Abstract
Background/Purpose
In patients with cryptogenic stroke (CS) and patent foramen ovale (PFO), the Risk of Paradoxical Embolism (RoPE) Score has been proposed as a method to estimate a patient-specific “PFO-attributable fraction”—the probability that a documented PFO is causally-related to the stroke, rather than an incidental finding. The objective of this research is to examine the relationship between this RoPE-estimated PFO-attributable fraction and the effect of closure in 3 randomized trials.
Methods
We pooled data from the CLOSURE-I, RESPECT and PC Trials. We examine the treatment effect of closure in high RoPE score (>=7) versus low RoPE score (<7) patients. We also estimated the relative risk reduction (RRR) associated with PFO closure across each level of the RoPE score using Cox proportional hazard analysis. We estimated a patient-specific attributable fraction using a PC Trial-compatible (9-point) RoPE equation (omitting the neuroradiology variable), as well as a two-trial analysis using the original (10-point) RoPE equation. We examined the Pearson correlation between the estimated attributable fraction and the RRR across RoPE strata.
Results
In the low RoPE score group (<7, n=912), the rate of recurrent strokes per 100 person-years was 1.37 in the device arm versus 1.68 in the medical arm (HR=0.82 ( 0.42 to 1.59) p=0.56; compared to 0.30 versus 1.03 (HR = 0.31 ( 0.11 to 0.85) p=0.02) in the high RoPE score group (>=7, n=1221); treatment-by-RoPE score group interaction, p=0.12. The RoPE score estimated attributable fraction anticipated the RRR across all levels of the RoPE score, in both the three-trial (r=0.95, p<0.001) and two-trial ( r=0.92, p<0.001) analyses.
Conclusions
The RoPE score estimated attributable fraction is highly correlated to the RRR of device versus medical therapy. This observation suggests the RoPE score identifies patients with CS who are likely to have a PFO that is pathogenic rather than incidental.
Keywords: Patent Foramen Ovale Closure, Patent Foramen Ovale, Ischemic Stroke, Ischemic stroke, meta-analysis
Introduction
Approximately 20–30% of ischemic strokes are “cryptogenic”, an etiologically heterogeneous class including many possible occult mechanisms. One-half of patients <60 years with cryptogenic stroke (CS) have a patent foramen ovale (PFO), twice the prevalence in the general population.1,2 For these patients, the stroke mechanism may be paradoxical transcardiac embolism (i.e., PFO-related), but may still be another occult etiology (i.e., PFO-unrelated).
We previously developed the Risk of Paradoxical Embolism (RoPE) score, which predicts the probability of discovering a PFO in a patient with CS.3 Since the probability of discovering a PFO in patients with CS is directly related to the probability that the stroke is caused by a PFO-related mechanism, we proposed that this “PFO propensity” could be used to stratify patients by the probability that a discovered PFO is pathogenic versus incidental (i.e., the attributable fraction). While the mathematical assumptions supporting this are well accepted (i.e., Bayes theorem), and while the RoPE score performance for predicting PFO was consistent across nine component databases3 (and independently validated),4 the RoPE score has not yet been shown to predict the degree of benefit from transcatheter PFO closure.
In theory, the attributable fraction—the proportion of index events that are PFO-related—represents the theoretical maximal benefit of PFO closure. That is, if device closure has a relative risk reduction (RRR) compared to medical therapy of 100% in patients with PFO-related stroke, and if the RoPE score accurately estimates attributable fraction, we would anticipate the RRR in each RoPE stratum to reflect the RoPE-estimated attributable fraction. Herein, we examine the treatment effect of closure in high RoPE Score versus low RoPE score patients in three pooled trials, and also examine the correlation between the RoPE-estimated attributable fraction and the RRR in recurrent stroke.
Methods
The data that support the findings are aggregated across 12 different studies (9 in the RoPE study plus 3 clinical trials). The corresponding author has separate data use agreements for each of the studies that does not include sharing the data with third parties. The data are potentially available from the original study PIs or the sponsoring companies. Proposals to the corresponding author can be considered by the RoPE Study team and the individual study investigators. We used individual participant data from 3 randomized trials testing percutaneous mechanical closure versus medical therapy: the CLOSURE-I5, RESPECT6 and PC Trials7. Details of these trials were reported in their original reports, and the 3-trial cohort used here is described in a prior individual patient data meta-analysis (IPDMA).8 The primary outcome was stroke recurrence and our primary analysis was intention-to-treat (ITT), in which randomized patients are analyzed according to their assigned treatment groups. Ethics approval and consent were waived by the local institutional review board.
Statistical Analysis
We used Cox proportional hazard regression to estimate the hazard ratio associated with device versus medical therapy in patients with high versus low RoPE score, dichotomizing the RoPE score at < versus >= 7. We tested the significance of the contrast in effect between the strata using a binary interaction term in the regression equation. Patients who were missing neuroimaging data (i.e. from the PC Trial) were excluded from this analysis only when the value of this variable may have resulted in reclassification into a different RoPE strata.
