Abstract
Background
Abdominal pain and opioid analgesic use are common in Crohn’s disease (CD).
Aims
We sought to identify factors associated with abdominal pain in CD and evaluate the impact of opioid analgesics on pain and quality-of-life scores in this setting.
Methods
We performed a longitudinal cohort study using a prospective, consented IBD natural history registry from a single academic center between 2009 and 2013. Consecutive CD patients were followed for at least 1 year after an index visit. Data were abstracted regarding pain experience (from validated surveys), inflammatory activity (using endoscopic/histologic findings), laboratory studies, coexistent psychiatric disorders, medical therapy, opioid analgesic, and tobacco use.
Results
Of 542 CD patients (56.6% women), 232 (42.8%) described abdominal pain. Individuals with pain were more likely to undergo surgery and were more frequently prescribed analgesics and/or antidepressants/anxiolytics. Elevated ESR (OR 1.79; 95%CI 1.11–2.87), coexistent anxiety/depression (OR 1.87; 95%CI 1.13–3.09), smoking (OR 2.08; 95%CI 1.27–3.40), and opioid use (OR 2.46; 95%CI 1.33–4.57) were independently associated with abdominal pain. Eighty patients (14.8%) were prescribed opioids, while 31 began taking them at or after the index visit. Patients started on opioids demonstrated no improvement in abdominal pain or quality-of-life scores on follow-up compared to patients not taking opioids.
Conclusions
Abdominal pain is common in CD and is associated with significant opioid analgesic utilization and increased incidence of anxiety/depression, smoking, and elevated inflammatory markers. Importantly, opioid use in CD was not associated with improvement in pain or quality-of-life scores. These findings reinforce the limitations of currently available analgesics in IBD and support exploration of alternative therapies.
Keywords: Abdominal pain, Crohn’s disease, Inflammatory bowel disease, Opioid, Opiate, Analgesic
Introduction
Abdominal pain is common in inflammatory bowel disease (IBD, Crohn’s disease (CD), ulcerative colitis (UC)), affecting a third or more of this population [1–5] and leads to a significant burden in quality of life [6, 7], higher rates of disability [8, 9], lost work hours, and increased healthcare resource utilization [10, 11]. Considering the high prevalence and impact of abdominal pain in IBD, and the limited number of safe analgesic options in this setting, clinicians, and patients often face challenging questions about appropriate pain management. Addressing the inflammatory process itself can be very effective in some patients but is not uniformly helpful [4]. An increasing body of evidence supports a relationship between chronic abdominal pain in IBD and coexistent psychiatric conditions, including anxiety and depression [12]. Previous studies have demonstrated a significant increase in the prevalence of psychiatric disorders in IBD [12, 13] and a distinct improvement in disease management when coincident states of anxiety or depression are appropriately treated [14–16]. Evaluating a large ulcerative colitis (UC) cohort, we demonstrated that disease activity correlated with pain scores but more frequent abdominal pain was also independently associated with mood disorders as well as a younger age and female gender [5].
Regardless of the cause of the pain, concerns about side effects or a possible impact on the underlying inflammatory processes complicate the use of analgesic agents in the IBD population. Non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with an increased incidence of IBD [17, 18]. Opioid analgesics come with the potential for addiction as well as constipation and many other adverse gastrointestinal side effects, including narcotic bowel syndrome [19–21]. Most importantly, the increased use of prescription opioid analgesics for chronic pain in benign disorders has been associated with rising rates of death due to unintentional drug overdoses [22, 23]. In CD, narcotic use has also been associated with poor outcomes [24, 25], which could be related to the potential adverse effects of these agents or their preferential use in more severe and difficult to control disease. Narcotics have also been associated with increased healthcare resource utilization in this setting [26, 27]. Despite these risks, a significant proportion of IBD patients (i.e., 30% or more) are still prescribed opioid analgesic medications to manage pain and related symptoms associated with their disease [28]. Interestingly, very little is known about the relative influence of opioid analgesics on abdominal pain experience and quality of life in CD, as no prior studies have directly evaluated these factors simultaneously in a longitudinal manner. It is possible that, despite the expectations of providers and patients, opioid analgesics may not even positively affect these factors. Improving our understanding of the impact that opioid analgesics have on individuals with CD is essential in order to inform providers and IBD patients about appropriate management options for those suffering from chronic pain and to help mitigate risks associated with the use of these medications, including death.
We undertook this study to re-evaluate the incidence of abdominal pain in CD and its impact on major clinical outcomes in these patients. We simultaneously evaluated the incidence of opioid analgesic medication use and its influence on abdominal pain and quality of life over time. Additionally, we sought to determine factors associated with opioid analgesic use in CD.
Methods
Patient Cohort
This study was undertaken in compliance with the principles and rules set forth by the United States Federal Policy on the Protection of Human Subjects. We used a prospective IBD natural history registry (approved by the University of Pittsburgh Institutional Review Board (IRB0309054)) to identify individuals with CD who were cared for at a single referral center (The Digestive Disorders Center of the University of Pittsburgh Medical Center). We included patients who were followed for at least 1 year after an index visit (i.e., their first visit) during a five-year period between 2009 and 2013. Of note, all study participants had established diagnosis of CD before the index visit. The electronic medical records of individuals included in this study were reviewed to retrieve all relevant clinical data (see below). Details of this IBD registry have been previously reported [29].
