Abstract
As the prevalence of asymptomatic COVID-19 continues to increase, there is an increasing possibility that patients with COVID-19 may presen with ST-segment elevation myocardial infarction (STEMI). With social distancing and restricted access to preventive healthcare and emergency services, the management of acute cardiac emergencies such as myocardial infarction has suffered collateral damage. Thus far, global trends suggest a decrease in STEMI activations with possible worse outcomes due to delayed presentation and management. In this review, we discuss the challenges to STEMI management in the COVID-19 era and provide potential solutions for adherence to evidence-based therapies as the pandemic progresses into the year 2021.
Abbreviations and acronyms: CAD, coronary artery disease; COVID-19, coronavirus disease 2019; HEPA, high-efficiency particulate air; LUCAS, Lund University cardiopulmonary assist system; PPCI, primary percutaneous coronary interventions; PCI, percutaneous coronary interventions; PPE, primary protective equipment; STEMI, ST-segment elevation myocardial infarction; ECG, electrocardiogram; WMA, wall motion abnormalities
Keywords: COVID-19, STEMI, PCI, Pandemic
Introduction
Coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization on March 11, 2020. Since the initial breakout from Wuhan district, China, the disease has spread to over 177 countries, with the United States having the maximum number of cases. There is a high prevalence of cardiovascular risk factors such as hypertension and diabetes mellitus among patients with COVID-19 [1,2]. These patients are also at an increased risk of death due to COVID-19 [2].
There are several caveats unique to the management of ST-segment elevation myocardial infarction (STEMI) during the COVID-19 pandemic. In patients with COVID-19, diverse conditions such as myo-pericarditis, coronary artery vasospasm, pulmonary embolism, or stress-induced cardiomyopathy may mimic STEMI [3]. Additionally, there is a delay in patient presentation, referral, and transport to the treatment facility [4,5]. Patients with STEMI and COVID-19 may have significant thrombus burden due to heightened hypercoagulability resulting in suboptimal results after primary percutaneous coronary interventions (PPCI) due to slow flow, or no-reflow warranting novel approaches for treatment [6]. In this review, we highlight the challenges and discuss potential approaches to optimize the treatment of STEMI in patients with COVID-19.
Trends in incidence of STEMI in the COVID-19 era
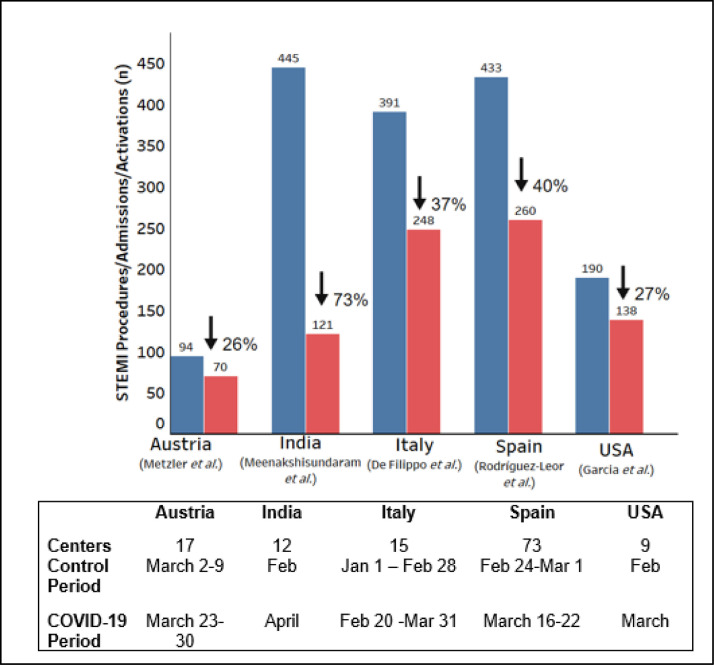
There has been a decrease in STEMI-related admissions in the COVID-19 period (Fig. 1 ) [7], [8], [9]–10]. STEMI activations in nine high-volume centers (>100 PPCI/year) in the United States decreased from >180/month in the before COVID-19 period to 138/month in the COVID-19 period [7]. Similarly, data from fifteen hospitals in Italy suggests a decrease in admission for STEMI from 8/day to 6.1/day in the COVID-19 period [8].
