Abstract
Parasympathetic signalling via muscarinic acetylcholine receptors (mAChRs) regulates gastrointestinal smooth muscle function. In most instances, the mAChR population in smooth muscle consists mainly of M2 and M3 subtypes in a roughly 80% to 20% mixture. Stimulation of these mAChRs triggers a complex array of biochemical and electrical events in the cell via associated G proteins, leading to smooth muscle contraction and facilitating gastrointestinal motility. Major signalling events induced by mAChRs include adenylyl cyclase inhibition, phosphoinositide hydrolysis, intracellular Ca2+ mobilisation, myofilament Ca2+ sensitisation, generation of non-selective cationic and chloride currents, K+ current modulation, inhibition or potentiation of voltage-dependent Ca2+ currents and membrane depolarisation. A lack of ligands with a high degree of receptor subtype selectivity and the frequent contribution of multiple receptor subtypes to responses in the same cell type have hampered studies on the signal transduction mechanisms and functions of individual mAChR subtypes. Therefore, novel strategies such as genetic manipulation are required to elucidate both the contributions of specific AChR subtypes to smooth muscle function and the underlying molecular mechanisms. In this article, we review recent studies on muscarinic function in gastrointestinal smooth muscle using mAChR subtype-knockout mice.
Keywords: muscarinic receptor subtypes, knockout mouse, smooth muscle, gastrointestinal tract, non-selective cationic channels, signal transduction pathways
1. Introduction
Muscarinic acetylcholine receptors (mAChRs) are widely expressed at presynaptic and postsynaptic sites throughout the body, where they regulate many critical neurological and physiological processes, including neural excitability, cardiac and smooth muscle contraction and endocrine and exocrine gland activity. The mAChR family consists of five molecularly distinct subtypes, M1–M5, all of which are coupled to membrane-associated GTP-binding proteins (G-proteins) that transduce binding of acetylcholine (ACh) and other muscarinic agonists into various intracellular signalling cascades [1,2,3,4]. Molecular biological studies have shown that the M2 and M4 subtypes are preferentially coupled to G-proteins of the pertussis toxin (PTX)-sensitive Gi/o family, whereas M1, M3 and M5 are selectively coupled to the PTX-insensitive Gq/11 family [3]. However, more precise identification of specific functions in individual cell types and tissues has been challenging due to the paucity of muscarinic receptor subtype-specific antibodies for immunohistochemistry [5], the lack of commercially available and highly subtype-selective ligands, the complex overlapping expression patterns of individual subtypes and the contributions of multiple subtypes to the same cellular or physiological response. To circumvent these problems, many mAChR subtype-specific knockout (KO) mouse strains have been generated by gene targeting technology [6,7]. As disruption of one specific mAChR gene appears to have little substantial effect on the expression levels of the remaining four mAChRs [8], these models are providing crucial evidence for the contributions of specific mAChR subtypes to various developmental, physiological and pathophysiological processes.
In the gastrointestinal tract and many other visceral organs, release of ACh from autonomic nerves triggers excitation and contraction of smooth muscle by activating mAChRs. Smooth muscle mAChRs are a mixture of M2 and M3 subtypes with M2 predominance (M2:M3 = 3–5:1) [1,9], although mRNAs encoding all five mAChR subtypes have been detected in gastrointestinal smooth muscle [10]. Activation of mAChRs triggers multiple biochemical and electrical signalling events that modulate contraction [11,12,13,14]. Traditional studies using various mAChR antagonists suggested that the M3 subtype is the primary mediator of contraction in visceral smooth muscles, while the contribution of the M2 subtype was considered less clear [15,16]. However, more recent studies have demonstrated that the M2 subtype modulates contraction, at least in part by inhibiting cyclic AMP (cAMP)-dependent relaxation [9] and by regulating smooth muscle ion channel activity [17,18,19]. Nevertheless, little is known as to which mAChR subtype mediates the individual cellular events that underlie or modulate the contractile response.
In this article, we review key results from studies using mAChR subtype-specific KO mice to examine contributions to the excitation and contraction of smooth muscle and the underlying molecular signalling pathways. In addition to the contributions of post-junctional mAChRs expressed by smooth muscle cells, pre-junctional mAChRs expressed by various enteric neurons contribute to regulation of smooth muscle activity through modulation of excitatory or inhibitory neurotransmitters, including ACh, substance P and nitric oxide (NO). The roles of neural mAChR subtypes have been studied using mAChR-mutant mice [20,21] and reviewed [22].
2. Adenylyl Cyclase Inhibition and Phosphoinositide Hydrolysis
A common feature of mAChR activation in gastrointestinal smooth muscle is the co-induction of two second messenger responses, adenylyl cyclase inhibition resulting in reduced accumulation of cAMP and stimulation of phospholipase C (PLC) hydrolysis of phosphoinositides resulting in formation of inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG). In turn, IP3 releases Ca2+ from intracellular stores and DAG activates protein kinase C (PKC), leading to the phosphorylation of various proteins [1,9,15]. Further, muscarinic subtypes demonstrate complex reciprocal modulation of second messenger pathways.
Sakamoto et al. (2007) [23] examined effects of the potent non-subtype-selective muscarinic agonist carbachol on accumulation of cAMP elicited by the β-adrenoceptor agonist isoprenaline, which activates adenylyl cyclases, in ileal longitudinal muscles from mAChR-KO and wild-type mice. Carbachol (1 µM) induced greater inhibition of isoprenaline-stimulated cAMP accumulation in ileal muscle from M3-KO mice than wild-type mice (50% vs. 26%). In M2-KO mice, however, carbachol enhanced isoprenaline-stimulated cAMP accumulation by ~30%, while M2/M3 double-KO mice exhibited only a slight increase. These results are consistent with a pharmacological study in rabbit stomach suggesting that M2 receptors are coupled to inhibition of adenylyl cyclases via Gαi3 and that M3 receptors are coupled to adenylyl cyclase activation via Gβγq/11 [24]. Thus, the cAMP response to carbachol in wild-type mice reflects the predominant inhibitory influence of M2 receptors.
Tran et al. (2006) [25] investigated the coupling of mAChR subtypes to phosphoinositide hydrolysis in ileum and urinary bladder smooth muscles from mAChR-KO mice by measuring agonist-mediated conversions of [3H]inositol-labelled phosphoinositides into [3H]inositol phosphates in response to the potent muscarinic agonist oxotremorine-M. Phosphoinositide hydrolysis in the urinary bladder did not differ markedly between M2-KO and wild-type mice during oxotremorine-M stimulation, and no measurable response was observed in bladder tissue from M3-KO or M2/M3 double-KO mice. These results are consistent with a previous study of guinea-pig urinary bladder suggesting that the muscarinic phosphoinositide response is exclusively M3-mediated [26]. On the other hand, in the ileum, M1, M2 and M3 subtypes appeared to participate in the oxotremorine-M-induced phophoinositide response [25]. In this tissue, M2-KO mice exhibited small and M3-KO mice displayed large decreases in phosphoinositide hydrolysis, while M2/M3 double-KO mice still demonstrated a substantial response, similar to an M1 profile in competitive antagonism experiments. The relative contributions of M1, M2 and M3 subtypes to the wild-type phosphoinositide response amounted to 15%, 5% and 80%, respectively, suggesting a major role for the M3 receptor and minor roles for the M1 and M2 receptors in mouse ileum, which is generally consistent with multiple pharmacological studies suggesting that M3 mediates phosphoinositide hydrolysis via coupling to Gq/11 proteins [9,27]. Alternatively, M2 likely stimulates PLC due to βγ dimer released from Gi proteins [28]. The M1-mediated response likely occurs in ileal smooth muscle cells rather than associated cells such as enteric neurons since oxotremorine-M also induced robust phosphoinositide hydrolysis in dissociated muscle cells from M2/M3 double-KO ileum [25]. However, the resultant second messengers InsP3 and DAG appear unlikely to be involved in contraction and other muscarinic effects, including intracellular Ca2+ release, membrane depolarisation, activation or inhibition of various ion channels and Ca2+ sensitisation of contractile proteins, because all were totally abolished by knockout of M2, M3 or both, as described below. Further study using M1-KO mice will be needed to confirm the contribution of M1-mediated phosphoinositide hydrolysis to gastrointestinal smooth muscle contraction.
3. Muscarinic Regulation of Ion Channel Activity
Multiple effects of mAChR activation on ion channels have been described in gastrointestinal and other visceral smooth muscles, including activation of non-selective cationic channels or chloride (Cl−) channels, inhibition or potentiation of voltage-dependent Ca2+ channels (VDCCs) and modulation of several K+ channel types [12,14,29,30]. These effects underlie muscarinic modulation of smooth muscle excitation and contraction, as evidenced by recent studies of ileal longitudinal smooth muscle cells from various mAChR-KO mice.
3.1. Activation of Non-Selective Cationic Channels
The opening of non-selective cationic channels is the primary mechanism by which mAChR activation produces smooth muscle depolarisation and contraction [11,31]. Early studies of guinea-pig ileal myocytes reported that the mAChR-mediated cationic current (mIcat) was sensitive to PTX and potentiated strongly by a rise in intracellular Ca2+ concentration ([Ca2+]i) [32,33,34], suggesting activation primarily by M2 receptors and potentiation by M3-mediated Ca2+ release. However, accumulating evidence now suggests that channel opening is a mixed M2/M3 response requiring co-activation of both receptors. Under conditions where [Ca2+]i was buffered to eliminate the influence of Ca2+ release, mIcat was inhibited by M2-preferring and M3-preferring antagonists, but also markedly depressed by anti-Gαo antibody and the PLC inhibitor U-73122 [17,35,36,37]. Furthermore, mIcat was blocked by YM-254890, a chromobacterium-derived peptide that specifically inhibits Gq/11 protein signalling activity [38] (see Figures 1 and 3 in Tanahashi et al., 2020 [39]). Sakamoto et al. (2006) [40] reported that mIcat in mouse ileal myocytes showed all of the typical pharmacological features of guinea pig mIcat. Therefore, it has been hypothesised that the mIcat of both species is activated through an interaction between M2 and M3 receptors and ensuing activation of downstream signalling pathways via coupling to Go and Gq/11 proteins, respectively.
To evaluate this hypothesis, Sakamoto et al. (2007) [23] measured carbachol-evoked mIcat in ileal myocytes from M2-KO, M3-KO, M2/M3 double-KO and wild-type mice under conditions where Ca2+ modulation was eliminated. In M2-KO and M3-KO myocytes, mIcat amplitudes evoked by a maximally effective carbachol dose (100 µM) were only 11% and 6% of wild-type amplitude, respectively, and current was undetectable in M2/M3 double-KO myocytes. However, all mutants demonstrated normal G-protein-cationic channel coupling as judged from their ability to generate cationic currents in response to infusion of the direct G-protein activator GTPγS. Taken together, these results provide direct evidence that the wild-type mIcat is not a simple additive response from M2 and M3 activation but results from a synergy between M2- and M3-mediated signalling pathways.
Single channel activity was also measured in outside-out membrane patches excised from M2-KO, M3-KO and wild-type ileal myocytes stimulated with carbachol [23]. Activated patches from wild-type myocytes showed three distinct patterns of cationic channel activity. One was characterised by 70-pS unitary conductance channels with three distinct open states (as distinguished by mean open times (Oτ values) = 0.25, 1.1 and 13.2 ms) and mimicked by active patches from M3-KO cells, indicating induction by M2. The second pattern consisted of mixed bursts of brief 70-pS and 120-pS unitary currents, each with single open states (Oτ = 0.46 and 0.32 ms, respectively) and mimicked by activated patches from M2-KO cells, indicating induction by M3. Finally, the third pattern was characterised by longer 70-pS unitary currents with four distinct open states (Oτ = 0.62, 2.7, 16.9 and 121.1 ms) undetected in active patches from M2-KO or M3-KO myocytes. Therefore, this channel activity requires M2/M3 interaction. These single channel analyses provide evidence that intact ileal myocytes are endowed with three distinct muscarinic cationic channel activation pathways initiated by activation of M2 or M3 and co-activation of M2 and M3, with the major contribution of the M2/M3 pathway to mIcat activity in intact ileal myocytes. Thus, deletion of M2 or M3 subtypes resulted in abolition of not only each separate pathway but also the M2/M3 synergistic pathway.
