19) pandemic, antiviral and antiinflammatory treatments are often offered to patients in real-world settings,1 despite recommendations by international agencies to reserve such treatments to randomized trials. Where real-world settings aim to not overwhelm mechanical ventilation (MV) resources and improve survival, they often do so by using promising, although unproven, treatments.
COVID-19 disease severity is hypothesized to result from cytokine release syndrome (CRS), marked by elevations in C-reactive protein,2 which may represent a key pathophysiological process that contributes to elevated morbidity and mortality.3 Although glucocorticoids showed promise in treating CRS in COVID-19 patients with ARDS, including possible reduced mortality,4 a systematic review in patients with severe acute respiratory syndrome/Middle Eastern respiratory syndrome found that this strategy resulted in lower survival.5 IL-6 seems to play a role in COVID-19-related CRS, and interruptions of the IL-6 pathway may influence outcomes.6, 7, 8, 9, 10 Consequently, IL-6 receptor (IL-6R) antagonists have been repurposed to treat COVID-19, but their exact role in treatment remains unclear. Tocilizumab, a humanized anti-IL-6R monoclonal antibody, is indicated for the treatment of several inflammatory conditions, including CRS caused by chimeric antigen receptor T-cell infusion.11 , 12
Small clinical case series of tocilizumab-treated patients with COVID-19, many who also received glucocorticoids, generally had improvements in oxygenation and inflammatory biomarkers and had high hospital discharge rates.10 , 13 , 14 These findings suggest tocilizumab may be a valuable treatment strategy in hospitalized patients, although little is known about its safety or how it influences MV and survival. The goal of the current study was to determine if tocilizumab benefits patients hospitalized with COVID-19.
Patients and Methods
Consecutive patients admitted with COVID-19 at a single academic hospital between March 10, 2020, and March 31, 2020, in New Haven, Connecticut, underwent standardized chart review; all patients had ≥ 21 days of follow-up data through April 21, 2020. The Yale School of Medicine Institutional Review Board (2000027792) approved the study.
Study Design and Participants
Consecutive adults aged ≥ 18 years with a polymerase chain reaction-confirmed severe acute respiratory syndrome coronavirus 2 infection over the 21-day observation period were identified by using electronic medical records. A subgroup of tocilizumab-treated patients underwent additional pre/post assessments.
With no controlled trials to guide treatment, a multidisciplinary team reviewed available literature to construct a COVID-19 algorithm-based monitoring and treatment strategy (e-Appendix 1). Because Connecticut has the fourth highest COVID-19 density per 100,000 US population, concerns about limited MV and high mortality guided the inclusion of tocilizumab, a nonproven treatment for COVID-19, into the treatment algorithm. It was selected for its scientific pathophysiologic plausibility and potential utility in treating CRS (including in COVID-19 patients), increased morbidity and mortality using glucocorticoids in patients with severe acute respiratory syndrome/Middle Eastern respiratory syndrome,5 and a favorable safety profile in non-COVID-19 patients.
The algorithm initially recommended tocilizumab for admitted patients who met criteria for severe disease, defined as receiving ≥ 3 L of supplemental oxygen to maintain oxygen saturation (Spo 2) > 93%; patients with critical disease (ie, requiring MV) were included in this group. As treatment experience evolved, clinicians increasingly prescribed tocilizumab to treat nonsevere patients with evolving CRS, manifested by increasing high-sensitivity C-reactive protein (hs-CRP) levels and oxygen requirements. Other recommendations for antiviral agents, hydroxychloroquine, and frequency of monitoring were also provided (e-Appendix 2). Patients admitted with severe disease could receive tocilizumab immediately; patients with nonsevere disease could receive it later if CRS evolved. Ultimately, treatment decisions were made by the provider based on clinical judgment.
Tocilizumab was administered 8 mg/kg intravenously, not to exceed 800 mg; a second dose could be given if the patient had a markedly elevated BMI.
Data Collection
Structured chart review included time points such as symptom onset, hospitalization, MV, discharge, and death. Death was assessed either as occurring during hospitalization or following discharge and included a 21-day observation period.
Definitions
We included parameters recorded either upon admission, or for repeated measures, as once or twice daily as listed in e-Appendix 3. For analysis purposes, disease severity was designated at admission, recognizing that some patients with nonsevere disease would progress. Laboratory toxicity was scored according to guidelines set forth by the US Food and Drug Administration, ranging from 0 (no toxicity) to 4 (life-threatening). Spo 2 was determined as the highest value measured for a 24-h period, irrespective of fluctuations. A 13-point scale was used to examine changes in oxygenation status over 14 days (e-Appendix 4) following tocilizumab administration, reported as either worse (higher oxygen requirement) or improved (no change or improved). For the pre- and post-tocilizumab outcomes, all pretreatment values were those immediately prior to tocilizumab administration. If alive and hospitalized, patients’ posttreatment biomarkers and safety data were collected over 14 days; violin plots were deployed to show changes in the outcomes over the 14 days.