Fig. 1.
Trends of STEMI activation across countries with a high burden of COVID-19. Blue and red bars represent the number of activations in the control and study period, respectively. The details of the control and study period are given in the table below the panel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Along with decreased activations, there is an increase in time to reperfusion. A high-volume center in Ireland reported an increase in ischemic time from 485 to 1,550 min, with an increase in the delay from the point of the first medical contact to the point of activation [5]. A recent study from a hospital offering 24/7 PPCI facilities in Hong Kong suggested an ~300% increase in time from symptom onset to first medical contact, a 30% increase in door-to-device time, and a 60% increase in time from arrival to the cardiac catheterization laboratory to PPCI [4]. This delay in reperfusion is due to both patient-related and systemic factors. Patients may avoid or delay seeking medical care or present at low-volume centers to minimize the risk of exposure to COVID-19 [3,11]. System-related factors may include an increase in emergency department (ED) time for additional COVID -19 testing/confirmation; a decrease in focus on cardiovascular disease and instead, an increased focus on COVID-19 and consequent time spent on performing additional diagnostic tests such as chest X-ray and computed tomography (CT); and possible high threshold to transfer patients with COVID-19 to the cardiac catheterization lab. Although current guidelines advocate avoiding any delays in PPCI in STEMI, it is important to note that a high proportion of patients with COVID-19 may not have significant epicardial coronary artery disease on angiography (Table 1 ).
Table 1.
Studies with COVID-19 patients presenting with ST-segment elevation on electrocardiogram and undergoing invasive coronary angiography.
| Author | Country | Sample size | Women (%) | Mean Age (years) | Hypertension (%) | Diabetes Mellitus (%) | Previous MI/ PCI/CABG (%) | Culprit lesion identified during invasive angiography n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Alaarag et al. [44] | Egypt | 26 | 30.8 | 57.7 | 42.3 | 38.5 | 15.3 | 18/26 (69) |
| Bangalore et al. [24] | United States | 18 | 17 | 63 | 65 | 35 | – | 6/9 (67) |
| Choudry et al. [22] | United Kingdom | 39 | 15.4 | 61.7 | 71.8 | 46.2 | 15.4 | 38/38 (100) |
| Hamadeh et al. [45] | Lithuania, Italy | 19 | 53 | 65 | 79 | 11 | 5 | 18/19 (95) |
| Spain, Iraq | ||||||||
| NACMI [46] | United States | 171 | 30 | – | 73 | 44 | 48 | 115/138 (83) |
| Siudak et al. [47] | Poland | 145 | 28.7 | 63 | 46.2 | 14.5 | 25.5 | 123/143 (86) |
| Secco et al. [48] | Italy | 31 | 22.6 | 72.3 | 71 | 38.7 | 35.4 | 8/10 (80) |
| Stefanini et al. [28] | Italy | 28 | 28.6 | 68 | 71.4 | 32.1 | 10.7 | 17/28 (61) |
CABG, coronary artery bypass grafting, MI, myocardial infarction, NACMI, North American COVID-19 STEMI Registry, PCI, Percutaneous coronary intervention.
The decreased hospitalization for acute care suggests that a large proportion of patients with STEMI may have died without seeking medical care [12]. Emergency Medical System Incident Dispatch data from the New York Emergency Medical Services suggest around a 400% increase in calls for cardiac arrest during March 2020 [13]. A recent analysis of cause-specific mortality in the United States suggested a larger than expected increase in death due to non-respiratory causes, with heart disease and diabetes mellitus as the most notable causes [14].
