Abstract
Currently, no single medication has been approved for the management of coronavirus disease-2019 (COVID-19) caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Therefore, drug repositioning by investigating the use of existing drugs for management of COVID-19 patients is considered a desperate need. Tramadol is a commonly prescribed analgesic drug for treatment of moderate to severe pain with less potential for dependence and respiratory depression. Multiple evidence support that tramadol is a promising drug for treatment of COVID-19 patients. Herein, we discuss the possible beneficial effects of using tramadol against SARS-CoV-2 infection and their underlying mechanism of action. The anti-inflammatory effect of tramadol may help to suppress the COVID-19 related cytokine storm through decreasing interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP). Besides, tramadol activates natural killer (NK) and T-cells and enhances IL-2 secretion, which produce immune-enhancing effect against SARS-CoV-2. Recent studies confirmed that COVID-19 patients with acute respiratory failure showed increased fibrin formation and polymerization that may lead to thrombosis. Tramadol owing to its hypocoagulable effect may protect against venous thromboembolism in these patients. Moreover, tramadol can exert a cardioprotective effect via decreasing lactate dehydrogenase (LDH) level which is elevated in most of patients with COVID-19. Furthermore, the severity and mortality of COVID-19 have been correlated with old age patients, which may be due to the lack of antioxidant mechanisms and increased oxidative damage. Tramadol could protect COVID-19 patient from disease complications by increases the antioxidant enzymes superoxide dismutase and glutathione peroxidase while diminished malondialdehyde. More interestingly, tramadol as an effective analgesic and antitussive may have a beneficial effect on COVID-19 patients suffering from cough, headache, ache, and pain. The tramadol anti-psychotic effect may also protect against psychiatric disorders associated with SARS-CoV-2 infection. Moreover, tramadol has bactericidal activity against a wide range of pathogens including Pseudomonas aeruginosa which is common in severe COVID-19 patients leading to pneumonia with worse clinical outcomes. Therefore, we hypothesize that tramadol might be a promising adjuvant therapeutic option against SARS-CoV-2 infection. Based on that, tramadol should be considered as adjuvant therapy for COVID-19 clinical trials.
Keywords: SARS-CoV-2, COVID-19, Tramadol, Anti-inflammatory, Immune-enhancing, Hypocoagulable effect
Background
The emergence and spread of the novel coronavirus disease-2019 (COVID-19) is a public health crisis threatening the world. This disease is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which had its origin in bats and was transmitted to humans in Wuhan, China in December 2019 [1], [2], [3]. According to WHO, there have been confirmed 50 676 072 reported cases of COVID-2019 and 1 261 075 reported deaths to date (10/11/2020). The disease is mild in most cases; in some patients, it may progress to acute respiratory distress syndrome (ARDS), pneumonia, and multi-organ failure [1], [3], [4]. Currently, no single medication has been approved for the management of COVID-19 [4], [5]. Therefore, drug repositioning by investigating the use of existing drugs for management of COVID-19 patients is considered a desperate need. Several attempts for drug repositioning in treatment of COVID-19 was reported [6].
The hypothesis
T-cells play a critical role in antiviral immunity, their level was dramatically reduced in COVID-19 patients [7]. There is a negative correlation between T-cell numbers and cytokines serum level in COVID-19 patients [7]. In those patients, there is up-regulation of inflammatory cytokines including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, and interferon γ [8]. This makes the use of tramadol reasonable in such patients because it has anti-inflammatory effect decreasing plasma level of TNF-α [9], which may result in a subsequent increase in T-cell numbers. Besides, COVID-19 patients with acute respiratory failure present with severe hypercoagulability due to hyperfibrinogenemia that may predispose to thrombosis [10]. It has been reported that tramadol has a remarkable hypocoagulable effect. Consequently, tramadol may be useful for patients who have a tendency toward a hypercoagulable status and thromboembolic complications [11].
On the other hand, the severity and mortality risk of COVID-19 have been associated with the age. This age-related mortality is attributed to the shortage of antioxidant mechanisms and increased oxidative damage [12]. Tramadol increased the antioxidant enzymes superoxide dismutase and glutathione peroxidase while diminished the oxidative stress marker malondialdehyde [13]. Owing to its antioxidant properties, tramadol could reduce complications in COVID-19 patients. Moreover, tramadol was reported to significantly lower lactate dehydrogenase (LDH) level and to provide a cardio-protective effect [14]. This seems beneficial as most of COVID-19 patients are presented with elevated LDH levels [15].
