Abstract
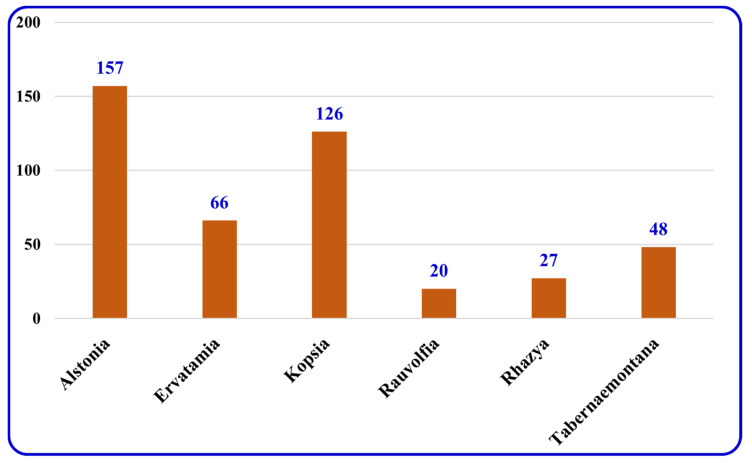
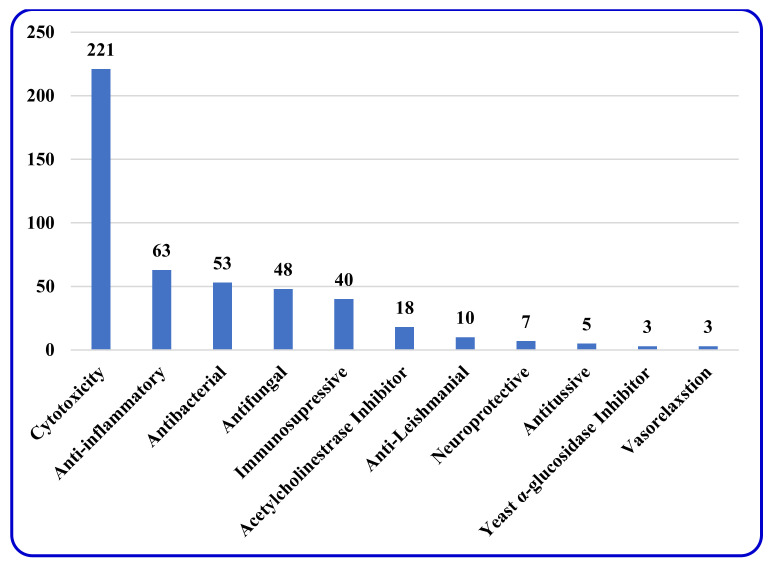
By the end of the twentieth century, the interest in natural compounds as probable sources of drugs has declined and was replaced by other strategies such as molecular target-based drug discovery. However, in the recent times, natural compounds regained their position as extremely important source drug leads. Indole-containing compounds are under clinical use which includes vinblastine and vincristine (anticancer), atevirdine (anti-HIV), yohimbine (erectile dysfunction), reserpine (antihypertension), ajmalicine (vascular disorders), ajmaline (anti-arrhythmic), vincamine (vasodilator), etc. Monoterpene Indole Alkaloids (MIAs) deserve the curiosity and attention of researchers due to their chemical diversity and biological activities. These compounds were considered as an impending source of drug-lead. In this review 444 compounds, were identified from six genera belonging to the family Apocynaceae, will be discussed. These genera (Alstonia, Rauvolfia, Kopsia, Ervatamia, and Tabernaemontana, and Rhazya) consist of 400 members and represent 20% of Apocynaceae species. Only 30 (7.5%) species were investigated, whereas the rest are promising to be investigated. Eleven bioactivities, including antibacterial, antifungal, anti-inflammatory and immunosuppressant activities, were reported. Whereas cytotoxic effect represents 47% of the reported activities. Convincingly, the genera selected in this review are a wealthy source for future anticancer drug lead.
Keywords: Apocynaceae, monoterpene, alkaloids, cytotoxicity, anti-inflammatory, antimicrobial
1. Introduction
Alkaloids are basic nitrogenous natural metabolites with structural diversity and molecular conformity. They displayed interesting bioactivities and are known to perform an important role in plant protection. The majority of them were discovered from plants and recently recorded Ca 21,000 [1,2]. The alkaloids are generally derived from amino acids that are containing one or more nitrogen atoms. These precursors are playing a rule in their classification. Also, the biosynthetic pathway of alkaloids can be named according the amino acid source [3]. Thus, they can be categorized into several groups based on associated moieties, including piperidine, pyrrolidine, pyrrole, pyridine, quinolone, isoquinoline, indole, quinolizidine, pyrrolizidine, tropane, benzylisoquinoline, purine, β-carboline, indolinics and quinolizidine.
Terpenoids are considered to be interesting natural products that have chemical diversity and different bioactivities. Common terpenoids have been reported from marine sources [4]. Whereas, the plants were listed as an important source of such metabolites. Terpenoids include several subclasses according to the number of carbo-skeleton; monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), and tetraterpenes (C40).
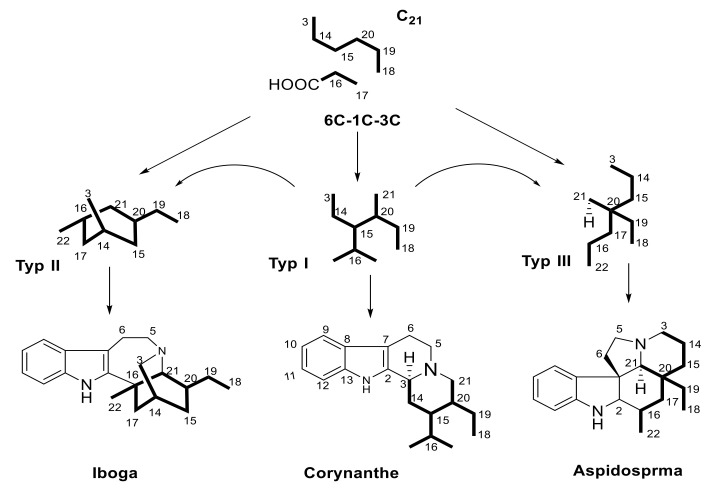
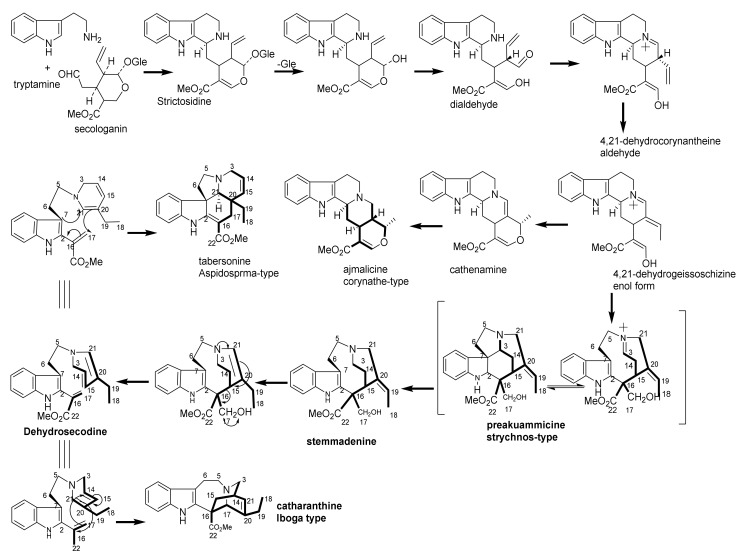
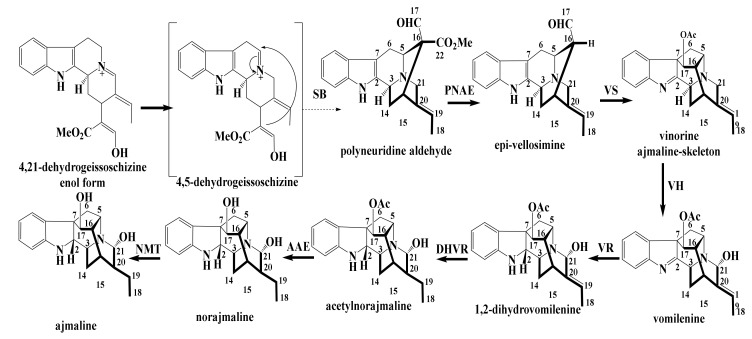
Monoterpene indole alkaloids (MIAs) are metabolites containing a bicyclic structure of a benzene ring fused to a five-membered pyrrole ring. It is a noteworthy that the occurrence of multipart alkaloids is largely restricted to limited number of plant families. (e.g., Apocynaceae, Loganiaceae, and Rubiaceae) [5,6,7,8]. These families are closely taxonomically related. Also, on the chemical aspect, they are recognized to have apparent uniformity in the building blocks of these alkaloids. MIAs have been proposed to be sourced from strictosidine, which originates from the condensation of tryptophan with secologanin (C10 or C9 part), which can be divided into linear six carbon (6 C), one carbon (1 C) and three carbon (3 C) units (Figure 1). The connection between them requires proving. The nine-carbons fragment may be formed by the loss at certain stage of one of the carbons from the 3 C unit, and there are also a few indole bases which appear to have ended up without the 3 C or the 1 C units. Three hypothetical building blocks, Types I, II and III. It is nevertheless a useful way of dividing indole alkaloids into groups based on their sub architecture. Since Type I alkaloids are by far the most numerous, they may be the source of Type II and III. It was suggested by LeMen and Tylor that the convention be extended to cover Type II and III alkaloids as illustrated in Figure 1. On these hypothetical bases, the MIAs categorized according to their biogenic pathway in three main groups, corynanthe, aspidosperma and iboga [9].
Figure 1.
Biogenetic numbering rule as adopted from LeMen and Tylor.
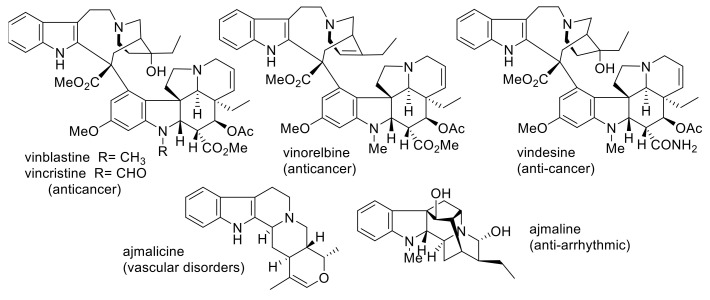
Recently, strictosidine has been considered as the building block of MIAs biosynthesis [10]. MIAs have been proposed to arise from strictosidine, which itself originates from the condensation of tryptophan with secologanin in a 1:1 ratio. Strictosidine has been elaborated to give an impressive array of structural variants. This type of alkaloids possess 18 (or 19) carbon atoms on its skeleton. Additionally, the MIAs could be produced from tryptophan and secologanin in 1:2 or 2:1 ratio. According to this arrangement, three types (classes) of monoterpenes were constructed, including, corynanthe (e.g., ajmalicine), aspidosperma (e.g., tabersonine) and iboga (e.g., catharanthine) [11,12,13].
Apocynaceae contains about 250 genera and 2000 species [14]. Five sub-families are classified under Apocynaceae, including, Apocynoideae, Asclepiadoideae, Periplocoideae, Rauvolfioideae, and Secamonoideae. Apocynaceae species ranged from shrubs to trees. The characteristic features of these plants include colorful flowers and opposite leaves. Traditionally, species of this family have been used for the treatment of fever, malaria, gastrointestinal ailments, diabetes, and pain [15]. Additionally, some species have shown antiplasmodial and anticancer activities [14]. Several Apocynaceae MIAs have been used as anticancer, analgesic, anti-inflammatory and anti-spasmodic agents. For example, vinblastine, vinorelbine, vincristine, and vindesine were utilized as anticancer agents, whereas ajmalicine and ajmaline were used in the treatment of cardiovascular disorders (Figure 2) [2]. Catharanthus roseus and Rauvolfia serpentine are members of Apocynaceae and are known as sources of bioactive indole alkaloids [16]. Reserpine has been used as a tranquillizer, whereas vinblastine and vincristine have been used as anti-leukemic agents [17]. Vincristine and vinblastine were among the earliest anti-tumor agents, and since 1965 have been used as tubulin polymerization inhibitors. They have been used in combination for the treatment of acute lymphoblastic leukemia and also against both Hodgkin’s and non- Hodgkin lymphoma. Additionally, strychnine is potent muscle contracting agent whereas, yohimbine has been used for the treatment of sexual dysfunction and investigated as a remedy for type-2 diabetes in animal and human models.
Figure 2.
Examples of well-known biologically active terpene indole alkaloids.