Statistical Analysis
We hypothesized that patients treated for CRS, irrespective of disease severity at the time of admission, would have improved outcomes relative to other case series and that tocilizumab-treated patients would have survival outcomes more like patients with less severe disease. The primary outcome was 14-day survival. Secondary outcomes included MV days and post-tocilizumab CRS response. Prespecified subgroup analyses were for those who received tocilizumab and for those who required MV. Because there are no data to guide response to tocilizumab use in COVID-19 patients, pre/post assessments of oxygenation status, biomarkers, and adverse consequences were made in tocilizumab-treated patients. Last, because of new reports of increased mortality in black and Hispanic subjects,15 a post hoc age-adjusted survival analysis of race/ethnicity was conducted.
We reported the mean and SD for nonskewed data and the median and interquartile range for skewed data. Overall survival was estimated by using the Kaplan-Meier estimator with 95% Greenwood CIs. The pre/post tocilizumab changes were examined by using either the McNemar test or the Wilcoxon signed-rank test for categorical and continuous variables, respectively. For severe and nonsevere subgroups, difference between these two groups used either χ2 or Wilcoxon rank sum testing for categorical and continuous variables. The log-rank test was used for survival data. No comparisons were made between patients treated and not treated with tocilizumab due to the nonrandomized study design.
Sample size justification used precision analysis in which computer simulation was employed to estimate the half-width of the 95% Greenwood CIs for survival data. Assumptions for the survival curve include exponential distribution with ≈10% attrition within 30 days. We simulated 2,000 times for each condition. With the proposed sample size (ie, 120-180), the half-width of the 95% Greenwood CI is ≤ 10%. A two-sided P value < .05 was considered statistically significant; all analyses were performed by using R 3.6.3 (R Foundation for Statistical Computing).
Results
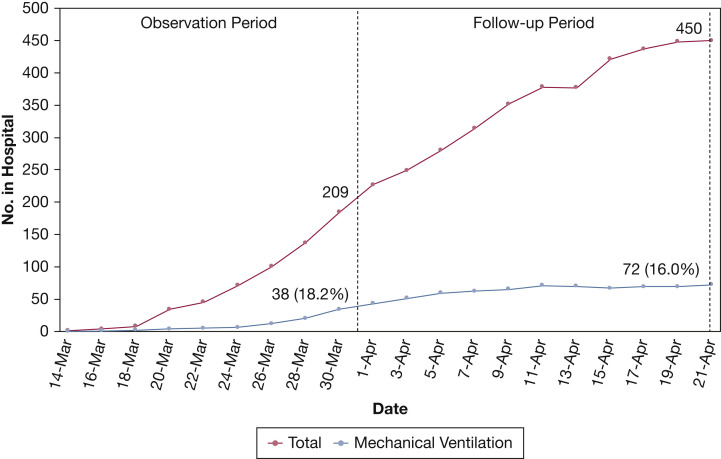
Over the 21-day observation period, 239 consecutive COVID-19 patients were admitted, with 104 (44%) meeting admission criteria for severe disease (Table 1 ). Figure 1 shows the daily census for all COVID-19 patients hospitalized and for those on MV for the 21-day observation period plus the 21-day follow-up period. Total census increased markedly to 209 after three weeks and was 450 three weeks later, after accounting for discharges and deaths. MV census increased early but flattened and never exceeded 18% of hospital census.
Table 1.
Baseline Characteristics of Patients Stratified According to COVID-19 Disease Severity (N = 239)
| Variable | No. | Entire Sample (N = 239) | Nonsevere (n = 135) | Severe (n = 104) | P Value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, median (range), y | 239 | 64 (22-99) | 62 (23-99) | 65 (22-93) | .21b |
| Sex | 238 | … | … | … | .09a |
| Female | 113/238 (47%) | 71/135 (53%) | 42/103 (41%) | … | |
| Male | 238 | 125/238 (53%) | 64/135 (47%) | 61/103 (59%) | … |
| Race/ethnicity | 238 | .82a | |||
| African American | 86/238 (36%) | 48/134 (36%) | 38/104 (37%) | … | |
| Hispanic | 45/238 (19%) | 28/134 (21%) | 17/104 (16%) | … | |
| White | 95/238 (40%) | 51/134 (38%) | 44/104 (42%) | … | |
| Other | 12/238 (5%) | 7/134 (5.2%) | 5/104 (4.8%) | … | |
| Days of symptoms prior to hospitalization, median (IQR) | 233 | 5.0 (2.0, 8.0) | 3.0 (2.0, 6.0) | 6.0 (2.0, 8.0) | < .001b |
| Days hospitalized, median (IQR) | 239 | 10 (7, 20) | 10 (6, 19) | 11 (8, 22) | .06b |
| Hospitalized at day 14 | 239 | 94/239 (39%) | 47/135 (35%) | 47/104 (45%) | .13a |
| Length of follow-up, median (IQR) | 239 | 10 (7, 20) | 10 (6, 19) | 11 (8, 22) | .06b |
| Medical comorbidities | |||||
| Diabetes mellitus | 239 | 91/239 (38%) | 46/135 (34%) | 45/104 (43%) | .19a |