Transient receptor potential canonical (TRPC) channels are widely assumed to mediate G-protein-coupled non-selective cationic currents in smooth muscle [41]. Recently, Tsvilovskyy et al. (2009) [42] provided compelling evidence that TRPC4 and TRPC6 channels are responsible for mIcat in ileal myocytes, with relative contributions of 84% and ~19%, respectively, at a holding potential of −50 mV based on electrophysiological studies of TRPC4-KO, TRPC6-KO and TRPC4/6 double-KO mice. A similar result was obtained in gastric myocytes [43]. Single channel recordings by Tsvilovskyy et al. (2009) [42] also revealed that activity of a mAChR-dependent 55-pS unitary current was absent in TRPC4-KO ileal myocytes, suggesting that TRPC4 corresponds to the muscarinic 70-pS channel described by Sakamoto et al. (2007) [23]. Alternatively, the TRPC6-mediated mIcat could also be activated by DAG, similar to the mIcat observed in PTX-treated wild-type mouse ileal myocytes [40,44], suggesting that the TRPC6 channel mediates the 120-pS unitary current activated through the M3 pathway [23].
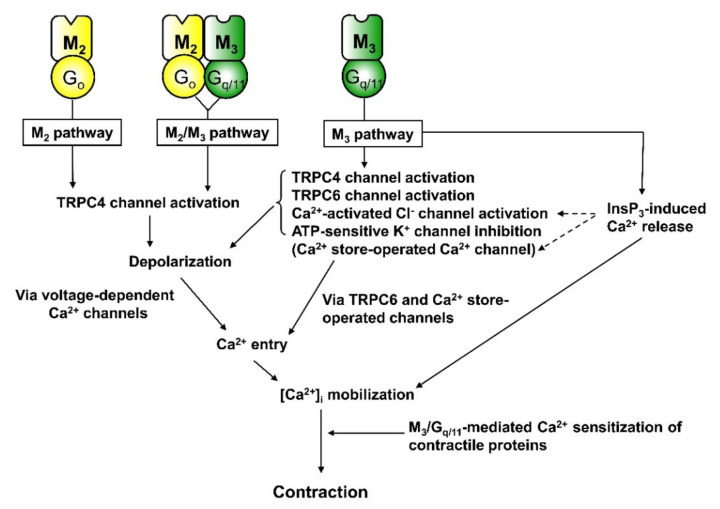
Figure 1 presents a scheme of the three distinct signal transduction pathways linking mAChR subtypes to cationic channel activation and mIcat generation. The M3 pathway activates the brief opening mode of 70-pS (TRPC4) and 120-pS (TRPC6) channels via Gq/11-PLC signalling. The lower conductance TRPC4 is regulated by the PLC substrate phosphatidylinositol 4,5-bisphosphate (PIP2), which stabilises the inactive conformation [45], while PIP2 depletion by M3 activity relieves channel inactivation, allowing transition of channel gating to the brief open mode. Alternatively, the higher conductance TRPC6 is opened by M3 via a direct action of DAG [40,44,45]. The M2 pathway activates the longer opening mode of TRPC4 and independently inhibits adenylyl cyclases via Gi/o proteins [36]. The M2/M3 pathway transmits distinct concurrent signals to TRPC4 channels, with the M3/Gq/11/PLC branch providing a permissive signal for channel gating through PIP2 depletion and the M2/Go branch allowing transition to a gating state with long openings and brief closings depending on activation strength of the receptor or G-protein [23,36,45,46]. Evidence has shown that the M2/M3 pathway, but not the separate M2 and M3 pathways, allows for potentiation of channel activation by Ca2+ [23], so this pathway is strengthened by M3/InsP3-dependent Ca2+ release. Whether the M2/M3 pathway can significantly stimulate InsP3/DAG formation and/or significantly inhibit cAMP accumulation remains unknown. A study in rabbit intestinal smooth muscle has suggested that Go protein is not involved in mAChR-mediated adenylyl cyclase inhibition [24].
Figure 1.
Three distinct muscarinic signalling pathways leading to transient receptor potential (TRP)-like cationic channel opening in ileal myocytes (redrawn from Tanahashi et al. 2020 [39]). The M3/Gq/11/phospholipase C (PLC) pathway activates brief opening states of 70-pS and 120-pS cationic channels and concurrently evokes InsP3-induced Ca2+ release. Opening of the lower conductance channel is induced by relief from PIP2 inhibition following PLC-mediated hydrolysis, while the higher conductance channel is activated by PLC-generated diacylglycerol (DAG). The M2 pathway transmits M2 signals via Gi/o proteins to the 70-pS channel, which shifts the gating state from the brief to a longer opening mode, and also inhibits adenylyl cyclase. The M2/M3 pathway transmits M2 signals via Go protein, and M3 signals via Gq/11/PLC, to the 70-pS cationic channel, resulting in channel gating with a much longer open mode. This pathway is the major contributor to the generation of mIcat, but is inactive when either the M2 or M3 receptor is absent, or when either Go, Gq/11 or PLC is inactivated. In other words, the activity of this pathway is conditional, occurring only when both M2/Go and M3/Gq/11 signalling pathways are activated. Studies of mAChR-KO mice [23,44] and TRPC-mutant mice [42] indicate that these 70-pS and 120-pS cationic channel activities are mediated by TRPC4 and TRPC6, respectively. The M2/M3 pathway, but not the M2 or M3 pathway, involves a signalling step in which Ca2+ has a potentiating effect on TRPC channel activation, suggesting that the M3 pathway may facilitate M2/M3 pathway function through InsP3-induced Ca2+ release. Whether the M2/M3 pathway has a significant role in stimulating InsP3/DAG formation or inhibiting cAMP accumulation is currently unclear. One study suggested that Go protein is not involved in adenylyl cyclase inhibition by M2 receptors of intestinal smooth muscle [24]. These three pathways may also converge on voltage-dependent Ca2+ channels (VDCCs) to suppress Ca2+ influx via the same G-protein pathways mediating cationic channel activation (see Figure 10B in Tanahashi et al., 2009 [49]).
The M2/M3 pathway appears to involve the formation of a tight connection among M2, M3 and cationic channels via G-proteins Go for the M2, and Gq/11 for the M3 [17,31,47], a hypothesis recently tested using mAChR-mutant mice [39]. As mentioned previously, carbachol-evoked mIcat in ileal myocytes was markedly reduced by M3-KO due to the absence of activated Gq/11 proteins. In M3-KO ileal myocytes, prostaglandin F2α (PGF2α) was able to stimulate the Gq/11/PLC/InsP3-induced Ca2+ release pathway as measured by Ca2+-activated K+ current activation, but it was unable to potentiate the reduced carbachol-evoked mIcat. Similar results were obtained using neuropeptide Y (NPY) in M2-KO myocytes, where carbachol-evoked mIcat was also reduced due to the absence of activated Go proteins. NPY was reported to stimulate the Gi/o/adenylyl cyclase system in intestinal smooth muscle [48]. However, it did not potentiate the reduced carbachol-evoked mIcat in M2-KO mice. If the Go and Gq/11 proteins involved in the M2/M3 pathway were activated by NPY and PGF2α, respectively, the reduced mIcat should have been increased toward the wild-type mIcat. However, there was no such potentiation, implying that the G-proteins (Go and Gq/11) involved in the M2/M3 pathway are specific for this signalling pathway and supporting segregation of the M2/M3 pathway by tight coupling among mAChRs (M2/M3), G proteins (Go/Gq/11), cationic channels (TRPC4) and other elements.
From these findings arises an enigma. Previous studies on guinea-pig ileal myocytes reported that an anti-Gαq/11 antibody did not significantly influence carbachol-evoked mIcat but did block carbachol-evoked K+ currents secondary to M3/Gq/11/PLC/InsP3-mediated Ca2+ release [35,37]. In contrast to the anti-Gαq/11 antibody, M3 receptor antagonists, the PLC inhibitor U-73122 and the Gq/11 inhibitor peptide YM-254890 have been shown to inhibit both carbachol-evoked currents. Although the exact reason for this unexpected behaviour of the anti-Gαq/11 antibody is unknown, a possible explanation is that the M2/M3 signalling complex is tight not only functionally but also structurally such that the associated Gq/11 proteins are inaccessible to the antibody, while the Gq/11 proteins involved in other signalling pathways such as the discrete M3 receptor pathway are accessible to the antibody. Indeed, Aslanoglou et al. (2015) [50] reported M2/M3 heteromers at the surface of cells stably expressing human wild-type M2 receptor and a Receptor Solely by Synthetic Ligand (RASSL) variant of the human M3 receptor. Therefore, M2/M3 receptor heterodimers may form a structural complex with Go and Gq/11, and this complex may then form specific associations with downstream signals that activate mIcat.
3.2. Regulation of K+ and Cl− Channels
K+ channels are important targets for ACh and secondary messengers in gastrointestinal smooth muscles as they are the main regulators of the resting membrane potential and critical modulators of excitation kinetics. Muscarinic modulation of various types of K+ channels has been reported [12,30], including the opening of large conductance Ca2+-activated K+ (BK) channels secondary to intracellular Ca2+ release through the M3/Gq/11/PLC/InsP3 signalling pathway [47,51]. Sakamoto et al. (2007) [23] measured BK currents evoked by carbachol in ileal myocytes isolated from mAChR-mutant and wild-type mice at a holding potential of 0 mV, near the reversal potential of mIcat. At this potential, cells exhibited spontaneous transient outward currents (STOCs) reflecting the opening of a population of BK channels in response to sporadic localised Ca2+ release from internal stores [52]. Application of maximally effective carbachol (100 µM) to wild-type cells produced a large transient BK current followed by abolition of STOCs due to depletion of Ca2+ stores. Current kinetics were similar in M2-KO cells, while M3-KO and M2/M3-KO cells exhibited no such carbachol-activated outward current. These results indicate that intracellular Ca2+ release required for BK current activation is triggered exclusively by M3 receptor-induced InsP3 generation in gut smooth muscle. In addition to BK channel activation, muscarinic stimulation is reported to suppress BK channel activity through a PTX-sensitive mechanism, suggesting mediation by M2 receptors, in canine gut and equine airway myocytes [53,54]. However, we observed that carbachol evoked neither BK currents nor significant changes in the size and shape of STOCs in M3-KO mouse ileal myocytes (unpublished data), indicating a lack of M2 receptor coupling to BK channels, at least in this cell type.
Visceral smooth muscle cells also expressed ATP-sensitive K+ (KATP) channels, which are inhibited by intracellular ATP or glibenclamide and activated by the K+ channel openers such as cromakalim. Muscarinic suppression of KATP channels has been reported in urinary bladder [55], oesophageal [56] and tracheal smooth muscles [57]. Pharmacological studies suggest that channel suppression is mediated by M3 receptors and involves PKC activity. Recently, Wang et al. (2018) [58] found that carbachol (100 µM) inhibited cromakalim-activated KATP current to a similar extent in ileal myocytes from wild-type and M2-KO mice but had no effect on ileal myocytes from M3-KO and M2/M3-double-KO mice, suggesting that this muscarinic suppression of KATP channels is mediated primarily by M3. Further, muscarinic suppression of the KATP current was blocked by the Gq/11 inhibitor YM-254890 and the PLC inhibitor U73122, but not by the PKC inhibitor calphostin C, suggesting the involvement of a Gq/11/PLC-dependent but PKC-independent signalling pathway. Wild-type ileal myocytes responded to cromakalim by hyperpolarisation and to glibenclamide by depolarisation, implying that KATP channels contribute significantly to the muscarinic regulation of cellular excitability and contraction.
Chloride channels are expressed in many smooth muscles and contribute to depolarisation, as the Cl− equilibrium potential is well above the resting potential [30]. Like BK channels, chloride channel opening is triggered by intracellular Ca2+ release. In tracheal smooth muscle, muscarinic agonist-evoked Cl− currents were blocked by M3 receptor antagonists or anti-Gq/11 antibody [59,60]. In wild-type mouse ileal myocytes, carbachol (100 µM) evoked an inward current consisting of an initial rapid peak followed by a smaller sustained phase, and the initial rapid current was mediated in part by opening of Ca2+-activated Cl− channels [40].
3.3. Inhibition of Voltage-Dependent Ca2+ Channels
Voltage-dependent Ca2+ channels (VDCCs) are the predominant pathway for Ca2+ entry into smooth muscle cells during depolarisation. Surprisingly, while mACh receptors can depolarise smooth muscle cells, receptor activation generally inhibits VDCC activity and suppresses the inward Ca2+ current (ICa) [12]. This effect may constitute a negative feedback mechanism to prevent cytosolic Ca2+ overload during muscarinic depolarisation. In guinea-pig ileal myocytes, carbachol suppressed ICa in a biphasic manner, with an initial rapid suppression lasting ~10 s followed by a more sustained suppression subsiding slowly only after agonist removal [61,62]. The initial transient suppression reflects Ca2+-induced channel inactivation by the M3/Gq/11/PLC/InsP3 pathway [61,62,63], while the mechanisms underlying the sustained phase are less clear. One group reported that both phases were insensitive to PTX, suggesting mediation via M3-Gq/11 [62], whereas another reported that the sustained phase was sensitive to PTX and thus potentially mediated via M2-Gi/o [19]. These contradictory results underscore the importance of experimental conditions (such as voltage clamp modality and pipette solution) for ICa measurements and for examining the effects of various modulators.