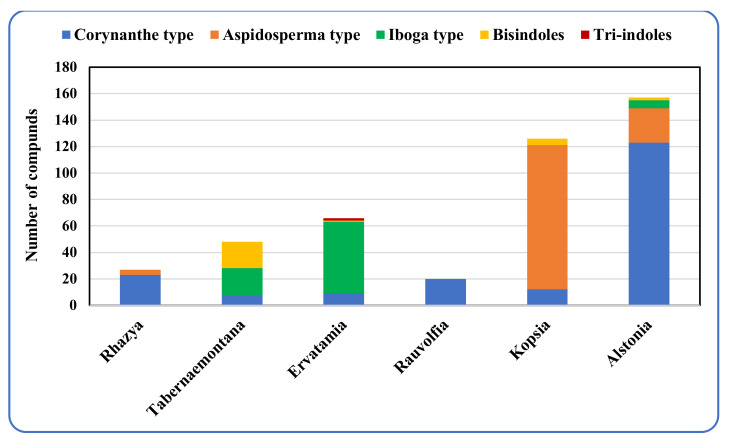
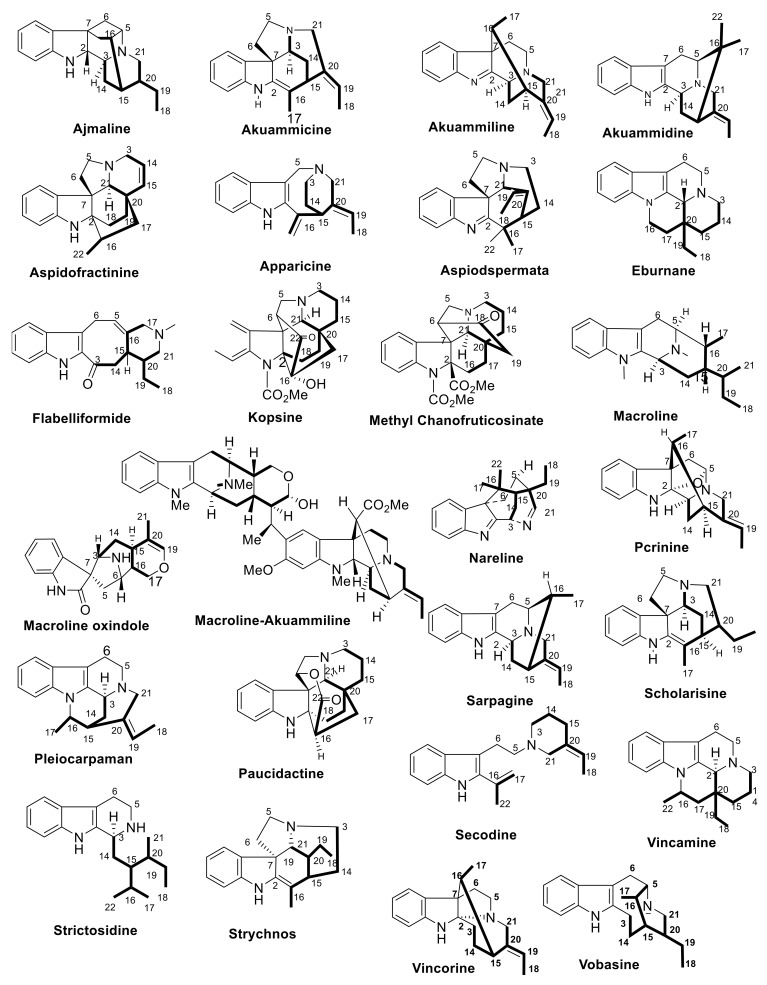
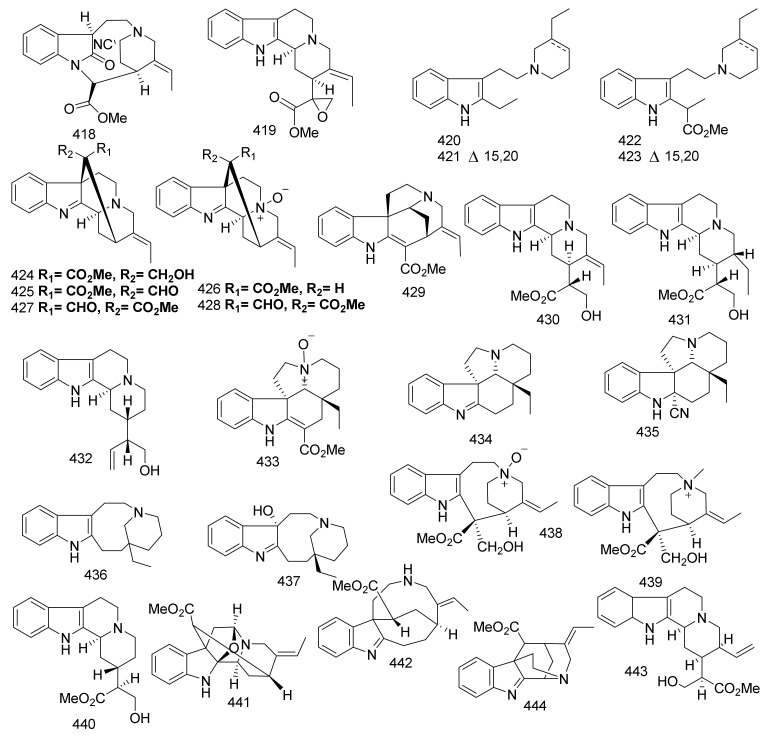
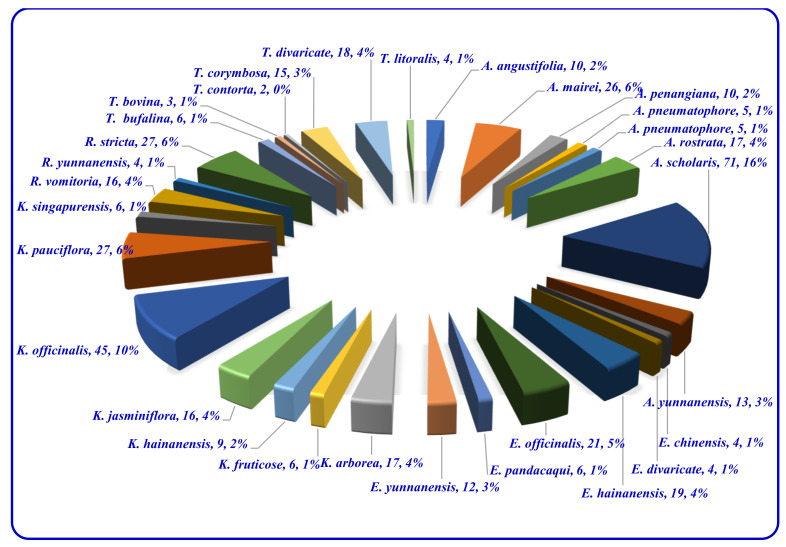
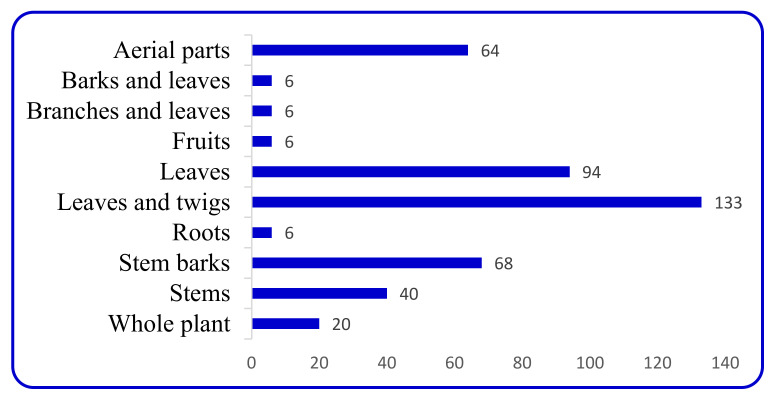
There are several publications interested in the terpene indole alkaloids of individual species of the family Apocynaceae. The current review organizes the reported MIAs considering the historical aspect in each selected genus. Moreover, these MIAs were biosynthetically classified according to the tepenoidal fragment, i.e., corynanthe, aspidosperma, or iboga. Also, it focuses on the origin, structural diversity and biological activities exerted by 444 (Table 1) monoterpene indole alkaloids which have been reported from selected six genera of the family Apocynaceae (Alstonia, Kopsia, Ervatamia, Rauvolfia, Tabernaemontana and Rhazya), in the period between 2010 and December 2020. The listed metabolites are categorized under 26 subclasses, ajmaline, akuamiline, akuammidine, akuammicine, apparicine, aspidofractinine, aspidospermatan, eburnane, flabelliformide, kopsine, macroline, macroline oxindole, macroline-akuammiline, methyl chanofruticosinate, nareline, paucidactine, picrinine, pleiocarpamine, sarpagine, scholaricine, secodine, strictosidine, strychnos, vincamine, vincorine and vobasine (Figure 3 and Figure 4).
Table 1.
Monoterpenoid indole alkaloids from the six species of Apocynaceae.
| Comp No | Compound Name | Class Type | Source | Part | Country | Activities |
|---|---|---|---|---|---|---|
| 1 | (14a,15a)-14,15-Epoxy Aspidofractinine | Aspidofractinine | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 2 | Maireine A | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 3 | Maireine B | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 4 | Venalstonine | Aspidofractinine | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 5 | (−)-Minovincinine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 6 | (−)-11-Methoxymino Vincinine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 7 | (−)-Echitovenine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 8 | Echitovenaldine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 9 | Echitovenidine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 10 | 11-Methoxyechitovenidine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 11 | Echitoveniline | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 12 | 11-Methoxyechitoveniline | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 13 | Echitoserpidine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 14 | 11-Methoxyechitoserpidine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 15 | Vindolinine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 16 | Lochnericine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 17 | Tabersonine | Aspidosperma | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 18 | Perakine | Ajmaline | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 19 | Picrinine | Picrinine | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 20 | Deacetylpicraline 3,4,5-Trimethoxybenzoate | picraline | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 21 | Picralinal | picraline | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 22 | Rhazimol | Akummidine | Alstonia mairei | Leaves and twigs | China | Cytotoxicity |
| 23 | Alsmaphorazines A | Scholaricine | Alstonia pneumatophore | Leaves | Malysia | Anti-inflammatory |
| 24 | Alsmaphorazine B | Scholaricine | Alstonia pneumatophore | Leaves | Malysia | Anti-inflammatory |
| 25 | Alstrostine A | Strictosidine | Alstonia rostrata | Leaves and twigs | China | Cytotoxicity |
| 26 | Alstrostine B | Strictosidine | Alstonia rostrata | Leaves and twigs | China | Cytotoxicity |
| 27 | Alstrostine C | Akummicine | Alstonia rostrata | Leaves and twigs | China | Cytotoxicity |
| 28 | Alstrostine D | Akummicine | Alstonia rostrata | Leaves and twigs | China | Cytotoxicity |
| 29 | Alstrostine E | Akummicine | Alstonia rostrata | Leaves and twigs | China | Cytotoxicity |
| 30 | Alstrostine F | Corynanthe | Alstonia rostrata | Leaves and twigs | China | Cytotoxicity |
| 31 | 11-Hydroxy-6,7-Epoxy-8-Oxo-Vincadifformine | Aspidosperma | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 32 | 14-Chloro-15-Hydroxyvinca Difformine | Aspidosperma | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 33 | Perakine N4-Oxide | Ajmaline | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 34 | Raucaffrinoline N4-Oxide | Ajmaline | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 35 | Vinorine N1,N4-Dioxide | Ajmaline | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 36 | Oxovincadifformine | Aspidosperma | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 37 | Vinorine N4-Oxide | Ajmaline | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 38 | Vinorine | Ajmaline | Alstonia yunnanensis | Whole plant | China | Cytotoxicity |
| 39 | Alsmaphorazine C | Octahydropyrrolo[2,3-b]pyrrole and 2-azabicyclo[3.3.1]nonane units | Alstonia pneumatophore | Leaves | Malaysia | Cytotoxicity |
| 40 | Alsmaphorazine D | Octahydropyrrolo[2,3-b]pyrrole and 2,8-diazabicyclo[3.3.1]nonane units | Alstonia pneumatophore | Leaves | Malaysia | Cytotoxicity |
| 41 | Alsmaphorazine E | Octahydropyrrolo[2,3-b]pyrrole and 2,8-diazabicyclo[3.3.1]nonane units | Alstonia pneumatophore | Leaves | Malaysia | Cytotoxicity |
| 42 | Scholarisin I | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory Antifungal |
| 43 | Scholarisin II | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory Antifungal |
| 44 | Scholarisin III | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 45 | Scholarisin IV | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 46 | Scholarisin V | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 47 | Scholarisin VI | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 48 | Scholarisin VII | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 49 | (3R,5S,7R,15R,16R,19E)-Scholarisine F | picrinine | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 50 | 3-Epi-Dihydrocorymine | Vincorine | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 51 | (E)-16-Formyl-5α-Methoxystrictamine | picraline | Alstonia scholaris | Leaves | China | Cytotoxicity, Anti-inflammatory, Antifungal |
| 52 | Alstolactine A | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 53 | Alstolactine B | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 54 | Alstolactine C | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 55 | Alistonitrine A | Corynanthe | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 56 | 6,7-Epoxy-8-Oxo-Vincadifformine | Aspidosperma | Alstonia rupestris | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 57 | 11-Acetyl-6,7-Epoxy-8-Oxo-Vincadifformine | Aspidosperma | Alstonia rupestris | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 58 | 11-Hydroxy-14-Chloro-15-Hydroxyvincadifformine | Aspidosperma | Alstonia rupestris | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 59 | Perakine N1,N4-Dioxide | Ajmaline | Alstonia rupestris | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 60 | 11-Hydroxy-6,7-Epoxy-8-Oxovincadifformine | Aspidosperma | Alstonia rupestris | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 61 | N(4)-Methyl-Talpinine | Sarpagine | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 62 | N(4)-Meth-Yl-N(4),21-Secotalpinine | Macroline | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 63 | Alstonerinal | Macroline | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 64 | Alstonerine | Macroline | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 65 | Macrocarpine B | Macroline | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 66 | Affinisine | Sarpagine | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 67 | Villalstonine | Macroline-Pleiocarpamine | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 68 | Villalstonine N(4)-Oxide | Macroline-Pleiocarpamine | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 69 | Villalstonidine D | Macroline-Pleiocarpamine | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 70 | Villalstonidine E | Macroline-Pleiocarpamine | Alstonia angustifolia | Stem bark |
Vietnam | Anti-inflammatory, Anti-Leishmanial |
| 71 | Normavacurine-21-One | Pleiocarpaman | Alstonia scholaris | Leaves | China | Antibacterial |
| 72 | 5-Hydroxy-19,20-E-Alschomine | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 73 | 5-Hydroxy-19,20-Z-Alschomine | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 74 | Alstoniascholarine A | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 75 | Alstoniascholarine B | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 76 | Alstoniascholarine C | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 77 | Alstoniascholarine D | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 78 | Alstoniascholarine E | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 79 | Alstoniascholarine F | Scholarisine | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 80 | Alstoniascholarine G | Scholarisine | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 81 | Alstoniascholarine H | Scholarisine | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 82 | Alstoniascholarine I | Scholarisine | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 83 | Alstoniascholarine J | Scholarisine | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 84 | Alstoniascholarine K | Scholarisine | Alstonia scholaris | Leaves | China | Antibacterial, Anti- Fungal |
| 85 | Alstoniascholarine L | Corynanthe | Alstonia scholaris | Leaves | China | Cytotoxicity |
| 86 | Alstoniascholarine M | Corynanthe | Alstonia scholaris | Leaves | China | Cytotoxicity |
| 87 | Alstoniascholarine N | Corynanthe | Alstonia scholaris | Leaves | China | Cytotoxicity |
| 88 | Alstoniascholarine O | Scholarisine | Alstonia scholaris | Leaves | China | Cytotoxicity |
| 89 | Alstoniascholarine P | Scholarisine | Alstonia scholaris | Leaves | China | Cytotoxicity |
| 90 | Alstoniascholarine Q | Scholarisine | Alstonia scholaris | Leaves | China | Cytotoxicity |
| 91 | Scholarisine H | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 92 | Scholarisine I | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 93 | Scholarisine J | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 94 | Scholarisine K | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 95 | Scholarisine L | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 96 | Scholarisine M | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 97 | Scholarisine N | Corynanthe | Alstonia scholaris | Leaves | China | Antibacterial |
| 98 | Melosline A | Corynanthe | Alstonia scholaris | Leaves and twigs | China | Cytotoxicity |
| 99 | Melosline B | Secodine | Alstonia scholaris | Leaves and twigs | China | Cytotoxicity |
| 100 | 1-[2-[2-(Carboxymethyl) Indole-3-Yl] Ethyl]-3-Ethylpyridinium Hydroxide Inner Salt | Secodine | Alstonia scholaris | Leaves and twigs | China | Cytotoxicity |
| 101 | Alstiyunnanenine A | Sarpagine | Alstonia Yunnanensis | Aerial parts |
China | Cytotoxicity |
| 102 | Alstiyunnanenine B | Picraline | Alstonia Yunnanensis | Aerial parts |
China | Cytotoxicity |
| 103 | Alstiyunnanenine C | Akummiline | Alstonia Yunnanensis | Aerial parts |
China | Cytotoxicity |
| 104 | Alstiyunnanenine D | Scholaricine | Alstonia Yunnanensis | Aerial parts |
China | Cytotoxicity |
| 105 | Alstiyunnanenine E | Scholaricine | Alstonia Yunnanensis | Aerial parts |
China | Cytotoxicity |
| 106 | Alstomairine A | Scholaricine | Alstonia Mairei | Leaves | China | Cytotoxicity |
| 107 | Alstomairine B | Scholaricine | Alstonia Mairei | Leaves | China | Cytotoxicity |