More interestingly, COVID-19 patient may experience intense emotional and behavioral reactions, such as fear, loneliness, and anxiety [16]. Tramadol is a centrally acting analgesic drug with a dual mechanism of action: binding to μ-opioid receptors and the inhibition of serotonin and norepinephrine reuptake [17]. Through its ability to inhibit serotonin and norepinephrine reuptake, tramadol may exhibit antidepressant activity [18]. In this context, the analgesic and antidepressant effects of tramadol may favor its use for COVID-19 patients. Tramadol also was found to have dose- and time-dependent bactericidal activity against Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa pathogens in vitro [19].
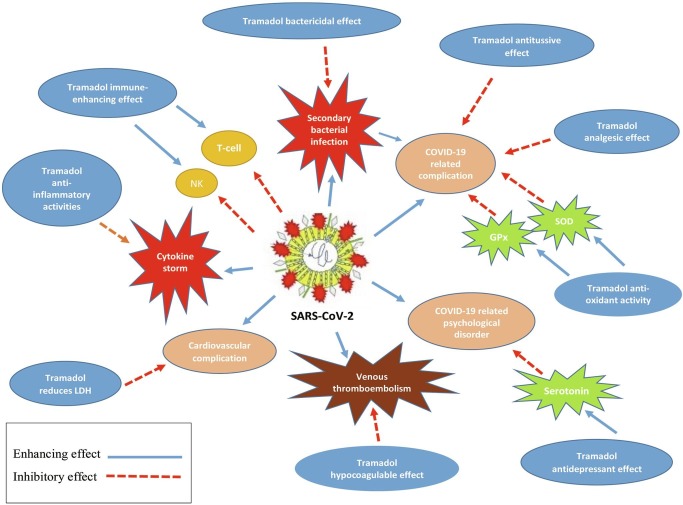
The rationale of the use of tramadol for COVID-19 patients is attributed to its anti-inflammatory, hypocagulatory, antioxidant, cardio-protective, analgesic, antitussive, bactericidal, and antidepressant effect (Fig. 1 ).
Fig. 1.
The plausible mechanisms of tramadol for treatment of COVID-19. GPx: glutathione peroxidase, LDH: lactate dehydrogenase, NK: natural killer, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, SOD: superoxide dismutase.
Support for hypothesis
Tramadol is a widely used opioid drug with less potential for dependance and respiratory depression
Tramadol is a centrally acting analgesic by two different mechanisms. It binds to μ-opioid receptors on cells, especially those responsible for feelings of pleasure and pain and in controlling heart rate, breathing, and sleeping [20], [21], [22]. Tramadol also inhibits serotonin and norepinephrine reuptake, exerting inhibitory effects on pain transmission in the spinal cord and regulating mood and social behavior [20], [21], [22]. The two synergistic mechanisms improve the analgesic efficacy and tolerability of tramadol [22].
In contrast to the other pure opioid agonists, tramadol has a minimum risk of respiratory depression, tolerance, and dependence [22], [23]. Previous studies confirmed that tramadol dependence may occur when it is used daily for more than a few weeks/months [24]. The use of tramadol is largely considered safe [22], [23]. The most common side effects reported for tramadol use are nausea, dizziness, and constipation [22]. However, the toxicity of tramadol may be underestimated [25], [26].
Tramadol is considered atypical opioid. This special character made tramadol one of the most prescribed analgesics for treatment of moderate to severe pain [23], [27]. It is recommended for the treatment of moderate to severe pain in patients who do not respond to oral therapies or who have a contraindication to nonselective COX inhibitors or selective COX-2 inhibitors [23]. Tramadol can be administrated orally, rectally, and in a solution for IM/IV administration. Oral administration of tramadol has fast absorption and distribution [23].
The anti-inflammatory and immune-modulatory effect of tramadol may have a beneficial effect against SARS-CoV-2 infection
SARS-CoV-2 infection is characterized by severe lymphopenia and eosinopenia, a cytokine storm leading to ARDS, and multiorgan dysfunction as well as an increased level of C-reactive protein (CRP) [28], [29]. Lymphopenia causes a disorder in antiviral and immune regulatory activity [29]. There is an elevation of inflammatory cytokines including IL-6, IL-1, TNF-α, and interferon γ in COVID-19 patients [4], [8].
Liu et al. [30] reported that tramadol suppresses pro-inflammatory cytokine production like IL-6 in a dose-dependent manner but doesn’t affect IL-2 levels, suggesting that it may suppress the inflammation and has a beneficial role in the modulation of IL-2 associated with cell-mediated immunity. Another study showed that adding tramadol to a caudal bupivacaine block can attenuate the pro-inflammatory response of cytokines (significantly decrease IL-6), cortisol, and CRP (markers of acute-phase and inflammatory mediator responses) in children undergoing surgery [31]. Moreover, plasma levels of TNF-α were reported to be decreased after treatment with tramadol (100 mg every 12 h for 10 days) [9].