| Uncontrolled diabetes mellitus defined as glycosylated hemoglobin ≥ 8% | 90 | 37/90 (41%) | 18/45 (40%) | 19/45 (42%) | > .99a |
| Immunosuppressed | 239 | 36/239 (15%) | 19/135 (14%) | 17/104 (16%) | .76a |
| Chronic lung disease | 239 | 91/239 (38%) | 48/135 (36%) | 43/104 (41%) | .44a |
| Hypertension | 237 | 142/237 (60%) | 79/133 (59%) | 63/104 (61%) | .96a |
| Chronic heart disease | 239 | 71/239 (30%) | 42/135 (31%) | 29/104 (28%) | .69a |
| Obesity (BMI ≥ 30 kg/m2) | 231 | 112/231 (48%) | 55/129 (43%) | 57/102 (56%) | .06a |
| No. of comorbidities | 239 | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | .36b |
| BMI, kg/m2, median (IQR) | 231 | 30 (25, 35) | 29 (24, 32) | 32 (27, 37) | < .001b |
| BMI, kg/m2 | 231 | … | … | … | .02a |
| < 30 | 119/231 (52%) | 74/129 (57%) | 45/102 (44%) | … | |
| 30.0-34.99 | 61/231 (26%) | 36/129 (28%) | 25/102 (25%) | … | |
| 35.0-39.99 | 30/231 (13%) | 12/129 (9.3%) | 18/102 (18%) | … | |
| ≥ 40 | 21/231 (9%) | 7/129 (5.4%) | 14/102 (14%) | … | |
| Temperature at admission, median (IQR), °C | 38.25 (37.57, 38.80) | 38.10 (37.40, 38.60) | 38.50 (37.80, 39.32) | < .001b | |
| hs-CRP at admission, mg/L, median (IQR) | 233 | 68 (20, 134) | 42 (11, 81) | 110 (64, 182) | < .001b |
| < 100 mg/L | 150/233 (64%) | 104/129 (81%) | 46/104 (44%) | < .001a | |
| ≥ 100 mg/L | 83/233 (36%) | 25/129 (19%) | 58/104 (56%) | ||
| Chest radiograph at baseline | 235 | … | … | … | < .001a |
| Normal | 70/235 (30%) | 53/132 (40%) | 17/103 (17%) | … | |
| Abnormal | 165/235 (70%) | 79/132 (60%) | 86/103 (83%) | … | |
| Hospital treatments, No. (%) | |||||
| Antiviral agents | 237 | 237/237 (100) | 135/135 (100) | 102/102 (100) | > .99a |
| Days from admission to antivirals | 115 | 2.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 2.0) | .06b |
| Hydroxychloroquine | 238 | 201/238 (84%) | 106/134 (79%) | 95/104 (91%) | .02a |
| Glucocorticoids | 239 | 48/239 (20%) | 12/135 (8.9%) | 36/104 (35%) | < .001a |
| Tocilizumab | 239 | 153/239 (64%) | 59/135 (44%) | 94/104 (90%) | < .001a |
| Tocilizumab (second dose) | 239 | 8/239 (3%) | 5/135 (3.7%) | 3/104 (2.9%) | > .99a |
| Days to tocilizumab from onset of symptoms | 135 | 7.0 (4.5, 10.0) | 7.0 (4.0, 9.0) | 6.5 (5.0, 10.0) | .36b |
| Days to tocilizumab from admission | 135 | 2.0 (2.0, 4.0) | 4.0 (2.0, 6.5) | 2.0 (1.0, 3.0) | < .001b |
| Patient outcomes | |||||
| Survivalc | 239 | … | … | … | .001d |
| 14-Day survival (95% CI) | 86% (80%, 91%) | 93% (88%, 99%) | 78% (69%, 87%) | … | |
| Mechanical ventilation | 239 | 53/239 (22%) | 7/135 (5.2%) | 46/104 (44%) | < .001a |
| Days mechanically ventilated | 53 | 4.5 (3.0, 7.5) | 1.0 (0.5, 2.0) | 5.5 (4.0, 7.9) | .003b |
Data are expressed as median (interquartile range [IQR]), No. (%). Survival probability (95% CI). COVID-19 = coronavirus disease 2019; hs-CRP = high-sensitivity C-reactive protein.
Test used, Pearson χ2 test.
Test used, Wilcoxon signed-rank test.
Seven and 21-day survival available in e-Table 1.
Test used, log-rank test; N is the number of nonmissing values.
Figure 1.
Hospital census for all patients with coronavirus disease 2019 and those who were mechanically ventilated from March 10, 2020, through April 21, 2020.
The demographic, clinical presentation, and concomitant treatment data are presented in Table 1, stratified according to disease severity; 64% of all patients received tocilizumab. Patients with severe and nonsevere disease did not differ by age, sex, race/ethnicity, or type or number of comorbidities. Those with severe disease were, however, significantly more likely to have higher admission hs-CRP and IL-6 levels, abnormal chest radiographs, and to receive adjuvant medications such as hydroxychloroquine, glucocorticoids, and tocilizumab. Relative to patients with nonsevere disease, those with severe disease were more likely to receive tocilizumab (90% vs 44%; P < .001) and have a shorter median time from admission to tocilizumab administration (2 vs 3 days; P < .001).