| 108 | Alstomairine C | Scholaricine | Alstonia Mairei | Leaves | China | Cytotoxicity |
| 109 | Alpneumine A | Scholaricine | Alstonia Mairei | Leaves | China | Cytotoxicity |
| 110 | Alstrostine G | Corynanthe | Alstonia rostrata | Bark and trunks | China | Cytotoxicity |
| 111 | Alstrostine H | Corynanthe | Alstonia rostrata | Bark and trunks | China | Cytotoxicity |
| 112 | Alstrostine I | Scholarisine | Alstonia rostrata | Bark and trunks | China | Cytotoxicity |
| 113 | Alstrostine J | Secodine | Alstonia rostrata | Bark and trunks | China | Cytotoxicity |
| 114 | Alstrostine K | Corynanthe | Alstonia rostrata | Bark and trunks | China | Cytotoxicity |
| 115 | Scholarisine T | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 116 | Scholarisine U | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 117 | Scholarisine V | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 118 | Scholarisine W | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 119 | Scholarisine A | Nareline | Alstonia scholaris | Leaves | China | Antibacterial |
| 120 | Scholarisine P | Nareline | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 121 | Scholarisine Q | Akuammiline | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 122 | Scholarisine R | Corynanthe | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 123 | Scholarisine S | Nareline | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 124 | (16R)-E-Isositsnikine | Corynanthe | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 125 | Nareline | Nareline | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 126 | 5-Methoxystrictamine | Akuammiline | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 127 | Leuconolam | Aspidosperma | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 128 | Epileuconolam | Aspidosperma | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 129 | Nb-Demethylalstogustine | Scholarisine | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 130 | 19-Epischolaricine | Scholarisine | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 131 | Scholaricine | Scholarisine | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 132 | Vallesamine | Corynanthe | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 133 | Akuammidine | Akuammidine | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 134 | 17-Nor-Excelsinidine | Corynanthe | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 135 | Strictosamide | Corynanthe | Alstonia scholaris | Leaves | China | Anti-inflammatory |
| 136 | Vincamaginine A | Ajmaline | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 137 | Vincamaginine B | Ajmaline | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 138 | Alstonisinine A | Macroline Oxindole | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 139 | Alstonisinine B | Macroline Oxindole | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 140 | Alstonisinine C | Macroline Oxindole | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 141 | Alstonoxine F | Macroline Oxindole | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 142 | Angustilongine A | Macroline-Akuammiline | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 143 | Angustilongine B | Macroline-Akuammiline | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 144 | Angustilongine C | Macroline-Akuammiline | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 145 | Angustilongine D | Macroline-Akuammiline | Alstonia penangiana | Leaves | Malaysia | Cytotoxicity |
| 146 | Winphyllines A | Vincorine | Alstonia rostrata | Twigs | China | Cytotoxicity |
| 147 | Winphyllines B | Scholarisine | Alstonia rostrata | Twigs | China | Cytotoxicity |
| 148 | Nb-Demethylechitamine | Vincorine | Alstonia rostrata | Twigs | China | Cytotoxicity |
| 149 | 17-O-Acetylnorechitamine | Vincorine | Alstonia rostrata | Twigs | China | Cytotoxicity |
| 150 | 12- Methoxyechitamidine | Scholarisine | Alstonia rostrata | Twigs | China | Cytotoxicity |
| 151 | N(4)-Demethylastogustine | Scholarisine | Alstonia rostrata | Twigs | China | Cytotoxicity |
| 152 | 17-Formyl-10-Demethoxyvincorine N(4)-Oxide | Vincorine | Alstonia scholaris | Leaves | China | _ |
| 153 | 10-Methoxyalstiphyllanine H | Ajmaline | Alstonia scholaris | Leaves | China | _ |
| 154 | 10-Demethoxyvincorine N(4)-Oxide | Vincorine | Alstonia scholaris | Leaves | China | _ |
| 155 | Alstoscholactine | Corynanthe | Alstonia scholaris | Leaves | Malaysia | Vasorelaxation Cytotoxicity |
| 156 | Alstolaxepine | Corynanthe | Alstonia scholaris | Leaves | Malaysia | Vasorelaxation Cytotoxicity |
| 157 | Alstobrogaline | Corynanthe | Alstonia scholaris | Leaves | Malaysia | Cytotoxicity |
| 158 | Kopsiyunnanines G | Aspidosperma | Kopsia arbora | Aerial parts |
China | _ |
| 159 | Kopsiyunnanines H | Aspidosperma | Kopsia arbora | Aerial parts |
China | _ |
| 160 | Kopsihainin A | Aspidosperma | Kopsia hainanensis | Stems | China | Antitussive |
| 161 | Kopsihainin B | Aspidofractinine | Kopsia hainanensis | Stems | China | Antitussive |
| 162 | Kopsihainin C | Aspidofractinine | Kopsia hainanensis | Stems | China | Antitussive |
| 163 | Kopsinine | Aspidofractinine | Kopsia hainanensis | Stems | China | Antitussive |
| 164 | Methyl Demethoxycarbonylchanofruticosinate | Methyl Chanofruticosinate | Kopsia hainanensis | Stems | China | Antitussive |
| 165 | Singaporentine A | Aspidofractinine | Kopsia singapurensis | Barks and leaves | Malaysia | _ |
| 166 | N(1)-Formylkopsininic Acid | Aspidofractinine | Kopsia singapurensis | Barks and leaves | Malaysia | _ |
| 167 | N(1)-Formylkopsininic Acid-N(4)-Oxide | Aspidofractinine | Kopsia singapurensis | Barks and leaves | Malaysia | _ |
| 168 | 15-Hydroxykopsamine | Aspidofractinine | Kopsia singapurensis | Barks and leaves | Malaysia | _ |
| 169 | 14α-Hydroxy-N(4)-Methylcondylocarpine | Aspidosoermata | Kopsia singapurensis | Barks and leaves | Malaysia | _ |
| 170 | Singaporentinidine | Corynanthe | Kopsia singapurensis | Barks and leaves | Malaysia | _ |
| 171 | Kopsininate | Aspidofractinie | Kopsia hainanensis | Leaves and stems | China | Antifungal, Antibacterial |
| 172 | N1-Decarbomethoxy Chanofruticosinic Acid | Methyl Chanofruticosinate | Kopsia hainanensis | Leaves and stems | China | Antifungal, Antibacterial |
| 173 | Methyl N1- Decarbomethoxy Chanofruticosinate N(4)-Oxide | Methyl Chanofruticosinate | Kopsia hainanensis | Leaves and stems | China | Antifungal, Antibacterial |
| 174 | Methyl Chanofruticosinate N(4)-Oxide | Methyl Chanofruticosinate | Kopsia hainanensis | Leaves and stems | China | Antifungal, Antibacterial |
| 175 | 5,6-Secokopsinine | Aspidofractinine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 176 | 5β-Hydroxykopsinine | Aspidofractinine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 177 | 16-Epi-Kopsinilam | Aspidofractinine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 178 | 5-Oxokopsinic Acid | Aspidofractinine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 179 | Na-Demethoxycarbonyl-12-Methoxykopsine | Kopsine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 180 | 14(S)-Hydroxy-19(R)- Methoxytubotaiwine | Strychnos | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 181 | 19-Oxo-(−)-Eburnamonine | Vincamine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 182 | 19(S)-Hydroxy-Δ14-Vincamone | Vincamine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 183 | Kopsinilam | Aspidofractinine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 184 | Kopsinic Acid | Aspidofractinine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 185 | 12-Methoxykopsine | Kopsine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 186 | Kopsanone | Kopsine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 187 | 19(R)- Methoxytubotaiwine | Strychnos | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 188 | (−)-Eburnamonine | Vincamine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
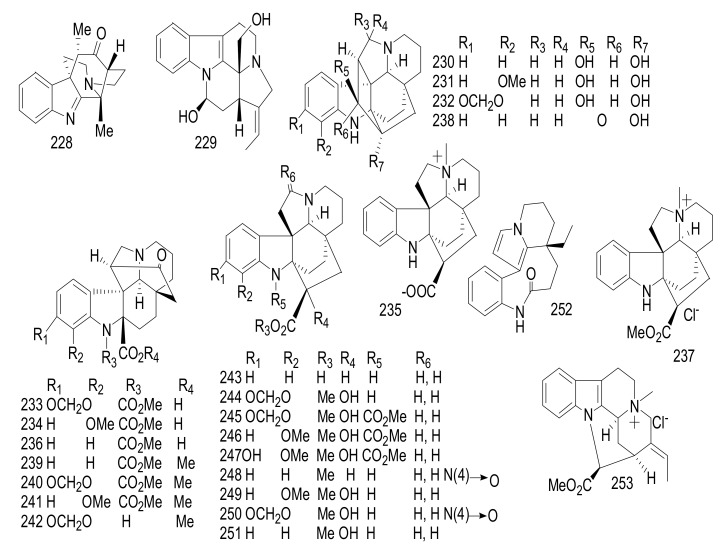
| 189 | 19-OH-(−)-Eburnamonine | Vincamine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity, Acetylcholinesterase inhibitor |
| 190 | Δ14-Vincamone | Vincamine | Kopsia jasminiflora | Stem barks | Thailand | Cytotoxicity |
| 191 | Phutdonginin | Eburnane | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 192 | Melodinine E | Aspidosperma | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 193 | Kopsilongine | Aspidofractinine | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 194 | Kopsamine | Aspidofractinine | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 195 | (−)-Methylenedioxy-11,12-Kopsinaline | Aspidofractinine | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 196 | Decarbomethoxykopsiline | Kopsine | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 197 | Vincadifformine | Aspidosperma | Kopsia arborea | Twigs | Thailand | Antibacterial, Acetylcholinesterase inhibition |
| 198 | Arboridinine | Corynanthe | Kopsia arborea | _ | Malaysia | Relaxation Effect |
| 199 | Kopsiyunnanines J1 and J2 | Aspidosoermata | Kopsia arborea | Aerial parts |
China | _ |
| 200 | Paucidirinine | Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 201 | Paucidirisine | Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 202 | Paucidactinine | Aspidosperma | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 203 | Pauciduridine | Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 204 | Paucidactine D | Paucidactine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 205 | Paucidactine E | Paucidactine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 206 | Paucidisine | Kopsine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 207 | (−)-19-Oxoisoeburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 208 | (−)-19(R)-Hydroxyeburnamenine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 209 | (−)-19(R)-Hydroxy-O-Ethylisoeburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 210 | Larutienine B | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 211 | Paucidactine A | Paucidactine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 212 | Paucidactine B | Paucidactine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 213 | Paucidactine C | Paucidactine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
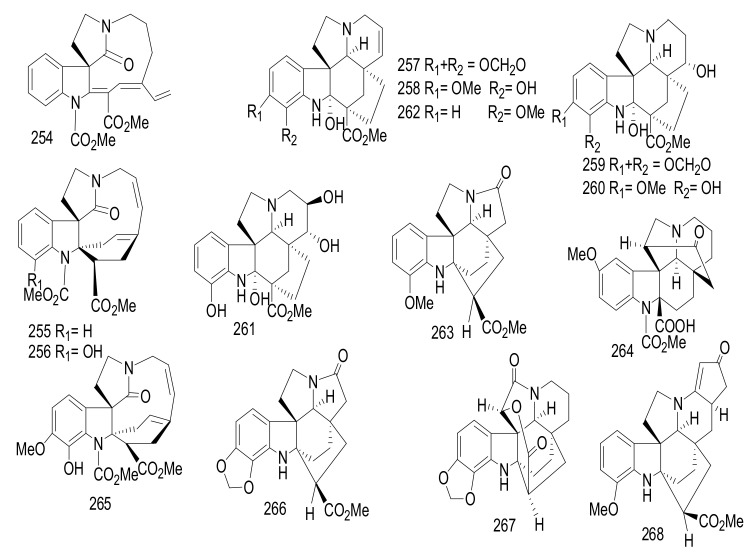
| 214 | 5, 22-Dioxokopsane | Kopsine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 215 | (+)-Eburnamonine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity, |
| 216 | Eburnamenine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 217 | (−)-Eburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 218 | (+)-Isoeburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 219 | (+)-19-Oxoeburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 220 | (−)-19(R)-Hydroxyisoeburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 221 | (+)-19(R)-Hydroxyeburnamine | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 222 | Larutienine A | Eburnane | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 223 | (−)-Norpleiomutine | Eburnane- Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 224 | (+)-Kopsoffinol | Eburnane- Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 225 | (−)-Demethylnorpleiomutine | Eburnane- Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 226 | (+)-Kopsoffine | Vincamine- Aspidofractinine | Kopsia pauciflora | Stem bark | Malaysia | Cytotoxicity |
| 227 | Kopsiyunnanine M | Scholarisine- Corynanthe | Kopsia arborea | Aerial partss |
China | _ |
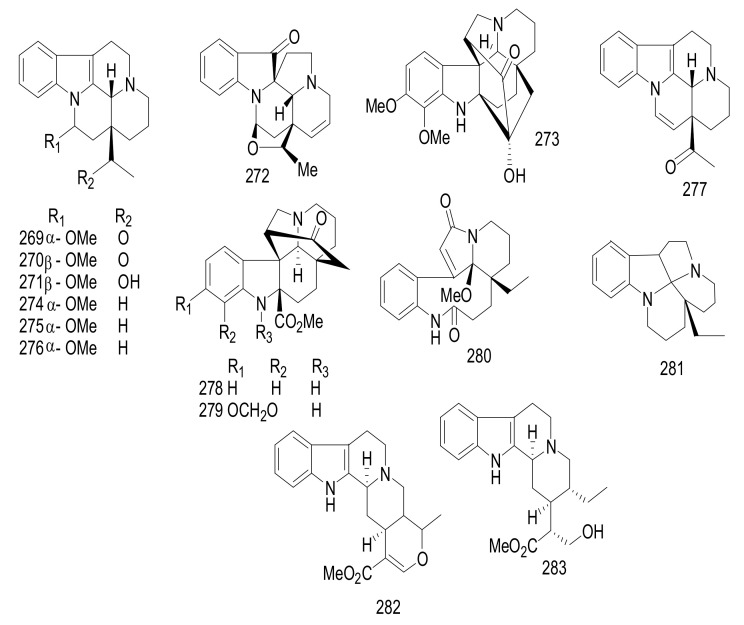
| 228 | Arborisidine | Pericine | Kopsia arborea | Whole plant | Malayan | Cytotoxicity |