Tanahashi et al. (2009) [49] attempted to link specific mAChR subtypes/G-protein combinations to carbachol-induced ICa inhibition in mouse ileal myocytes using mAChR-KO mice and the pharmacological tools PTX and YM-254890. In wild-type cells, carbachol caused a biphasic inhibition of ICa, as described for guinea-pig ileal ICa (see above). In contrast, M2-KO cells showed a marked reduction in the sustained inhibition but no deficit in the initial transient inhibition, whereas M3-KO cells were deficient in the initial transient inhibition and displayed a marked reduction in the later sustained inhibition and no ICa inhibition occurred in the absence of M2 and M3 receptors. Accordingly, the muscarinic inhibition of ICa is mediated by both M2 and M3 receptors, but not other mAChR subtypes. The M2-mediated suppression in M3-KO ileal myocytes was abolished by PTX but unaffected by the Gq/11 blocker YM-254890, while M3-mediated sustained suppression in M2-KO myocytes was unaffected by PTX but blocked by YM-254890. The magnitude of sustained suppression (% inhibition of ICa) by carbachol in wild-type myocytes was much greater than the sum of M2- and M3-mediated inhibition, indicating that a considerable part of the inhibition in wild-type cells (~56%) requires co-activation of M2 and M3 receptors. Moreover, the sustained inhibition in wild-type cells was profoundly reduced by PTX or YM-254890, and the residual inhibition closely resembled M3- and M2-mediated inhibition, respectively. When [Ca2+]i was strongly buffered by intracellular EGTA perfusion, M2- and M3-mediated inhibition remained unaffected, but carbachol-induced sustained inhibition in wild-type myocytes was markedly reduced, and the remaining ICa component resembled the sum of M2- and M3-mediated inhibition.
Taken together, these results indicate that M2 receptors mediate ICa suppression via Gi/o proteins in mouse ileal myocytes, while M3 receptors mediate both fast transient and slow sustained ICa suppressions via Gq/11, and another pathway involving M2–M3 interaction is the major contributor to sustained carbachol-induced ICa suppression. This M2/M3 synergistic pathway, unlike the separate M2 and M3 pathways, involves a process where Ca2+ has permissive and/or potentiating action(s) on carbachol-induced sustained ICa suppression. These three distinct muscarinic pathways for ICa suppression show the typical features of mIcat activation (see Figure 1). Therefore, it is plausible that all three converge on both TRPC4/6 cationic channels and VDCCs. Activation of the former triggers depolarisation and Ca2+ entry via VDCCs, while the latter acts as a feedback brake to prevent cytosolic Ca2+ overload during depolarisation.
In contrast to ICa suppression by mAChRs, Jin et al. (2002) [64] reported that the muscarinic agonist methacholine enhanced ICa in rabbit colonic myocytes under conditions where M3 signalling was disrupted by an M3 receptor antagonist or anti-Gαq antibody, suggesting a possible pathway linking M2-receptor activation to ICa enhancement. However, in M3-KO mouse ileal myocytes, carbachol caused no potentiation of ICa but rather induced phasic suppression. One possible explanation for this discrepancy in ICa potentiation by M2 signalling is that both inhibitory and facilitatory pathways operate in parallel, with the balance modified by currently unknown factors.
Studies on ileal myocytes from mAChR-KO mice indicate that the M2 subtype participates in regulation of TRPC4 channels and VDCCs, but not TRPC6, BK, KATP or Cl− channels, whereas the M3 subtype appears to regulate all of these ionic channels.
4. Depolarisation
Numerous studies have examined membrane potential changes induced by muscarinic agonists and by enteric nerve stimulation in mAChR-KO mice. In general, results are in agreement with macroscopic mIcat and single channel current studies.
Changes in membrane potential produced by carbachol were recorded in ileal myocytes from mAChR-KO and wild-type mice using the nystatin-perforated patch-clamp technique [23] under physiological ionic conditions (high extracellular Na+ and intracellular K+), where the resting membrane potentials were similar among the different mouse strains. The wild-type cells responded to 0.1–0.3 µM carbachol with a moderate depolarisation of 10–20 mV and to higher concentrations (>1 µM) by a depolarisation of 40–50 mV, reaching the equilibrium potential for muscarinic depolarisation (−10 mV) [65,66]. In M2-KO cells, 0.1 µM of carbachol was without effect, but 1 µM was equipotent to the effect of 0.1 µM carbachol on wild-type cells and 30–100 µM evoked a full depolarisation, indicating that the M2 receptor contributes substantially to depolarisation at low agonist concentrations. In M3-KO cells, 1–3 µM carbachol caused only a slight depolarisation, and even 30–100 µM evoked a depolarisation of up to only 10 mV. In the M2/M3 double-KO cells, carbachol induced no depolarisation, but PGF2α caused a prominent depolarisation. Taken together, these observations suggest that the three muscarinic pathways depolarise the membrane with the following rank order of efficacy: M2/M3 > M3 >> M2 pathway. Both M2/M3 and M2 pathways lead to depolarisation only through activation of TRPC4 channels, while the M3 pathway depolarises the membrane through a combination of (at least) TRPC4, TRPC6 and Cl− channel activation and KATP channel inhibition (see Figure 2).
Figure 2.
Signal transduction mechanisms underlying muscarinic contraction of mouse intestinal smooth muscle. The M3 pathway activates multiple intracellular Ca2+ mobilisation events, including Ca2+ influx via VDCCs and voltage-independent Ca2+-permeable channels and intracellular Ca2+ release. Voltage-dependent Ca2+ influx is initiated by depolarisation from opening of TRPC4 and TRPC6 cationic channels, opening of Ca2+-activated Cl− channels and inhibition of KATP channels, while voltage-independent Ca2+ entry is mediated by opening of TRPC6 cationic channels and Ca2+ store-operated Ca2+ channels. In addition to Ca2+ mobilisation, Ca2+ sensitisation of contractile proteins is elicited, thereby increasing the efficiency of contraction–[Ca2+]i coupling. The M2 or M2/M3 pathways induce contraction through a simple Ca2+ mobilisation mechanism in which Ca2+ entry via VDCCs is activated by TRPC4 channel-induced depolarisation. These pathways play a major role in mediating muscarinic contraction, with the M2/M3 pathway making a relatively greater contribution at low agonist concentrations. The M2 pathway also induces contraction indirectly by inhibiting cAMP-dependent relaxation in response to adenylyl cyclase-activating agonists.
Excitatory junction potentials (EJPs) evoked by enteric nerve stimulation [67] have also been measured in smooth muscle cells from mAChR-KO mice. Matsuyama et al. (2013) [67] compared EJPs evoked by electrical field stimulation (EFS) in longitudinal muscle strips from the ileum of mAChR-KO and wild-type mice using intracellular microelectrodes and found that responses in the wild-type strips were strongly inhibited, but not abolished, by PTX and by the cationic channel blocker SK&F96365 [68]. Alternatively, cholinergic EJPs evoked in M2-KO strips were only 20%–30% of the wild-type amplitude and markedly reduced but not abolished by SK&F96365. In contrast, no EJP was elicited in M3-KO or M2/M3 double-KO strips. Taken together, these results clearly demonstrate that both M2 and M3 receptors contribute to cholinergic EJPs in ileal myocytes and further suggest that wild-type cholinergic EJPs occur through both M2/M3 and M3 pathways with a greater contribution from the M2/M3 pathway. In contrast, M2 receptors alone do not elicit large EJPs. Note, however, that the application of drugs to prevent muscle contraction (nifedipine and papaverine) or simultaneous inhibitory neurotransmission (L-NAME and guanethidine) may influence these responses.
5. Ca2+ Sensitisation of Contraction
Muscarinic receptors control contractile tension in smooth muscle primarily by inducing elevations in [Ca2+]i. In addition, however, mAChRs can also regulate smooth muscle contraction by enhancing the Ca2+ sensitivity of contractile proteins. This mAChR-mediated Ca2+ sensitisation occurs through two distinct pathways involving the small G-protein Rho/Rho kinase and the DAG target PKC, respectively [69,70]. Both pathways may be initiated by M3 receptors [28], and possible positive or negative regulation of Ca2+ sensitivity by M2 receptors via other kinases has also been suggested [28,71,72].
Suguro et al. (2010) [73] characterised the mAChR subtypes mediating carbachol-induced Ca2+ sensitisation by comparing the [Ca2+]i (pCa)–tension relationship among α-toxin-permeabilised ileal muscle strips from mAChR-KO and wild-type mice. In strips from wild-type mice, isometric tension responses to Ca2+ applied cumulatively (pCa 7.0–5.0) were increased by carbachol (100 µM) as indicated by shifts in both the 50% effective concentration (EC50) and the maximum response (Emax). The M2-KO strips exhibited similar changes, while M3-KO and M2/M3 double-KO strips showed little or no change in pCa–tension curves. As expected from mAChR-mediation, the direct G-protein activator GTPγS altered both EC50 and Emax in all KO and wild-type strips without additional stimulation, and carbachol-induced Ca2+ sensitisation in the wild-type and M2-KO strips was totally blocked by the Gq/11 inhibitor YM-254890. These results provide strong evidence that mAChR-mediated Ca2+ sensitisation of contractility in intestinal smooth muscle occurs through coupling of M3 receptors to Gq/11 proteins, consistent with the pharmacological study of Murthy et al. (2003) [28], while there is currently no evidence for involvement of M2 receptors in regulation of myofilament Ca2+ sensitivity.
6. Heterologous Desensitisation of Contraction
It is well known that prolonged exposure of gastrointestinal smooth muscle to a muscarinic agonist decreases the contractile response evoked by subsequent exposure to a muscarinic agonist or other spasmogen such as PGF2α and histamine. In guinea-pig ileum, ACh-induced desensitisation of the contractile response to histamine was prevented by disruption of M2/Gi/o signalling with PTX or by M3 receptor inactivation with 4-DAMP mustard [74]. This and other pharmacological results suggest that this mAChR-mediated heterologous desensitisation depends on activation of both M2 and M3 receptors. Griffin et al. (2004) [75] investigated the mAChR subtypes mediating heterologous desensitisation in the isolated ileum using mAChR-KO and wild-type mice. Prolonged ACh treatment of wild-type mouse ileum (30 µM for 20 min) desensitised the subsequent contractile response to both PGF2α and oxotremorine-M as indicated by significant increases in EC50 measured 5 min after ACh wash-out. The desensitisation to PGF2α was prevented by either M2-KO or M3-KO and that to oxotremorine-M by M2-KO. These results indicate that muscarinic agonist-induced heterologous desensitisation requires activation of both M2 and M3 receptors and that activation of either receptor by itself is insufficient, in accordance with a previous study in guinea-pig ileum [74].
The requirement of M2 and M3 receptor co-activation for heterologous desensitisation further hints at the underlying mechanism. As described earlier, synergy between M2 and M3 receptors (or M2/M3 pathway activation) results in extensive opening of TRPC4 non-selective cation channels. This may result in intracellular Na+ accumulation and loss of intracellular K+, thereby accelerating transmembrane Na+-K+ pump activity. The resulting hyperpolarisation from increased pumping activity (as the exchanger expels 3 Na+ for every 2 K+ pumped back in) may reduce the sensitivity to depolarising agents [76]. The inhibition of VDCC activity, which occurs through M2/M3 interaction, may also participate in heterologous desensitisation since it has been shown to subside slowly after cessation of mAChR stimulation [19].
7. Smooth Muscle Contraction
Pharmacological evidence has shown that the less abundant M3 receptor is largely responsible for mediating direct contractile responses to applied muscarinic agonists or neurogenic ACh [1,9], while most (but not all) studies have found no or little evidence for involvement of the more abundant M2 receptor in contractile responses [16,77,78]. However, M2 receptor activation induces contraction indirectly by preventing the cAMP-dependent relaxation effects of forskolin and isoprenaline in the presence of a stimulatory receptor agonist such as histamine [79]. The availability of mAChR-KO mice has promoted studies to definitively determine which mAChR subtype(s) mediate direct or indirect contraction and to elucidate muscarinic contractile mechanisms in gastrointestinal smooth muscle.
7.1. Contraction Evoked by Applied Muscarinic Agonists
In many studies, carbachol or oxotremorine-M was applied with cumulative or single dose application protocols, and concentration–response curves for the agonist-evoked contraction were constructed to yield Emax and EC50. The contributions of individual mAChR subtypes in contraction have been characterised based on concentration–response changes in KO mice. Table 1 presents Emax values for different mutant strains expressed relative to the corresponding wild-type Emax garnered either from published values or extracted from concentration–response curves, while Table 2 presents published pEC50 values (negative logarithm of EC50). Parameters obtained from other visceral smooth muscles are also included in Table 1 and Table 2.