More interestingly, other studies reported that tramadol may have an immune enhancing effect by increasing secretion of IL-2 and mediating activation of natural killer (NK) cells [17], [32]. NK cells are lymphocytes with characteristic ability to eradicate tumor and virus-infected cells, it is now well established that NK cells also have a critical role in regulating immune cell homeostasis [33]. IL-2 is a cytokine that enhances NK cytolytic activity, activates T-cell growth, and drives the differentiation of regulatory T-cells. In normal conditions, immunologic response does not show IL-2 in the blood [34].
On the other hand, T-cells play a serious role in antiviral immunity, number of T-cells was dramatically decreased in COVID-19 patients [7], [35]. There is a negative correlation between number of T-cells and circulating cytokines level in COVID-19 patients [7]. This makes the use of tramadol reasonable in such patients because plasma levels of TNF-α and IL-6 were reported to be decreased after treatment with tramadol which may result in a subsequent increase in T-cell numbers according to the negative correlation mentioned above [7], [8], [9], [30], [31].
Tramadol’ hypocoagulable and cardioprotective effect may prevent venous thromboembolism in COVID-19 patients
COVID-19 patients with acute respiratory failure showed a remarkable elevation in plasma fibrinogen and hypercoagulability [10]. This hyperfibrinogenemia leads to increase fibrin formation and polymerization which may complicate to venous thromboembolism [10]. A previous study found that tramadol exhibits a hypocoagulable activity in the blood of females with gynecologic malignancies in vitro [11]. Consequently, tramadol could have a beneficial effect in patients with a tendency to a hypercoagulable status and thromboembolic complications [11]. Moreover, tramadol could affect hemostatic parameters in favor of bleeding tendency in rats [36].
Moreover, tramadol was reported to significantly lower LDH level and to provide a cardioprotective effect [14]. This effect of tramadol seems useful since around 60% of COVID-19 patients showed elevated LDH levels [15].
Tramadol as a potent antioxidative agent may decrease complications in COVID-19 patients
The severity and mortality of COVID-19 disease have been correlated with age. The mean case fatality rate for adult patients below 60 years old is found to be <0.2%, compared with 9.3% in patient aged over 80 [4], [12]. This age-related mortality is due to the lack of antioxidant mechanisms together with increased oxidative damage during aging process [12]. It was suggested that age related oxidative damage and attenuated antioxidant defense mechanisms may result in elevated reactive oxygen species [12].
Tramadol administration increased superoxide dismutase (an enzyme that helps breakdown of potentially harmful oxygen molecules in cells and prevents damage to tissue) and glutathione peroxidase (an enzyme family whose main biological role is to protect the organism from oxidative damage) while diminished malondialdehyde levels (indicator for oxidative stress) in testicular ischemia–reperfusion injury [13]. These results proposed that tramadol might be a potent antioxidant agent [13]. Therefore, we hypothesize that using tramadol as a potent antioxidant may reduce complications in old age COVID-19 patients.
Tramadol’ anti-depressant effect may protect against psychiatric disorders associated with COVID-19 infection
As the COVID-19 was recognized by the World Health Organization (WHO) as a pandemic, psychiatric fear was increased in the population [16]. Studies reported that patients infected with SARS-CoV-2 may experience severe behavioral and emotional reactions, such as fear, loneliness, boredom, insomnia, anxiety, or anger [16], [37], [38]. Such conditions can complicate to disorders, including depressive, anxiety, psychotic, or paranoid, and can even lead to suicide [37], [38]. These conditions can be especially prevalent in quarantined patients, whose psychological stress tends to be higher [16], [38]. Tramadol is considered a centrally acting analgesic drug with a dual mechanism of action. It can bind to μ-opioid receptors and inhibit norepinephrine and serotonin reuptake [20]. Through its capability to inhibit norepinephrine and serotonin reuptake, tramadol may possess antidepressant activity [18], [39], [40]. In this context, the antidepressant activities of tramadol may favor its indication for COVID-19 patients.
Tramadol’ analgesic and antitussive effect may have a beneficial effect on COVID-19 symptoms
One of the most common signs of SARS-CoV-2 infection is the cough which is reported in 45.8% of infected patients [41]. Headache, aches, pains and sore throat are also reported in some COVID-19 patients [41]. Previous studies showed that tramadol has antitussive properties [23]. Besides, tramadol is a special analgesic exhibiting moderate, dose-related pain relieving effect with less potential for respiratory depression and abuse [23], [42].