The personal experience of the authors suggests an increased incidence of STEMI-related mechanical complications such as development of ventricular septal defects and papillary muscle rupture. The treating team needs to be aware of these complications, especially in those presenting late after onset of chest pain.
Pathophysiology of STEMI unique to COVID-19
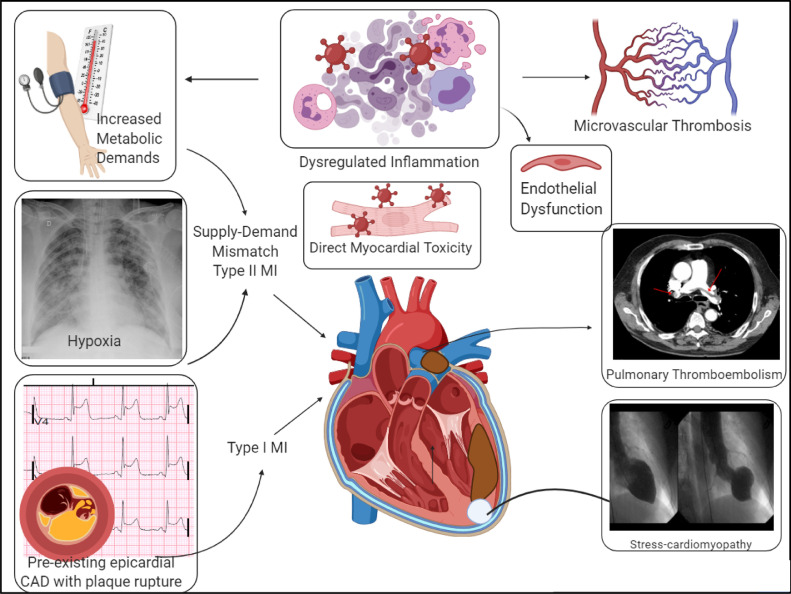
Infectious illnesses like influenza are associated with systemic inflammation and hypercoagulability, leading to an increased risk of atherosclerotic plaque rupture and thrombosis [15]. However, thromboembolic complications in patients with severe COVID-19 occur at a higher incidence compared to other acute infections [16]. Multiple pathogenic mechanisms contribute to hypercoagulability in COVID-19. The dysregulated systemic immune response to viral replication results in the activation of the complement and coagulation cascade [17] (Fig. 2 ). Endothelial cell dysfunction mediated by direct viral invasion through angiotensin converting enzyme-2 receptors and secondary to systemic inflammation has been found to cause endothelitis and thrombosis in micro-circulation in autopsy studies [18,19]. In particular, the formation of microthrombi in multiple organ systems including the lung and the myocardium may result in troponin elevation even in the absence of ST-elevation, making diagnosis challenging. Systemic inflammation along with profound hypercoagulability predisposes to both arterial and venous thrombosis [20], even without traditional risk factors [21]. Thrombosis increases with worse disease severity and patients with thrombotic events have a significantly elevated risk of all-cause mortality.
Fig. 2.
Potential mechanisms of myocardial injury in COVID-19.
Patients with severe COVID-19 and risk factors are at an increased risk of adverse cardiac events such as cardiogenic shock and cardiovascular death [3]. In a study of 3,334 adult patients admitted to a New York health system with COVID-19, myocardial infarction occurred in 8.9% and accounted for a majority of the arterial thrombotic events [20]. The incidence of myocardial infarction and arterial thrombosis was higher in patients in the intensive care unit vs. others. Another single-center observational study revealed that patients presenting with a STEMI with concurrent COVID-19 had a higher incidence of multivessel thrombosis and stent thrombosis when compared to STEMI patients without COVID-19 [22]. Patients with COVID-19 also had a higher modified thrombus grade resulting in increased utilization of GP IIb/IIIa inhibitors and aspiration thrombectomy [22].