Inclusion Criteria Participants had to meet the following criteria: (1) age equal or greater than 17 years; (2) established diagnosis of CD based on standard clinical criteria incorporating historical, laboratory, endoscopic, and histo-logical evaluation; (3) no coexisting condition which could explain abdominal pain, including pregnancy, trauma, malignancy, infection or non-IBD-associated inflammatory disorder. Exclusion Criteria Indeterminate forms of IBD, microscopic colitis, inflammatory enteritis/colitis not associated with IBD or missing information about pain at the time of index visit.
Data Abstraction
Age, gender, disease location (according to Montreal classification), disease/inflammation severity based upon endoscopic and histologic findings of colonoscopies and/or upper endoscopies performed within 1 year of the associated visit (unless otherwise indicated), C-reactive protein (CRP; mg/dL), sedimentation rate (ESR; mm/h), the presence of strictures based upon review of the medical record including endoscopic, imaging and surgery findings, surgeries related to the underlying IBD, disease duration, disease treatment (steroid, immunomodulator, biologic agent), the presence of a coexisting mood and/or somatoform disorder as listed in the record, the use of prescription opioid analgesics, antidepressant and/or anxiolytic therapy, NSAID use, other pain medication use (including acetaminophen and/or anti-spasmodic), and smoking status were recorded. Pain ratings were based on responses to the fourth question in this questionnaire (“How often over the past 2 weeks have you experienced abdominal pain?”). Patients respond using a frequency-based inverse Likert scale, with 1 representing pain “all of the time” and 7 representing pain “none of the time”. Abdominal pain severity data were also collected on the day of visit using patient responses to the second item from the Harvey–Bradshaw Index (HBI) which included potential responses of 0 (“no abdominal pain”), 1 (“mild”), 2 (“moderate”) and 3 (“severe”). The SIBDQ pain score (hereafter referred to as the “SPS”) significantly correlated in an inverse fashion with the HBI pain score (r = − 0.63; P < 0.001). Due to the larger number of individuals who completed a SIBDQ compared to the HBI and larger scale, the SPS was subsequently used as the value to define the presence and frequency of pain. For the purposes of this study, clinically relevant abdominal pain was operationally defined as a numeric rating of < 5 on the SPS in order to identify patients with moderate to severe symptoms and to exclude those with mild or spurious abdominal pain experiences [30]. The first visit that fell into the study period was used as the “index visit”. A single clinic visit following the index visit was used for the “follow-up” visit. There were at least 12 months between the index visit and follow-up visit. For both the index and follow-up visits, medication use, pain frequency, and SIBDQ scores were obtained. Individuals who were taking opioid analgesics before the index visit and continued to do so to the follow-up visit were not included in any analysis evaluating outcomes associated with the use of these agents.
Statistical Analysis
The primary endpoints were (1) clinically relevant abdominal pain (as defined above) and (2) opioid analgesic use. Secondary endpoints were quality of life (SIBDQ) and negative outcomes as defined by death or need for operative intervention during the time of follow-up. We started by calculating descriptive statistics for the whole study group. We then compared demographic and clinical characteristics for separate groups at the index visit [e.g., (a) patients with or without clinically relevant pain, and (b) taking or not taking opioid analgesic medications] using the unpaired student’s t-test or Mann–Whitney test to compare continuous variables or Chi-square or Fisher’s exact tests for categorical variables. Each of these analyses was performed using the program Prism (GraphPad Software, San Diego, CA). In order to identify potential predictors of clinically relevant abdominal pain, variables with a p value of 0.1 or less were then entered into a logistic regression analysis using Statistical Analysis Software (SAS, Cary, NC). To assess the longitudinal impact of opioid analgesic medications in this setting, we also compared demographic and clinical characteristics in CD patients that did or did not start opioid analgesics at or after the index visit. Finally, we evaluated the impact of opioid analgesics on CD patients over time by evaluating the relative changes in SIBDQ (quality of life) and SPS (abdominal pain) scores between the index and follow-up visits using the Wilcoxon rank-sum test. In order to evaluate for relative influences from other commonly employed medical interventions, we compared these same factors in CD patients that did or did not undergo escalation of IBD-associated medical therapy (defined by initiation of biologic therapy at or after the index visit). Unless indicated otherwise, all data are given as mean values with standard error of the mean. P values < 0.05 were considered statistically significant.
Results
Patient Characteristics
During the five-year study period, 542 patients with CD enrolled in the research registry were seen at least twice over a minimum follow-up time of 12 months. The average interval between the index and last visit was approximately 541 days (Table 1). The cohort was predominantly female (56.6%) with a mean age of 40 years. Most (61.5%) had colonic involvement (L2 or L3). Almost two-thirds (63.5%) of the cohort had undergone at least one surgery for their underlying IBD. Most (56.3%) of the patients received immunomodulator and/or anti-TNF therapy, the latter primarily in the form of infliximab (Table 1). About one-fourth had been diagnosed with a psychiatric disorder (anxiety and/or depression) and approximately 20% took antidepressants (including selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, tetracyclics, buspirone, and/or bupropion) and/or anxiolytics (including benzodiazepines and barbiturates). Of the patients who had undergone a colonoscopy before or after the index visit (n = 288), approximately one third (34%) had at least moderate to severe inflammation. The mean SIBDQ score obtained during the index visit was 49.2 (Table 1). Of note, no study participant died during the study period.
Table 1.