Table 1.
Emax values for muscarinic agonist-induced contraction of visceral smooth muscle from various mAChR-mutant mice.
| Tissue | Protocol/Agonist b | Emax (% of Wild Type) a | References | |||
|---|---|---|---|---|---|---|
| M2-KO | M3-KO | M2/M3-KO | M4-KO | |||
| Stomach fundus | Cumulative/CCh | 96 | 44 * | 95 | Stengel et al. (2000; 2002) [80,81] | |
| Single dose/CCh | 102 | 66 * | 0 (relax.) | Kitazawa et al. (2007) [82] | ||
| Cumulative/CCh | 114 | 49 * | 0 (relax.) | Kitazawa et al. (2007) [82] | ||
| Stomach antrum | Single dose/CCh | 84 | 64 * | 0 (relax.) | Kitazawa et al. (2007) [82] | |
| Cumulative/CCh | 86 | 57 * | 0 (relax.) | Kitazawa et al. (2007) [82] | ||
| Stomach body | Cumulative/CCh | 95 | 42 * | Ruggieri and Braverman (2013) [83] | ||
| Ileum | Cumulative/CCh | 75 | 25 | 0 | Matsui et al. (2000; 2002) [84,85] | |
| Cumulative/Oxo.M | 98 | Matsui et al. (2003) [86] | ||||
| Single dose/CCh | 103 | 62 * | 0 (relax.) | Unno et al. (2005) [87] | ||
| Single dose/ACh | 102 | 43 | Takeuchi et al. (2007) [88] | |||
| Cumulative/Oxo.M | 101 | 36 * | Griffin et al. (2009) [89] | |||
| Colon proximal | Single dose/CCh | 66 * | 34 | 0 (relax.) | Kondo et al. (2011) [90] | |
| Colon distal | Single dose/CCh | 65 * | 21 | 0 (relax.) | Kondo et al. (2011) [90] | |
| Trachea | Cumulative/CCh | 86 * | 44 * | 82 | Stengel et al. (2000; 2002) [80,81] | |
| Cumulative/Oxo.M | 88 | Matsui et al. (2003) [86] | ||||
| Gallbladder | Cumulative/CCh | 79 | 21 | 100 | Stengel and Cohen (2002) [91] | |
| Urinary bladder | Cumulative/CCh | 95 | 5 | 0 | Matsui et al. (2000; 2002) [84,85] | |
| Cumulative/Oxo.M | 84 | Matsui et al. (2003) [86] | ||||
| Cumulative/CCh | 82 | 6 * | 97 | Stengel et al. (2000; 2002) [80,81] | ||
| Cumulative/Oxo.M | 89 | 15 * | 0 | Ehlert et al. (2005) [92] | ||
| Single dose/CCh | 77 * | 7 * | 0 | # | ||
| Uterus | Cumulative/CCh | 66 * | 0 | 0 | Kitazawa et al. (2008) [93] | |
a Obtained directly from reported Emax values for mACh-mutant knockout (KO) strains and corresponding wild-type strain or estimated from concentration–response curves published for the KO and wild-type strains. b CCh: carbachol; ACh: acetylcholine; Oxo.M: oxotremorine-M. These agents were applied using a cumulative or single dose protocol. * Significant difference between mutant and wild-type mice. # Unpublished data (Komori, Tanahashi, Matsuyama, Unno).
Table 2.
pEC50 values for muscarinic agonist-induced contraction of visceral smooth muscle from various mAChR-mutant mice.
| Tissue | Protocol/Agonist | pEC50 | References | ||
|---|---|---|---|---|---|
| Wild Type: M2-KO | Wild Type: M3-KO | Wild Type: M4-KO | |||
| Stomach fundus | Cumulative/CCh | 6.68:6.39 * | 6.54:6.71 | 6.76:6.70 | Stengel et al. (2000; 2002) [80,81] |
| Single dose/CCh | 6.93:6.51 * | 7.03:6.97 | Kitazawa et al. (2007) [82] | ||
| Cumulative/CCh | 6.56:6.18 * | 6.67:6.73 | Kitazawa et al. (2007) [82] | ||
| Stomach antrum | Single dose/CCh | 6.60:6.20 * | 6.50:6.90 | Kitazawa et al. (2007) [82] | |
| Cumulative/CCh | 6.82:6.08 * | 6.92:7.10 | Kitazawa et al. (2007) [82] | ||
| Stomach body | Cumulative/CCh | 6.1:5.7 * | 6.1:6.5 * | Ruggieri and Braverman (2013) [83] | |
| Ileum | Single dose/CCh | 6.39:5.93 * | 6.14:6.18 | Unno et al. (2005) [87] | |
| Cumulative/Oxo.M | 6.75:6.26 * | 6.75:6.99 * | Griffin et al., (2009) [89] | ||
| Cumulative/Oxo.M | 6.70:6.38 * | Matsui et al. (2003) [86] | |||
| Colon proximal | Single dose/CCh | 6.90:6.34 * | Kondo et al. (2011) [90] | ||
| Colon distal | Single dose/CCh | 5.90:6.03 | Kondo et al. (2011) [90] | ||
| Trachea | Cumulative/CCh | 6.56:6.27 * | 6.52:6.51 | 6.46:6.62 | Stengel et al. (2000; 2002) [80,81] |
| Cumulative/Oxo.M | 6.94:6.86 | Matsui et al. (2003) [86] | |||
| Urinary bladder | Cumulative/CCh | 6.27:6.07 * | 6.02:5.71 * | 6.30:6.20 | Stengel et al. (2000; 2002) [80,81] |
| Cumulative/Oxo.M | 6.54:6.31 * | 6.54:6.60 | Ehlert et al. (2005) [92] | ||
| Cumulative/Oxo.M | 6.58:6.41 | Matsui et al. (2003) [86] | |||
| Single dose/CCh | 6.22:5.96 | 6.22:6.10 | # | ||
* Significant difference from the corresponding wild-type value. # Unpublished data (Komori, Tanahashi, Matsuyama, Unno). CCh: carbachol; Oxo.M: oxotremorine-M.
Knockout of M3 receptors markedly reduced Emax in the stomach (fundus, antrum and body), ileum and colon (proximal and distal), with estimated Emax values ranging from 21% to 66% of the wild-type Emax among different tissues and studies of the same tissue. Similar effects of M3-KO were also found in other visceral smooth muscle tissues, with reductions to 5–15% of the wild-type Emax in the urinary bladder, 44% in the trachea, 21% in the gallbladder and complete elimination in the uterus. Alternatively, there were few significant changes in pEC50 or agonist potency except for a two-fold increase in the ileum and stomach bodies and a two-fold decrease in the urinary bladder (Table 2).
Unlike M3 receptor KO, M2 receptor KO did generally not markedly alter Emax (65–86% of wild-type Emax in the colon, trachea, urinary bladder and uterus; Table 1). In line with the results in the urinary bladder, a highly selective but not commercially available M2 antagonist THRX-182087 had little effect on the amplitude of the CCh-induced contractions in the rat urinary bladder [94]. Alternatively, a significant decrease in pEC50 was observed, with most agonist concentration–response curves demonstrating a 2- to 3-fold decrease in pEC50 in M2-KO mice (Table 2). Further, no tissues from M2/M3 double-KO mice exhibited detectable contraction, and gastrointestinal tissues actually showed relaxation in response to the agonist (Table 1). Therefore, it is highly probable that M2 and M3 receptors, but not other mAChR subtypes, mediate contraction in all visceral smooth muscle studied. Indeed, M4-KO had little effect on Emax and pEC50 for carbachol in the stomach fundus, urinary bladder and trachea [80]. In contrast, M4-KO in the gallbladder did cause a dextral shift in the carbachol concentration–response curve, suggesting that the M4 receptor may provide a signal for optimal carbachol potency in this particular tissue (see Figure 1 in Stengel and Cohen, 2002 [91]). However, the gallbladder contractile response to carbachol is unique in that it is associated with release of a cyclooxygenase (COX) product [91].
The kinetics of contraction evoked by a single dose of carbachol differ considerably between M2-KO and M3-KO mice, features particularly apparent in the stomach antrum and fundus, longitudinal muscle of the ileum and both distal and proximal colon [82,87,90]. Specifically, contraction in M3-KO tissues (M2-mediated) exhibited a phasic form characterised by an initial rapid peak in tension followed by a gradual decline even in the continued presence of the agonist, while contraction in M2-KO tissues (M3-mediated) exhibited a rapid rise to peak and persistence in the continued presence of the agonist. The later form resembled that of wild-type tissues, consistent with the documented predominance of M3 in mediating the contractile response to carbachol. As mentioned above, although pharmacological studies have found no or little evidence for involvement of the M2 receptor in contractile responses [16,77,78], these studies using the muscarinic receptor subtype-KO mice revealed that the M2 can directly induce contraction in gastrointestinal smooth muscles.
A contribution of M1 receptor-mediated NO release has been suggested in the relaxation of gastrointestinal smooth muscle [95], as the phasic carbachol-induced contraction in M3-KO colon was changed to a sustained contraction by the NOS inhibitor L-NAME or TTX [90]. A similar mechanism may also contribute to carbachol-evoked relaxation in M2/M3 double-KO gastrointestinal tissue [82]. In addition, Stengel and Cohen (2003) [96] reported that M3 receptor KO unmasked neurogenic NO-dependent relaxation evoked by carbachol in the mouse stomach fundus, resulting in a bell-shaped concentration–response curve for the contractile effect of carbachol. Thus, NO released from nerve endings by stimulation of muscarinic receptors other than M2 and M3 receptors may affect the muscarinic contractions in gastrointestinal smooth muscles.
The use of mAChR-mutant mice has revealed that muscarinic agonist-induced contraction in visceral smooth muscles is mediated predominantly by a combination of M2 and M3 receptors, with a greater contribution of M3, generally consistent with previous pharmacological studies. It has also been demonstrated that activation of M2 receptors can evoke a direct contraction in most M3-KO visceral tissues studied (except for the gallbladder, in which muscarinic contraction involves prostaglandins), whereas pharmacological studies have found no or little evidence for involvement of the M2 receptor in contractile responses (see above). The Emax of carbachol varies considerably among different tissues of M3-KO mice (ranking: stomach, ileum and colon = trachea < urinary bladder ≪ uterus) (see Table 1). This variation in M2-dependent Emax may reflect different coupling efficiencies between M2 receptors and downstream contractile mechanisms, but could also indicate distinct M2-mediated contractile mechanisms. In the uterus, for example, M2 receptors have no or little contractile activity in M3-KO mice, but can enhance M3-mediated contraction in the wild-type uterus [93].
As expected, PTX treatment markedly depressed carbachol-induced contraction in the M3-KO mouse ileum, but had no effect on the M2-KO mouse ileum [87]. The involvement of M2/Gi/o signalling in the contraction of the wild-type ileum was then characterised using the toxin. Changes in the concentration–response curve to carbachol by PTX treatment indicated a significant contribution of M2/Gi/o signalling only at relatively low agonist concentrations (by ~70% at 0.1 µM but only 40% at 1 µM and no reduction at 10 µM carbachol), while M3/Gq/11 signalling was completely dominant at higher agonist concentrations. These findings and those from the aforementioned electrophysiological studies indicate that M2/Gi/o signalling, probably via a synergy with M3/Gq/11 signalling, may produce contraction at relatively low agonist concentrations in intact gastrointestinal smooth muscle co-expressing M2 and M3 receptors.
7.2. Cholinergic Nerve-Mediated Contraction
Studies using mAChR-KO mice have also helped define the mAChR subtypes mediating contraction in gastrointestinal smooth muscles evoked by cholinergic nerve stimulation [82,88,97]. In the wild-type ileum, EFS evoked a rapid phasic contraction followed by a sustained contraction lasting for 2–8 min after cessation. The initial contraction was arrested by the anticholinergic atropine, confirming cholinergic nerve dependence. Cholinergic contractions evoked by EFS at 5 to 50 Hz were also significantly reduced by M2- or M3-KO. In both tissues, the residual contraction strength was ~80% of the wild-type control regardless of stimulus frequency [97]. Similar results were also obtained by Takeuchi et al. (2007) [88] under similar experimental conditions, with 1–10 Hz EFS evoking contractions in the ileum from M2-KO mice resembling those in the wild-type and corresponding contractions in M3-KO mice reaching 70 to 80% of wild-type contraction strength. Kitazawa et al. (2007) [82] reported that the cholinergic contractions in the gastric fundus and antrum induced by 32 Hz stimulation were reduced by both M2-KO (71% of wild-type response in the fundus, 75% in the antrum) and M3-KO (54% of wild-type in the fundus and 54% in the antrum), while M2/M3 double-KO completely eliminated EFS-evoked cholinergic contraction in the stomach and ileum. Collectively, these results clearly demonstrate that cholinergic nerve-mediated contractions in gastrointestinal smooth muscle are mediated by a combination of M2 and M3 receptors, with M3 predominance.