Multiple laboratory markers have been evaluated to identify patients at a higher risk of thrombosis and adverse events with COVID-19. Initial elevation and rising levels of d-dimer during the hospital stay has been consistently associated with an increased risk of thrombosis, mortality, and adverse clinical outcomes [20,22,23]. In addition to d-dimer, troponin elevation can also help identify patients at higher risk. In COVID-19 positive patients with ST-segment elevation, those with a myocardial infarction had higher mean troponin levels vs. those without [24]. Higher troponin levels are independently associated with an increased risk of mortality after adjusting for baseline characteristics [25].
Differential diagnosis of ST-segment elevation in patients with COVID-19
Besides myocardial infarction, elevated troponin levels may be due to myocarditis, stress-induced cardiomyopathy, myopericarditis, spontaneous coronary artery dissection, systemic microthrombi, and pulmonary embolism [1,3,26,27]. In a case series of 28 patients with STEMI and COVID-19 from the Lombardy region in Italy, ~40% had non-obstructive CAD [28]. In another case series of 18 patients with COVID-19 who presented with ST-segment elevations, eight patients had a myocardial infarction and ten had a non-coronary myocardial injury [24]. Of the nine patients who underwent invasive coronary angiography, only six had obstructive CAD. Troponin-T was elevated in both patients with and without myocardial infarction but peak levels were higher in those with myocardial infarction [24]. Examples of ST-segment elevation patterns in patients with COVID-19 was reported recently by Bangalore and colleagues [24].
Pre-hospital logistics and initial management of patients with COVID-19 and STEMI
Regardless of the COVID-19 status, timely PPCI is the standard of care for patients with STEMI [29]. The ED physicians play an essential role in the timely diagnosis and triage of patients, especially those where the diagnosis of STEMI is unclear due to atypical EKG findings, delayed presentation, or high clinical suspicion for alternate diagnoses. ED evaluation should be focused on rapid risk stratification of COVID-19 status and utilization of point-of-care ultrasound (POCUS) or portable echocardiography to determine the likelihood of coronary occlusion [26]. In COVID-19 positive patients or a person under investigation, assessment of regional wall motion using POCUS may help differentiate STEMI from myocarditis [29]. In a case series of 28 patients with STEMI and COVID-19 from Italy, 22 (82.1%) patients had localized wall-motion abnormalities [28]. The presence of regional wall motion abnormalities suggests STEMI whereas global hypokinesis usually suggests myocarditis. Although there is some evidence to support the possible diagnostic role of computed tomography (CT) coronary angiography in patients with COVID-19 to rule out significant epicardial CAD, the majority of the society guidelines recommend early catheterization laboratory transfer for all patients with suspicion for STEMI [26,30].
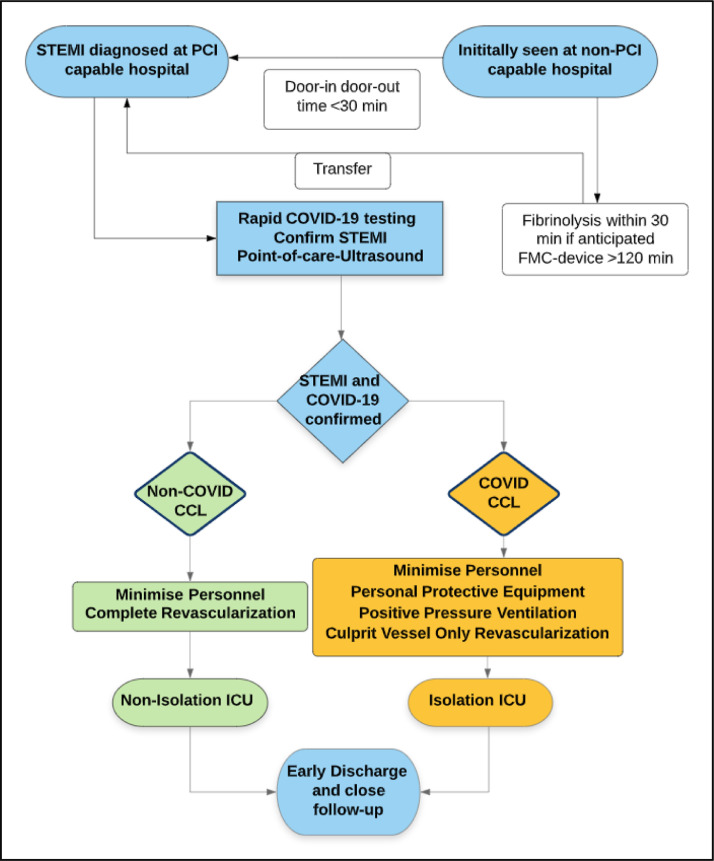
The management of patients with STEMI at non-PPCI capable facility may be challenging and needs a multi-system coordinated effort. If initial contact with a PPCI capable facility is >120 min, fibrinolysis should be considered based on physician experience, when appropriate, followed by immediate transfer to a PPCI-capable facility. Timely communication between emergency medical services personnel and physicians on diagnosis, COVID-19 status, fibrinolysis therapy, and early activation of cardiac catheterization laboratory and isolation cardiac intensive care unit beds can help reduce system delays in PPCI (Fig. 3 ).