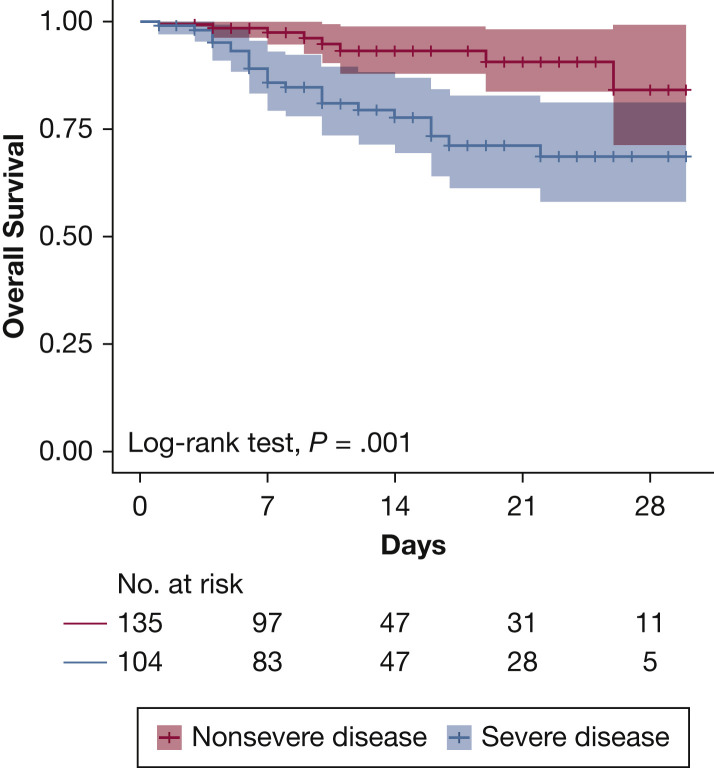
Fourteen-day survival for the 239 patients was 86% and was lower (78% vs 93%; P < .001) for patients with severe disease (Fig 2 ). Overall, 53 patients (22%) required MV, higher among patients with severe disease (44 vs 5%; P < .001). Fourteen-day survival for patients receiving MV was 72%.
Figure 2.
Overall survival according to disease severity.
Among tocilizumab-treated patients, no differences were observed for age, sex, race/ethnicity, or medical comorbidities when stratified according to disease severity (Table 2 ). Patients with severe disease had significantly higher admission hs-CRP levels and abnormal chest radiographs. Although median hs-CRP levels were higher for patients with severe disease upon admission (120 vs 71 mg/L; P = .002), they were similar when tocilizumab was administered (137.75 vs 131.9 mg/L; P = .34). For tocilizumab-treated patients, unlike the overall sample, 14-day survival was 87% and did not differ (83% vs 91%; P = .11) according to disease severity. Survival at 7 and 21 days is shown in e-Table 1. MV was used in 48 (31%) tocilizumab-treated patients; they spent a median of 5.5 days on the ventilator, and their survival was 75%.
Table 2.
Characteristics of COVID-19 Patients Treated With Tocilizumab, Stratified According to Disease Severity (N = 153)
| Variable | No. | Entire Sample (N = 153) | Nonsevere (n = 59) | Severe (n = 94) | P Value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, median (range), y | 153 | 65 (23-92) | 65 (27-88) | 64 (23-92) | .40b |
| Sex | 152 | … | … | … | .37a |
| Female | 64/152 (42%) | 28/59 (47%) | 36/93 (39%) | … | |
| Male | 88/152 (58%) | 31/59 (53%) | 57/93 (61%) | … | |
| Race/ethnicity | 153 | … | … | … | .92a |
| African American | 61/153 (40%) | 25/59 (42%) | 36/94 (38%) | … | |
| Hispanic | 26/153 (17%) | 9/59 (15%) | 17/94 (18%) | … | |
| White | 59/153 (39%) | 22/59 (37%) | 37/94 (39%) | … | |
| Other | 7/153 (4·6%) | 3/59 (5·1%) | 4/94 (4·3%) | … | |
| Days of symptoms prior to hospitalization, median (IQR) | 151 | 5.0 (2.0, 8.0) | 3.0 (1.0, 6.0) | 6.0 (3.0, 8.0) | < .001b |
| Days hospitalized, median (IQR) | 153 | 12 (8,22) | 11 (9, 22) | 12 (8, 22) | .8b |
| Hospitalized at day 14 | 153 | 73/153 (48%) | 27/59 (46%) | 46/94 (49%) | .83a |
| Length of follow-up, median (IQR) | 153 | 12 (8,22) | 11 (9,22) | 12 (8,22) | .8b |
| Medical comorbidities | |||||
| Diabetes mellitus | 153 | 72/153 (47%) | 31/59 (53%) | 41/94 (44%) | .36a |
| Uncontrolled diabetes mellitus defined as glycosylated hemoglobin ≥ 8% | 71 | 29/71 (41%) | 11/30 (37%) | 18/41 (44%) | .71a |
| Immunosuppressed | 153 | 26/153 (17%) | 12/59 (20%) | 14/94 (15%) | .51a |
| Chronic lung disease | 153 | 58/153 (38%) | 20/59 (34%) | 38/94 (40%) | .52a |
| Hypertension | 152 | 97/152 (64%) | 40/58 (69%) | 57/94 (61%) | .39a |
| Chronic heart disease | 153 | 46/153 (30%) | 21/59 (36%) | 25/94 (27%) | .32a |
| Obesity (BMI ≥ 30 kg/m2) | 149 | 83/149 (56%) | 29/57 (51%) | 54/92 (59%) | .44a |