| 229 | Arbornamine | Arbornane | Kopsia arborea | Whole plant | Malayan | Cytotoxicity |
| 230 | Kopsinidine C | Kopsine | Kopsia officinalis$ | Twigs and leaves | China | Immunosuppressive activity |
| 231 | Kopsinidine D | Kopsine | Kopsia officinalis$ | Twigs and leaves | China | Immunosuppressive activity |
| 232 | Kopsinidine E | Kopsine | Kopsia officinalis$ | Twigs and leaves | China | Immunosuppressive activity |
| 233 | 11,12-Methylenedioxychanofruticosinic Acid | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 234 | 12-Methoxychanofruticosinic Acid | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 235 | N(4)-Methylkopsininate | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 236 | Chanofruticosinic Acid | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 237 | Kopsinine Methochloride | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 238 | Demethoxycarbonylkopsin | Kopsine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 239 | Methyl Chanofruticosinate | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 240 | Methyl 11,12-Methylenedioxychanofruticosinate | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 241 | Methyl 12-Methoxychanofruticosinate | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 242 | Methyl 11,12-Methylenedioxy-N1-Decarbomethoxychanofruticosinate | Methyl Chanofruticosinate | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
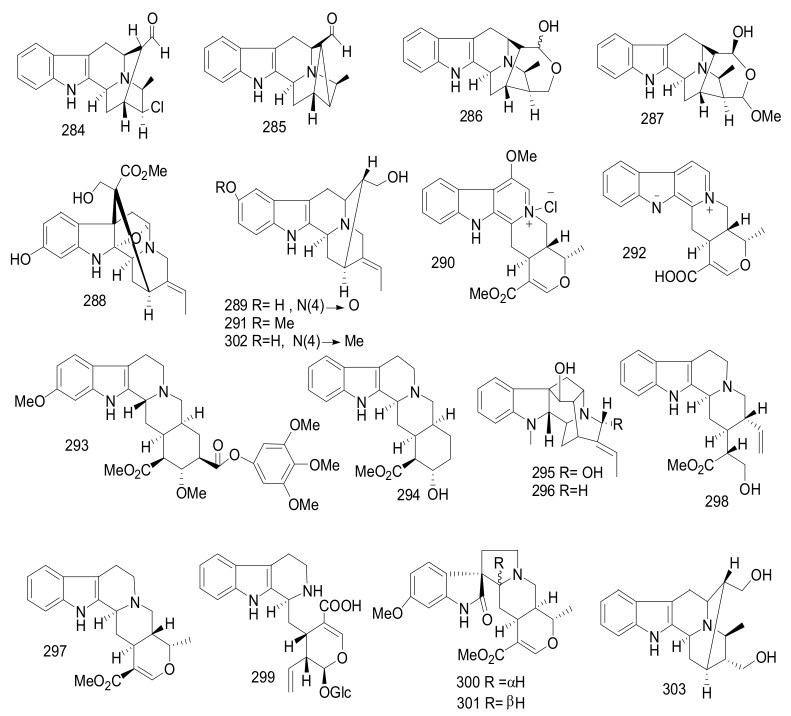
| 243 | Kopsininic Acid | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 244 | (−)-11,12-Methylenedioxykopsinaline | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 245 | (−)-N-Methoxycarbonyl-11,12-Methylenedioxykopsinaline | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 246 | (−)-N-Methoxycarbonyl- 12-Methoxykopsinaline | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 247 | N-Carbomethoxy-11-Hydroxy-12- Methoxykopsinaline | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 248 | Kopsinoline | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 249 | (−)-12-Methoxykopsinaline | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 250 | 11,12-Methylenedioxykopsinaline N(4)- Oxide | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 251 | Kopsinine B | Aspidofractinine | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 252 | Rhazinilam | Aspidosperma | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 253 | Pleiocarpamine Methochloride | Corynanthe | Kopsia officinalis | Twigs and leaves | China | Immunosuppressive activity |
| 254 | Kopsioffine A | Aspidosperma | Kopsia officinalis | Leaves and stems | China | Yeast α-glucosidase inhibitory |
| 255 | Kopsioffine B | Aspidosperma | Kopsia officinalis | Leaves and stems | China | Yeast α-glucosidase inhibitory |
| 256 | Kopsioffine C | Aspidosperma | Kopsia officinalis | Leaves and stems | China | Yeast α-glucosidase inhibitory |
| 257 | Kopsifoline G | Aspidosperma | Kopsia fruticose | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 258 | Kopsifoline H | Aspidosperma | Kopsia fruticose | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 259 | Kopsifoline I | Aspidosperma | Kopsia fruticose | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
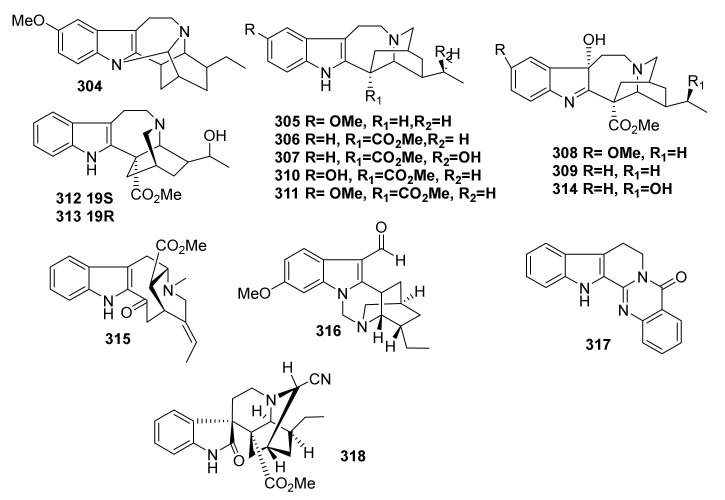
| 260 | Kopsifoline J | Aspidosperma | Kopsia fruticose | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 261 | Kopsifoline K | Aspidosperma | Kopsia fruticose | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 262 | Kopsifoline A | Aspidosperma | Kopsia fruticose | Aerial parts |
China | Cytotoxicity, Antifungal, Antibacterial |
| 263 | Kopsiarborine A | Aspidofractinine | Kopsia arborea | Aerial parts | China | Cytotoxicity |
| 264 | Kopsiarborine B | Methyl Chanofruticosinate | Kopsia arborea | Aerial parts |
China | Cytotoxicity |
| 265 | Kopsiarborine C | Aspidosperma | Kopsia arborea | Aerial parts | China | Cytotoxicity |
| 266 | Kopsiaofficine A | Aspidofractinine | Kopsia officinalis | Aerial parts | China | Cytotoxicity |
| 267 | Kopsiaofficine B | Paucidactine | Kopsia officinalis | Aerial parts | China | Cytotoxicity |
| 268 | Kopsiaofficine C | Aspidofractinine | Kopsia officinalis | Aerial parts | China | Cytotoxicity |
| 269 | Kopsiofficine H | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 270 | Kopsiofficine I | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
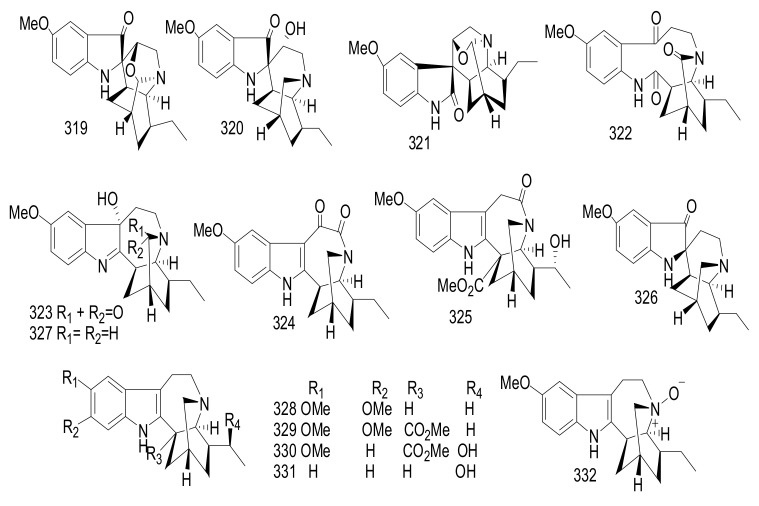
| 271 | Kopsiofficine J | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 272 | Kopsiofficine K | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 273 | Kopsiofficine L | Kopsine | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 274 | (+)-O-Methyleburnamine | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 275 | (−)-O-Methylisoeburnamine | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 276 | 16-Isoeburnamine | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 277 | 20-Oxoeburnamenine | Eburnane | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 278 | Methyl 11, 12-Methylenedioxychanofruticosinate | Methyl Chanofruticosinate | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 279 | Methyl N-(Decarbomethoxy)-11,12-(Methylenedioxy) Chanofruticosinate | Methyl Chanofruticosinate | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 280 | O-Methylleuconolam | Aspidosperma | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 281 | Leuconodine D | Aspidosperma | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 282 | Oxayohimban-16-Carboxylic Acid | Corynanthe | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 283 | 19, 20-Dihydroisositsirikine | Corynanthe | Kopsia officinalis | Stems | China | Anti-inflammatory |
| 284 | Rauvomine A | Sarpagine | Rauvolfia vomitoria | Aerial parts |
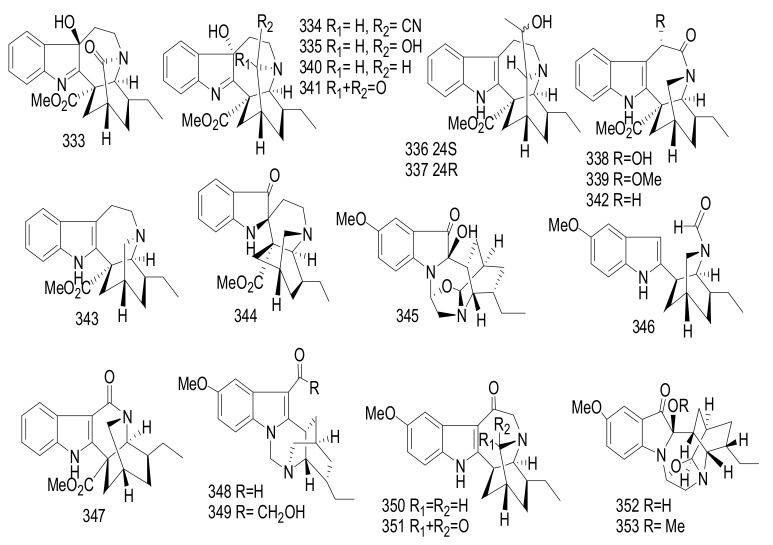
China | Anti-inflammatory |
| 285 | Rauvomine B | Sarpagine | Rauvolfia vomitoria | Aerial parts | China | Anti-inflammatory |
| 286 | Peraksine | Sarpagine | Rauvolfia vomitoria | Aerial parts | China | Anti-inflammatory |
| 287 | Alstoyunine A | Sarpagine | Rauvolfia vomitoria | Aerial parts | China | Anti-inflammatory |
| 288 | 11-Hydroxyburnamine | Picraline | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 289 | Rauvoyunnanine A | Sarpagine | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 290 | Rauvoyunnanine B | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 291 | Lochnerine | Sarpagine | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 292 | Serpentinic Acid | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 293 | Reserpine | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 294 | (−)-Yohimbine | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 295 | Ajmaline | Ajmaline | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 296 | Mauiensine | Ajmaline | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 297 | Ajmalicine | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 298 | Sitsirikine | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 299 | Strictosidinic Acid | Strictosidine | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 300 | Caboxine B | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 301 | Isocaboxine B | Corynanthe | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 302 | Spegatrine | Sarpagine | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 303 | 19(S),20(R)-Dihydroperaksine | Sarpagine | Rauvolfia yunnanensis | Whole plant | China | Cytotoxicity Immunosuppressive |
| 304 | Ervataine | Iboga | Ervatamia yunnanensis | Stems | China | _$ |
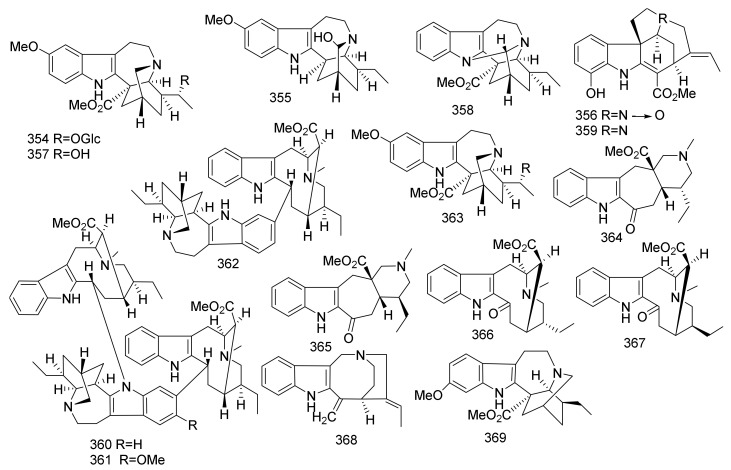
| 305 | Ibogaine | Iboga | Ervatamia yunnanensis | Stems | China | _$ |
| 306 | Coronaridine | Iboga | Ervatamia yunnanensis | Stems | China | Acetylcholinesterase Inhibition |
| 307 | Heyneanine | Iboga | Ervatamia yunnanensis | Stems | China | _ |
| 308 | Voacangine Hydroxyindolenine | Iboga | Ervatamia yunnanensis | Stems | China | _ |
| 309 | Coronaridine Hydroxyindolenine | Iboga | Ervatamia yunnanensis | Stems | China | _ |
| 310 | 10-Hydroxycoronaridine | Iboga | Ervatamia hainanensis | Stems | China | Acetylcholinesterase inhibition |
| 311 | Voacangine | Iboga | Ervatamia hainanensis | Stems | China | Acetylcholinesterase inhibition |
| 312 | 19(S)-Heyneanine | Iboga | Ervatamia hainanensis | Stems | China | Acetylcholinesterase inhibition |
| 313 | 19(R)-Heyneanine | Iboga | Ervatamia hainanensis | Stems | China | Acetylcholinesterase inhibition |
| 314 | Heyneanine Hydroxyindolenine | Iboga | Ervatamia hainanensis | Stems | China | Acetylcholinesterase inhibition |
| 315 | Vobasine | Vobasine | Ervatamia hainanensis | Stems | China | Acetylcholinesterase inhibition |
| 316 | Ervachinine E | Iboga | Ervatamia chinensis | Whole plants | China | Cytotoxicity |
| 317 | Rutaecarpine | Corynanthe | Ervatamia chinensis | Whole plants | China | Cytotoxicity |
| 318 | Ervahainine A | Iboga | Ervatamia hainanensis | Leaves and twigs | China | Cytotoxicity |
| 319 | Ervaoffine A | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 320 | Ervaoffine B | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
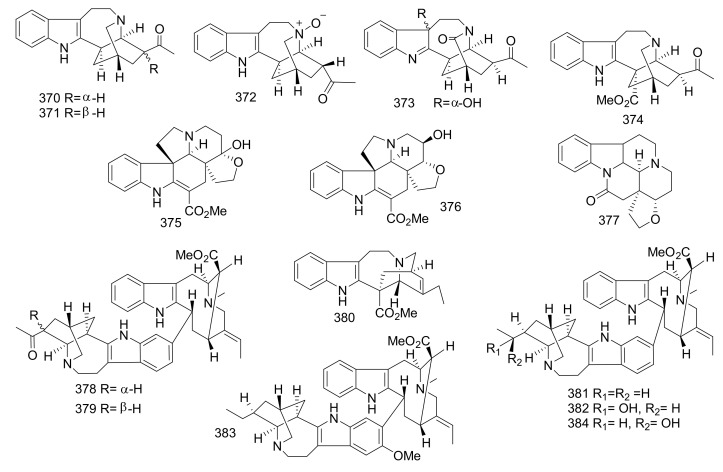
| 321 | Ervaoffine C | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 322 | Ervaoffine D | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 323 | (7S)-3-Oxoibogaine Hydroxyindolenine | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 324 | Ibogaine- 5,6-Dione | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 325 | 19-Epi-5-Oxovoacristine | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 326 | Iboluteine | Ervatamia officinalis | Leaves and twigs | China | _ | |
| 327 | (7S)- Ibogaine Hydroxyindolenine | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 328 | Ibogaline | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 329 | Conopharyngine | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 330 | Voacristine | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 331 | 19S -Hydroxyibogamine | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 332 | Ibogaine N4-Oxide | Iboga | Ervatamia officinalis | Leaves and twigs | China | _ |
| 333 | 3-Oxo-7r-Coronaridine Hydroxyindolenine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 334 | 3S-Cyano-7S-Coronaridine Hydroxyindolenine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 335 | 3R-Hydroxy-7S-Coronaridine Hydroxyindolenine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
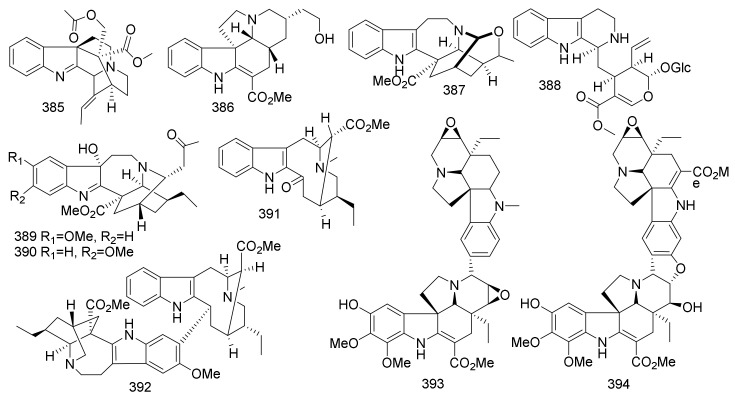
| 336 | 3S -(24S-Hydroxyethyl)-Coronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 337 | 3S -(24R-Hydroxyethyl)-Coronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 338 | 5-Oxo-6S-Hydroxycoronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 239 | 5-Oxo-6S -Methoxy-Coronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 340 | 7S-coronaridine hydroxyindolenine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 341 | 3-Oxo-7S-Coronaridine Hydroxyl Indolenine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 342 | 5-Oxocoronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 343 | 3-Oxocoronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 344 | Pseudoindoxyl Coronaridine | Iboga | Ervatamia hainanensis | Leaves and twigs | China | _ |
| 345 | Ervaoffine E | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
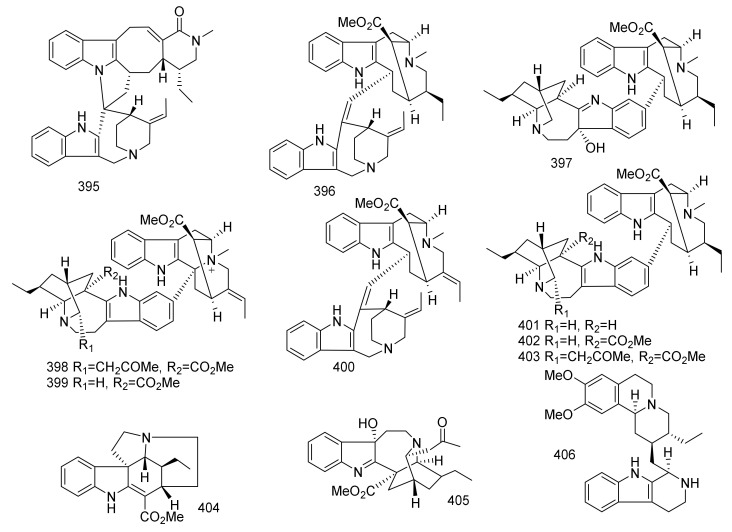
| 346 | Ervaoffine f | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
| 347 | Ervaoffine G | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
| 348 | Lirofoline A | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
| 349 | Lirofoline B | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
| 350 | 6-Oxo-Ibogaine | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