Patient demographic and clinical characteristics
| Variable | Cohort (n = 542) | Infrequent to No Pain (SPS > 4) (n = 310) | Pain (SPS < 5) (n = 232) | p |
|---|---|---|---|---|
| Gender (% females) | 56.6% | 53.5% | 60.3% | 0.12 |
| Follow-up (days ± SEM) | 540.7 ± 7.4 | 547.6 ± 9.3 | 531.6 ± 11.9 | 0.28 |
| Age (years ± SEM) | 40.4 ± 0.5 | 40.4 ± 0.8 | 40.4 ± 0.9 | 0.96 |
| Disease duration (years ± SEM) | 18.2 ± 0.5 | 18.1 ± 0.6 | 18.3 ± 0.7 | 0.83 |
| Disease localization | 0.68 | |||
| L1(ileal) | 207 (38.2%) | 84 | 123 | |
| L2(colonic) | 29 | 44 | ||
| L3(ileocolonic) | 73 (13.5%) | 119 | 143 | |
| 262 (48.3%) | ||||
| Stricturing disease | 97 (17.9%) | 44 | 53 | < 0.01 |
| Prior bowel surgery | 344 (63.5%) | 176 | 168 | < 0.001 |
| Surgery during the follow-up period | 67 (12.4%) | 36 | 31 | 0.51 |
| Active smoker | 125 (23.1%) | 70 | 55 | < 0.0001 |
| Colonoscopy | 288 (53.1%) | 140 | 148 | 0.46 |
| Inflammation > 1 | 98 (18.1%) | 51 | 47 | |
| Histology | 265 (48.9%) | 176 | 89 | 0.49 |
| Inflammation > 1 | 64 (11.8%) | 36 | 28 | |
| ESR (mm/h ± SEM) | 20.3 ± 0.8 | 17.1 ± 0.9 | 24.4 ± 1.4 | < 0.0001 |
| CRP (mg/dL ± SEM) | 1.25 ± 0.1 | 0.8 ± 0.1 | 1.8 ± 0.3 | < 0.001 |
| Medical therapy | ||||
| Corticosteroid | 100 (18.5%) | 44 | 56 | < 0.01 |
| Immunomodulator | 147 (27.1%) | 94 | 53 | 0.06 |
| Anti-TNF agents | 191 (35.2%) | 117 | 74 | 0.17 |
| Psychiatric disorder | 159 (29.3%) | 69 | 90 | < 0.0001 |
| Antidepressant/anxiolytic use | 128 (23.6%) | 59 | 69 | < 0.01 |
| Opiate use | 80 (14.8%) | 22 | 58 | < 0.0001 |
| NSAID use | 48 (8.9%) | 29 | 19 | 0.76 |
| Other pain medication use | 51 (9.4%) | 20 | 31 | < 0.01 |
| Mean SIBDQ index (± SEM) | 49.2 ± 0.6 | 57.0 ± 0.5 | 38.9 ± 0.7 | < 0.0001 |
| Mean SIBDQ follow-up (± SEM) | 51.5 ± 0.4 | 56.4 ± 0.6 | 45.0 ± 0.8 | < 0.0001 |
Values listed in bold within the table are considered to represent statistically significant differences (i.e., p < 0.05)
In this analysis, “Psychiatric disorder” included clinical diagnoses of major depressive disorder or generalized anxiety disorder. “Antidepressant/anxiolytic use” included patient use of selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, tetracyclics, buspirone, bupropion, benzodiazepines, and/or bupropion. “Other pain medication use” included acetaminophen and antispasmodics. SEM standard error measurement, SIBDQ short inflammatory bowel disease questionnaire, SPS SIBDQ pain score
Incidence and Association with Abdominal Pain
Based on our operational definition, 232 patients (42.8%) described clinically relevant abdominal pain. Gender distribution, age, and time to follow-up were comparable for the cohorts with and without pain (Table 1). As expected, due to the impact of pain on quality of life and the fact that the pain scale is part of the SIBDQ, patients with pain had a significantly lower quality of life compared to those without (Table 1).
Patients with clinically relevant abdominal pain were much more likely to receive opioid analgesics at or after the index visit (25.0% vs. 7.1%; P < 0.0001). Several disease-related variables demonstrated a significant association with frequent abdominal pain on univariate analysis. Abdominal pain was associated with an increased likelihood of prior bowel surgery, stricturing disease, elevated CRP and ESR, and more frequent corticosteroid use (Table 1). In addition to increased opioid analgesic use, individuals with pain were also more likely to use other non-NSAID pain medications (13.3% vs. 6.5% respectively; p < 0.01) (Table 1). Considering the known influence of anxiety and depression on pain perception, we also examined the incidence of these disorders as well as antidepressant and/or anxiolytic use and found that these factors were more common in patients describing significant abdominal pain (Table 1).
To determine independent predictors of abdominal pain, we entered the previously identified variables into a logistic regression analysis. Focusing on the 434 (80.1%) individuals with concurrent laboratory findings available, elevated ESR (> 20 mm/h), comorbid psychiatric conditions (anxiety and/or depression), active smoking, and opioid analgesic use significantly correlated with clinically relevant pain (Table 2).
Table 2.