We then used Pearson’s correlation coefficient to characterize the association between the RoPE-estimated attributable fraction and the degree (i.e. hazard ratio [HR]) of stroke recurrence reduction with PFO closure. To estimate the HR of recurrent stroke for closure versus medical therapy across patients with different RoPE scores, we again used Cox proportional hazard regression including 3 terms: device closure versus medical therapy; RoPE score as a continuous variable; and the interaction between RoPE Score (as a continuous variable) and device closure. To include the PC Trial, for which no neuroradiological data were collected, we used a 9-point RoPE score (i.e., the originally reported RoPE score without a point for neuroradiology). We also performed a two-trial analysis (including only CLOSURE-I and RESPECT) using the full 10-point RoPE Score. The 10-point RoPE score and the RoPE logistic regression equation from which it was derived are shown in the Supplement (Supplemental Tables I and II). We used cubic splines to examine potential non-linearity in the effect of closure across RoPE scores but these did not improve model fit and are not shown. As a stability analysis, we repeated the above correlation analyses using an “as treated” analysis, where patients who received the device are contrasted with patients who did not, regardless of treatment assignment.
To estimate the attributable fraction, we used the RoPE logistic regression equation, which predicts the prevalence of a PFO among patients with CS, conditional on patient characteristics.3 While PFOs are a congenital defect distributed randomly across the population, among patients diagnosed with CS a correlation is induced between PFO and other risk factors via index event (or collider) bias.9 Specifically, in CS patients, PFOs are associated with the absence of conventional stroke risk factors (age, hypertension, hypercholesterolemia, and diabetes), the absence of a prior stroke and the presence of a superficial infarct. The RoPE-estimated attributable fraction can be calculated from the predicted PFO prevalence according to Bayes theorem, as follows:
Attributable fraction:
Where ProbPFO is the probability of discovering a PFO based on the RoPE logistic regression model conditioned on patient characteristics and CR represents the control rate (i.e. the PFO prevalence in the general population; here we used 0.25). The attributable fraction is the probability that a CS is PFO-related given that a CS patient has a PFO. Patients with CS but a very low RoPE score have a PFO prevalence near that in the general population and so a near-zero attributable fraction (even when a PFO is found). CS patients with a high RoPE score have a high PFO prevalence and therefore a high attributable fraction when a PFO is found. A PC-Trial-compatible equation was derived excluding the neuroimaging variable for the 3-trial analysis (Supplemental Table III); the published RoPE logistic regression model was used for the 2-trial analysis. We graphically examined the RRR (1-HR) compared to the attributable fraction estimated in each RoPE stratum.
Results
The pooled trials included 2303 randomized patients. This analysis included 2289 patients with complete data followed for 5926 person years (Supplemental Figure I). There were 58 stroke outcomes. Dichotomizing at a RoPE score of < 7 versus >= 7, the rate of recurrent strokes per 100 person-years in the low RoPE score group (n=912) was 1.37 in the device arm versus 1.68 in the medical therapy arm (HR=0.82 ( 0.42 to 1.59) p=0.56) and 0.30 versus 1.03 (HR = 0.31 ( 0.11 to 0.85) p=0.02) in the high RoPE score group (n=1221). The treatment-by-RoPE score group interaction did not reach statistical significance (p=0.12).
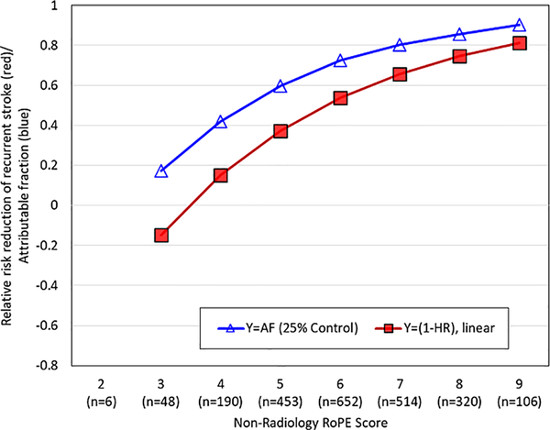
Figure 1 graphs both the RoPE-estimated attributable fraction and the RRR (1-HR) across different levels of the 9-point RoPE Score. The Pearson correlation showed a strong association (r=0.95, p<0.001). Similar results were obtained in the two-trial analysis using the 10-point RoPE score (Pearson correlation=0.92, p<0.001; Supplemental Figure II) as well as with an “as treated” analysis (Pearson correlation =0.94, p<0.001; Supplemental Figure III).