| No. of comorbidities, median (IQR) | 153 | 3·0 (2·0, 4·0) | 3·0 (2·0, 4·0) | 3·0 (2·0, 4·0) | .35b |
| BMI, median (IQR) | 149 | 31 (26, 35) | 30 (25, 32) | 33 (27, 37) | .004b |
| BMI, kg/m2 | 149 | … | … | … | .03a |
| < 30 | 66/149 (44%) | 28/57 (49%) | 38/92 (41%) | … | |
| 30.0-34.99 | 44/149 (30%) | 21/57 (37%) | 23/92 (25%) | … | |
| 35.0-39.99 | 24/149 (16%) | 7/57 (12%) | 17/92 (18%) | … | |
| ≥ 40 | 15/149 (10%) | 1/57 (1·8%) | 14/92 (15%) | … | |
| Clinical indicators | |||||
| Temperature at baseline, median (IQR), °C | 153 | 38.4 (37.8, 39.2) | 38.3 (37.69, 38.8) | 38.6 (37.82, 39.4) | .04b |
| hs-CRP at admission, median (IQR), mg/L | 153 | 97 (59, 156) | 71 (35, 126) | 120 (69, 191) | .002b |
| hs-CRP at admission, stratified, mg/L | 153 | … | … | … | |
| < 100 | 77/153 (50%) | 38/59 (64%) | 39/94 (41%) | .009a | |
| ≥ 100 | 76/153 (50%) | 21/59 (36%) | 55/94 (59%) | … | |
| hs-CRP at time of tocilizumab administration, median (IQR), mg/L | 153 | 135 (92, 194) | 133 (85, 162) | 137 (102, 218) | .18b |
| Chest radiograph at baseline | 152 | … | … | … | .02a |
| Normal | 39/152 (26%) | 22/59 (37%) | 17/93 (18%) | … | |
| Abnormal | 113/152 (74%) | 37/59 (63%) | 76/93 (82%) | … | |
| Hospital treatments | |||||
| Antiviral agents | 151 | 151/151 (100%) | 59/59 (100%) | 92/92 (100%) | > .99a |
| Days to antiviral receipt from admission, median (IQR) | 91 | 2.0 (1.0, 2.0) | 2.0 (1.0, 2.5) | 2.0 (1.0, 2.0) | .26b |
| Hydroxychloroquine | 153 | 141/153 (92%) | 53/59 (90%) | 88/94 (94%) | .54a |
| Glucocorticoids | 153 | 47/153 (31%) | 11/59 (19%) | 36/94 (38%) | .02a |
| Days to tocilizumab from symptom onset, median (IQR) | 135 | 7.0 (4.5, 10.0) | 7.0 (4.0, 9.0) | 6·5 (5.0, 10.0) | .36b |
| Days to tocilizumab from admission, median (IQR) | 135 | 2.0 (2.0, 4.0) | 3.0 (2.0, 5.5) | 2·0 (1.0, 3.0) | < .001b |
| Patient outcomes | |||||
| Survivalc | 153 | … | … | … | .10d |
| 14-day survival (95% CI) | 86% (80-93) | 91% (83-100) | 83% (75-92) | … | |
| Mechanical ventilation | 153 | 48/153 (31%) | 6/59 (10%) | 42/94 (45%) | < .001a |
| Survivalc in those mechanically ventilated | 48 | … | … | … | .8d |
| 14-day survival (95% CI) | 75% (64-89) | 83% (58-100) | 74% (61-89) | … | |
| Days mechanically ventilated, median (IQR) | 48 | 5.5 (3.9, 7.6) | 1.0 (0.6, 2.5) | 5.8 (4.0, 8.0) | .006b |
| Status at last follow-up | 153 | … | … | … | .02a |
| Deaths | 23/153 (15%) | 5/59 (8.5) | 18/94 (19) | … | |
| Discharged | 96/153 (63%) | 42/59 (71) | 54/94 (57) | … | |
| In the hospital, not on mechanical ventilation | 26/153 (17%) | 12/59 (20) | 14/94 (15) | … | |
| In the hospital, on mechanical ventilation | 8/153 (5.2%) | 0/59 (0) | 8/94 (8.5) | … |
Data are median (interquartile range, IQR), No. (%). Survival probability (95% CI). See Table 1 legend for expansion of abbreviations.
Test used, Pearson χ2 test.
Test used, Wilcoxon signed-rank test.
Seven- and 21-day survival available in e-Table 1.
Test used, log-rank test; No. is the number of nonmissing values.