Independent predictors of abdominal pain in CD
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Prior bowel surgery | 1.47 | 0.95–2.26 | 0.0834 |
| Elevated CRP | 1.37 | 0.81–2.30 | 0.2388 |
| Elevated ESR | 1.79 | 1.11–2.87 | 0.0158 |
| Psychiatric disorder | 1.87 | 1.13–3.09 | 0.0148 |
| Antidepressant/anxiolytic use | 0.91 | 0.53–1.57 | 0.7458 |
| Opiate use | 2.46 | 1.33–4.57 | 0.0043 |
| Steroid use | 1.42 | 0.84–2.39 | 0.1923 |
| Anti-TNF use | 0.71 | 0.46–1.10 | 0.1280 |
| Active smoking | 2.08 | 1.27–3.40 | 0.0035 |
“Psychiatric disorder” included clinical diagnoses of major depressive disorder or generalized anxiety disorder. “Antidepressant/anxiolytic use” included patient use of selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, tri- or tetracyclic antidepressants, monoamine oxidase inhibitors, buspirone, bupropion, benzodiazepines and/or bupropion. Values listed in bold within the table are considered to represent statistically significant differences (i.e., P < 0.05)
Opioid Analgesic Use, Quality of Life, and Abdominal Pain
As indicated above, CD patients experiencing clinically relevant abdominal pain were significantly more likely to use opioid analgesics or other pain medications (except for NSAIDs) (Table 1). In order to assess the longitudinal impact of opioid analgesic medications in this setting, after excluding individuals who had been on opioid analgesics before the index visit, we evaluated demographic and disease characteristics of CD patients at the follow-up visit who started opioid analgesics at or after the index visit (n = 31) and compared them to CD patients who had not used opioid analgesics during that time (n = 431). Of note, there was no difference in incidence of opioid analgesic use when comparing study participants in the lower and upper halves of time to follow-up after the index visit (14 vs. 17 respectively, p = 0.70). However, opioid analgesic users had a longer mean disease duration (Table 3). They were also more likely to undergo surgery during the follow-up period (25.8% vs. 12.3%; p < 0.05). Notably, none of the opiate users in this context had surgery within two months of the follow-up visit.
Table 3.
Characteristics of opiate and non-opiate users
| Variable | No opiate use (n = 431) | Opiate started (n = 31) | p |
|---|---|---|---|
| Gender (% females) | 55.5% | 61.3% | 0.40 |
| Age (years ± SEM) | 39.9 ± 0.7 | 42.4 ± 2.5 | 0.37 |
| Disease Duration (years ± SEM) | 17.7 ± 0.5 | 20.3 ± 1.4 | < 0.05 |
| Disease localization | 0.45 | ||
| L1 (ileal) | 170 | 9 | |
| L2 (colonic) | 59 | 4 | |
| L3 (ileocolonic) | 202 | 18 | |
| Stricturing disease | 72 | 7 | 0.40 |
| Prior bowel surgery | 256 | 25 | 0.06 |
| Bowel surgery during follow-up period | 53 | 8 | < 0.05 |
| Smoker | 81 | 7 | 0.87 |
| Colonoscopy | 220 | 18 | |
| Inflammation > 1 | 80 | 4 | 0.31 |
| Histology | 199 | 17 | |
| Inflammation > 1 | 48 | 5 | 0.57 |
| ESR (mm/h ± SEM) | 19.7 ± 0.9 | 22.1 ± 4.3 | 0.53 |
| CRP (mg/dL ± SEM) | 1.2 ± 0.2 | 0.8 ± 0.3 | 0.51 |
| Medical therapy | |||
| Corticosteroid | 71 | 4 | 0.60 |
| Immunomodulator | 124 | 10 | 0.29 |
| Anti-TNF agent | 151 | 11 | 0.96 |
| Psychiatric disorder | 109 | 11 | 0.21 |
| Antidepressant/anxiolytic use | 85 | 6 | 1.0 |
| Mean SPS index (± SEM) | 5.1 ± 0.1 | 4.0 ± 0.3 | < 0.001 |
| Mean SIBDQ index (± SEM) | 51.7 ± 0.6 | 49.6 ± 2.1 | < 0.001 |
| Mean SPS change (± SEM) | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.79 |
| Mean SIBDQ change (± SEM) | 1.9 ± 0.5 | 2.1 ± 2.0 | 0.88 |
Values listed in bold within the table are considered to represent statistically significant differences (i.e., p < 0.05)
SEM standard error measurement, SIBDQ short inflammatory bowel disease questionnaire, SPS SIBDQ pain score
Consistent with results based on the entire cohort, opioid analgesic users (n = 31) had lower SIBDQ and SPS scores at the index visit (Table 3). Of note, opioid analgesic users demonstrated no significant difference in SIBDQ (43.6 vs. 45.7; p = 0.48) or SPS scores (4.0 vs. 4.3; p = 0.55) between the index and follow-up visits. This contrasted with CD patients who did not use opioids (n = 431), as they demonstrated significant improvements in both SIBDQ (51.7 vs. 53.6, p < 0.05) and SPS (5.1 vs. 5.4, p < 0.01) (Figs. 1, 2). Opioid users also demonstrated no significant difference in the change of SIBDQ (2.1 vs. 1.9, p = 0.88) or change in SPS (0.2 vs. 0.3, p = 0.79) when compared to CD patients who did not use opioid analgesics.
Fig. 1.