| 351 | 8-Oxo-Ibogaine Lactam | Iboga | Ervatamia officinalis | Leaves and twigs | China | Neuroprotective |
| 352 | Erchinine A | Iboga | Ervatamia chinensis | Roots | China | Antibacterial, Antifungal |
| 353 | Erchinine B | Iboga | Ervatamia chinensis | Roots | China | Antibacterial, Antifungal |
| 354 | Ervapandine A | Iboga | Ervatamia pandacaqui | Leaves and twigs | China | Cytotoxicity |
| 355 | 3R-Hydroxyibogaine | Iboga | Ervatamia pandacaqui | Leaves and twigs | China | Cytotoxicity |
| 356 | 12-Hydroxyakuammicine N4-Oxide | Akuammicine | Ervatamia pandacaqui | Leaves and twigs | China | Cytotoxicity |
| 357 | 19-Epi-Voacristine | Iboga | Ervatamia pandacaqui | Leaves and twigs | China | Cytotoxicity |
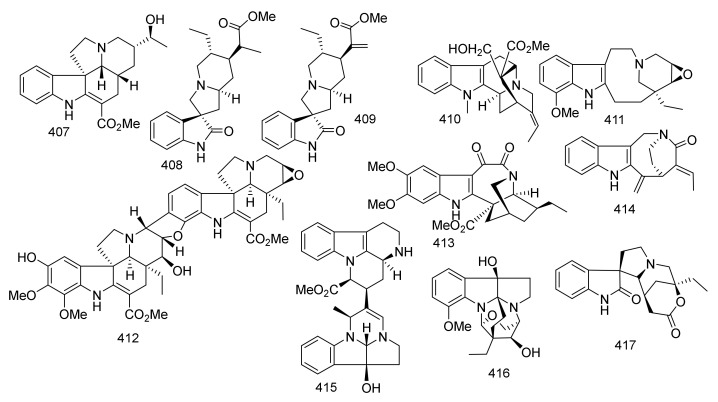
| 358 | Taberdivarine I | Iboga | Ervatamia pandacaqui | Leaves and twigs | China | Cytotoxicity |
| 359 | 12-Hydroxyakuamicine | Akuammicine | Ervatamia pandacaqui | Leaves and twigs | China | Cytotoxicity |
| 360 | Ervadivamine A | Vobasine-Iboga-Vobasine | Ervatamia divaricate | Roots | China | Cytotoxicity |
| 361 | Ervadivamine B | Vobasine-Iboga-Vobasine | Ervatamia divaricate | Roots | China | Cytotoxicity |
| 362 | 19,20-Dihydroervahanine A | Vobasine-Iboga | Ervatamia divaricate | Roots | China | Cytotoxicity |
| 363 | Ibogamine | Iboga | Ervatamia divaricate | Roots | China | Cytotoxicity |
| 364 | Ervatamine | Flabelliformide | Ervatamia yunnanensis | Stems | China | _ |
| 365 | 20-Epi-Ervatamine | Flabelliformide | Ervatamia yunnanensis | Stems | China | _ |
| 366 | Dregamine | Vobasine | Ervatamia yunnanensis | Stems | China | _ |
| 367 | Tabernaemontanine | Vobasine | Ervatamia yunnanensis | Stems | China | _ |
| 368 | Apparicine | Iboga | Ervatamia yunnanensis | Stems | China | _ |
| 369 | Isovoacangine | Apparicine | Ervatamia yunnanensis | Stems | China | _ |
| 370 | Conodusine A | Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 371 | Conodusine B | Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 372 | Conodusine C | Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 373 | Conodusine D | Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 374 | Conodusine E | Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 375 | Apocidine A | Aspidosperma | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 376 | Apocidine B | Aspidosperma | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 377 | Conoduzidine A | Vincamine | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 378 | Tabernamidine A | Vobasine-Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 379 | Tabernamidine B | Vobasine-Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 380 | (+)-Catharanthine | Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 381 | Tabernamine | Vobasine-Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 382 | 19′(S)-Hydroxytabernamine | Vobasine-Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 383 | 19′(R)-Hydroxytabernamine | Vobasine-Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 384 | 16′-Decarbomethoxyvoacamine | Vobasine-Iboga | Tabernaemontana corymbosa | Stem bark | Malaysia | Cytotoxicity |
| 385 | Isoakuammiline | Corynanthe | Tabernaemontana litoralis | Fruits | USA | _ |
| 386 | 18-Hydroxypseudovincadifformine | Iboga | Tabernaemontana litoralis | Fruits | USA | _ |
| 387 | 3,19-Oxidocoronaridine | Iboga | Tabernaemontana litoralis | Fruits | USA | _ |
| 388 | Strictosidine | Strictosidine | Tabernaemontana litoralis | Fruits | USA | _ |
| 389 | $Tabervarine A | Iboga | Tabernaemontana divaricate | Leaves and twigs | China | Cytotoxicity |
| 390 | $Tabervarine B | Iboga | Tabernaemontana divaricate | Leaves and twigs | China | Cytotoxicity |
| 391 | Vobasidine C | Vobasine | Tabernaemontana divaricate | Leaves and twigs | China | Cytotoxicity |
| 392 | Ervadivaricatine B | Vobasine-Iboga | Tabernaemontana divaricate | Leaves and twigs | China | Cytotoxicity |
| 393 | Pedunculine | Aspidosperma- Aspidosperma | Tabernaemontana divaricate | Leaves and twigs | China | Cytotoxicity |
| 394 | Polyervine | Aspidosperma- Aspidosperma | Tabernaemontana divaricate | Leaves and twigs | China | Cytotoxicity |
| 395 | Flabellipparicine | Flabelliformide-Apparicine | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 396 | 19,20-Dihydrovobparicine | Vobasine-Apparicine | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 397 | 10′- Demethoxy-19,20-Dihydrovobatensine D | Vobasine-Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 398 | 3′-(2-Oxopropyl)Ervahanine A | Sarpagine-Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 399 | Ervahanine A | Sarpagine-Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 400 | Vobparicine | Vobasine-Apparicine | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 401 | 19,20-Dihydrotabernamine | Vobasine-Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 402 | 19,20-Dihydrotabernamine A | Vobasine-Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 403 | Taberdivarines E | Vobasine-Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 404 | Tubotaiwine | Strychnos | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 405 | Hydroxy-3-(2-Oxopropyl) Coronaridine Indolenine | Iboga | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 406 | Deoxytubulosine | Corynanthe bisindole | Tabernaemontana divaricate | Stems | China | Cytotoxicity |
| 407 | (3R,7S,14R,19S,20R)-19-Hydroxypseudovincadifformine | Iboga | Tabernaemontana bufalina | Branches and leaves | China | Cytotoxicity |
| 408 | 17-Demethoxy-Hydroisorhyn Chophylline | Corynanthe | Tabernaemontana bufalina | Branches and leaves | China | Cytotoxicity |
| 409 | 17-Demethoxy-Isorhynchophylline | Corynanthe | Tabernaemontana bufalina | Branches and leaves | China | Cytotoxicity |
| 410 | Voachalotine | Akuammidine | Tabernaemontana bufalina | Branches and leaves | China | Cytotoxicity |
| 411 | 12-Methoxyl-Voaphylline | Aspidosperma | Tabernaemontana bufalina | Branches and leaves | China | Cytotoxicity |
| 412 | Conophylline | Aspidosperma- Aspidosperma | Tabernaemontana bufalina | Branches and leaves | China | Cytotoxicity |
| 413 | 5,6-Dioxo-11-Methoxy Voacangine | Iboga | Tabernaemontana contorta | Fruits | Cameroon | Anti-inflammatory |
| 414 | (−)-Apparicin-21-One | Apparicine | Tabernaemontana contorta | Fruits | Cameroon | Anti-inflammatory |
| 415 | Tabernabovine A | Corynanthe bisindole | Tabernaemontana bovina | Leaves | China | Anti-inflammatory |
| 416 | Tabernabovine B | Aspidosperma | Tabernaemontana bovina | Leaves | China | Anti-inflammatory |
| 417 | Tabernabovine C | Iboga | Tabernaemontana bovina | Leaves | China | Anti-inflammatory |
| 418 | Secopleiocarpamine A | Corynanthe | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 419 | 16,17-Epoxyisositsirikine | Corynanthe | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 420 | 2-Ethyl-3[2-(3-Ethyl-1,2,3,6-Tetrahydropyridine)Ethyl]-Indole | Secodine | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 421 | 2-Ethyl-3[2-(3-Ethylpiperidine)Ethyl]-Indole | Secodine | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 422 | Tetrahydrosecodine | Secodine | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 423 | 16,17-Dihydrosecodine | Secodine | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 424 | Deacetylakuammilin | Akuammiline | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 425 | Rhazimal | Akuammiline | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 426 | Strictamine-N-Oxide | Akuammiline | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 427 | Rhazinaline | Akuammiline | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 428 | Rhazinaline Nb-Oxide | Akuammiline | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 429 | Akuammicine | Akummicine | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 430 | 16R-E-Isositsirikine | Corynanthe | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 431 | Dihydrositsirikine | Corynanthe | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 432 | Antirhine | Corynanthe | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 433 | Vincadifformine N(4)-Oxide | Aspidosperma | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 434 | Eburenine | Aspidosperma | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 435 | Winchinine B | Aspidosperma | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 436 | Quebrachamine | Aspidosperma | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 437 | Strictanol | Aspidosperma | Rhazya stricta | Aerial parts | Pakistan | Antifungal |
| 438 | 16-Epi-Stemmadenine-N-Oxide | Corynanthe | Rhazya stricta | Leaves | Saudi Arabia | Cytotoxicity |
| 439 | Stemmadenine-N-Methyl | Corynanthe | Rhazya stricta | Leaves | Saudi Arabia | Cytotoxicity |
| 440 | 20-Epi-Antirhine | Corynanthe | Rhazya stricta | Leaves | Saudi Arabia | Cytotoxicity |
| 441 | Isopicrinine | Picrinine | Rhazya stricta | Leaves | Saudi Arabia | Cytotoxicity |
| 442 | Epirhazyaminine | Rhazya stricta | Aerial parts | Saudi Arabia | Cytotoxicity | |
| 443 | 20-Epi-sitsirikine | Rhazya stricta | Aerial parts | Saudi Arabia | Cytotoxicity | |
| 444 | Strictamine | Rhazya stricta | Aerial parts | Saudi Arabia | Cytotoxicity |
Figure 3.
The types of the structures identified monoterpenoid alkaloids from the six genera.
Figure 4.
Common monoterpenoid indole alkaloidal skeletons of the six genera.
Additionally, the future prospective and emphasizing the research gaps and highlighting the roadmap to discover the potent bioactive monoterpenoid alkaloids, which could be a drug lead from the six genera. Also, this review will discuss the reported structural activity relationships.
2. Alstonia
Plants of the genus Alstonia are grown in Africa and Asia. It includes 60 species, which were recognized as rich source of heterocyclic monoterpene indole alkaloids. It has different names according to the geographical sources, including Devil tree, Australian fever bush, dita bark, Australian quinine, fever bark and palimara. Alstonia bark shows potent therapeutic effects including anti-inflammatory, antirheumatic, analgesic, antidiabetic, antimalarial, antipyretic, antihelminthic, antibiotic, antimicrobial, anticancer, antibacterial and antitussive effects [18,19,20].
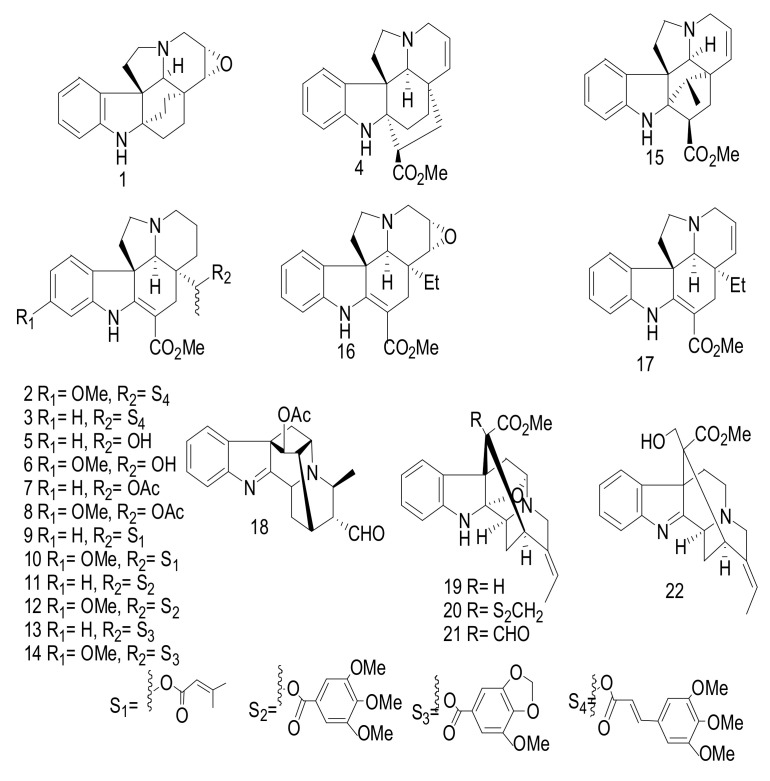
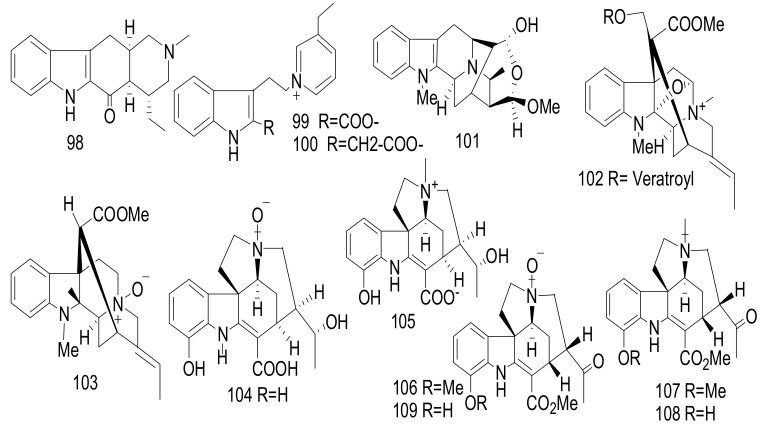
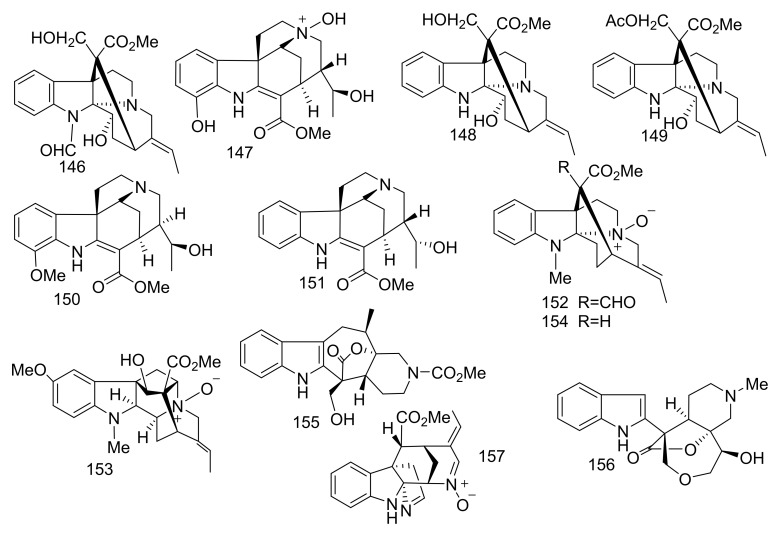
Three monoterpene indole alkaloids (MIAs) derivatives, (14α,15α)-14,15-epoxyaspidofractinine (1) and maireines A (2) and B (3) have been isolated from the leaves and twigs of A. mairei [21]. Additionally, venalstonine (4) [22], (−)-minovincinine (5) [23], (−)-11-methoxyminovincinine (6) [24], (−)-echitovenine (7) [25], echitovenaldine (8) [26], echitovenidine (9), 11-methoxyechitovenidine (10) [27], echitoveniline (11), 11-methoxyechitoveniline (12) [24], echitoserpidine (13) [28],11-methoxyechitoserpidine (14) [29], (19S)-vindolinine (15) [22], lochnericine (16), tabersonine (17) [30], perakine (18) [31], picrinine (19) [32], F (20) [33], picralinal (21) [34] and rhazimol (22) [35] were isolated from the same species (Figure 5). These compounds were elucidated through the interpretation of different spectroscopic measurements including 1D and 2D NMR and MS. Interesting in compound (1) was the interpretation of the Rotating Frame Overhauser Enhancement Spectroscopy (ROSY) spectrum led to the establishment of the α-orientation of the epoxy moiety. Compounds 1–22 were evaluated against five human cancer cells, hepatocellular carcinoma (SMMC-7721), breast (SK-BR-3), pancreatic (PANC-1), human myeloid leukemia (HL-60), and lung (A-549) with IC50 values > 40 μM [21].