Fig. 3.
Multi-disciplinary collaboration of systems for the management of STEMI diagnosed at non-PCI capable locations. CCL, cardiac catheterization laboratory, COVID, Coronavirus disease, FMC, first medical contact, ICU, intensive care unit, PCI, percutaneous coronary intervention, POCUS, point of care ultrasound, STEMI, ST-elevation myocardial infarction.
Management of cardiac arrest in COVID-19 patients presenting with STEMI
Management of cardiac arrest can be a major challenge in a suspected/confirmed COVID-19 patient. Consensus statements released from various societies support the dawning of appropriate primary protective equipment (PPE) including N95 masks, long-sleeve gowns, gloves, and face shields to minimize exposure to healthcare workers [29]. For proper protection, it is critical to understand the correct technique of dawning and doffing PPE [31]. Oxygenation and ventilation strategies should be prioritized to minimize aerosolization, and early endotracheal intubation with connection to high-efficiency particulate air filter enabled airway system should be performed in patients with impending respiratory failure [32]. Immediate defibrillation of a shockable rhythm reduces the risk of aerosolization more than chest compressions in patients with an unprotected airway and should be considered where indicated [29]. Wherever feasible, use of the Lund University Cardiopulmonary Assist System (LUCAS) device, a mechanical device that provides automatic chest compressions should be encouraged. Institutions should establish policies to address goals of care discussion with patients to help guide providers for the appropriateness of resuscitation efforts [33]. In patients with known or high probability of COVID-19, placement of venous-venous extracorporeal membrane oxygenation should be considered in the setting of respiratory failure and inability to oxygenate. Whenever appropriate, mechanical circulatory support and extracorporeal membrane oxygenation should be placed at the bedside to avoid the risk of exposure to catheterization laboratory personnel [26].
Reperfusion strategy in patients with COVID-19 and STEMI
PPCI is associated with better outcomes in patients with STEMI relative to thrombolysis and should be the preferred reperfusion strategy even during the ongoing pandemic [34]. PPCI has been shown in multiple trials to have a better success rate in terms of achieving Thrombolysis In Myocardial Infarction-3 flow [35]. Furthermore, most patients treated with thrombolysis at non-PPCI eventually require either rescue or definitive PCI. PPCI, therefore, eventually leads to better outcomes [36]. Moreover, there is also a higher prevalence of coagulation abnormalities in patients with COVID-19 [17]; thus, patients treated with thrombolysis would be at an increased risk of fatal complications such as hemorrhagic cardiac tamponade, intracranial bleeding, or hemorrhagic shock [37]. Taken together, even in the setting of COVID-19, the risk-benefit profile favors PPCI over thrombolysis. The risk of exposure to health care workers in the cardiac catheterization laboratories can be significantly minimized by effective use of PPE [26].