Tramadol’ bactericidal activity may prevent COVID-19 complications
A recent study showed that the prevalence of fungal and Pseudomonas aeruginosa colonization is higher in severe COVID-19 patients compared to non-COVID-19 cases [43]. This association might be due to the over-activation of the immune system causing the defect in the regulation of the defenses against pathogens other than SARS-CoV-2, and the progression to co-infections and subsequent lung injury [35], [43] . Fungi are the main causes of mortality in immunocompromised COVID-19 patients, while Pseudomonas aeruginosa is the most popular gram-negative bacteria causing pneumonia with worse clinical outcomes [43]. Tramadol was found to have dose-and time-dependent bactericidal effect against a wide range of pathogens including Escherichia coli, Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa pathogens in vitro [19].
Conclusion
Reposition of tramadol for treatment of COVID-19 infection is an innovative therapeutic approach based on the aforementioned plausible mechanisms of tramadol. Most of clinical manifestations of COVID-19 patients can be addressed by oral tramadol administration. Of particular interest, tramadol has anti-inflammatory, hypocagulatory, cardioprotective, antioxidant, antitussive, analgesic, antidepressant, and bactericidal effect. In conclusion, tramadol with its multiple mechanisms of action could be an effective therapeutic option for COVID-19 patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N.a., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haggag Y.A., El-Ashmawy N.E., Okasha K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? Med Hypotheses. 2020;144:109957. doi: 10.1016/j.mehy.2020.109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diao B, Wang C, Tan Y, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol 2020;11:827. https://doi.org/10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed]
- 8.Feldmann M., Maini R.N., Woody J.N. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. The Lancet. 2020;395(10234):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraychete D.C., Sakata R.K., Issy A.M., Bacellar O., Jesus R.S., Carvalho E.M. Proinflammatory Cytokines in Patients with Neuropathic Pain Treated with Tramadol. Brazilian Journal of Anesthesiology. 2009;59(3):297–303. doi: 10.1590/S0034-70942009000300004. [DOI] [PubMed] [Google Scholar]
- 10.Spiezia L., Boscolo A., Poletto F. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb Haemost. 2020;120(06):998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceyhan D., Andıc N., Bilir A., Akay M.O. Does tramadol affect coagulation status of patients with malignancy? Indian J Pharmacol. 2014;46(4):413. doi: 10.4103/0253-7613.135954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado-Roche L., Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch Med Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asghari A., Akbari G., Beigi A.M., Mortazavi P. Tramadol reduces testicular damage of ischemia-reperfusion rats. Anim Reprod. 2016;13:811–819. doi: 10.21451/1984-3143-AR823. [DOI] [Google Scholar]
- 14.Bilir A., Erkasap N., Koken T. Effects of tramadol on myocardial ischemia-reperfusion injury. Scand Cardiovasc J. 2007;41(4):242–247. doi: 10.1080/14017430701227747. [DOI] [PubMed] [Google Scholar]
- 15.Lo I.L., Lio C.F., Cheong H.H. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16(10):1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ornell F, Schuch JB, Sordi AO, Kessler FHP. “Pandemic fear” and COVID-19: mental health burden and strategies. Brazilian J Psychiatry 2020;42:232–5. [DOI] [PMC free article] [PubMed]
- 17.Sacerdote P., Bianchi M., Manfredi B., Panerai A.E. Effects of tramadol on immune responses and nociceptive thresholds in mice: Pain. 1997;72(3):325–330. doi: 10.1016/S0304-3959(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 18.Kalra B.S., Tayal V., Chawla S. Antidepressant-like activity of tramadol in mice. Indian J Psychiatry. 2008;50(1):51. doi: 10.4103/0019-5545.39760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamanai-Shacoori Z., Shacoori V., Jolivet-Gougeon A. The Antibacterial Activity of Tramadol Against Bacteria Associated with Infectious Complications After Local or Regional Anesthesia: Anesth Analg. 2007;105(2):524–527. doi: 10.1213/01.ane.0000267525.51017.b8. [DOI] [PubMed] [Google Scholar]
- 20.Dayer P., Collart L., Desmeules J. The Pharmacology of Tramadol: Drugs. 1994;47(Supplement 1):3–7. doi: 10.2165/00003495-199400471-00003. [DOI] [PubMed] [Google Scholar]