Figure 5.
Compounds 1–22.
The majority of reported alkaloids from A. scholaris, were of the picrinine type whereas, those isolated from A. yunnanensis were either picrinine or aspidospermine types.
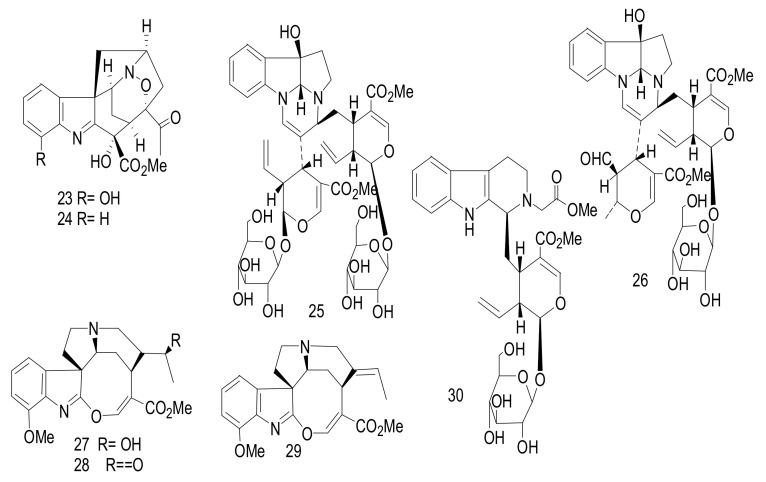
Alsmaphorazines A (23) and B (24) (Figure 6) were identified from the leaves of malaysian A. pneumatophore. The chemical structures were determined on the basis of 2D NMR and MS spectral analysis. These compounds had an unprecedented skeleton containing an 1,2-oxazine (six-member ring) and an isoxazolidine (five-member ring) [36]. The absolute configuration of alsmaphorazine B was determined using CD spectral analysis. The absolute configuration of alsmaphorazine B (24) was studied by comparing its experimental CD spectrum with the calculated CD spectrum, with the CD calculations performed by Turbomole 6.1using the Time-Dependent Density Functional Theory (TD-DFT-B3LYP/TZVPP) level of theory on RI-DFTBP386LYP/TZVPP optimized geometries. Compound 23 inhibited the production of nitric oxide (NO) in an LPS-stimulated J774.1 cell with an IC50 value = 49.2 μM, without affecting the cell viability, whereas compound 24 showed no inhibitory effect at 50.0 μM. Compound 23 was more potent as an anti-inflammatory agent due to the presence of a hydroxyl group at C-12 [36].
Figure 6.
Compounds 23–30.
Alstrostines A (25) and B (26) were determined as derived from the condensation of tryptophan and secologanin in a ratio of 1:2. They were isolated from Alstonia rostrata [37]. The structures were established by measuring 1H, 13C, HSQC, HMBC, 1H-1H COSY and ROESY. Compounds, 25 and 26, exhibited a weak cytotoxicity against five human cancer cells, hepatocellular carcinoma (SMMC-7721), breast (MCF-7), colon (SW480), myeloid leukemia (HL-60) and lung (A-549), with IC50 values > 40 μM [37].
Alstrostines C-F (27–30) (Figure 6) were isolated from the leaves and twigs of Chinese A. rostrata [38]. Compounds 27–30 showed a characteristic UV absorption at 326, 275 and 214 nm, which indicated the presence of an indole alkaloid with a β-anilineacrylate system. The chemical structure elucidation was confirmed by 1D and 2D NMR. Compounds 27–30 showed weak cytotoxicity against five human cancer cells, breast (SK-BR-3), human myeloid leukemia (HL-60), pancreatic (PANC-1), hepatocellular carcinoma (SMMC-7721) and lung (A-549) cells, with IC50 values > 40 μM [38].
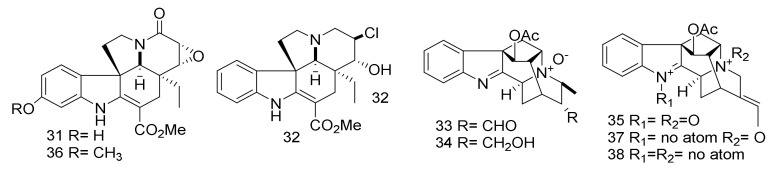
Five MIAs, 11-hydroxy-6,7-epoxy-8-oxo-vincadifformine (31), 14-chloro-15-hydroxyvinca difformine (32), perakine N4-oxide (33), raucaffrinoline N4-oxide (34), and vinorine N1,N4-dioxide (35) (Figure 7) have been reported from A. yunnanensis. Additionally, three compounds, 11-methoxy-6,7-epoxy-8-oxovincadifformine (36), vinorine N4-oxide (37) and vinorine (38) have also been found from the same plant [39]. The chemical structures were established based on 1D and 2D (1H-1H-COSY, HMQC, HMBC, and ROESY) NMR spectroscopy. Compounds 33, 34, and 37 showed cytotoxicity against astrocytoma and glioma cells (CCF-STTG1, CHG-5, SHG-44 and U251) with IC50 values ranging from 9.2 to 17.4 μM. Adriamycin was used as positive control and showed cytotoxicity with an IC50 value ranging from 21.8 to 33.7 μM. These compounds exhibited a cytotoxic effect against breast cancer (MCF-7) and human skin cancer (SK-MEL-2) with IC50 values ranging from 28.1 to 35.5 μM. Adriamycin was used as positive control and exhibited a cytotoxic effect with IC50 values ranging from 14.1 to 37.6 μM [39]. Alkaloids 35 and 38 displayed no cytotoxic activities or selective inhibition of COX-2 comparable to those of 33, 34 and 37 although they possess the same monoterpene indole skeleton. The observations indicated that a N4-oxide functionality was essential for cytotoxic and anti-inflammatory properties, while a N1-oxide maybe weaken the cytotoxic activities for this type of alkaloids. The observations indicated that the presence of oxide in N4 was essential for cytotoxic and anti-inflammatory activities, while the presence of the oxide on N1-oxide led to decreasing the cytotoxicity.
Figure 7.
Compounds 31–38.
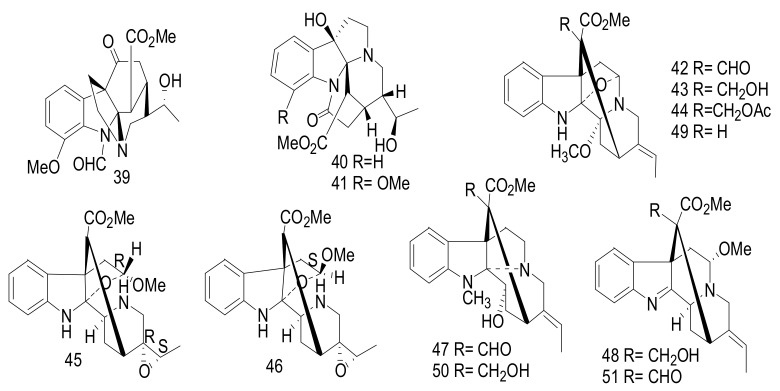
Alsmaphorazines (C) (39), (D), (40), and (E) (41) (Figure 8) were elucidated from A. pneumatophore [40]. The planar structure of 39 was elucidated by 2D NMR and MS. This alkaloid possesses a novel ring skeleton containing an octahydropyrrolo[2,3-b]pyrrole unit. The absolute configuration of (39) was determined by the modified Mosher’s method and also confirmed by measuring the CD spectrum, which fully agreed with the CD calculations. Compounds 39–41 showed no cytotoxicity and also weak anti-melanogenesis activity against HL-60 and B16F10 cells with IC50 values >100 μM [40].
Figure 8.
Compounds 39–51.
New scholarisins I-VII (42–48), and (3R,5S,7R,15R,16R,19E)-scholarisine F (49) [41], along with three known indoles: 3-epi-dihydrocorymine (50), and (E)-16-formyl-5α-methoxystrictamine (51) were identified from the leaves of Alstonia rupestris (Figure 8) [42]. Compounds 42, 47, and 51 showed significant cytotoxicity against cancer cells, A-549, BGC-823, HepG2, HL-60, MCF-7, SMMC-7721, and SW480 with IC50 values < 30 μM. These compounds exhibited selective inhibition effect of COX-2 with IC50 values ranging between 92.0 and 96.4 μM, while compounds 43, 44, and 48–50 displayed a weak cytotoxicity towards the tested tumor cells with IC50 values > 40 μM. Furthermore, alkaloids 45 and 46 showed a weak cytotoxicity with IC50 values > 80 μM. Doxorubicin was used as a positive control and showed with IC50 value < 35 µM. These activities of 45 and 46, indicated that the bond connection between C-5 and N-4 was essential for the cytotoxicity [41]. Compounds 42, 43, 44 and 49 showed antifungal activity against Gibberella pulicaris (KZN 4207) and Colletotrichum nicotianae (SACC-1922) with MIC values of 0.64 and 0.69 mM; 1.37 and 1.44 mM; 1.80 and 1.91 mM and 1.55 and 1.71 mM, respectively. Nystatin was implemented as a positive control and showed MIC values of 0.007 and 0.006 mM. These bioactivities may be due to the presence of a formyl group at C-16 in the alkaloids subclasses picrinine in 42, vincorine in 47, and akuammiline in 51, respectively and also may play a role in anti-inflammatory activity [41].
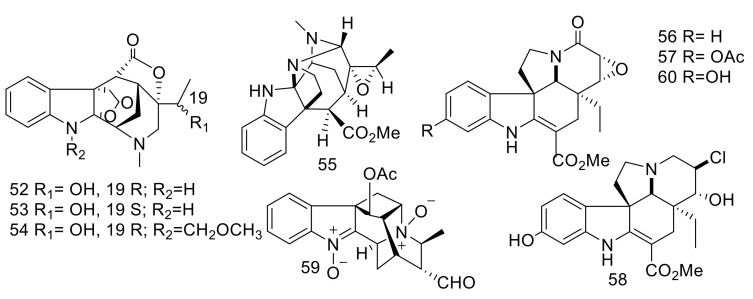
Alstolactines A (52), B (53), and C (54) (Figure 9) were isolated from the leaves of chines A. scholaris [43]. The structures were identified by extensive spectroscopic data analyses and X-ray diffraction analyses. The absolute stereochemistry was deduced from crystal X-ray diffraction. These compounds are biosynthetically originated from picrinine, which is the main metabolite in A. scholaris. Compounds 52–54 exhibited no effects against four bacterial strains: Klebsiella pneumonia, Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus [43].
Figure 9.
Compounds 52–60.
Moreover, Alistonitrine A (55) (Figure 9) had an unprecedented caged carbon skeleton with a unique 6/5/6/5/5/6 ring system and also contained three nitrogen atoms. It was isolated from the same species [12]. Its structure and absolute configuration were established by extensive spectroscopic analyses and electron circular dichroism calculations. Compound 55 exhibited no activity as an anti-inflammatory in both NF-κB and HIF-α models [12].
The MIAs, 6,7-epoxy-8-oxo-vincadifformine (56), 11-acetyl-6,7-epoxy-8-oxo-vincadifformine (57), 11-hydroxy-14-chloro-15-hydroxyvincadifformine (58) and perakine N1,N4-dioxide (59) were identified from the aerial parts of A. rupestris. Additionally, 11-hydroxy-6,7-epoxy-8-oxovincadifformine (60) and 35 were isolated from the same species [44].
Compounds 56, 57 and 60 exhibited potent cytotoxic effects against head and neck squamous cancer (SCL-1, Detroit-562, UMSCC-1, CAL-27, TCA-83, HepG2 and SCC-PKU) cells, with IC50 values < 20 μM. Doxorubicin was implemented as a positive control and showed cytotoxicity, with IC50 values ≤ 35.4 µM. Compound 56 exhibited potent effect, with IC50 values ≤ 13.7 μM. This may be due to the absence of any substitution at the phenolic ring. This can be explained by the fact that the attachment of electron-donating groups (OH and OAc) led to a reduction in the cytotoxicity [44]. Compounds 56, 57, and 60 displayed significant antifungal activities against Alternaria alternata and Phytophthora capsici, with MIC values = 0.66 & 0.99 mM, 0.87 & 1.10 mM and 1.53 & 1.64 mM, respectively. Nystatin was implemented as positive control and showed effect with MIC values 0.007 and 0.061 mM. Compounds 56, 57, and 60 displayed moderate activity against Staphylococcus aureus, with MIC values of 15.72, 16.33 and 14.91 mM. Meanwhile, compounds 59 and 35 exhibited potent effects against Staphylococcus aureus, with MIC values of 0.49 and 0.83 mM. Rifampicin was used as a positive control and showed an effect at MIC valued = 0.003 mM for bacteria. Additionally, compound 59 showed higher antibacterial effects toward S. aureus than compound 35. The present of a formyl group at the C-20 position might increase the activities for ajmaline indole alkaloids [44].
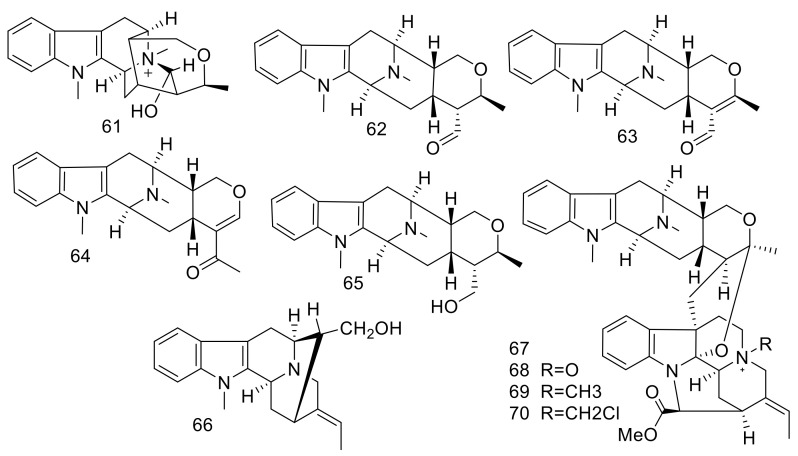
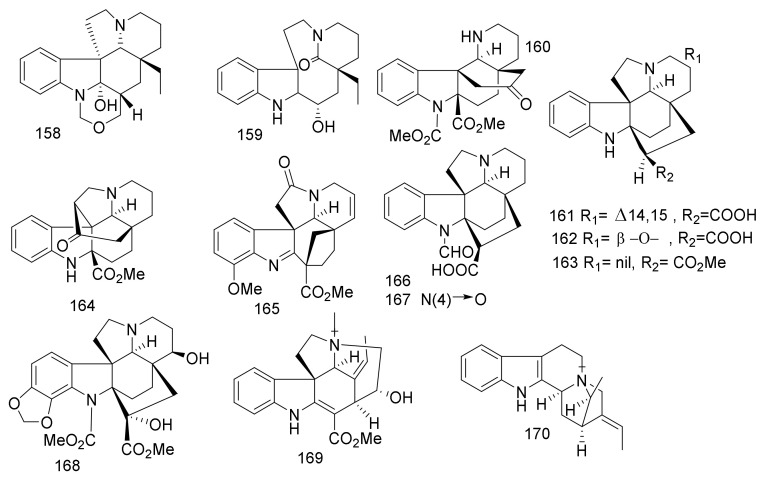
The bioassay-guided fractionation of the stem bark of Vietnamese Alstonia angustifolia using the HT-29 human colon cancer cells, led to the reporting of six MIAs, N(4)-methyl-talpinine (61) [45], N(4)-meth-yl-N(4),21-secotalpinine (62) [46], alstonerinal (63) [47], alstonerine (64) [48], macrocarpine B (65) [46], affinisine (66) [49], from the stem bark of A. angustifolia. Additionally, villalstonine (67), villalstonine N(4)-oxide (68) [50], villalstonidine D (69) and villalstonidine E (70) [51] (Figure 10) were identified from the same plant.
Figure 10.
Compounds 61–70.