Catheterization laboratory management of STEMI in patients with COVID-19
In compliance with the World Health Organization guidelines for high-exposure procedures, all healthcare workers in the cardiac catheterization laboratory must wear appropriate PPE, including gown, gloves, goggles or face shields, and N95 masks [29]. The number of personnel in the laboratory, the amount of equipment should be minimized, and stratified to avoid over-exposure to COVID-19. Radial artery access helps the early discharge of patients and should be encouraged [38]. If femoral artery access is utilized, closure devices should be favored over manual compression to further minimize patient contact and staff exposure [39]. In the current scenario where critical care beds are extremely valuable, efforts should be made by catheterization laboratory operators to stratify low-risk STEMI patients using scoring systems, such as the CADILLAC and the Zwolle scores, to identify patients who may be safe for an early discharge without the requirement of cardiac intensive care [40], [41]–42].
Post-PPCI management of COVID-19 patients with STEMI
Post-PPCI management should focus on minimizing exposure and early transfer to an intermediate care observation unit for 24–48 h followed by an early discharge for low-risk patients (see above). This would require a careful assessment of procedural success, hemodynamic stability, and the ability of the patient to follow-up in case of adverse events. Interestingly, there may be a role of serial measurements of biomarkers such as cardiac troponins and N-Terminal Pro-B-Type Natriuretic Peptide in the post-PPCI setting to stratify patients who may more likely achieve sustained revascularization and have a lower risk for short term adverse cardiac events, and consequently plan them for early discharge [43]. For high risk or hemodynamically unstable patients, isolation critical care beds are required for a more prolonged stay. Furthermore, as hospital systems open and family members can visit in a controlled setting, wearing masks, maintaining social distancing and routine handwashing should be strictly enforced. Regardless of the risk, the role of telemedicine and out-patient follow-up, including potentially home-based rehabilitation would be crucial to improve overall outcomes [29].
Conclusion
During the ongoing COVID-19 pandemic, there is a need to further educate patients with symptoms of MI and the need to seek medical attention early. Coordinated care among emergency medical service, ED, and catheterization laboratory personnel is needed to ensure least time to revascularization. All PPCI-capable hospitals should designate a catheterization laboratory with appropriate ventilation for patients with confirmed or suspected COVID-19, and the use of PPE should be enforced when caring for these patients. Along with early discharge, patients should be monitored for electro-mechanical complications. Furthermore, heightened knowledge of potential drug-drug interactions when newer COVID-19 specific therapies are used is needed.
Footnotes
Disclosures: Dr. Qamar reports receiving institutional grant support from the NorthShore Auxiliary research scholar fund, Daiichi-Sankyo, American Heart Association, and fees for educational activities from the American College of Cardiology, Society for Vascular Medicine, Society for Cardiovascular Angiography and Interventions, Janssen and Janssen, Pfizer, Medscape, and Clinical Exercise Physiology Association. The other authors have no conflict of interest related to this work.
Ethical statement: The paper is not under consideration elsewhere. None of the paper's contents have been previously published. Author disclosures are explicitly stated in the manuscript text. All authors have read and approved the manuscript and meet guidelines for authorship.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam C.C.F., Cheung K.S., Lam S., Wong A., Yung A., Sze M., et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circul: Cardiovas Qual Outcomes. 2020;13(4):e006631. doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlan J.J., Chongprasertpon N., Arockiam S., Arnous S., Kiernan T.J. COVID-19 and STEMI: a snapshot analysis of presentation patterns during a pandemic. Int J Cardiol Heart Vasc. 2020;30 doi: 10.1016/j.ijcha.2020.100546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia S., Albaghdadi M.S., Meraj P.M., Schmidt C., Garberich R., Jaffer F.A., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Filippo O., D'Ascenzo F., Angelini F., Bocchino P.P., Conrotto F., Saglietto A., et al. Reduced rate of hospital admissions for ACS during covid-19 outbreak in northern Italy. N Engl J Med. 2020;383(1):88–89. doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzler B., Siostrzonek P., Binder R.K., Bauer A., Reinstadler S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Leor O., Alvarez-Álvarez B., Ojeda S., Martín-Moreiras J., Ramón Rumoroso J., López-Palop R., Serrador A., Cequier Á., Romaguera R., Cruz I., Pérez de Prado A., Moreno R., l on behalf of all the participants of the ACI-SEC Infarction Code Registry Impact of the COVID-19 pandemic on interventional cardiology activity in Spain. REC: Interv Cardiol. 2020;2:82–89. [Google Scholar]
- 11.G.W V. Poll Results: Another Take on STEMI During the Pandemic: American College of Cardiology; 2020 [Available from: https://www.acc.org/latest-in-cardiology/articles/2020/04/27/09/38/poll-results-another-take-on-stemi-during-the-pandemic.