- 21.Grond S., Sablotzki A. Clinical Pharmacology of Tramadol: Clin Pharmacokinet. 2004;43(13):879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kaye A.D. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18:395–400. [PubMed] [Google Scholar]
- 23.Subedi M., Bajaj S., Kumar M.S., YC M. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019;111:443–451. doi: 10.1016/j.biopha.2018.12.085. [DOI] [PubMed] [Google Scholar]
- 24.Committee E., Dependence D., Update T. WHO (ECDD) Tramadol Update Review. 2015:1–39. [Google Scholar]
- 25.Ripple M.G., Pestaner J.P., Levine B.S., Smialek J.E. Lethal combination of tramadol and multiple drugs affecting serotonin. Am J Forensic Med Pathol. 2000;21(4):370–374. doi: 10.1097/00000433-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Tjäderborn M., Jönsson A.K., Hägg S., Ahlner J. Fatal unintentional intoxications with tramadol during 1995–2005. Forensic Sci Int. 2007;173(2-3):107–111. doi: 10.1016/j.forsciint.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 27.le Roux P.J., Coetzee J.F. Tramadol today. Curr Opin Anaesthesiol. 2000;13(4):457–461. doi: 10.1097/00001503-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Shanthanna H., Strand N.H., Provenzano D.A. Caring for patients with pain during the COVID-19 pandemic: consensus recommendations from an international expert panel. Anaesthesia. 2020 doi: 10.1111/anae.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terpos E., Ntanasis-Stathopoulos I., Elalamy I. Hematological findings and complications of COVID-19. Am J Hematol. 2020;20(395):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y.-M., Zhu S.-M., Wang K.-R., Feng Z.-Y., Chen Q.-L. Effect of tramadol on immune responses and nociceptive thresholds in a rat model of incisional pain. J. Zhejiang Univ. Sci. B. 2008;9(11):895–902. doi: 10.1631/jzus.B0820039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayed J.A., Abd Elshafy S.K., Kamel E.Z., Fathy Riad M.A., Mahmoud A.A., Khalaf G.S. The impact of caudally administrated tramadol on immune response and analgesic efficacy for pediatric patients: a comparative randomized clinical trial. Korean J Pain. 2018;31(3):206. doi: 10.3344/kjp.2018.31.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z., Gao F., Tian Y. Effects of morphine, fentanyl and tramadol on human immune response. J Huazhong Univ Sc Technol. 2006;26(4):478–481. doi: 10.1007/s11596-006-0427-5. [DOI] [PubMed] [Google Scholar]
- 33.Björkström N.K., Ljunggren H.-G., Michaëlsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16(5):310–320. doi: 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- 34.Liao W., Lin J.-X., Leonard W.J. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23(5):598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9. https://doi.org/10.1172/JCI137244. [DOI] [PMC free article] [PubMed]
- 36.Roshdy H., Abdel-Rahman R., Azzam H., El-Bakary A. Repeated Tramadol Administration Induced Bleeding in Albino Rats. Mansoura J Forensic Med Clin Toxicol. 2018;26(2):169–177. doi: 10.21608/mjfmct.2018.47201. [DOI] [Google Scholar]
- 37.Raony Í, de Figueiredo CS, Pandolfo P, Giestal-de-Araujo E, Oliveira-Silva Bomfim P, Savino W. Psycho-neuroendocrine-immune interactions in COVID-19: potential impacts on mental health. Front Immunol 2020;11:1170. [DOI] [PMC free article] [PubMed]
- 38.Yao H., Chen J.-H., Xu Y.-F. Patients with mental health disorders in the COVID-19 epidemic. The Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojas-Corrales M.O., Gibert-Rahola J., Micó J.A. Tramadol induces antidepressant-type effects in mice. Life Sci. 1998;63(12):PL175–PL180. doi: 10.1016/S0024-3205(98)00369-5. [DOI] [PubMed] [Google Scholar]
- 40.Osman M., Mustafa M. Tramadol-Induced Mood Elevation in a Patient with No Previous Psychiatric History. Case Reports in Psychiatry. 2018;2018:1–3. doi: 10.1155/2018/9574395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian S., Hu N., Lou J. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aronson M.D. Nonsteroidal anti-inflammatory drugs, traditional opioids, and tramadol: constrating therapies for the treatment of chronic pain. Clin Ther. 1997;19(3):420–432. doi: 10.1016/S0149-2918(97)80127-0. [DOI] [PubMed] [Google Scholar]
- 43.Intra J., Sarto C., Beck E., Tiberti N., Leoni V., Brambilla P. Bacterial and fungal colonization of the respiratory tract in COVID-19 patients should not be neglected. Am J Infect Control. 2020;48(9):1130–1131. doi: 10.1016/j.ajic.2020.06.185. [DOI] [PMC free article] [PubMed] [Google Scholar]