Compounds 61 and 66 are sarpagine-type and compounds 62–65 are macroline-derived alkaloids whereas macroline-pleiocarpamine bisindole alkaloids are present in compounds 67–70.
Compound 61 showed significant inhibitory activity toward NF-κB (p65), with an ED50 value = 1.2 μM. Rocaglamide was employed as a positive control, with ED50 value = 0.9 μM. Compounds 61–64, 66 and 68–70 showed anti-leishmanial activity toward the promastigotes of Leishmania Mexicana, with IC50 values < 183.5 μM. Compound 62 exhibited a potent effect, with IC50 value = 57.8 μM. Amphotericin B was employed as a positive control and exhibited potent effect against L. mexicana promastigote, with an IC50 value = 0.09 μM. The dimeric compounds 68–70, which contain quaternary ammonium cation at N(4), exhibited potent effect than compound 67. Additionally, compound 67 has not function group at N(4) [45]. Also, the presence of formyl and acetyl groups in 62–64. These moieties may enhance the effects of compounds belonging to macroline indole alkaloids compared with 65.
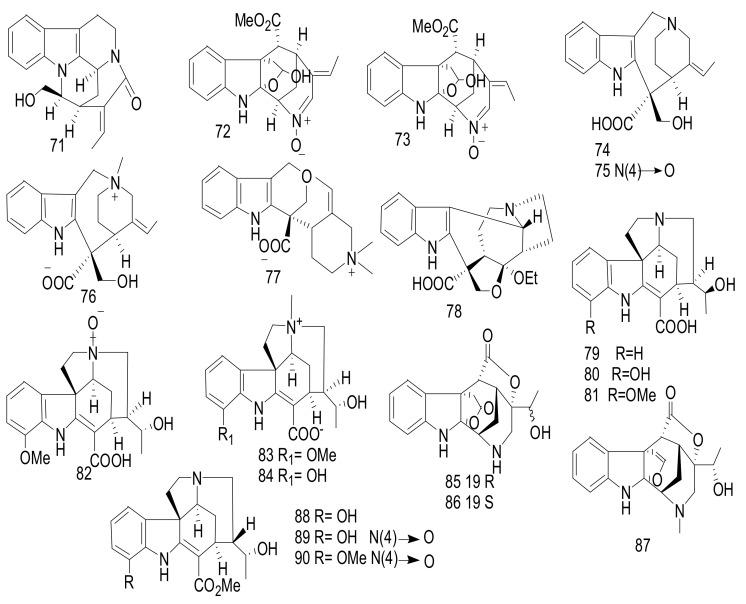
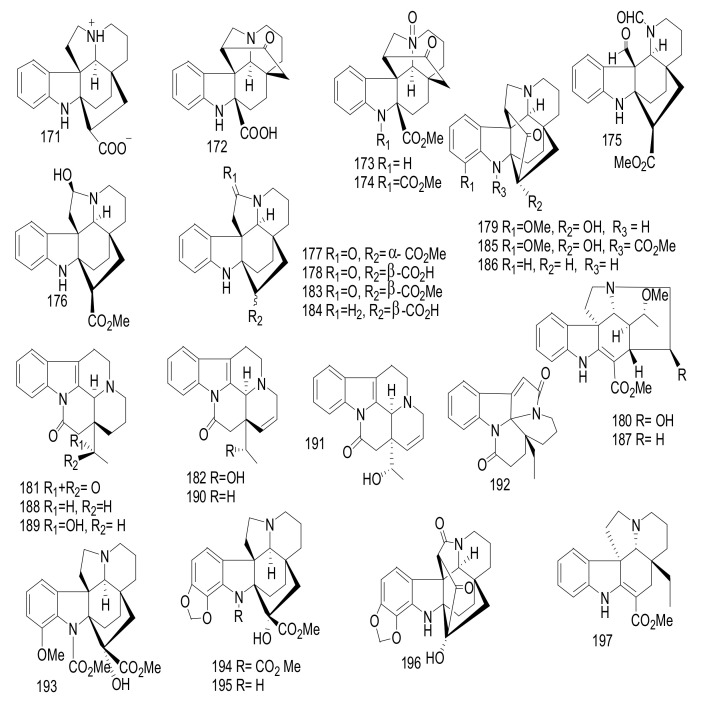
Normavacurine-21-one (71), 5-hydroxy-19, 20-E-alschomine (72), and 5-hydroxy-19, 20-Z-alschomine (73) (Figure 11), were isolated from the leaves of Alstonia scholaris cultivated in Kunming, China [52]. Compound 71 exhibited a significant antimicrobial effect against Enterococcus faecalis ATCC 10541, with an MIC = 0.78 μg/mL, whereas compound 73 showed a significant effect against Pseudomonas aeruginosa ATCC 27853, with an MIC value = 0.781 μg/mL. Cefotaxime was used as a positive control, with an MIC = 0.19 μg/mL [52]. Alstoniascholarines A-Q (74–90), were identified from the leaves of A. scholaris collected from Yunnan [53,54]. Compounds 79 and 83 showed a potent antibacterial activity against Pseudomonas aeruginosa ATCC 27853, with MIC value = 3.13 mg/mL. Gentamycin was applied as a Positive control and showed an inhibitory effect, with an MIC value = 0.78 mg/mL. Additionally, compounds 77, 80, and 83 exhibited moderate antifungal activities toward Epidermophyton floccosum CBS 566.94, with MIC value s= 31.25 mg/mL. Griseofulvin was applied as a positive control and showed an inhibitory effect, with an MIC value = 7.81 mg/mL [53]. Compounds 85–90 showed no cytotoxicity against five tumor cell: MCF-7, A-549, HL-60, SW-480, and SMMC-7721[54].
Figure 11.
Compounds 71–90.
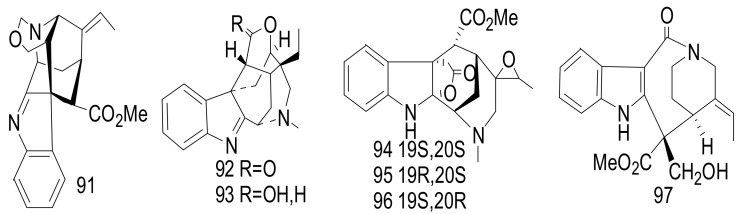
Scholarisines H-O (91–97) (Figure 12) were isolated from the leaves of the Chinese A. scholaris [55]. The chemical structures were elucidated on the basis of comprehensive spectroscopic data and X-ray diffraction. Compounds 91–97 showed weak antibacterial activities against five strains: Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25922, Escherichia coli ATCC 11775, Providencia smartii ATCC 29916, and Enterococcus faecalis ATCC 10541), with MIC values = 100 μg/mL. Gentamycin was used as a positive control, with an MIC value < 2.00 μg/mL [55].
Figure 12.
Compounds 91–97.
A further study on the leaves and twigs of A. scholaris [56] led to identification of melosline A (98), B (99) and 1-[2-[2-(carboxymethyl) indole-3-yl] ethyl]-3-ethylpyridinium hydroxide inner salt (100) (Figure 13) [57]. Melosline A (98) was an unprecedented indole alkaloid, with a 6/5/6/6 tetracyclic ring skeleton. The structures were established by spectroscopic analyses. The absolute configuration of 98 was confirmed by the comparison of experimental data with the calculated electronic circular dichroism (ECD). Compound 98 showed a moderate cytotoxic activity against breast cancer (MCF-7), with an IC50 value = 39.78 μM. Cisplation was employed as a positive control [56].
Figure 13.
Compounds 98–109.
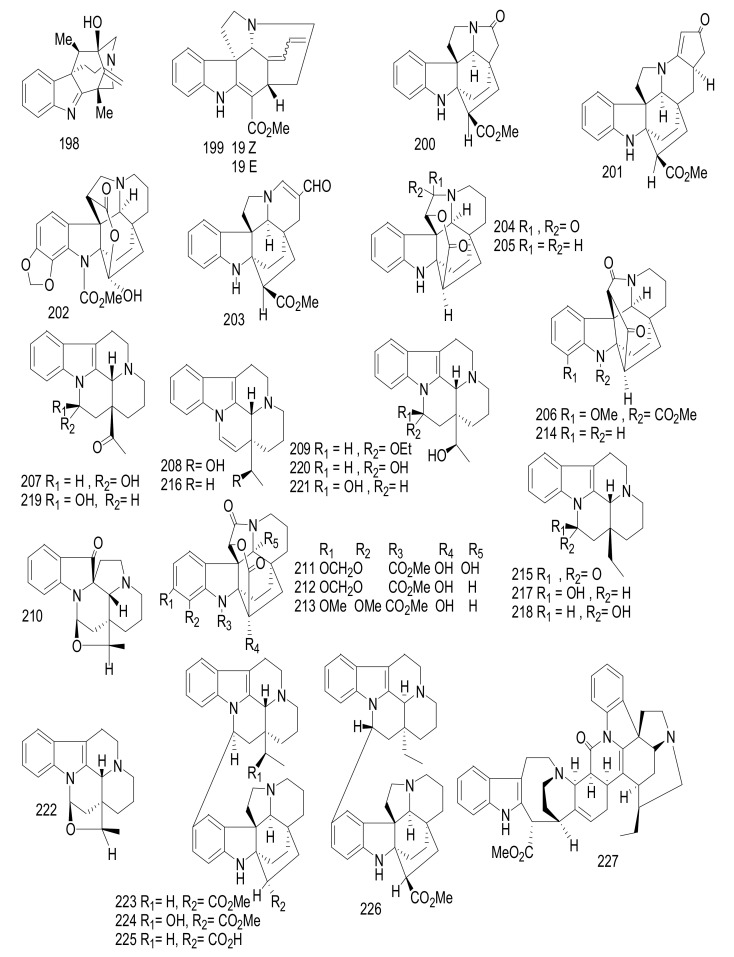
Alstiyunnanenines A-E (101–105) (Figure 13) and alstoniascholarine I (82) (Figure 11), were isolated from A. yunnanensis [54,58]. Compounds 104, 105, and 82 displayed potent cytotoxicity against human gastric carcinoma (BGC-823 cells), human hepatocellular, (HepG2 cells), human myeloid leukemia (HL-60), human breast cancer (MCF-7), and osteosarcoma (SOSP-9607, MG-63, Saos-2, M663), with IC50 values ranging between 3.2 and 5.8 μM. Adriamycin was used as a positive control and exhibited cytotoxicity, with an IC50 value < 0.04 μM [58]. Three monoterpenoid indoles, alstomairines A-C (106–108) [59], together with alpneumine A (109) [60] were identified from the leaves of the chines A. mairei. Compounds 107 and 108 showed potent cytotoxic effects against osteosarcoma cells (U2-OS, Mg-63, Saos-2, and SOSP-9607) with IC50 values ranging from 9.2 to 13.0 μM, whereas compounds 106 and 109 had IC50 values < 15.0 μM. The presence of the methyl group on N-4 indicate increasing the cytotoxicity in that scholaricine-type (Figure 4) than the presence of N(4)-oxide moiety. Doxorubicin was used as Positive control and showed cytotoxicity, with an IC50 value < 0.03 μM [59].
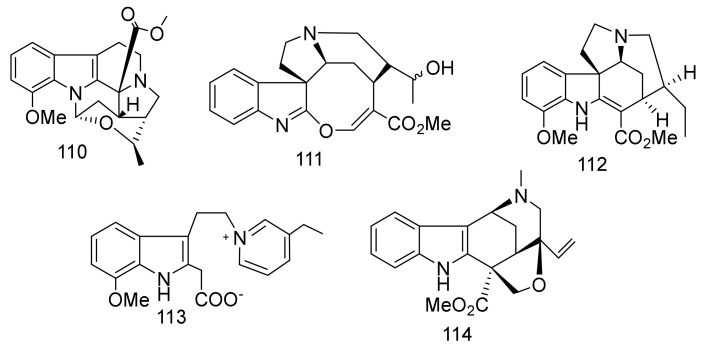
Alstrostine G-K (110–114) (Figure 14), were identified from the Chinese A. rostrata [61]. Compounds 110–114 showed no cytotoxicity against HeLa, SGC-7901 gastric cancer, and A-549 lung cancer at 20 µM [61].
Figure 14.
Compounds 110–114.
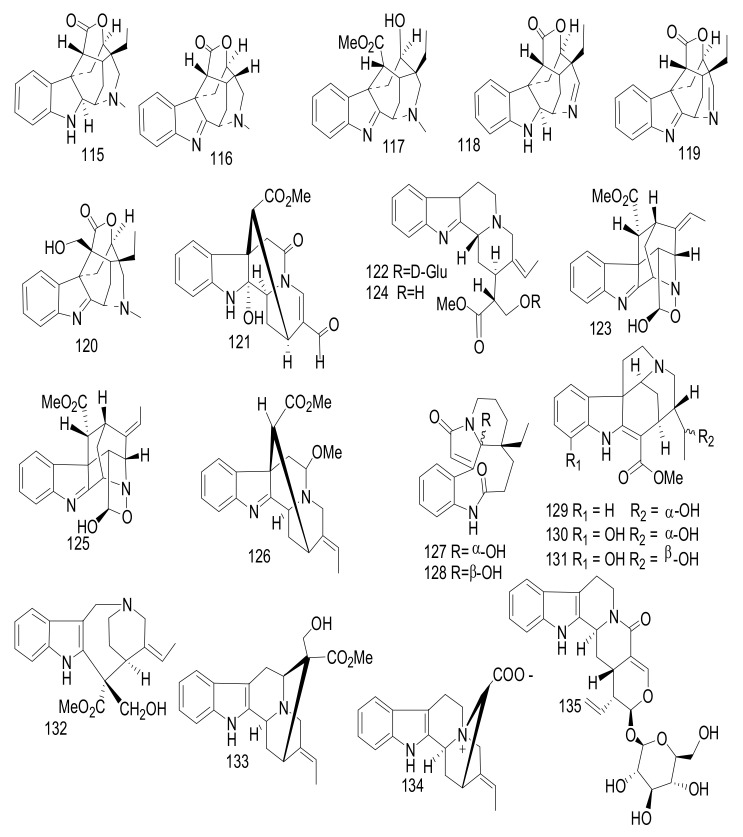
Six nareline-type indoles including three cage-like skeletons, scholarisines T-V (115–117) [62] (Figure 15), and three previously identified analogues scholarisine W (118), scholarisine A (119), and scholarisine I (92) [55], were isolated from the leaves of the Chinese A. scholaris [56]. Compounds 115–117 displayed anti-bacterial effects against Escherichia coli ATCC 8739, with an MIC value = 0.78 μg/mL. Additionally, compound (116) inhibited the growth of Bacillus subtilis ATCC 6633 bacterium with an MIC value = 3.12 μg/mL and was referenced with cefotaxime as a positive control. The absence of the ethyl group at C-20 position indicated an increase in the anti-bacterial activities as in 116, compared with compounds (115 and 117) [63]. Cefotaxime was used as a positive control and exhibited an inhibitory effect, with an MIC value of 0.39 μg/mL. There were scholarisines P-S (120–123), (16R)-E-isositsnikine (124) [64], nareline (125) [65], 5-methoxystrictamine (126), leuconolam (127), epileuconolam (128) [66], and Nb-demethylalstogustine (129) [67]. Also, 19-epischolaricine (130), scholaricine (131), vallesamine (132) [68], akuammidine (133) [69], 17-nor-excelsinidine (134) [70], strictosamide (135) [71,72] and compounds 19 and 21, were isolated from the same species. Compounds 123, 19, 21, 130 and 133 exhibited significant NF-κB inhibitory activity with IC50 < 25 μM. Furthermore, compounds 19, 126 and 130 inhibited TNFα-induced NF-κB activation in the same dose. Three nareline-type MIAs, compounds (120, 123 and 125) were identified from A. scholaris [73].
Figure 15.
Compounds 115–135.