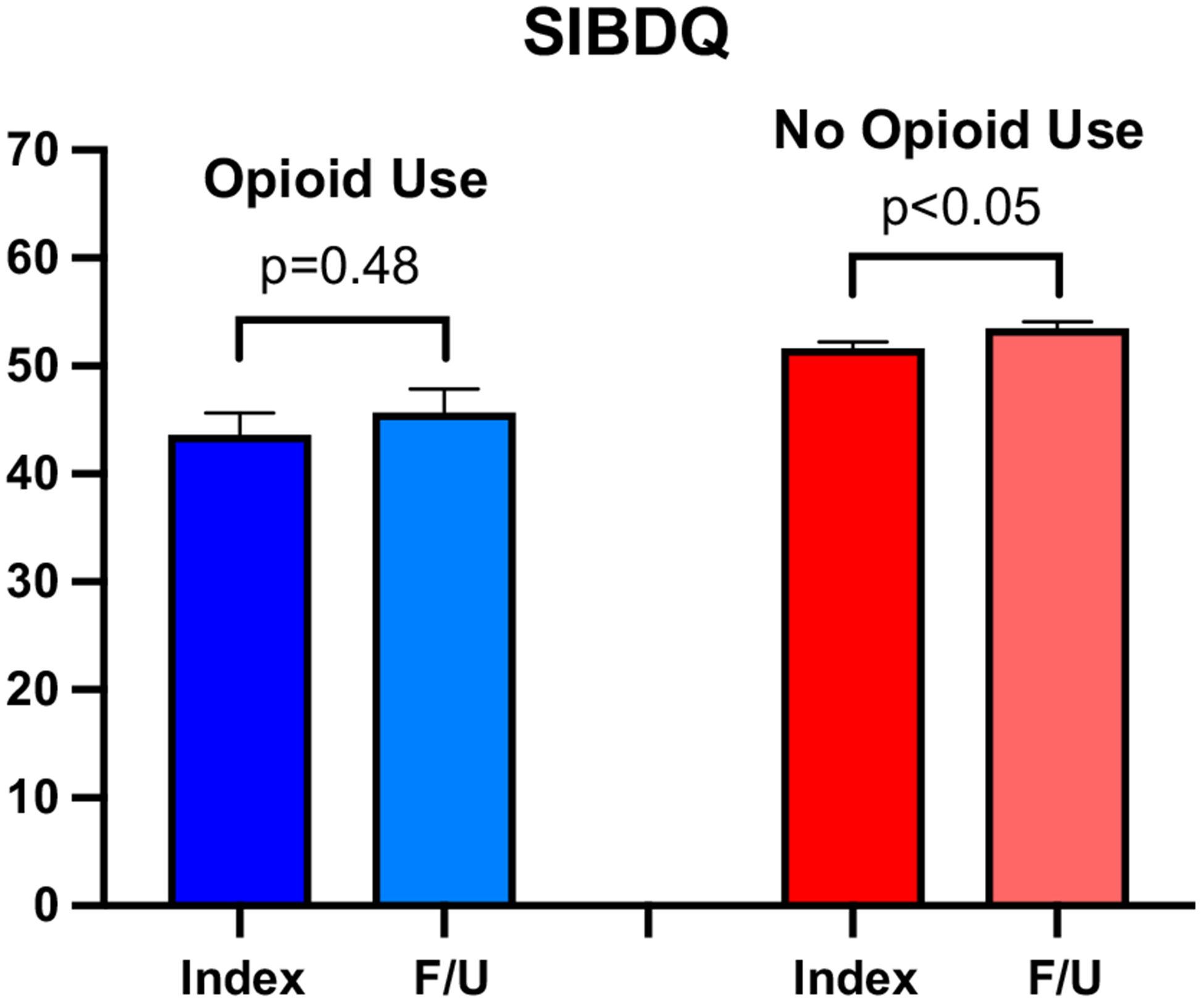
Quality of life scores over time. Mean short inflammatory bowel disease questionnaire (SIBDQ) values (0–70) at index and follow-up (F/U) visits for CD opioid users (blue) and CD patients not using opioids (red)
Fig. 2.
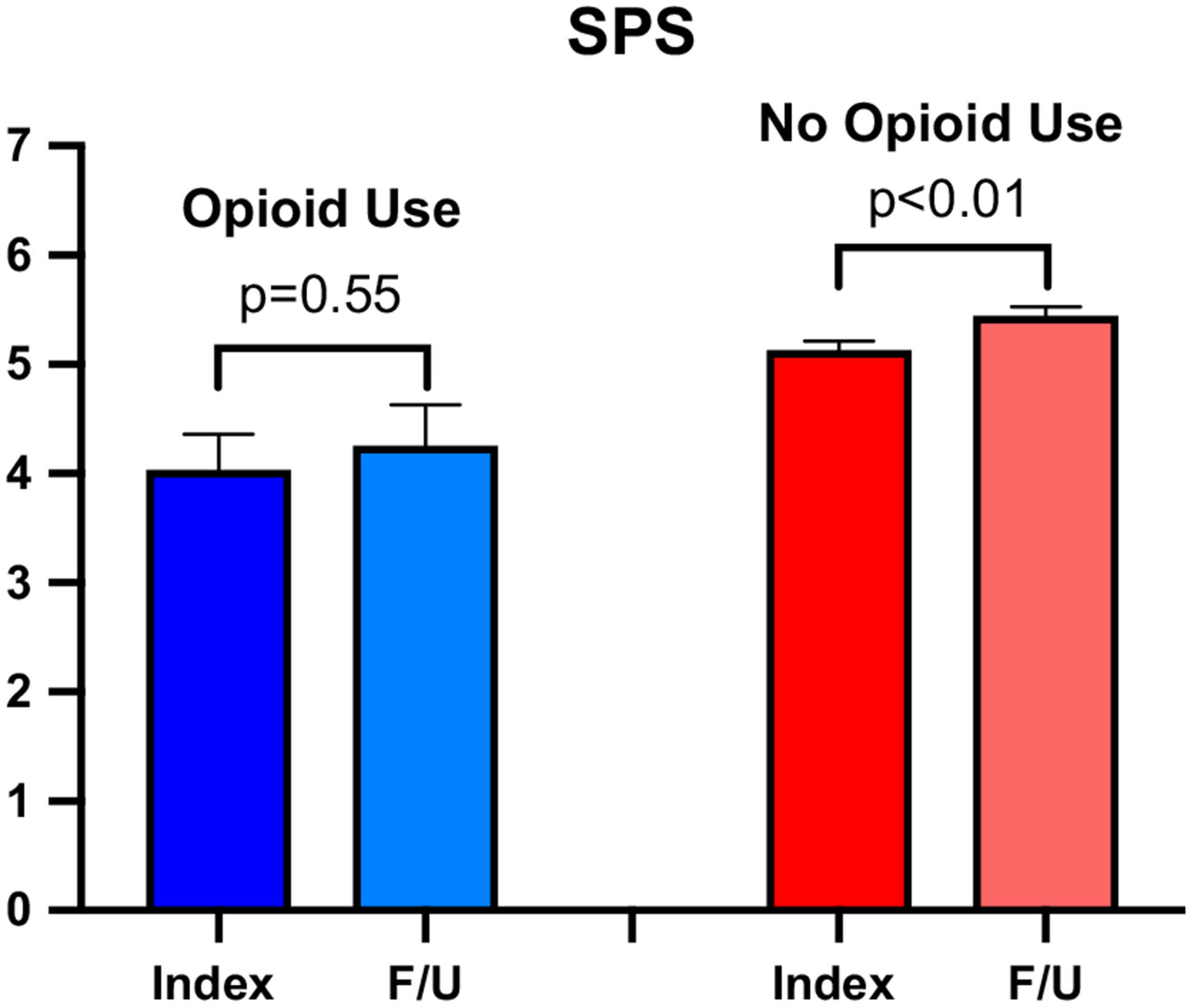
Abdominal pain scores over time. Mean short inflammatory bowel disease questionnaire pain scores (SPS) values (0–7) at index and follow-up (F/U) visits for CD opioid users (blue) and CD patients not using opioids (red)
In order to help assess for potential objective changes in disease status between these cohorts, we evaluated inflammatory markers over time from the index to follow-up visit. CRP decreased significantly in the opioid users (0.9 mg/dL vs. 0.5 mg/dL, p < 0.05) but did not significantly change in non-opioid users (1.1 mg/dL vs. 1.1 mg/dL, p = 0.80). ESR demonstrated a non-significant decrease in opioid users (21.9 mm/h vs. 17.3 mm/h, p = 0.13) while it significantly dropped in non-opioid users (21.1 mm/h vs. 17.9 mm/h, p < 0.05). Of note, the mean changes exhibited in the opioid and non-opioid users for CRP (− 0.4 mg/dL vs. − 0.1 mg/dL respectively, p = 0.78) and ESR (− 4.6 mm/h vs. − 2.2 mm/h respectively, p = 0.57) were not significantly different from one another.
Disease‑Specific Therapy, Quality of Life, and Abdominal Pain
In order to evaluate the impact of different medical interventions on abdominal pain and quality-of-life scores, and considering the role of inflammatory markers as independent predictors of pain frequency, we examined the effect of intensified treatment of this underlying inflammation. Patients who started medications targeting disease activity (n = 123), such as anti-TNF biologics, demonstrated improvements in both the SIBDQ (45.6 ± 1.5 vs. 49.5 ± 1.5; p < 0.05) and SPS (4.0 ± 0.2 vs. 4.8 ± 0.2; p < 0.05) between the index and follow-up visits when compared to CD patients that did not change to these therapies (n = 419).
Conclusions
Using a large and well-characterized cohort of CD outpatients followed in a tertiary center, our data clearly demonstrate that abdominal pain is common in CD, affecting over 40% of all of the patients in this study. We also reaffirmed that abdominal pain negatively impacts CD patient quality of life. Variables independently associated with abdominal pain in CD included smoking, comorbid anxiety and/or depression, elevated inflammatory marker (ESR) and opioid analgesic use. All of these findings are consistent with previously published data [31–34]. Opioid analgesic use was common in our study population, with about 15% of our patient cohort receiving these medications. These results are also similar to previously reported data for IBD and other gastrointestinal disorders [20, 34–37]. Finally, it is notable that although we found that opioid analgesic use was independently associated with abdominal