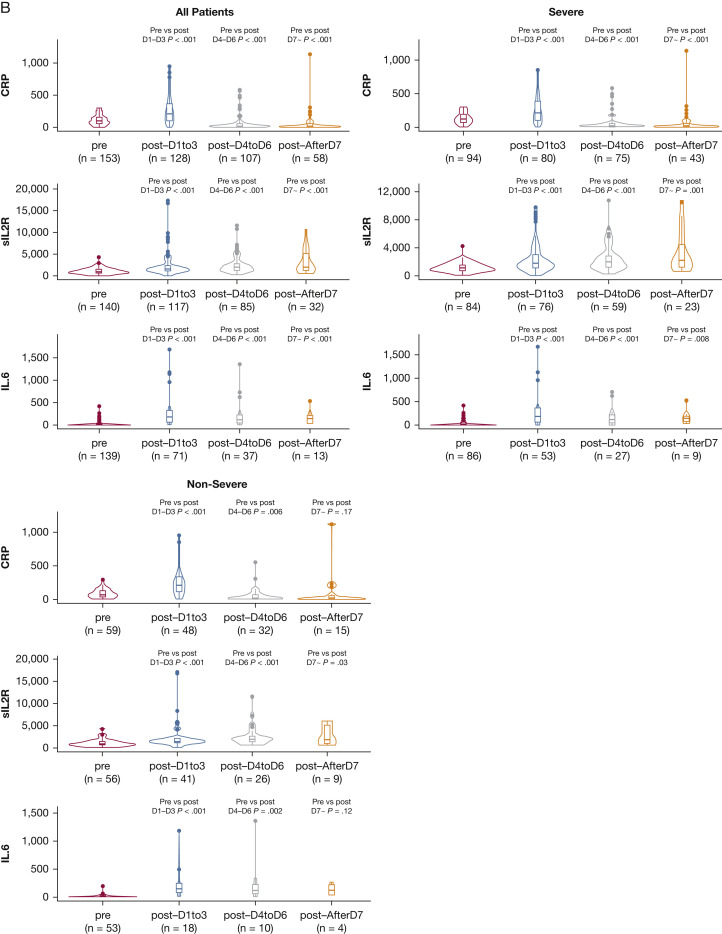
Figure 3 shows the 14-day trajectory following tocilizumab administration. Oxygenation improved over 14 days but less so over the first 3 to 4 days. Temperature decreased immediately, but hs-CRP levels decreased toward normal over 14 days. Soluble IL-2 receptor (sIL2R; also termed CD25) and D-dimer levels increased significantly. Although pretreatment D-dimer levels were not statistically significantly different between these two groups, levels in both groups increased significantly after treatment but more so in the severe disease group (e-Table 2).
Figure 3.
Changes in oxygenation and inflammatory biomarkers over 14 days following tocilizumab administration. A, Oxygenation status relative to pre-tocilizumab administration over 14 days (n = 153). Throughout the 14 days following tocilizumab administration, the proportion whose oxygenation levels improved or remained the same initially declined but more so in patients with severe disease, followed by steady improvements in both groups. B, Inflammatory biomarker status relative to pre-tocilizumab administration over 14 days. High-sensitivity CRP and IL-6 levels significantly decreased over 14 days, initially with an increase in IL-6 levels during the first 72 hours after administration. sIL2R levels, however, significantly increased over time for patients with both severe and nonsevere disease. D-dimer levels (not depicted here) significantly increased for nonsevere disease (0.67; 95% CI, 0.31 to 1.3; P < .001) and severe disease (1.09; 95% CI, 0.62 to 1.9; P < .001). Temperature also significantly decreased a similar amount in both nonsevere and severe cases (–1.35; 95% CI, –1.65 to –1; P < .001). CRP = C-reactive protein; sIL2R = soluble interleukin-2 receptor.
Few adverse consequences were observed following tocilizumab treatment (e-Table 3). Six patients had posttreatment neutropenia; two had neutropenia prior to treatment. Four patients experienced bacteremia; none had neutropenia, and bacteremia occurred > 10 days following administration of tocilizumab. Although patients’ transaminase levels generally increased in grade after tocilizumab treatment, no patient experienced grade 4 hepatotoxicity. No tocilizumab infusion reactions were observed.
A post hoc analysis of tocilizumab-treated patients showed that survival was significantly lower in white subjects relative to both black and Hispanic subjects after controlling for age (Fig 4 ), with no significant differences between black and Hispanic subjects.
Figure 4.
Survival stratified according to race/ethnicity.
Discussion
To the best of our knowledge, this study is the largest clinical series of hospitalized patients with COVID-19 disease who were administered tocilizumab, guided by a hospital-based treatment algorithm that initially focused on patients with severe disease but evolved to address CRS. Although the study design is unable to establish causality, several key findings were observed. Despite an asymptotic surge in the number of patients admitted with COVID-19, a parallel increase in MV did not occur. This finding extended beyond the observation period and into the 21-day follow-up period when the hospital hit its peak census. Several modelers have projected the expected need for MV as hospitals experience an epidemic surge and lead to parallel increases in hospital and MV occupancy, in the absence of an intervention.16 , 17
The two early case series from China reporting tocilizumab outcomes included only patients with severe disease, many on MV. These observations informed the initial treatment algorithm, but treating CRS increasingly became the focus of therapy and was based on increasing oxygenation requirements and bioinflammatory markers, especially increasing hs-CRP levels. This finding is affirmed by the increase in median hs-CRP in all patients with nonsevere disease (70 to > 130 mg/L) just before tocilizumab was administered. The elevated hs-CRP and the longer time to tocilizumab administration in the nonsevere group support disease progression while in the hospital. Our data confirm findings from New York City in which 31% of patients who initially presented with nonsevere disease progressed to MV,18 suggesting the need for continual monitoring of inflammatory biomarkers of CRS.