Figure 1:
RoPE – Estimated Attributable Fraction and Relative Risk Reduction (RRR) across Different RoPE scores
This figure depicts the RoPE-estimated attributable fraction (open blue triangles) and the RRR as estimated from a Cox model (red squares) against different RoPE scores. Higher RoPE scores are associated with much higher attributable fractions and much higher RRR. The estimated RRR correlated highly with the RoPE score estimated attributable fraction (r=0.88, p<0.001). Both the RoPE Equation and the 9-point RoPE score omit the neuroradiology variable to permit inclusion of the PC Trial data.
AF=attributable fraction; HR=hazard ratio.
Discussion
In this 3-trial analysis, the estimated RRR was 69% in patients with a RoPE score of >= 7, but only 18% in those with a RoPE score of <7. While this contrast in effects did not reach statistical significance, we nevertheless showed that the RRR for recurrent stroke in patients treated with closure is highly correlated to the calculated attributable fraction based on the RoPE score. This observation adds important empirical support to the compelling theoretical claim that the RoPE score identifies patients with CS likely to have a PFO that is pathogenic versus incidental. We note that the magnitude of the effect of closure, and the effect modification of the RoPE score on the relative benefits of closure, is approximately what one might expect based on theory—assuming PFO closure is a near-perfect therapy to prevent recurrence in patients whose index event is PFO-related. The gap between the RoPE-estimated attributable fraction and the actual benefit observed in the 3-trials may represent a combination of: 1) closure is less than 100% effective in preventing PFO-related stroke recurrence (e.g., due to incomplete closure in some patients10), 2) recurrent strokes in patients with cryptogenic stroke and PFO may be from other mechanisms11, and 3) estimation error.
We acknowledge several limitations. While different devices were used across the trials and different closure rates were achieved, we considered the treatment effect of closure as consistent across trials, in part due to a lack of power to consider between-trial heterogeneity. We note our analysis applies only to patients meeting trial enrollment criteria (e.g. age ≤ 60). The potential usefulness of the RoPE score for predicting effects in patients not qualifying for the trials remains unknown. Also, the methods used to derive the RoPE score do not permit inclusion of anatomic features of the PFO (e.g. atrial septal aneurysm, shunt size), which might impact recurrence risk and closure benefits.
Despite the highly significant correlation between the RRR in recurrent stroke estimated from the Cox model and the RoPE-estimated attributable fraction, the RoPE score-by-closure interaction did not reach conventional thresholds of statistical significance. We attribute this to the paucity of outcomes (only 58 recurrent strokes) and that treatment interactions require substantial statistical power. We also note that, while RRR is the appropriate scale to examine the influence of attributable fraction, absolute risk reductions are often considered more important from a clinical decision making perspective. Since the recurrence rate of strokes due to PFOs appears to be low, and lower in high RoPE-score8 patients, the score may identify large relative reductions that translate to only low-to-moderate absolute reductions. However, given that PFO-related stroke is prevalent amongst young stroke patients, small absolute risk reductions may become magnified over time. Further, knowledge of the probable biological mechanism for individual stroke patients can be very influential for mechanism-specific therapeutic decisions, such as whether to close a PFO or treat with medication alone. Additional studies (including recent trials12) are necessary to better motivate decision rules for patient selection.
In conclusion, the RoPE score estimated attributable fraction is highly correlated to the RRR of device therapy versus medical therapy. More thorough analysis with more comprehensive databases may be needed to better determine how this should influence clinical decision making.
Supplementary Material
Acknowledgements
We would like to acknowledge the other investigators of the Risk of Paradoxical Embolism (RoPE) Study for contributing the data and supporting the predictive modeling on which this current analysis relies:
Emanuele Di Angelantonio, MD, MSc, Cambridge University; Marco DiTullio, MD, Columbia University; Mitchell S. V. Elkind, MD, MS, Columbia University; Shunichi Homma, MD, FACC, Columbia University; Cheryl Jaigobin, MD, FRCP, MSc, University of Toronto; Jean-Louis Mas, MD, Hôpital Sainte-Anne, Paris-Descartes University; Patrik Michel, MD, Centre Hospitalier Universitaire Vaudois; Marie-Luise Mono, MD, Triemli Municipal Hospital, Zurich; Krassen Nedeltchev, MD, FESC, Triemli Municipal Hospital, Zurich; Federica Papetti, MD, University of Rome; Joaquín Serena, MD, PhD, Hospital Universitari Doctor Josep Trueta Institut d’Investigació Biomèdica de Girona, Spain; Christian Weimar, MD, Professor of Neurology, University of Duisburg-Essen.
Funding
This work was supported by two Patient Centered Outcomes Research (PCORI) contracts: [SA.Tufts.PARC.OSCO.2018.01.25] and [RS-SCOPE-2019-001].