pain, it was not associated with a significant improvement in pain or quality-of-life scores. This stable pain rating stands in contrast with the decrease in incidence of abdominal pain scores associated with treatments that target the underlying inflammatory process (i.e., anti-TNF agents), suggesting that our approach did allow us to detect differences between the two time points included in our analysis. This is particularly remarkable, considering that proxy measures of inflammation (e.g., CRP) appeared to improve over time in CD opioid users.
Opioid analgesic use in benign disorders rose significantly during the last two decades, correlating with the introduction, marketing and increased availability of various slow-release formulations [38, 39]. Opioid analgesics have become one of the most commonly prescribed medications in the United States [40]. Our study was not designed nor powered to examine time trends in opioid analgesic use. Interestingly, the only cohort study addressing opioid analgesic use before the year 2000 described a significantly lower prevalence with 2.7% of IBD patients on narcotics [41], while the most recent analyses of opioid analgesic use in IBD patients (including our own study) suggested current use of these medications had more than doubled in this population [42].
Concerns about opioid analgesics often focus on dependency, abuse, and overdose potential [43]. Coexisting psychiatric illness [41] and smoking [25, 35] have been identified as predictors of prescription opioid analgesic use (and abuse). Interestingly, these variables closely correlate with the choice of analgesic therapy in chronic back pain [44] and also suggest a higher abuse potential [45, 46]. However, concerns about opioid analgesic use in IBD go beyond abuse, as narcotics have been linked with worsening treatment outcomes [24]. Opioid analgesics significantly impact gastrointestinal function and may negatively impact perception of gastrointestinal symptoms but also the course of IBD and its management [15, 47, 48]. Chronic opioid analgesic use has previously been associated with increased healthcare resource use in CD [26, 27]. Thus, altered perception of symptoms may lead to misinterpretations of disease severity and affect implementation of medical and/or surgical therapy, leading to inappropriate interventions. Finally, intensified symptomatic therapy may simply be a surrogate marker of more severe disease. Given the association between abdominal pain and psychiatric conditions in this population, and the lack of evidence for efficacy of opioid analgesics or other standard analgesic medications in addressing abdominal pain in IBD, strong consideration of alternative interventions including psychotherapy should be made as these modalities have demonstrated a significant impact on disease course and pain modifying factors in selected populations [15, 49].