The EFS-evoked cholinergic contractions in the M3-KO ileum were completely arrested by PTX treatment, indicating that M2/Gi/o signalling activity is essential in the absence of M3 receptors. In the wild-type ileum, EFS-evoked contractions at 10–50 Hz were depressed by 20–30% by PTX treatment, consistent with mediation by a combination of M2 and M3 receptors. Curiously, EFS-evoked contractions at 2 Hz were increased and those at 5 Hz unchanged by PTX. To explain these observations, Unno et al. (2006) [97] speculated that the loss of M2 signalling by PTX treatment was overcome or balanced by increased M3 receptor signalling, probably owing to suppressed presynaptic autoinhibition of ACh release mediated by M2/Gi/o [20].
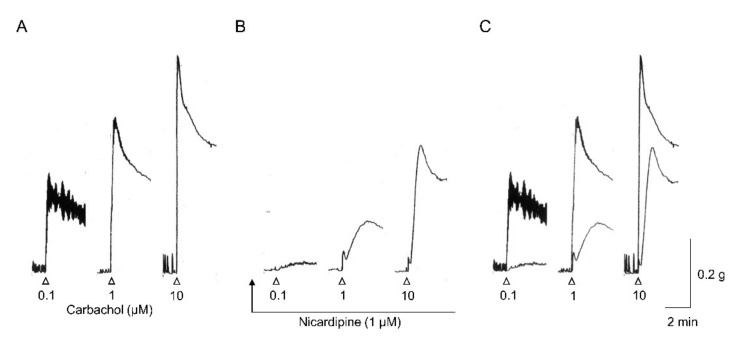
Under conditions where non-adrenergic neural inputs were pharmacologically minimised, contractions evoked by 20 Hz, 5 s EFS trains in the wild-type mouse ileum were almost abolished by atropine (93% reduction) or the VDCC blocker nifedipine (95% reduction) (Komori, Tanahashi, Matsuyama, Unno; unpublished data). These results indicate that cholinergic contractions in the mouse ileum depend largely on Ca2+ entry via VDCCs, similar to contraction evoked by low-dose muscarinic agonists (see Figure 3).
Figure 3.
Effects of nicardipine on carbachol-evoked contractions in a wild-type mouse ileal longitudinal muscle strip. Carbachol was applied for 3 min at the indicated concentrations before (A) and after nicardipine treatment to block VDCCs (B). In (C), the recording traces in A and B are superimposed. Note that when muscarinic stimulation is weak, contraction is relatively more dependent on voltage-dependent Ca2+ entry and is more sensitive to PTX. This finding indicates that the M2/M3 (and M2) pathway has a major role in mediating the contractile response to weak muscarinic stimulation. When receptor stimulation is stronger, the M3 pathway predominates by activating multiple mechanisms for Ca2+ mobilisation as well as myofilament Ca2+ sensitisation.
Tsvilovskyy et al. (2009) [42] also reported a 64% reduction in cholinergic contraction among TRPC4-deficient mice compared to wild-type controls. However, PTX treatment reduced these cholinergic contractions in the wild-type ileum by only ~30%, an inconsistency that remains to be clarified.
Interstitial cells of Cajal (ICCs) distributed within the smooth muscle layer have been implicated in enteric neurotransmission based on their close proximity to the varicosities of enteric motor neurons and expression of receptors for enteric neurotransmitters (see Sanders et al. 2014 [98]). Quantitative PCR revealed expression of both M2 and M3 muscarinic receptor transcripts in intramuscular ICCs (ICC-IMs) of the murine colon [99] and deep muscle plexus ICCs (ICC-DMPs) of the murine small intestine [100]. In contrast to smooth muscles (see above), Chrm3 (M3) expression was higher than Chrm2 (M2) expression in ICC-IMs and ICC-DMPs. Drumm et al. (2020) reported that EFS increased Ca2+ transients in colonic ICC-IMs, a response abolished by atropine and the M3 receptor antagonist DAU 5884 but not the M2 antagonist AF-DX 116. Furthermore, pharmacological inhibition of the anoctamin-1 (ANO1) Ca2+-activated Cl− channel expressed selectively in ICCs suppressed EFS-induced cholinergic contractions in the murine colon. These observations suggest that enhancement of Ca2+ transients by acetylcholine stimulation of M3 receptors activates ANO1 channels in colon ICC-IMs resulting in membrane depolarization. The depolarization can be conducted to adjacent smooth muscle cells through gap junctions, leading to excitation of the smooth muscle cells. Alternatively, the physiological functions of M2 receptors expressed by ICC are still unknown. Groneberg et al. (2006) [101] suggested parallel neurotransmission of enteric NO to ICCs and smooth muscle cells in the murine fundus. Thus, it will be of particular interest to reveal the effects of each muscarinic receptor subtype expressed by ICCs on muscarinic contraction and to compare the underlying signal transduction pathways with those of smooth muscle cells. Mutant mice generated with mAChR subtype-specific KO (e.g., Gautam et al. 2006 [102]) in ICCs and smooth muscle cells [101] using the Cre/LoxP recombination system should be useful to address these issues.
7.3. Indirect Contraction by Inhibiting Cyclic AMP-Dependent Relaxation
Pharmacological studies of visceral smooth muscles across species have demonstrated that M2 receptors contribute to contraction by inhibiting relaxation caused by agents that increase cAMP [9,15]. To establish an indirect role of M2 receptors in smooth muscle contraction, Matsui et al. (2003) [86] investigated the ability of the adenylyl cyclase activators forskolin and isoprenaline to inhibit oxotremorine-M-induced contraction in smooth muscles of the the ileum, urinary bladder and trachea from wild-type and M2-KO mice. The relaxant effects of forskolin against oxotremorine-M contraction were greatly increased in all three tissues from M2-KO mice compared to wild-type controls. Under similar conditions, the relaxant effects of isoprenaline were also enhanced in the ileum and urinary bladder from M2-KO mice. These results strongly suggest that M2 receptors suppress cAMP-dependent relaxation by inhibiting adenylyl cyclases [103]. In the trachea, however, no difference in isoprenaline-induced relaxant effect was found between wild-type and M2-KO mice. A similar conclusion was reached in pharmacological studies of guinea-pig and bovine trachea [104,105]. The difference in response between the trachea and other smooth muscle tissues remains to be explained [15]. In accord with agonist treatment, isoprenaline also induced a relaxant effect on the cholinergic contraction evoked by EFS in the urinary bladder from wild-type mice [106], while this effect was reduced by M2-KO mice, suggesting that M2 receptors indirectly promote contraction of gastrointestinal smooth muscle in response to cholinergic nerve stimulation.
Collectively, M2 receptors appear to facilitate contractility of smooth muscles by counteracting the relaxant effects of agents that increase intracellular cAMP. In visceral smooth muscle, the cholinergic muscarinic system serves to suppress sympathetically mediated relaxation through M2-mediated adenylyl cyclase inhibition.
7.4. Muscarinic Contractile Mechanisms in Mouse Intestinal Smooth Muscle
The signal transduction pathways linking mAChR activation to contraction of mouse intestinal smooth muscle are summarised in Figure 2. Unno et al. (2005) [87] characterised Ca2+ sources associated with pure M2- and pure M3-mediated contractions in ileal longitudinal muscle of M2-KO and M3-KO mice using VDCC blockers such as nicardipine and nifedipine, depolarising high-K+ medium for depolarization block of VDCCs and Ca2+-free medium for block of Ca2+ influxes. The pure M2-mediated contraction in M3-KO muscle strips was dependent exclusively on Ca2+ entry through VDCCs, especially influx associated with action potentials initiated or enhanced by TRPC4 channel activation (see Figure 1). On the other hand, the pure M3-mediated contraction in M2-KO muscle strips involved multiple Ca2+ mobilisation mechanisms, including both voltage-dependent and -independent Ca2+ entry and InsP3-induced Ca2+ release from intracellular stores. Voltage-dependent Ca2+ entry appears to be mediated by action potentials and sustained depolarisation caused by concomitant opening of TRPC4, TRPC6 and Cl− channels and closing of KATP channels, while voltage-independent Ca2+ entry appears to be mediated directly by influx through TRPC6 channels and other Ca2+-permeable channels activated by Ca2+ store depletion [107]. In addition to Ca2+ mobilisation, pure M3 contraction is also associated with Ca2+ sensitisation of contractility, which serves to increase contraction efficiency.
As mentioned above, the M2 receptor has direct, potent contractile activity in the mouse ileum. Griffin et al. (2009) [89] explored whether such M2 activity was shared by the guinea-pig ileum using the irreversible M3 receptor antagonist 4-DAMP mustard. First, the M3 selectivity of the antagonist was confirmed in the ileum from mAChR-mutant and wild-type mice. Following 4-DAMP mustard treatment, the concentration–response curve for oxotremorine-M-evoked contraction in the wild-type ileum closely resembled that in the M3-KO ileum as indicated by similar pEC50 and Emax values and inhibition by common muscarinic antagonists. Thus, 4-DAMP mustard appears to inactivate M3 receptors selectively. Similar experiments were then conducted on the guinea-pig ileum. Following mustard treatment, the contractile response to oxotremorine-M exhibited a competitive antagonism profile consistent with an M3 response, suggesting that the guinea-pig ileum lacks a direct, potent M2-contractile component. It should be noted again that TRPC4 channel activation is the primary mechanism underlying M2-mediated contraction in the mouse ileum (see Figure 2), and there is abundant evidence that the gating properties and activation mechanism of TRPC4 channels are shared by both the mouse and guinea-pig ileum [23,36,40,45,46,108]. Contraction mediated by M2 receptors depends entirely on voltage-dependent Ca2+ entry, especially influx associated with action potentials, but does not involve Ca2+ sensitisation of contractility, so there is a tendency for attenuation of contraction by physiological relaxation factors such as neuronal NO and by deterioration of action potential amplitude during prolonged higher-frequency mAChR stimulation [82,87,90,96]. Hence, before it is established that the guinea-pig ileum lacks the direct, potent M2 contractile component, further study is needed to test for the contributions of factors likely to diminish M2-mediated contraction.
In addition to independent M2 and M3 pathways, the M2/M3 pathway also functions in the contraction of the wild-type mouse ileum. This pathway leads to TRPC4 channel activation and mIcat generation, which in turn depolarises the cell and stimulates voltage-dependent Ca2+ influx through VDCCs, similar to contraction induced by the M2 pathway. However, this pathway is much more potent than the M2 pathway at activating TRPC4 channels and generating mIcat. Effects of nicardipine, a VDCC blocker, were examined on carbachol-evoked contraction in isolated longitudinal muscle strips from the wild-type mouse ileum. As shown in Figure 3 (Komori, Tanahashi, Matsuyama, Unno; unpublished data), nicardipine abolished contraction in response to low concentrations of carbachol (0.1 µM), indicating that this contractile response is mediated primarily by voltage-dependent Ca2+ entry. Alternatively, nicardipine blockade was only moderate at 1 µM and markedly reduced at 10 µM. This agonist dose-dependence resembles that of PTX treatment, which blocked contraction substantially at 0.1 µM carbachol (~70%) and moderately at 1 µM (40%) but had no effect at 10 µM carbachol [87]. In addition, deletion of InsP3 receptor 1 dramatically reduced contraction induced by 10 µM carbachol in colonic circular smooth muscles, suggesting the importance of InsP3-dependent Ca2+ mobilization [109]. Collectively, these findings suggest that the M2/M3 pathway (and the M2 pathway) are major inducers of contraction under weak mAChR stimulation, while the M3 pathway predominates under stronger mAChR stimulation.
These predicted muscarinic pathways for contraction, especially the M2/M3 and M3 pathways, are analogous to those in a model proposed by Sawyer and Ehlert (1999) [16] who studied the interaction between M2 and M3 receptors in contraction of the guinea-pig colon. In their model (Model II), occupation of M3 receptors activates two parallel signalling pathways, one causing simple M3-mediated contraction and one with no effect alone but activated in the presence of occupied M2 receptors (a conditional pathway).
8. In Vivo and In Vitro Gastrointestinal Motility
Ultimately, the relevance of these muscarinic pathways depends on their effects on gastrointestinal motility in vivo. Yamada et al. (2001) [110] examined the effects of M3-KO on gut smooth muscle activity in vivo by administering intragastric charcoal and monitoring the distance travelled, which revealed no effect on gut motor activity. Kitazawa et al. (2007) [82] also found that estimated gastric emptying rates as assessed by measuring the weight of food intake relative to that in the stomach 30 min later were similar between M2/M3 double-KO and wild-type mice, again indicating no significant change in gastric motility in the absence of M2 and M3 receptors. These results are seemingly at odds with in vivo pharmacological studies reporting that atropine injection delayed and/or decreased gastrointestinal tract motility, leading to constipation [9]. In mouse gastric and ileal tissues, the atropine-insensitive component of EFS-evoked neurogenic contraction was significantly increased in M2/M3 double-KO mice compared to wild-type mice [82,85,97], suggesting possible compensatory upregulation of non-cholinergic innervation, likely tachykinergic innervation.