Footnotes
Disclosures
Dr. Saver is an employee of the University of California, Regents. Neither the University of California, Regents nor Dr. Saver received any direct support for work on the current study. For topics broadly related to this study, the University of California Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled in multicenter clinical trials from Abbott and Boehringer Ingelheim; Dr. Saver served as an unpaid site investigator under these contracts. Dr. Saver has received contracted hourly payments and travel reimbursement for service as a scientific consultant advising on rigorous trial design and conduct to Abbott and Boehringer Ingelheim. For topics completely unrelated to the current study, the University of California Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled in multicenter clinical trials sponsored by Medtronic, Stryker, Cerenovus, NoNo Inc, Diffusion Pharmaceuticals, and Rapid Medical. Dr. Saver served as an unpaid site investigator under these contracts. Dr. Saver has received contracted hourly payments and travel reimbursement for services as a scientific consultant advising on rigorous trial design and conduct to Medtronic, Stryker, Cerenovus, NoNo Inc, and Diffusion Pharmaceuticals. Dr. Saver has received contracted stock options for services as a scientific consultant advising on rigorous trial design and conduct to Rapid Medical. Dr. Saver has not participated as a medicolegal expert in any litigation regarding stroke management.
Dr. Anthony Furlan was PI of CLOSURE NMT Boston.
Dr. John Carroll was a member of the Steering Committee for the RESPECT trial sponsored by Abbott. He was a local site investigator for the REDUCE trial sponsored by Gore. He also served as a local site investigator in TRILUMINATE, COAPT, EVEREST 2, SUMMIT, and EXPAND clinical trials, on the Steering Committee for the AMULET IDE project, and as Chair of the DSMB for the TENDYNE EFS project, all associated with Abbott. Dr. Carroll was also a consultant for Holistick.
Dr. Richard Smalling was a member of the Steering Committee for the RESPECT trial, sponsored by Abbott at St Jude’s Hospital and AGA Medical. He has also served as a consultant for Proctor for PFO closure.
Dr. Peter Jüni serves as unpaid member of the steering group of trials funded by Abbott Vascular, Astra Zeneca, Biotronik, Biosensors, St. Jude Medical, Terumo and The Medicines Company, has received research grants to the institution from Astra Zeneca, Biotronik, Biosensors International, Eli Lilly and The Medicines Company, and honoraria to the institution for participation in advisory boards and/or consulting from Amgen, Ava and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer.
Dr. Heinrich Mattle has been the Neurology PI of the PC-Trial, and has received honoraria for consulting from Covidien/Medtronic, Neuravi/Cerenovus, Servier, and Bayer outside the submitted work.
Dr. Bernhard Meier has received speaker fees from Abbott.
Dr. David Thaler was a member of the Steering Committee for the RESPECT Trial sponsored by Abbott.
Dr. Kent, Ms. Ruthazer, and Dr. Reisman have no disclosures to report.
References
- 1.Homma S, Di Tullio MR. Patent Foramen Ovale and Stroke. J Cardiol. 2010;56:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40:2349–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent DM, Ruthazer R, Weimar C, Mas JL, Serena J, Homma S, Di Angelantonio E, Di Tullio MR, Lutz JS, Elkind MS, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prefasi D, Martinez-Sanchez P, Fuentes B, Diez-Tejedor E. The utility of the RoPE score in cryptogenic stroke patients </=50 years in predicting a stroke-related patent foramen ovale. International journal of stroke : official journal of the International Stroke Society. 2016;11:NP7–8. [DOI] [PubMed] [Google Scholar]
- 5.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. The New England journal of medicine. 2012;366:991–999. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL, Investigators R. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. The New England journal of medicine. 2013;368:1092–1100. [DOI] [PubMed] [Google Scholar]
- 7.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. The New England journal of medicine. 2013;368:1083–1091. [DOI] [PubMed] [Google Scholar]
- 8.Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Saver JL, Smalling RW, Juni P, Mattle HP, et al. Device Closure of Patent Foramen Ovale After Stroke: Pooled Analysis of Completed Randomized Trials. J Am Coll Cardiol. 2016;67:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahabreh IJ, Kent DM. Index event bias: an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305:822–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng W, Yin S, McMullin D, Inglessis-Azuaje I, Elmariah S, Hung J, Lo EH, Palacios IF, Buonanno FS, Ning M. Residual Shunt After Patent Foramen Ovale Closure and Long-Term Stroke Recurrence. Ann Intern Med. 2020;172:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mono ML, Geister L, Galimanis A, Jung S, Praz F, Arnold M, Fischer U, Wolff S, Findling O, Windecker S, et al. Patent foramen ovale may be causal for the first stroke but unrelated to subsequent ischemic events. Stroke. 2011;42:2891–2895. [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Mattle HP, Thaler D. Patent Foramen Ovale Closure Versus Medical Therapy for Cryptogenic Ischemic Stroke: A Topical Review. Stroke. 2018;49:1541–1548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.