As a retrospectively designed study, our investigation has limitations. Biological markers of inflammation and endoscopic or histologic assessment of disease activity were not consistently obtained for the entire cohort. Moreover, pain as the primary endpoint was assessed and rated primarily based on a scale that measured frequency rather than severity. However, the measure correlated very well with a severity scale that was obtained in a subset of individuals. We also based the diagnosis of anxiety and depression on a recorded comorbid condition rather than systematic assessment with a validated diagnostic tool, which may lead to an underestimate of clinically relevant anxiety and/or depression. Additionally, although many patients included in our study cohort were prescribed opioid analgesics, a minority of the study participants involved in this study were started on these agents during the study period. This may have limited our ability to identify other more subtle influences on the development of abdominal pain and opioid analgesic use. Notably, while all of the records we reviewed suggested that the opioid prescriptions were provided for management of CD, it is possible that they were provided, at least in part, to manage EIMs or other non-gastrointestinal symptoms thought to be related to CD. Finally, although we can demonstrate associations among various factors, it is impossible to determine cause-and-effect relationships using the study design that we employed.
Despite the limitations noted above, our results clearly demonstrate the importance of abdominal pain in CD. They also suggest that opioid analgesics, while frequently prescribed in CD, do not improve abdominal pain or patient quality of life. Considering the potentially negative impact of opioid analgesics on IBD disease course (including the possibility of inducing narcotic bowel syndrome [21, 50]) as well as their serious side effects and risk for addiction, a critical review of approaches to chronic analgesic therapy and alternative options is needed. If opioid analgesics are prescribed in this setting, the provider should incorporate strategies that minimize the potential for harm or abuse. These strategies include, but are not limited to, short term prescriptions, close observation and follow-up, and a clear plan to de-escalate dosing safely when adverse effects and/or abuse occurs. In view of increasing concerns about chronic opioid analgesic use, our results highlight the relevance of potentially modifiable factors ranging from smoking to anxiety or depression as determinants and/or surrogate markers of pain and analgesic use. With this in mind, it is clearly important to evaluate for other possible underlying contributing factors, including psychiatric disorders, opioid analgesic use/abuse and other deleterious behaviors such as smoking. We need to continue to refine our understanding of the underlying mechanisms perpetuating abdominal pain in this population and broaden the search for novel and/or underappreciated pain modifiers and therapies, as even the myriad of pharmacological options we have available today frequently are inadequate for the job. Doing so will enable physicians and integrated healthcare systems to target such individuals, with the goal to proactively intervene and improve quality of life without relying as much on relatively inefficacious therapies associated with significant comorbidity.
Acknowledgments
The authors would like to acknowledge Drs. Leonard Baidoo, Richard Duerr, Marc Schwartz and Jason Swoger for their assistance in gathering relevant clinical data for this study.
Funding This work was supported by the National Institutes of Health grants R01DK 122364 and T32DK063922, and a grant from the IBD Working Group.
Footnotes
Conflict of interest The authors of this manuscript have no relevant conflicts of interest to disclose.
Ethical approval This study was approved by the University of Pittsburgh Institutional Review Board (IRB0309054) before data collection began. This study was undertaken in compliance with the principles and rules set forth by the United States Federal Policy on the Protection of Human Subjects.
References
- 1.Isgar B, Harman M, Kaye MD, et al. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut. 1983;24:190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minderhoud IM, Oldenburg B, Wismeijer JA, et al. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469–474. 10.1023/b:ddas.0000020506.84248.f9. [DOI] [PubMed] [Google Scholar]
- 3.Farrokhyar F, Marshall JK, Easterbrook B, et al. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12:38–46. [DOI] [PubMed] [Google Scholar]
- 4.Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates MD, Lahoti M, Binion DG, et al. Abdominal pain in ulcerative colitis. Inflamm Bowel Dis. 2013;19:2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirbel A, Reichert A, Roll S, et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J Gastroenterol. 2010;16:3168–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IsHak WW, Pan D, Steiner AJ, et al. Patient-reported outcomes of quality of life, functioning, and gi/psychiatric symptom severity in patients with inflammatory bowel disease (IBD). Inflamm Bowel Dis. 2017;23:798–803. [DOI] [PubMed] [Google Scholar]
- 8.van der Valk ME, Mangen MJ, Leenders M, et al. Risk factors of work disability in patients with inflammatory bowel disease—a Dutch nationwide web-based survey: work disability in inflammatory bowel disease. J Crohns Colitis. 2014;8:590–597. [DOI] [PubMed] [Google Scholar]
- 9.Tew GA, Jones K, Mikocka-Walus A. Physical activity habits, limitations, and predictors in people with inflammatory bowel disease: a large cross-sectional online survey. Inflamm Bowel Dis. 2016;22:2933–2942. [DOI] [PubMed] [Google Scholar]
- 10.Hay AR, Hay JW. Inflammatory bowel disease: medical cost algorithms. J Clin Gastroenterol. 1992;14:318–327. [DOI] [PubMed] [Google Scholar]