However, the contributions of M2 and M3 receptors appear necessary in the colon for both propulsive motility and defecation. Kondo et al. [90] reported that atropine reduced 3 h faeces output in wild-type mice and that faeces evacuation during a 3 h period was clearly reduced in M3-KO and M2/M3 double-KO mice and more modestly reduced in M2-KO mice compared to wild-types. The time required to expel a 2 mm glass bead from the colon as an indication of propulsion force was also increased in KO mice (rank order of propulsion force: wild-type = M2-KO > M3-KO ≥ M2/M3 double-KO), suggesting that mAChRs, especially the M3 subtype, are necessary for colon propulsion and defecation in mice.
In a related study [42], the lack of TRPC4 and TRPC6 channels coupling mAChRs to depolarisation and contraction reduced the rate of charcoal transit within the small intestine in vivo, a result seemingly contradicting the null phenotype of M3-KO and M2/M3 double-KO mice (see above). This may be explained by compensatory TRPC4/TRPC6 channel stimulation by tachykinergic inputs to stimulate gastrointestinal motility.
Tanahashi et al. (2013) [111] found complex differences in the peristaltic movements among isolated small intestine from mAChR-KO and wild-type mice. Changes in intraluminal pressure (IP; representing circular muscle activity) and longitudinal muscle tension (LT) produced by luminal distension were simultaneously measured in the gut segments. The wild-type preparations responded to luminal distension with peristaltic movements characterised by synchronous rises in IP and LT occurring at a constant interval during the stimulus and sensitivity to the mAChR blocker atropine and the sodium channel blocker TTX, indicating the involvement of muscarinic transmission from enteric neurons. In contrast, only small and irregular fluctuations in IP and LT were produced by luminal distension in the majority of M2-KO preparations, and there was no organised peristalsis. This finding is consistent with the report of Schwörer and Klibinger (1988) [112] that pharmacological block of M2 receptors suppressed peristaltic activity in the guinea-pig small intestine. On the other hand, the peristaltic responses elicited in M3-KO preparations were characterised by repeated synchronous rises in IP and LT but with irregular periodicity. As in wild-type mice, these responses were sensitive to atropine and TTX. The M2/M3 double-KO preparations showed atropine-insensitive and TTX-sensitive peristaltic responses to luminal distension. Taken together, these results suggest that M2 and M3 receptors have distinct roles in peristaltic movement of the gut, with M2 receptors pivotal for generation of peristalsis and M3 receptors contributing to control of periodicity. It has been suggested that M2 and M4 receptors are located in the myenteric plexus of the mouse ileum and play an auto-inhibitory role in the release of acetylcholine [20,113]. It is well known that tackykinins are co-transmitters of excitatory motor neurons with ACh [114], implying existence of muscarinic auto-inhibition of the release of tackykinins as well as ACh. In the guinea-pig ileum, exogenous ACh stimulated peristalsis at low concentrations, but inhibited it at higher ones, suggesting the importance to maintain the appropriate concentrations of ACh and other neurotransmitters at the synaptic cleft [115]. Collectively, the autoinhibition of neurotransmitters by M2 may be essential to generate the peristalsis. Of note, the perturbation of peristaltic periodicity associated with M3 deficiency was mimicked by elimination of ICCs in the myenteric plexus (ICC-MYs) [111], suggesting that M3 receptors expressed by ICC-MYs are responsible for periodicity control. It has also been reported that ICC-MYs are involved in the propagation of peristaltic contraction rings along the mouse small intestine from the oral to the anal end [116]. Further study is needed to clarify where these M2 and M3 receptors are located since gut motility is regulated by activation of mAChRs in not only smooth muscle but also other cell types including enteric neurons and ICCs. However, there is a limitation of studies using global knockout of muscarinic receptor subtypes to address the issues because deficiency of muscarinic receptor subtypes in other cells may confound interpretation of the results. Mutant mice with mAChR subtype KO in specific cells should be useful to address these issues.
9. Concluding Remarks
The past decade has seen substantial progress in our understanding of M2 and M3 receptor functions in smooth muscle excitation and contraction due to the development of mAChR-KO and other genetically modified mice. The present article reviews many of those studies focusing on gastrointestinal smooth muscle. A major outcome of this research is the identification of three distinct pathways linking M2 and M3 receptor activation to TRPC cationic channel opening, the primary mechanism by which muscarinic agonists evoke depolarisation and contraction of gastrointestinal smooth muscle. The three signalling pathways identified are depicted in Figure 1. It is noteworthy that they all also converge on another effector of VDCCs to inhibit its activity and ensuing Ca2+ entry. Another major outcome is the elucidation of definitive roles for M2 and M3 receptors in inducing direct contraction or modulating contraction. A scheme depicting the predicted muscarinic mechanisms that elicit contraction is shown in Figure 2. It is of interest that both M2 and M2/M3 pathways activate Ca2+ entry only via VDCCs as a Ca2+ source for contraction, while the M3 pathway activates multiple mechanisms for mobilisation of intracellular Ca2+ and in addition increases the Ca2+ sensitivity of contractile proteins to enhance excitation–contraction coupling efficiency. Both M2 and M2/M3 pathways elicit contraction at low agonist concentrations, while the M3 pathway becomes predominant at higher agonist concentrations. Studies of mAChR-KO mice have also defined distinct roles for M2 and M3 receptors in heterologous desensitisation of contraction and gastrointestinal motility. Future progress can be expected through studies addressing whether the three muscarinic pathways (each organised with mAChRs, G-proteins and effectors) are compartmented separately, whether the M2/M3 pathway leads to significant InsP3/DAG formation and/or cyclic AMP inhibition and whether M2/M3 heterodimers actually exist. The roles of ICCs and neuronal mAChRs in regulating smooth muscle contraction also remain undefined. Extrapolating these results to human physiology and disease remains the ultimate goal. We believe these studies will facilitate the development of treatments for disorders of the gastrointestinal tract and other visceral smooth muscle organs.
Acknowledgments
We thank Jürgen Wess (Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA) for providing muscarinic receptor KO mice. The authors would like to thank Enago (www.enago.jp) for the English language review.
Abbreviations
| ACh | acetylcholine |
| BK channels | large conductance Ca2+-activated K+ channels |
| [Ca2+]i | intracellular Ca2+ concentration |
| cAMP | cyclic AMP |
| DAG | diacylglycerol |
| EC50 | 50% effective concentration |
| EFS | electrical field stimulation |
| EJP | excitatory junction potential |
| Emax | maximum response |
| G-proteins | GTP binding proteins |
| Ica | Ca2+ current |
| ICCs | interstitial cells of Cajal |
| InsP3 | inositol 1,4,5-trisphosphate |
| KATP channels | ATP-sensitive K+ channels |
| KO | knockout |
| mIcat | mAChR-mediated cationic current |
| muscarinic acetylcholine receptors | mAChRs |
| NO | nitric oxide |
| NPY | neuropeptide Y |
| pEC50 | negative logarithm of EC50 |
| PGF2α | prostaglandin F2α |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKC | protein kinase C |
| PLC | phospholipase C |
| PTX | pertussis toxin |
| RASSL | Receptor Solely by Synthetic Ligand |
| STOCs | spontaneous transient outward currents |
| TRPC channels | Transient receptor potential canonical channels |
| VDCCs | voltage-dependent Ca2+ channels |
Author Contributions
Conceptualisation, S.K., Y.T. and T.U.; writing—original draft preparation, Y.T. and S.K.; writing—review and editing, H.M., T.K. and T.U.; Y.T. and S.K. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
The authors’ work was partly supported by the JSPS Research Fellows for Young Scientists (No. 203080) and JSPS KAKENHI Grants (No. 16380199, 17580253, 22380159, 25870891, 26450402, 15K07765, 17K08076) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
No conflict of interest, financial or otherwise, are declared by the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caulfield M.P. Muscarinic receptors-characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-B. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield M., Birdsall N. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 3.Felder C.C. Muscarinic acetylcholine-receptors-signal-transduction through multiple effectors. FASEB J. 1995;9:619–625. doi: 10.1096/fasebj.9.8.7768353. [DOI] [PubMed] [Google Scholar]
- 4.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit. Rev. Neurobiol. 1996;10:69–99. doi: 10.1615/CritRevNeurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 5.Jositsch G., Papadakis T., Haberberger R., Wolff M., Wess J., Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch. Pharmacol. 2009;379:389–395. doi: 10.1007/s00210-008-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wess J., Duttaroy A., Zhang W., Gomeza J., Cui Y., Miyakawa T., Bymaster F.P., McKinzie L., Felder C.C., Lamping K.G., et al. M-1-M-5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Recept. Channels. 2003;9:279–290. doi: 10.3109/10606820308262. [DOI] [PubMed] [Google Scholar]
- 7.Matsui M., Yamada S., Oki T., Manabe T., Taketo M.M., Ehlert F.J. Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci. 2004;75:2971–2981. doi: 10.1016/j.lfs.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Wess J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- 9.Eglen R.M., Hegde S.S., Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 10.Sol I., Yang D.K., Kim H.J., Min K.W., Kang T.M., Kim S.J., Kim K.W., Park K.H., Jeon J.H., Choi K.H., et al. Five subtypes of muscarinic receptors are expressed in gastric smooth muscles of guinea pig. Exp. Mol. Med. 2003;35:46–52. doi: 10.1038/emm.2003.7. [DOI] [PubMed] [Google Scholar]
- 11.Bolton T.B. Mechanisms of action of transmitters and other substances on smooth-muscle. Physiol. Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- 12.Beech D.J. Actions of neurotransmitters and other messengers on Ca2+ channels and K+ channels in smooth muscle cells. Pharmacol. Ther. 1997;73:91–119. doi: 10.1016/S0163-7258(97)87271-3. [DOI] [PubMed] [Google Scholar]
- 13.Ehlert F.J., Thomas E.A. Functional-role of M(2) muscarinic receptors in the guinea-pig ileum. Life Sci. 1995;56:965–971. doi: 10.1016/0024-3205(94)00035-Q. [DOI] [PubMed] [Google Scholar]
- 14.Bolton T.B., Prestwich S.A., Zholos A.V., Gordienko D.V. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- 15.Ehlert F.J., Ostrom R.S., Sawyer G.W. Subtypes of the muscarinic receptor in smooth muscle. Life Sci. 1997;61:1729–1740. doi: 10.1016/S0024-3205(97)00433-5. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer G.W., Ehlert F.J. Muscarinic M-3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea pig colon: Evidence for M-2/M-3 receptor interactions. J. Pharmacol. Exp. Ther. 1999;289:464–476. [PubMed] [Google Scholar]
- 17.Zholos A.V., Bolton T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komori S., Unno T., Nakayama T., Ohashi H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998;76:213–218. doi: 10.1254/jjp.76.213. [DOI] [PubMed] [Google Scholar]