Fourteen-day survival seems better than observed elsewhere and extends across the many groups of patients hospitalized, including those who underwent MV. Our survival estimates are conservative relative to most clinical series that incur more time bias. We addressed time bias by ensuring 21 days of follow-up, and we reduced reporting bias by including patients discharged to skilled nursing facilities and home. Mortality from COVID-19 has been highly variable (10%-45%), with most estimates about 30%. Geographic differences may also influence mortality, with some of the highest mortality reported in Italy, 2.2-fold higher than in China,13 and the United States being somewhere in between. Some of these variations may be related to age differences, health-care delivery, and a higher prevalence of medical comorbidities (eg, elevated prevalence of obesity in the United States).
A nationwide survey of patients from 575 hospitals in China observed an overall mortality of 24.5% in patients with COVID-19, which reached as high as 31.5% within Hubei Province.14 Reports from across Europe are similar,19 with mortality of 31% in hospitalized patients in Spain15 and 26% from Italy20; none included postdischarge assessments.
In hospitalized patients in the United States, mortality in New York City was 10.2% (the proportion needing MV was 33%, which is similar to our finding). This low mortality may, in part, be explained by both time and reporting biases because no mortality was reported for the 66% of discharged patients (ie, treated as survival), and 24% did not record any disposition data. Unlike our findings, however, only 18% of patients needing MV were extubated.18 In a 12-hospital assessment of hospitalized patients in New York City, within-hospital mortality was 21% (without postdischarge assessment), but unlike our study, only 12% received MV, suggesting a less severe patient population. Among their MV subgroup, 25% died and 72% were still receiving MV, a poor outcome.21 Survival in our sample was 72%.
Elsewhere, mortality in patients needing MV ranges from 50% to 90%.20, 21, 22, 23, 24 In the current tocilizumab-treated patients, including those needing MV, oxygenation improved overall within 14 days, similar to findings from Italy.14 Unlike the observations in Italy, however, our more granular assessment of oxygenation shows that improvements may not increase as rapidly until after 3 to 4 days. These findings tracked with hs-CRP and IL-6 levels (potentially reflecting an interruption in the CRS-related inflammatory process postulated to occur in COVID-19 patients)2 and the parallel finding of a median of 5.5 MV days, much lower than reported elsewhere.20 , 23 , 25 This finding suggests that, especially for those with severe disease who received tocilizumab as they were rapidly progressing toward needing MV, the incurred pulmonary inflammation required several days to improve.
Our observation that D-dimer levels increased in tocilizumab-treated patients, unlike the experience in Italy,17 is concerning and suggests that IL-6R antagonism may interrupt only part of the hyperinflammatory response of CRS. Thromboembolic events are increasingly reported in patients with COVID-19 disease, even in younger people without CRS, suggesting that some of these patients may become predisposed to a hypercoagulable state preceded by D-dimer level elevations.26 Unlike in Italy,17 we observed an expected early IL-6 level elevation but that was followed by rapid normalization. The observed increase in sIL2R is intriguing and may, in part, explain the incomplete immunomodulatory response from IL-6R blockade alone, leaving other inflammatory pathways operating in a subset of patients whose disease continued to progress. This observation may offer some clues to the mechanism of severe disease. High sIL2R levels are found in hemophagocytic syndromes, lymphoma, autoimmune lymphoproliferative syndrome, and other diseases associated with T-cell activation or dysregulation.27 Although this study cannot disentangle the added benefit of steroids after tocilizumab treatment in patients who clinically progressed possibly due to ongoing inflammation, there could be a role for other immunomodulators. Future studies of patients treated with IL-6R antagonists, including larger case series and randomized trials, should examine the extent to which sIL2R levels potentiate adverse outcomes.
Similar to other series, patients with severe and nonsevere disease differed markedly in survival, with large numbers of patients with severe disease progressing to MV. Survival in these patients, however, was still 78% (which included one-third requiring MV). Of interest was the observation that survival for tocilizumab-treated patients with severe and nonsevere disease did not differ (83% vs 91%; P = .10), perhaps suggesting that the treatment of CRS, rather than disease severity at admission, may play a role in survival and does so based on the pattern of changes in biomarkers and oxygenation following tocilizumab administration. The finding of similar survival regardless of admission disease severity for those treated with tocilizumab suggests that tocilizumab equalizes treatment outcomes (ie, a return to an improved status) by targeting CRS. This theory is further supported by high survival in patients requiring MV.