- 12.Baldi E., Sechi G.M., Mare C., Canevari F., Brancaglione A., Primi R., et al. Out-of-hospital cardiac arrest during the covid-19 outbreak in Italy. N Engl J Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NYC OpenData: EMS Incident Dispatch Data: City of New York; [updated August 13, 2020. Available from: https://data.cityofnewyork.us/Public-Safety/EMS-Incident-Dispatch-Data/76xm-jjuj.
- 14.Woolf S.H., Chapman D.A., Sabo R.T., Weinberger D.M., Hill L. Excess deaths from COVID-19 and other causes. JAMA. 2020;324(15):1562–1564. doi: 10.1001/jama.2020.19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong J.C., Schwartz K.L., Campitelli M.A., Chung H., Crowcroft N.S., Karnauchow T., et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 16.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Pulmonary embolism in patients with COVID-19. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 17.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R., et al. Pathophysiology of SARS-CoV-2: Targeting of Endothelial Cells Renders a Complex Disease with Thrombotic Microangiopathy and Aberrant Immune Response. The Mount Sinai COVID-19 Autopsy Experience. medRxiv. 2020:2020.05.18.20099960.
- 20.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fifi J.T., Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol. 2020;19(9):713–715. doi: 10.1016/S1474-4422(20)30272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudry F.A., Hamshere S.M., Rathod K.S., Akhtar M.M., Archbold R.A., Guttmann O.P., et al. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2020;76(10):1168. doi: 10.1016/j.jacc.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal A., Singh A.D., Jain V., Aggarwal M., Gupta S., Padappayil R.P., et al. The association of d-dimers with mortality, intensive care unit admission or acute respiratory distress syndrome in patients hospitalized with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Heart Lung: J Crit Care. 2021;50(1):9–12. doi: 10.1016/j.hrtlng.2020.08.024. S0147-9563(20)30380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., et al. ST-segment elevation in patients with covid-19 – a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michela S., Barbara B., Cioffi S.M.G., Morenghi E., Leone F.P., Maura F., et al. Association between cardiac troponin I and mortality in patients with COVID-19. Biomarkers. 2020;25(8):634–640. doi: 10.1080/1354750X.2020.1831609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmud E., Dauerman H.L., Welt F.G.P., Messenger J.C., Rao S.V., Grines C., et al. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(11):1375–1384. doi: 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation 2020 Jun 23;141(25):2113-2116. [DOI] [PMC free article] [PubMed]
- 29.The European Society for Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic 2020 [updated 10 June 2020. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance.