- 19.Pucovsky V., Zholos A.V., Bolton T.B. Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. Eur. J. Pharmacol. 1998;346:323–330. doi: 10.1016/S0014-2999(98)00059-4. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi T., Fujinami K., Goto H., Fujita A., Taketo M.M., Manabe T., Matsui M., Hata F. Roles of M-2 and M-4 muscarinic receptors in regulating acetylcholine release from myenteric neurons of mouse ileum. J. Neurophysiol. 2005;93:2841–2848. doi: 10.1152/jn.00986.2004. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi T., Toyoshima M., Mukai K., Hagi K., Matsui M., Nakajima H., Azuma Y.T., Hata F. Involvement of M-2 muscarinic receptors in relaxant response of circular muscle of mouse gastric antrum. Neurogastroenterol. Motil. 2006;18:226–233. doi: 10.1111/j.1365-2982.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 22.Harrington A.M., Hutson J.M., Southwell B.R. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog. Histochem. Cytochem. 2010;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto T., Unno T., Kitazawa T., Taneike T., Yamada M., Wess J., Nishimura M., Komori S. Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells. J. Physiol. Lond. 2007;582:41–61. doi: 10.1113/jphysiol.2007.133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy K.S., Makhlouf G.M. Differential coupling of muscarinic m(2) and m(3) receptors to adenylyl cyclases V/VI in smooth muscle. J. Biol. Chem. 1997;272:21317–21324. doi: 10.1074/jbc.272.34.21317. [DOI] [PubMed] [Google Scholar]
- 25.Tran J.A., Matsui M., Ehlert F.J. Differential coupling of muscarinic M-1, M-2, and M-3 receptors to phosphoinositide hydrolysis in urinary bladder and longitudinal muscle of the ileum of the mouse. J. Pharmacol. Exp. Ther. 2006;318:649–656. doi: 10.1124/jpet.106.103093. [DOI] [PubMed] [Google Scholar]
- 26.Noronhablob L., Lowe V., Patton A., Canning B., Costello D., Kinnier W.J. Muscarinic receptors-relationships among phosphoinositide breakdown, adenylate-cyclase inhibition, in vitro detrusor muscle contractions and invivo cystometrogram studies in guinea-pig bladder. J. Pharmacol. Exp. Ther. 1989;249:843–851. [PubMed] [Google Scholar]
- 27.Candell L.M., Yun S.H., Tran L.L.P., Ehlert F.J. Differential coupling of subtypes of the muscarinic receptor to adenylate-cyclase and phosphoinositide hydrolysis in the longitudinal muscle of the rat ileum. Mol. Pharmacol. 1990;38:689–697. [PubMed] [Google Scholar]
- 28.Murthy K.S., Zhou H.P., Grider J.R., Brautigan D.L., Eto M., Makhlouf G.M. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via G(i3), Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem. J. 2003;374:145–155. doi: 10.1042/bj20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carl A., Lee H.K., Sanders K.M. Regulation of ion channels in smooth muscles by calcium. Am. J. Physiol. Cell Physiol. 1996;271:C9–C34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- 30.Zholos A.V. Muscarinic effects on ion channels in smooth muscle cells. Neurophysiology. 1999;31:173–187. doi: 10.1007/BF02515064. [DOI] [Google Scholar]
- 31.Unno T., Matsuyama H., Okamoto H., Sakamoto T., Yamamoto M., Tanahashi Y., Yan H.D., Komori S. Muscarinic cationic current in gastrointestinal smooth muscles: Signal transduction and role in contraction. Auton. Autacoid Pharm. 2006;26:203–217. doi: 10.1111/j.1474-8673.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 32.Inoue R., Isenberg G. Acetylcholine activates nonselective cation channels in guinea-pig ileum through a g-protein. Am. J. Physiol. 1990;258:C1173–C1178. doi: 10.1152/ajpcell.1990.258.6.C1173. [DOI] [PubMed] [Google Scholar]
- 33.Pacaud P., Bolton T.B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth-muscle cells. J. Physiol. Lond. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komori S., Kawai M., Takewaki T., Ohashi H. Gtp-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. Lond. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan H.D., Okamoto H., Unno T., Tsytsyura Y.D., Prestwich S.A., Komori S., Zholos A.V., Bolton T.B. Effects of G-protein-specific antibodies and G beta gamma subunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 2003;139:605–615. doi: 10.1038/sj.bjp.0705289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zholos A.V., Tsytsyura Y.D., Gordienko D.V., Tsvilovskyy V.V., Bolton T.B. Phospholipase C, but not InsP(3) or DAG, -dependent activation of the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 2004;141:23–36. doi: 10.1038/sj.bjp.0705584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto H., Unno T., Arima D., Suzuki M., Yan H.D., Matsuyama H., Nishimura M., Komori S. Phospholipase C involvement in activation of the muscarinic receptor-operated cationic current in guinea pig ileal smooth muscle cells. J. Pharmacol. Sci. 2004;95:203–213. doi: 10.1254/jphs.FP0030635. [DOI] [PubMed] [Google Scholar]
- 38.Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. A novel G alpha(q/11)-selective inhibitor. J. Biol. Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 39.Tanahashi Y., Katsurada T., Inasaki N., Uchiyama M., Sakamoto T., Yamamoto M., Matsuyama H., Komori S., Unno T. Further characterization of the synergistic activation mechanism of cationic channels by M-2 and M-3 muscarinic receptors in mouse intestinal smooth muscle cells. Am. J. Physiol. Cell Physiol. 2020;318:C514–C523. doi: 10.1152/ajpcell.00277.2019. [DOI] [PubMed] [Google Scholar]
- 40.Sakamoto T., Unno T., Matsuyama H., Uchiyama M., Hattori M., Nishimura M., Komori S. Characterization of muscarinic receptor-mediated cationic currents in longitudinal smooth muscle cells of mouse small intestine. J. Pharmacol. Sci. 2006;100:215–226. doi: 10.1254/jphs.FP0050973. [DOI] [PubMed] [Google Scholar]
- 41.Beech D.J., Muraki K., Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP (vol 559, pg 685, 2004) J. Physiol. Lond. 2004;560:949, 950. doi: 10.1113/jphysiol.2004.560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsvilovskyy V.V., Zholos A.V., Aberle T., Philipp S.E., Dietrich A., Zhu M.X., Birnbaumer L., Freichel M., Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee K.P., Jun J.Y., Chang I.Y., Suh S.H., So I.S., Kim K.W. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol. Cells. 2005;20:435–441. [PubMed] [Google Scholar]
- 44.Sakamoto T., Matsuyama H., Yamamoto M., Tanahashi Y., Kitazawa T., Taneike T., Komori S., Unno T. A non-selective cationic channel activated by diacylglycerol in mouse intestinal myocytes. Eur. J. Pharmacol. 2008;599:54–57. doi: 10.1016/j.ejphar.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 45.Otsuguro K.I., Tang J.S., Tang Y.F., Xiao R., Freichel M., Tsvilovskyy V., Ito S., Flockerzi V., Zhu M.X., Zholos A.V. Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2008;283:10026–10036. doi: 10.1074/jbc.M707306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dresviannikov A.V., Bolton T.B., Zholos A.V. Muscarinic receptor-activated cationic channels in murine ileal myocytes. Br. J. Pharmacol. 2006;149:179–187. doi: 10.1038/sj.bjp.0706852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okamoto H., Prestwich S.A., Asai S., Unno T., Bolton T.B., Komori S. Muscarinic agonist potencies at three different effector systems linked to the M-2 or M-3 receptor in longitudinal smooth muscle of guinea-pig small intestine. Br. J. Pharmacol. 2002;135:1765–1775. doi: 10.1038/sj.bjp.0704642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misra S., Mahavadi S., Grider J.R., Murthy K.S. Differential expression of Y receptors and signaling pathways in intestinal circular and longitudinal smooth muscle. Regul. Pept. 2005;125:163–172. doi: 10.1016/j.regpep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Tanahashi Y., Unno T., Matsuyama H., Ishii T., Yamada M., Wess J., Komori S. Multiple muscarinic pathways mediate the suppression of voltage-gated Ca2+channels in mouse intestinal smooth muscle cells. Br. J. Pharmacol. 2009;158:1874–1883. doi: 10.1111/j.1476-5381.2009.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aslanoglou D., Alvarez-Curto E., Marsango S., Milligan G. Distinct agonist regulation of muscarinic acetylcholine M-2-M-3 heteromers and their corresponding homomers. J. Biol. Chem. 2015;290:14785–14796. doi: 10.1074/jbc.M115.649079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komori S., Bolton T.B. Calcium release induced by inositol 1,4,5-trisphosphate in single-rabbit intestinal smooth-muscle cells. J. Physiol. Lond. 1991;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benham C.D., Bolton T.B. Spontaneous transient outward currents in single visceral and vascular smooth-muscle cells of the rabbit. J. Physiol. Lond. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole W.C., Carl A., Sanders K.M. Muscarinic suppression of Ca2+-dependent K-current in colonic smooth-muscle. Am. J. Physiol. 1989;257:C481–C487. doi: 10.1152/ajpcell.1989.257.3.C481. [DOI] [PubMed] [Google Scholar]
- 54.Kume H., Kotlikoff M.I. Muscarinic inhibition of single kca channels in smooth-muscle cells by a pertussis-sensitive G-protein. Am. J. Physiol. 1991;261:C1204–C1209. doi: 10.1152/ajpcell.1991.261.6.C1204. [DOI] [PubMed] [Google Scholar]
- 55.Bonev A.D., Nelson M.T. Muscarinic inhibition of atp-sensitive K+ channels by protein-kinase-C in urinary-bladder smooth-muscle. Am. J. Physiol. 1993;265:C1723–C1728. doi: 10.1152/ajpcell.1993.265.6.C1723. [DOI] [PubMed] [Google Scholar]
- 56.Hatakeyama N., Wang Q., Goyal R.K., Akbarali H.I. Muscarinic suppression of atp-sensitive K+ channel in rabbit esophageal smooth-muscle. Am. J. Physiol. Cell Physiol. 1995;268:C877–C885. doi: 10.1152/ajpcell.1995.268.4.C877. [DOI] [PubMed] [Google Scholar]
- 57.Nuttle L.C., Farley J.M. Muscarinic receptors inhibit ATP-sensitive K+ channels in swine tracheal smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;273:L478–L484. doi: 10.1152/ajplung.1997.273.2.L478. [DOI] [PubMed] [Google Scholar]
- 58.Wang B., Murakami Y., Ono N., Fujikawa S., Matsuyama H., Unno T., Naitou K., Tanahashi Y. Muscarinic suppression of ATP-sensitive K+ channels mediated by the M-3/G(q/11)/phospholipase C pathway contributes to mouse ileal smooth muscle contractions. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G618–G630. doi: 10.1152/ajpgi.00069.2018. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y.X., Fleischmann B.K., Kotlikoff M.I. M-2 receptor activation of nonselective cation channels in smooth muscle cells: Calcium and G(i)/G(0) requirements. Am. J. Physiol. Cell Physiol. 1997;273:C500–C508. doi: 10.1152/ajpcell.1997.273.2.C500. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y.X., Kotlikoff M.I. Muscarinic signaling pathway for calcium release and calcium-activated chloride current in smooth muscle. Am. J. Physiol. Cell Physiol. 1997;273:C509–C519. doi: 10.1152/ajpcell.1997.273.2.C509. [DOI] [PubMed] [Google Scholar]
- 61.Beech D.J. Inhibitory Effects of histamine and bradykinin on calcium current in smooth-muscle cells isolated from guinea-pig ileum. J. Physiol. Lond. 1993;463:565–583. doi: 10.1113/jphysiol.1993.sp019611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unno T., Komori S., Ohashi H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth-muscle cells of guinea-pig ileum. J. Physiol. Lond. 1995;484:567–581. doi: 10.1113/jphysiol.1995.sp020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komori S., Bolton T.B. Inositol trisphosphate releases stored calcium to block voltage-dependent calcium channels in single smooth-muscle cells. Pflug. Arch. Eur. J. Physiol. 1991;418:437–441. doi: 10.1007/BF00497770. [DOI] [PubMed] [Google Scholar]
- 64.Jin X.C., Morsy N., Shoeb F., Zavzavadjian J., Akbarali H.I. Coupling of M-2 muscarinic receptor to L-type Ca2+ channel via c-src kinase in rabbit colonic circular smooth muscle. Gastroenterology. 2002;123:827–834. doi: 10.1053/gast.2002.35388. [DOI] [PubMed] [Google Scholar]
- 65.Bolton T.B. Depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J. Physiol. Lond. 1972;220:647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Unno T., Inaba T., Ohashi H., Takewaki T., Komori S. Role of Ca2+ mobilization in muscarinic receptor-mediated membrane depolarization in guinea pig ileal smooth muscle cells. Jpn. J. Pharmacol. 2000;84:431–437. doi: 10.1254/jjp.84.431. [DOI] [PubMed] [Google Scholar]
- 67.Matsuyama H., Tanahashi Y., Kitazawa T., Yamada M., Komori S., Unno T. Evidence for M-2 and M-3 muscarinic receptor involvement in cholinergic excitatory junction potentials through synergistic activation of cation channels in the longitudinal muscle of mouse ileum. J. Pharmacol. Sci. 2013;121:227–236. doi: 10.1254/jphs.12231FP. [DOI] [PubMed] [Google Scholar]
- 68.Zholos A.V., Tsytsyura Y.D., Philyppov I.B., Shuba M.F., Bolton T.B. Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365. Br. J. Pharmacol. 2000;129:695–702. doi: 10.1038/sj.bjp.0703115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itagaki M., Komori S., Unno T., Syuto B., Ohashi H. Possible involvement of a small G-protein sensitive to exoenzyme C3 of clostridium-botulinum in the regulation of myofilament Ca2+ sensitivity in beta-escin skinned smooth-muscle of guinea-pig ileum. Jpn. J. Pharmacol. 1995;67:1–7. doi: 10.1254/jjp.67.1. [DOI] [PubMed] [Google Scholar]