There were no unanticipated adverse consequences. Among tocilizumab-treated patients, there were few (6%) cases of neutropenia. In the absence of tocilizumab, bacteremia was reported in 8% of hospitalized patients in China28 and 6% in New York City.18 No tocilizumab-treated patients experienced grade 4 hepatotoxicity, but a number of patients experienced worsening transaminases following treatment. It is not clear whether this increase is due to COVID-19 disease progression (either viral or CRS-related processes),28 antiviral medications, or from tocilizumab itself. Data from tocilizumab-treated patients with other conditions have substantially lower levels of transaminase elevations, perhaps suggesting it may be part of the COVID-19 disease itself or a synergistic process between tocilizumab, concomitant medications, and COVID-19 infection. Prospective randomized trials should be able to disentangle the contributions of tocilizumab toward hepatotoxicity.
Last, an important observation from this tocilizumab-treated series is that after controlling for age, black and Hispanic patients had higher survival relative to white patients, unlike data reported in untreated patients in the United States15 , 29 and the United Kingdom.24 One potential explanation is that the standardized treatment algorithm used to guide treatment here was blind to race/ethnicity.
This study provides important insights to guide the potential role of tocilizumab in altering the course of hospitalization (specifically MV) and death when focusing on CRS. Although these outcomes may have been influenced by treatment with other medications (eg, hydroxychloroquine, antiviral agents, glucocorticoids) and not assessed here, randomized trials are needed to provide the necessary data to create an evidence basis for treating patients with COVID-19.
Conclusions
In the absence of randomized trials, findings from the largest sample to date of COVID-19 patients, who were treated using a standardized algorithm that included off-label tocilizumab to treat CRS rather than disease severity, suggest that MV and survival trajectories may be altered when trying to avoid resource-limited services. Tocilizumab targets a specific pathway in CRS, but other immunomodulators, including glucocorticoids, should be assessed for additional benefit in larger studies. Although a large proportion of patients in this series received tocilizumab early in their hospitalization, more precise identification of predictors of disease progression may help establish the ideal time for tocilizumab treatment. Consequently, this early report has generated interesting insights for future randomized trials. Until such trials are completed, however, use of tocilizumab may result in lower-than-expected mortality in a subgroup of patients with evidence of CRS.
Acknowledgments
Author contributions: C. C. P., J. E. T., C. D. C., and M. M. had the idea for and designed the study; they had full access to all data in the study and take responsibility for the integrity of the data analysis. C. C. P. and R. A. wrote the first full draft of the report. M. M., M. M. A., Y. S., G. C., A. I. L., P. M. L., N. K., R. B., J. E. T., and C. D. C. contributed to critical revision of the report. Y.S. and S. E. C. contributed to the statistical analysis. All authors contributed to data acquisition, data analysis, or data interpretation, and they all reviewed and approved the final version.
Financial/nonfinancial disclosures: None declared.
Data sharing: The data that support the findings of this study are available from the corresponding author on reasonable request. Participant data without names and identifiers will be made available after approval from the corresponding author. After publication of study findings, the data will be available for others to request. The research team will provide an e-mail address for communication once the data are approved to be shared with others. The proposal with detailed description of the study objectives and statistical analysis plan will be needed for evaluation of the reasonability to request for our data. The corresponding author will make a decision based on these materials. Additional materials may also be required during the process.
Other contributions: The authors acknowledge all the health care workers at Yale-New Haven Hospital. They also appreciate the Smilow Cancer Center and Phase I Clinical trials team.
Additional information: The e-Appendixes and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Price, Altice, and Shyr contributed equally to this manuscript as first author.
Drs Topal, Dela Cruz, and Malinis contributed equally to this manuscript as senior author.
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 2.Wang G., Wu C., Zhang Q., et al. C reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis. 2020;7(5):ofaa153. doi: 10.1093/ofid/ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 4.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michot J.M., Albiges L., Chaput N., et al. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020 doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Han M., Li T., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro G., Taroumian S., Barroso N., Duan L., Furst D. Tocilizumab in rheumatoid arthritis: a meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum. 2014;43(4):458–469. doi: 10.1016/j.semarthrit.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen H., Wang F., Zhang P., et al. Management of cytokine release syndrome related to CAR-T cell therapy. Front Med. 2019;13(5):610–617. doi: 10.1007/s11684-019-0714-8. [DOI] [PubMed] [Google Scholar]
- 13.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sciascia S., Aprà F., Baffa A., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020 [PubMed] [Google Scholar]
- 15.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissman G.E., Crane-Droesch A., Chivers C., et al. Locally informed simulation to predict hospital capacity needs during the COVID-19 pandemic. Ann Intern Med. 2020 doi: 10.7326/L20-1062. [DOI] [PubMed] [Google Scholar]
- 17.Wells C.R., Fitzpatrick M.C., Sah P., et al. Projecting the demand for ventilators at the peak of the COVID-19 outbreak in the USA. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients With COVID-19 in Washington state. JAMA. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Intensive Care National Audit & Research Center (ICNARC) Report on 2249 patients critically ill with COVID-19. 2020. https://www.icnarc.org/About/Latest-News/2020/04/04/Report-On-2249-Patients-Critically-Ill-With-Covid-19
- 25.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin L.A., Nelson D.L. The soluble interleukin-2 receptor: biology, function, and clinical application. Ann Intern Med. 1990;113(8):619–627. doi: 10.7326/0003-4819-113-8-619. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.APM Research Lab The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. 2020. https://www.apmresearchlab.org/covid/deaths-by-race
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.