- 30.Linde J.J., Kelbæk H., Hansen T.F., Sigvardsen P.E., Torp-Pedersen C., Bech J., et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;75(5):453. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Ortega R., Gonzalez M., Nozari A., Canelli R. Personal protective equipment and covid-19. N Engl J Med. 2020;382(26):e105. doi: 10.1056/NEJMvcm2014809. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan E.H., Gibson L.E., Berra L., Chang M.G., Bittner E.A. In-hospital airway management of COVID-19 patients. Crit Care. 2020;24(1):292. doi: 10.1186/s13054-020-03018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thapa S.B., Kakar T.S., Mayer C., Khanal D. Clinical outcomes of in-hospital cardiac arrest in COVID-19. JAMA Intern Med. 2020:e204796. doi: 10.1001/jamainternmed.2020.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 35.Andersen H.R., Nielsen T.T., Rasmussen K., Thuesen L., Kelbaek H., Thayssen P., et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349(8):733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 36.Huynh T., Perron S., O'Loughlin J., Joseph L., Labrecque M., Tu J.V., et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction: Bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation. 2009;119(24):3101–3109. doi: 10.1161/CIRCULATIONAHA.108.793745. [DOI] [PubMed] [Google Scholar]
- 37.Gurwitz J.H., Gore J.M., Goldberg R.J., Barron H.V., Breen T., Rundle A.C., et al. Risk for intracranial hemorrhage after tissue plasminogen activator treatment for acute myocardial infarction. Ann Intern Med. 1998;129(8):597–604. doi: 10.7326/0003-4819-129-8-199810150-00002. [DOI] [PubMed] [Google Scholar]
- 38.Mason P.J., Shah B., Tamis-Holland J.E., Bittl J.A., Cohen M.G., Safirstein J., et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circul: Cardiovasc Interv. 2018;11(9) doi: 10.1161/HCV.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 39.Hamid T., Choudhury T.R., Clarke B., Mahadevan V.S. Pre-closure of large-sized arterial access sites in adults undergoing transcatheter structural interventions. Cardiol Ther. 2015;4(1):59–63. doi: 10.1007/s40119-014-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halkin A., Singh M., Nikolsky E., Grines C.L., Tcheng J.E., Garcia E., et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol. 2005;45(9):1397–1405. doi: 10.1016/j.jacc.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 41.De Luca G., Suryapranata H., van 't Hof A.W., de Boer M.J., Hoorntje J.C., Dambrink J.H., et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109(22):2737–2743. doi: 10.1161/01.CIR.0000131765.73959.87. [DOI] [PubMed] [Google Scholar]
- 42.Lopez J.J., Ebinger J.E., Allen S., Yildiz M., Henry T.D. Adapting STEMI care for the COVID-19 pandemic: the case for low-risk STEMI triage and early discharge. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2020:10.1002/ccd.28993. [DOI] [PMC free article] [PubMed]
- 43.Heeschen C., Hamm C.W., Mitrovic V., Lantelme N.H., White H.D. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110(20):3206–3212. doi: 10.1161/01.CIR.0000147611.92021.2B. [DOI] [PubMed] [Google Scholar]
- 44.Alaarag A., Hassan T., Samir S., Naseem M. Clinical and angiographic characteristics of patients with STEMI and confirmed diagnosis of COVID-19: an experience of Tanta University Hospital. Egypt Heart J. 2020;72(1):68. doi: 10.1186/s43044-020-00103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamadeh A., Aldujeli A., Briedis K., Tecson K.M., Sanz-Sánchez J., Al Dujeili M., et al. Characteristics and outcomes in patients presenting with COVID-19 and ST-segment elevation myocardial infarction. Am J Cardiol. 2020;131:1–6. doi: 10.1016/j.amjcard.2020.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry T.D. Initial Outcomes from NACMI the North American COVID-19 STEMI Registry. Cardiovascular Reseaech Foundation TCT CONNECT 2020.
- 47.Siudak Z., Grygier M., Wojakowski W., Malinowski K.P., Witkowski A., Gąsior M., et al. Clinical and procedural characteristics of COVID-19 patients treated with percutaneous coronary interventions. Catheter Cardiovasc Interv. 2020;96(6):E568–E575. doi: 10.1002/ccd.29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Secco G.G., Tarantini G., Mazzarotto P., Garbo R., Parisi R., Maggio S., et al. Invasive strategy for COVID patients presenting with acute coronary syndrome: the first multicenter Italian experience. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2020 doi: 10.1002/ccd.28959. [DOI] [PMC free article] [PubMed] [Google Scholar]