- 70.Somlyo A.P., Somlyo A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 71.Cook A.K., Carty M., Singer C.A., Yamboliev I.A., Gerthoffer W.T. Coupling of M-2 muscarinic receptors to ERK MAP kinases and caldesmon phosphorylation in colonic smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G429–G437. doi: 10.1152/ajpgi.2000.278.3.G429. [DOI] [PubMed] [Google Scholar]
- 72.Ihara E., Moffat L., Ostrander J., Walsh M.P., MacDonald J.A. Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G699–G710. doi: 10.1152/ajpgi.00214.2007. [DOI] [PubMed] [Google Scholar]
- 73.Suguro M., Matsuyama H., Tanahashi Y., Unno T., Kitazawa T., Yamada M., Komori S. Muscarinic receptor subtypes mediating Ca2+ sensitization of intestinal smooth muscle contraction: Studies with receptor knockout mice. J. Vet. Med. Sci. 2010;72:443–451. doi: 10.1292/jvms.09-0458. [DOI] [PubMed] [Google Scholar]
- 74.Shehnaz D., Ansari K.Z., Ehlert F.J. Acetylcholine-induced desensitization of the contractile response to histamine in guinea pig ileum is prevented by either pertussis toxin treatment or by selective inactivation of muscarinic M-3 receptors. J. Pharmacol. Exp. Ther. 2001;297:1152–1159. [PubMed] [Google Scholar]
- 75.Griffin M.T., Matsui M., Shehnaz D., Ansari K.Z., Taketo M.M., Manabe T., Ehlert F.J. Muscarinic agonist-mediated heterologous desensitization in isolated ileum requires activation of both muscarinic M-2 and M-3 receptors. J. Pharmacol. Exp. Ther. 2004;308:339–349. doi: 10.1124/jpet.103.055327. [DOI] [PubMed] [Google Scholar]
- 76.Bolton T.B. Role of electrogenic sodium pumping in response of smooth-muscle to acetylcholine. J. Physiol. Lond. 1973;228:713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Unno T., Kwon S.C., Okamoto H., Irie Y., Kato Y., Matsuyama H., Komori S. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br. J. Pharmacol. 2003;139:337–350. doi: 10.1038/sj.bjp.0705267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wrzos H.F., Tandon T., Ouyang A. Mechanisms mediating cholinergic antral circular smooth muscle contraction in rats. World J. Gastroenterol. 2004;10:3292–3298. doi: 10.3748/wjg.v10.i22.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas E.A., Ehlert F.J. Pertussis toxin blocks M(2) muscarinic receptor-mediated effects on contraction and cyclic-amp in the guinea-pig ileum, but not M(3)-mediated contractions and phosphoinositide hydrolysis. J. Pharmacol. Exp. Ther. 1994;271:1042–1050. [PubMed] [Google Scholar]
- 80.Stengel P.W., Gomeza J., Wess J., Cohen M.L. M-2 and M-4 receptor knockout mice: Muscarinic receptor function in cardiac and smooth muscle in vitro. J. Pharmacol. Exp. Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- 81.Stengel P.W., Yamada M., Wess J., Cohen M.L. M-3-receptor knockout mice: Muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1443–R1449. doi: 10.1152/ajpregu.00486.2001. [DOI] [PubMed] [Google Scholar]
- 82.Kitazawa T., Hashiba K., Cao J.S., Unno T., Komori S., Yamada M., Wess J., Taneike T. Functional roles of muscarinic M-2 and M-3 receptors in mouse stomach motility: Studies with muscarinic receptor knockout mice. Eur. J. Pharmacol. 2007;554:212–222. doi: 10.1016/j.ejphar.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Ruggieri M.R., Braverman A.S. Gastric body cholinergic contractile signal transduction in M-2 and M-3 receptor knockout mice. J. Recept. Signal Transduct. 2013;33:249–254. doi: 10.3109/10799893.2013.802803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsui M., Motomura D., Karasawa H., Fujikawa T., Jiang J., Komiya Y., Takahashi S., Taketo M.M. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M-3 subtype. Proc. Natl. Acad. Sci. USA. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsui M., Motomura D., Fujiwara T., Jiang J., Takahashi S., Manabe T., Taketo M.M. Mice lacking M-2 and M-3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J. Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsui M., Griffin M.T., Shehnaz D., Taketo M.M., Ehlert F.J. Increased relaxant action of forskolin and isoproterenol against muscarinic agonist-induced contractions in smooth muscle from M-2 receptor knockout mice. J. Pharmacol. Exp. Ther. 2003;305:106–113. doi: 10.1124/jpet.102.044701. [DOI] [PubMed] [Google Scholar]
- 87.Unno T., Matsuyama H., Sakamoto T., Uchiyama M., Izumi Y., Okamoto H., Yamada M., Wess J., Komori S. M-2 and M-3 muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br. J. Pharmacol. 2005;146:98–108. doi: 10.1038/sj.bjp.0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeuchi T., Tanaka K., Nakajima H., Matsui M., Azuma Y.T. M-2 and M-3 muscarinic receptors are involved in enteric nerve-mediated contraction of the mouse ileum: Findings obtained with muscarinic-receptor knockout mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G154–G164. doi: 10.1152/ajpgi.00173.2006. [DOI] [PubMed] [Google Scholar]
- 89.Griffin M.T., Matsui M., Ostrom R.S., Ehlert F.J. The guinea pig ileum lacks the direct, high-potency, M-2-muscarinic, contractile mechanism characteristic of the mouse ileum. Naunyn Schmiedebergs Arch. Pharmacol. 2009;380:327–335. doi: 10.1007/s00210-009-0434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kondo T., Nakajima M., Teraoka H., Unno T., Komori S., Yamada M., Kitazawa T. Muscarinic receptor subtypes involved in regulation of colonic motility in mice: Functional studies using muscarinic receptor-deficient mice. Eur. J. Pharmacol. 2011;670:236–243. doi: 10.1016/j.ejphar.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 91.Stengel P.W., Cohen M.L. Muscarinic receptor knockout mice: Role of muscarinic acetylcholine receptors M-2, M-3, and M-4 in carbamylcholine-induced gallbladder contractility. J. Pharmacol. Exp. Ther. 2002;301:643–650. doi: 10.1124/jpet.301.2.643. [DOI] [PubMed] [Google Scholar]
- 92.Ehlert F.J., Griffin M.T., Abe D.M., Vo T.H., Taketo M.M., Manabe T., Matsui M. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinary bladder. J. Pharmacol. Exp. Ther. 2005;313:368–378. doi: 10.1124/jpet.104.077909. [DOI] [PubMed] [Google Scholar]
- 93.Kitazawa T., Hirama R., Masunaga K., Nakamura T., Asakawa K., Cao J., Teraoka H., Unno T., Komori S.I., Yamada M., et al. Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377:503–513. doi: 10.1007/s00210-007-0223-1. [DOI] [PubMed] [Google Scholar]
- 94.Witte L., de Haas N., Mammen M., Stangeland E., Steinfeld T., Aiyar J., Michel M. Muscarinic receptor subtypes and signalling involved in the attenuation of isoprenaline-induced rat urinary bladder relaxation. Naunyn Schmiedebergs Arch. Pharmacol. 2011;384:555–563. doi: 10.1007/s00210-011-0689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olgart C., Iversen H.H. Nitric oxide-dependent relaxation induced by M-1 muscarinic receptor activation in the rat small intestine. Br. J. Pharmacol. 1999;127:309–313. doi: 10.1038/sj.bjp.0702529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stengel P.W., Cohen M.L. M-1 receptor-mediated nitric oxide-dependent relaxation unmasked in stomach fundus from M-3 receptor knockout mice. J. Pharmacol. Exp. Ther. 2003;304:675–682. doi: 10.1124/jpet.102.042283. [DOI] [PubMed] [Google Scholar]
- 97.Unno T., Matsuyama H., Izumi Y., Yamada M., Wess J., Komori S. Roles of M-2 and M-3 muscarinic receptors in cholinergic nerve-induced contractions in mouse ileum studied with receptor knockout mice. Br. J. Pharmacol. 2006;149:1022–1030. doi: 10.1038/sj.bjp.0706955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanders K.M., Ward S.M., Koh S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drumm B.T., Rembetski B.E., Huynh K., Nizar A., Baker S.A., Sanders K.M. Excitatory cholinergic responses in mouse colon intramuscular interstitial cells of Cajal are due to enhanced Ca(2+)release via M(3)receptor activation. FASEB J. 2020;34:10073–10095. doi: 10.1096/fj.202000672R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baker S.A., Drumm B.T., Skowronek K.E., Rembetski B.E., Peri L.E., Hennig G.W., Perrino B.A., Sanders K.M. Excitatory neuronal responses of Ca2+ transients in interstitial cells of cajal in the small intestine. Eneuro. 2018;5 doi: 10.1523/ENEURO.0080-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Groneberg D., Lies B., Konig P., Jager R., Seidler B., Klein S., Saur D., Friebe A. Cell-specific deletion of nitric oxide-sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterology. 2013;145:188–196. doi: 10.1053/j.gastro.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 102.Gautam D., Han S.J., Hamdan F.F., Jeon J., Li B., Li J.H., Cui Y.H., Mears D., Lu H.Y., Deng C.X., et al. A critical role for beta cell M-3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 103.Ostrom R.S., Ehlert F.J. M(2) muscarinic receptor inhibition of agonist-induced cyclic adenosine monophosphate accumulation and relaxation in the guinea pig ileum. J. Pharmacol. Exp. Ther. 1997;280:189–199. [PubMed] [Google Scholar]
- 104.Roffel A.F., Meurs H., Elzinga C.R.S., Zaagsma J. Muscarinic M(2) receptors do not participate in the functional antagonism between methacholine and isoprenaline in guinea-pig tracheal smooth-muscle. Eur. J. Pharmacol. 1993;249:235–238. doi: 10.1016/0014-2999(93)90438-N. [DOI] [PubMed] [Google Scholar]
- 105.Roffel A.F., Meurs H., Elzinga C.R.S., Zaagsma J. No evidence for a role of muscarinic M(2) receptors in functional antagonism in bovine trachea. Br. J. Pharmacol. 1995;115:665–671. doi: 10.1111/j.1476-5381.1995.tb14984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ehlert F.J., Ahn S., Pak K.J., Park G.J., Sangnil M.S., Tran J.A., Matsui M. Neuronally released acetylcholine acts on the M-2 muscarinic receptor to oppose the relaxant effect of isoproterenol on cholinergic contractions in mouse urinary bladder. J. Pharmacol. Exp. Ther. 2007;322:631–637. doi: 10.1124/jpet.107.121756. [DOI] [PubMed] [Google Scholar]
- 107.Ohta T., Kawai K., Ito S., Nakazato Y. Ca2+ entry activated by emptying of intracellular Ca2+ stores in ileal smooth-muscle of the rat. Br. J. Pharmacol. 1995;114:1165–1170. doi: 10.1111/j.1476-5381.1995.tb13329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zholos A.V. Regulation of TRP-like muscarinic cation current in gastrointestinal smooth muscle with special reference to PLC/InsP(3)/Ca2+ system. Acta Pharmacol. Sin. 2006;27:833–842. doi: 10.1111/j.1745-7254.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 109.Wang H., Jing R., Trexler C., Li Y., Tang H., Pan Z., Zhu S., Zhao B., Fang X., Liu J., et al. Deletion of IP(3)R1 by Pdgfrb-Cre in mice results in intestinal pseudo-obstruction and lethality. J. Gastroenterol. 2019;54:407–418. doi: 10.1007/s00535-018-1522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamada M., Miyakawa T., Duttaroy A., Yamanaka A., Moriguchi T., Makita R., Ogawa M., Chou C.J., Xia B., Crawley J.N., et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 111.Tanahashi Y., Waki N., Unno T., Matsuyama H., Iino S., Kitazawa T., Yamada M., Komori S. Roles of M2 and M3 muscarinic receptors in the generation of rhythmic motor activity in mouse small intestine. Neurogastroenterol. Motil. 2013;25:e687–e697. doi: 10.1111/nmo.12194. [DOI] [PubMed] [Google Scholar]
- 112.Schworer H., Kilbinger H. Enhancement of guinea-pig intestinal peristalsis by blockade of muscarinic M1-receptors. Br. J. Pharmacol. 1988;93:715–720. doi: 10.1111/j.1476-5381.1988.tb10330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.North R., Slack B., Surprenant A. Muscarinic-M1 and Muscarinic-M2 Receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous-system. J. Physiol. Lond. 1985;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Furness J.B. The Enteric Nervous System. Blackwell Publishing, Inc.; Malden, MA, USA: 2007. Pharmacology of transmission and sites of drug action in the enteric nervous system; pp. 103–131. [Google Scholar]
- 115.Bulbring E., Lin R.C. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J. Physiol. 1958;140:381–407. [PMC free article] [PubMed] [Google Scholar]
- 116.Nakagawa T., Misawa H., Nakajima Y., Takaki M. Absence of peristalsis in the ileum of W/W(V) mutant mice that are selectively deficient in myenteric interstitial cells of Cajal. J. Smooth Muscle Res. 2005;41:141–151. doi: 10.1540/jsmr.41.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.