- 11.Kappelman MD, Porter CQ, Galanko JA, et al. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodhand JR, Wahed M, Mawdsley JE, et al. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis. 2012;18:2301–2309. [DOI] [PubMed] [Google Scholar]
- 13.Chan W, Shim HH, Lim MS, et al. Symptoms of anxiety and depression are independently associated with inflammatory bowel disease-related disability. Dig Liver Dis. 2017;49:1314–1319. [DOI] [PubMed] [Google Scholar]
- 14.Deter HC, Keller W, von Wietersheim J, et al. Psychological treatment may reduce the need for healthcare in patients with Crohn’s disease. Inflamm Bowel Dis. 2007;13:745–752. [DOI] [PubMed] [Google Scholar]
- 15.Norton C, Czuber-Dochan W, Artom M, et al. Systematic review: interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:115–125. [DOI] [PubMed] [Google Scholar]
- 16.Regueiro M, Greer JB, Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:e4. [DOI] [PubMed] [Google Scholar]
- 17.Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long MD, Kappelman MD, Martin CF, et al. Role of nonsteroidal anti-inflammatory drugs in exacerbations of inflammatory bowel disease. J Clin Gastroenterol. 2016;50:152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou R, Ballantyne JC, Fanciullo GJ, et al. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:e15. [DOI] [PubMed] [Google Scholar]
- 20.Crocker JA, Yu H, Conaway M, et al. Narcotic use and misuse in Crohn’s disease. Inflamm Bowel Dis. 2014;20:2234–2238. [DOI] [PubMed] [Google Scholar]
- 21.Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5:1126–1139. (quiz 1121–2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohnert AB, Valenstein M, Bair MJ, et al. ASsociation between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. [DOI] [PubMed] [Google Scholar]
- 23.Ray WA, Chung CP, Murray KT, et al. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA. 2016;315:2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT Registry. Clin Gastroenterol Hepatol. 2006;4:621–630. [DOI] [PubMed] [Google Scholar]
- 25.Long MD, Barnes EL, Herfarth HH, et al. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis. 2012;18:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanford D, Thornley P, Teriaky A, et al. Opioid use is associated with decreased quality of life in patients with Crohn’s disease. Saudi J Gastroenterol. 2014;20:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alley K, Singla A, Afzali A. Opioid use is associated with higher health care costs and emergency encounters in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1990–1995. [DOI] [PubMed] [Google Scholar]
- 28.Burr NE, Smith C, West R, et al. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol. 2018;16:e6. [DOI] [PubMed] [Google Scholar]
- 29.Anderson AJ, Click B, Ramos-Rivers C, et al. Development of an inflammatory bowel disease research registry derived from observational electronic health record data for comprehensive clinical phenotyping. Dig Dis Sci. 2016;61:3236–3245. 10.1007/s10620-016-4278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos-Rivers C, Regueiro M, Vargas EJ, et al. Association between telephone activity and features of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh S, Blanchard A, Walker JR, et al. Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2011;9:769–775. [DOI] [PubMed] [Google Scholar]
- 32.Morrison G, Van Langenberg D, Gibson S, et al. Chronic pain in inflammatory bowel disease: characteristics and associations of a hospital-based cohort. Inflamm Bowel Dis. 2013;19:1210–1217. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman L, Srinath A, Goyal A, et al. The overlap of functional abdominal pain in pediatric Crohn’s disease. Inflamm Bowel Dis. 2013;19:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2809–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal N, Bielefeldt K. Diagnostic stringency and healthcare needs in patients with biliary dyskinesia. Dig Dis Sci. 2013;58:2799–2808. 10.1007/s10620-013-2719-5. [DOI] [PubMed] [Google Scholar]
- 37.Rogal SS, Winger D, Bielefeldt K, et al. Healthcare utilization in chronic liver disease: the importance of pain and prescription opioid use. Liver Int. 2013;33:1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9–ES38. [PubMed] [Google Scholar]
- 39.Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the united states, 2000–2010. Med Care. 2013;51:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong W, Maradit-Kremers H, Sauver JL, et al. Age and sex patterns of drug prescribing in a defined American population. Mayo Clinic Proc 2013;88:697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol. 2001;16:1235–1238. [DOI] [PubMed] [Google Scholar]
- 42.Narula N, Borges L, Steinhart AH, et al. Trends in narcotic and corticosteroid prescriptions in patients with inflammatory bowel disease in the United States ambulatory care setting from 2003 to 2011. Inflamm Bowel Dis. 2017;23:868–874. [DOI] [PubMed] [Google Scholar]
- 43.Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459. [DOI] [PubMed] [Google Scholar]
- 44.Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4:344–350. [DOI] [PubMed] [Google Scholar]
- 45.Tetrault JM, Desai RA, Becker WC, et al. Gender and non-medical use of prescription opioids: results from a national US survey. Addiction. 2008;103:258–268. [DOI] [PubMed] [Google Scholar]
- 46.Becker WC, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug and Alcohol Dependence 2008;94:38–47. [DOI] [PubMed] [Google Scholar]
- 47.Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126:21–38. [DOI] [PubMed] [Google Scholar]
- 48.Sternini C, Patierno S, Selmer IS, et al. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16:3–16. [DOI] [PubMed] [Google Scholar]
- 49.McCombie AM, Mulder RT, Gearry RB. Psychotherapy for inflammatory bowel disease: a review and update. J Crohns Colitis. 2013;7:935–949. [DOI] [PubMed] [Google Scholar]
- 50.Kurlander JE, Drossman DA. Diagnosis and treatment of narcotic bowel syndrome. Nat Rev Gastroenterol Hepatol. 2014;11:410–418. [DOI] [PubMed] [Google Scholar]