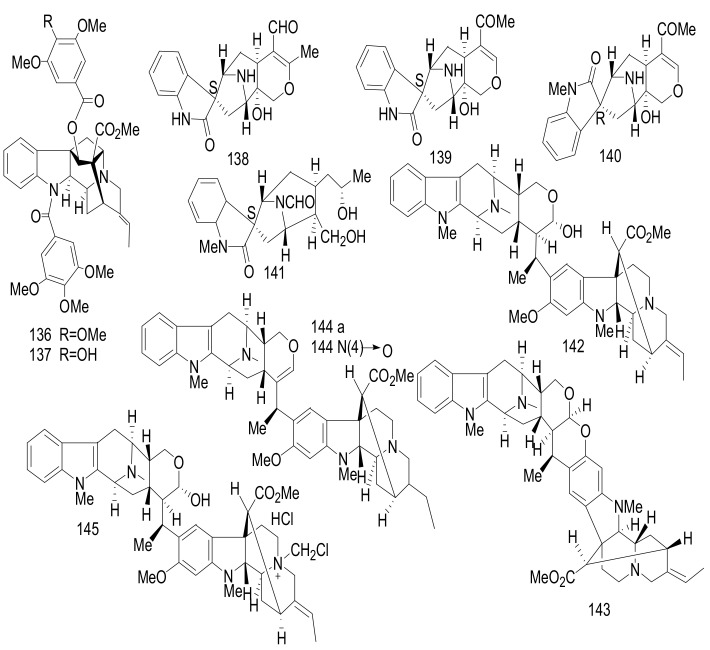
Two ajmaline type MIAs, vincamaginine A (136), and vincamaginine B (137); four macroline oxindole- alstonisinines A (138) and B (139), alstonisinine C (140), and alstonoxine F (141); four bisindole compounds of macroline-akuammiline type; angustilongine A–D (142–145) (Figure 16) were reported from Malaysian Alstonia penangiana [73]. The structures of these alkaloids were determined by the interpretation of spectroscopic data and compounds 141–142, were confirmed by X-ray diffraction analysis. Compounds 142 and 143 showed growth inhibitory activity against human prostate carcinoma (LNCaP and PC-3), human breast adenocarcinoma (MDA-MB-231 and MCF7), human colorectal carcinoma (HCT 116 and HT-29) and human lung carcinoma (A549). Furthermore, the potent effects of 142 and 143 against HT-29 cells were evaluated, with IC50 values = 0.7 ± 0.1 μM and 0.3 ± 0.0 μM, respectively (Cisplatin, IC50 >10 μM). Compound 143 exhibited an effect against vincristine-resistant KB cells, with an IC50 value of 0.7 ± 0.3 μM (Vincristine 0.3 ± 0.1 μM) [73].
Figure 16.
Compounds 136–145.
Winphyllines A (146), B (147) [74], Nb-demethylechitamine (148) [75], 17-O-acetylnorechitamine (149) (Figure 17) [76], 12-methoxyechitamidine (150) [67], and N(4)-demethylastogustine (151) [77] were isolated from the collected twigs of the Chinese A. rostrata. Compounds 146–151 exhibited cytotoxicity against cancer cells (HL-60, SMMC-7721, A-549, MCF-7, and SW480), with IC50 values = 40 μM [74]. A vincorine-type, 17-formyl-10-demethoxyvincorine N(4)-oxide (152), an ajmaline-type 10-methoxyalstiphyllanine H (153), and 10-demethoxyvincorine N(4)-oxide (154) were obtained from the leaves of A. scholaris [78]. The phytochemical investigation of A. scholaris led to the publication of alstoscholactine (155) and alstolaxepine (156) [79]. A further investigation on the leaves of Malaysian A. scholaris led to the reporting of alstobrogaline (157) [80]. Compounds 155 and 156 exhibited no cytotoxic effects, whereas 156 induced marked vasorelaxation in reported rat aortic rings precontracted with phenylephrine, with EC50 = 6.58 ± 3.66 μM and Emax = 93.9 ± 4.3% (cf. verapamil, EC50 = 0.55 ±0.19 μM and Emax = 106.4 ± 3.4%) [74]. Compound 157 showed weak cytotoxic activity against breast cancer cells MDA-MB-231 and MCF7, with IC50 values = 25.3 and 24.1 μM, respectively [80].
Figure 17.
Compounds 146–157.
The scaffold of the reported monoterpene indole compounds from A. scholaris is affected by the geographical environment. Indian, Pakistanian and Thai. A. scholaris are rich with picrinine-type indole compounds, whereas, those identified from Indonesia and the Philippine, are rich in angustilobine-type [81]. Genus Alstonia was addressed as a source of angustilongines A (142) and B (143). These compounds showed more potent anticancer activities than those recognized from A. penangiana, although all of them belong to macroline- and akuammiline-type bisindole alkaloids.
A review entitled "Alstonia scholaris and Alstonia macrophylla: A comparative review on traditional uses, phytochemistry and pharmacology" was published in 2014 and mentioned the compounds obtained from A. scholaris from 1976 to 2009, and from A. macrophylla from 1987 to 2013 [82]. A review published in 2018 entitled "The alstoscholarisine compounds: isolation, structure determination, biogenesis, biological evaluation and synthesis" studied the alstoscholarisine compounds obtained from A. scholaris [83]. Furthermore, a review published in 2016 called "An overview phytochemistry and chromatographic analysis of Alstonia scholaris used as a traditional medicine" discussed A. scholaris compounds which were reported between 1965 and 2009 [84].
The identified metabolites from Alstonia were categorized under two main classes: corynanthe and aspidosprma. Corynanthe contains eight subclasses: ajmaline-type (18, and 33–35), picrinine-type (19–21 and 42–44), akummiline-type (22), vincorine-type (47, 50 and 148–149), sarpagine-type (61, 66 and 101), macroline-type (62–65), scholaricine-type (104–109) and macroline oxindole-type (138–141). Meanwhile,, aspidosprmia contains six subclasses: aspidosprma-type (31 and 32) vincamine-type (82–84 and 88–90), aspidofractinine-type (1 and 4), bisindole alkaloids macroline-pleiocarpamine-type (67–70), and macroline- akuammiline-type (142–145). Ajmaline derivatives with formyl group and/or a quaternary ammonium cation N(4) showed an interesting bioactivity.
3. Kopsia
Kopsia (Family Apocynaceae) contained 30 species with a distribution in China, India, Southeast Asia, and Australia. Sixteen species were grown in Malaysia [85], and five species were grown in Thailand [86]. These plants are considered as rich sources of indole-containing compounds. Traditionally, some of the species have been used for the treatment of tonsillitis, dropsy and rheumatoid arthritis. Several species have been reported to have antitumor, antimanic, antitussive and antileishmanial effects [87,88,89]. A review published in 2017 was interested in reporting indole alkaloids from genus kopsia plants regarding reversing multidrug resistance in vincristine-resistant KB cells for example, kopsirensine B, arboloscine A [90], grandilodines A and C, and lapidilectine B [91,92].
Kopsiyunnanines G (158) and kopsiyunnanines H (159) (Figure 18) with an aspidosperma-containing skeleton were isolated from the aerial part of the Chinese Kopsia arborea [93]. Kopsihainins A (160), B (161), and C (162) were isolated as new compounds from K. hainanensis [89], along with the known compounds, kopsinine (163) [87] and methyl demethoxycarbonylchanofruticosinate (164) [94] were isolated from the stems of Chinese K. hainanensis. Compounds 163 and 164 showed significant antitussive activity, these compounds are within the aspidofractinine-type and methyl chanofruticosinate-type indoles, respectively. Compounds 163 and 164 inhibited coughing by 88% and 76%, respectively [83]. Compound 163 was more active, with an ID50 value = 0.11 mmoL/kg, whereas compound 164 exhibited an effect, with an ID50 value = 0.45 mmol/kg, (Codeine, ID50 = 0.1 mmol/kg) [90]. The link from C-2 to C-20 in compound 163 and the attachment of the methoxy carbonyl group at C-16 position promote the antitussive activity.
Figure 18.
Compounds 158–170.
Four alkaloids of aspidofractinine-type, singaporentine A (165), N(1)-formylkopsininic acid (166), N(1)-formylkopsininic acid-N(4)-oxide (167), and 15-hydroxykopsamine (168), along with an aspidospermatan-type, 14α-hydroxy-N(4)-methylcondylocarpine (169), and singaporentinidine (170) (Figure 18) were identified from the barks and leaves of Malaysian K. singapurensis [95].
From the leaves and stems of the Chinese medicinal plant K. hainanensis, four compounds, kopsininate (171), N1-decarbomethoxy chanofruticosinic acid (172), methyl N1- decarbomethoxy chanofruticosinate N(4)-oxide (173) and methyl chanofruticosinate N(4)-oxide (174) (Figure 19) were reported [96]. Compound 172 was the most effective against Erwinia carotovora bacterium, with an MIC of 7.8 mg/mL. Furthermore, compound 172 showed antifungal activities against four plant pathogenic fungi: Penicillium italicum, Fusarium oxysporum f. sp. Niveum, Rhizoctonia solani and Fusarium oxysporum. Cubense had an EC50 = from 15.2 to 43.8 μg/mL dose values. Compound 172 showed a potent effect towards F. oxysporum f. sp. Cubense, with an EC50 = 15.2 mg/mL. A comparison of this result with the positive control Midlothian, with an EC50 = 57.0 mg/mL showed compound 172 to be more active. The presence of carboxylic group attached to the C-2 position in 172 is important for antifungal activity, particularly, in methyl chanofruticosinate-type indoles [96].
Figure 19.
Compounds 171–197.
Three aspidofractinie-type compounds, 5,6-secokopsinine (175), 5β-hydroxykopsinine (176), 16-epi-kopsinilam (177) [97], two kopsine-type metabolites, 5-oxokopsinic acid (178), and Na-demethoxycarbonyl-12-methoxykopsine (179) [97], a strychnos-type, 14(S)-hydroxy-19(R)- methoxytubotaiwine (180), and vincamine-type, and strychnos type 19-oxo-(−)-eburnamonine (181), 19(S)-hydroxy-Δ14-vincamone (182) [97], along with ten known compounds, 163 [87], kopsinilam (183) [98], kopsinic acid (184), 12-methoxykopsine (185) [99], kopsanone 186), 19(R)- methoxytubotaiwine (187) [88], (−)-eburnamonine (188), 19-OH-(−)-eburnamonine (189), and Δ14-vincamone (190) [97] were yielded from the stem bark of the Thai Kopsia jasminiflora (Figure 19). Compounds 163, 183, and 184 belong to aspidofractinie-type, 185 and 186 belong to Kopsine-type, 187 belongs to strycno-type, 188–190 belonging to the vincamine- type MIAs.
The vincamine-type compound 182 showed a potent inhibitory activity against HT29, HCT116, and A549 cancer cells, with IC50 values = 0.36, 0.40, and 0.51 μM, respectively. Meanwhile, compounds 188 and 189 showed moderate activities with IC50 values ranging from 2.00 to 2.61 μM (Docetaxel, IC50 < 0.0005 μM). These results indicated the structural features that are necessary for the presence of a vincamine-type carbonyl group at the C-16 position, forming an amide function group, and a methylene group or hydroxyl methine at C-19 position in 182, 188, and 189 [97]. The presence of a double bond in the piperidine ring between C-15 and C-16 may be responsible for increasing the activity of compound 182.
A study on the content of twigs of K. arborea grown in Thailand revealed the isolation of a new MIA, phutdonginin (191) [100], an eburnane-type compound, together with eight known compounds, among them, 164 [87], 189 [88] melodinine E (192) [101], kopsilongine (193), kopsamine (194) [94], (−)-methylenedioxy-11,12-kopsinaline (195) [87], decarbomethoxykopsiline (196) [102], and vincadifformine (197) [103]. Only 194 and 196 displayed AChE inhibition activity with MIR values 12.5 and 6.25 μg, respectively, compared with reference drug galanthamine MIR = 0.004 μg. In addition, compounds 194 and 198 also displayed the weak acetylcholinesterase (AChE) inhibition of 23.3% and 45.7% in a microplate test at 1 mM. Compounds 191 and 189 showed moderate inhibition of bacterium toward Escherichia coli TISTR 780 with MIC = 32 μg/mL, with vancomycin and gentamycin references drugs with MIC values 0.125–0.25 μg/mL [100].
Malaysian Kopsia arborea was investigated and arboridinine (198) [85] was reported (Figure 20). The further investigation of the aerial parts of K. arborea led to the isolation of kopsiyunnanines J1 and J2 (199a and 199b) [104]. Compound 198 exhibited a moderate relaxation effect that was dependent on the contraction of phenylephrine-induced in the rat aortic rings, with an EC50 of 4.98 μM, and an Emax 60.6 ± 7.8% with the reference control isoprenaline with an EC50 value = 0.08 μM, and an Emax 79.7 ± 4.2% [85].
Figure 20.
Compounds 198–227.
Seven aspidofractinine -type alkaloids, paucidirinine (200), paucidirisine (201), paucidactinine (202), pauciduridine (203), paucidactine D (204), paucidactine E (205), and paucidisine (206), along with Additionally, four eburnane skeleton, (−)-19-oxoisoeburnamine (207), (−)-19(R)-hydroxyeburnamenine (208), (−)-19(R)-hydroxy-O-ethylisoeburnamine (209), and larutienine B (210) were isolated from Kopsia pauciflora [91]. Moreover, twelve compounds, paucidactine A (211), paucidactine B (212) [105], paucidactine C (213) [88], 5, 22-dioxokopsane (214) [98], (+)-eburnamonine (215) [94], (+)-eburnamenine (216) [106], (−)-eburnamine (217), (+)-isoeburnamine (218) [94], (+)-19-oxoeburnamine (219) [105], (−)-19(R)-hydroxyisoeburnamine (220), (+)-19(R)-hydroxyeburnamine (221) [87], and larutienine A (222) [90] were published. Furthermore, three bisindole compounds have been identified, (−)-norpleiomutine (223), (+)-kopsoffinol (224) [107], and (−)-demethylnorpleiomutine (225) [87] and (+)-kopsoffine (226) (Figure 20) [107], were identified from the same species. A bisindole alkaloid were isolated by Kitajima at et from Yunnan Kopsia arborea, named Kopsiyunnanine M (227) [108].
Compounds 223 and 224 exhibited growth inhibitory activity against MCF-7, PC-3, A549, and HCT-116, with IC50 values ranging between 11.5 and 25.1 μM (Cisplatin, IC50 value in the range of 5.0–14.3 μM). The obliteration of the biological activity in 225 may be due to the presence of a carboxylic group in C-16, instead of a methoxycarbonyl group in 223 [91]. Arborisidine (228) and arbornamine (229) were isolated from a Malaysian K. arborea. Compound 228 represented a unique skeleton [109]. Compounds 228 and 229 exhibited no activities against KB, PC-3, HCT116, A549 and HT-29 cells [109].
Six new Kopsinidine C-E (230–232), 11,12-methylenedioxychanofruticosinic acid (233), 12-methoxychanofruticosinic acid (234), and N(4)-methylkopsininate (235), in addition to chanofruticosinic acid (236) as new natural compound [110], along with compounds 163, 164, 178, 183, 179, and 215 (Figure 21) were isolated from K. officinalis. Additionally, Kopsinine methochloride (237), demethoxycarbonylkopsin (238) [111], methyl chanofruticosinate (239), methyl 11,12-methylenedioxychanofruticosinate (240) [94], methyl 12-methoxychanofruticosinate (241), methyl 11,12-methylenedioxy-N1-decarbomethoxychanofruticosinate (242) [112], kopsininic acid (243), and (−)-11,12-methylenedioxykopsinaline (244) [98] were identified from the same species. Furthermore, (−)-N-methoxycarbonyl-11,12-methylenedioxykopsinaline (245) [98], (−)-N-methoxycarbonyl- 12-methoxykopsinaline (246), N-carbomethoxy-11-hydroxy-12- methoxykopsinaline (247) [113], kopsinoline (248) [114], (−)-12-methoxykopsinaline (249) [98], 11,12-methylenedioxykopsinaline N(4)- oxide (250) [87], kopsinine B (251) [115], rhazinilam (252) [66], and pleiocarpamine methochloride (253) [116] were all isolated from the twigs and leaves of chines K. officinalis. Compound 252 displayed a significantly inhibition effect of the human T cell proliferation, which was activated by using anti-CD3/anti-CD28 antibodies, with an IC50 = 1.0 μM, showing stimulation, with an IC50 = 1.1 μM [110]. Compound 252 was indicated to have the highest cytotoxic effect due to the presence of a hydroxyl group in C-14 and C-15 position [110].
Figure 21.
Compounds 228–253.
Kopsioffines A-C (254–256) [117] (Figure 22) were isolated from the leaves and stems of K. officinalis. These compounds possess a relatively novel ten-membered lactam ring [117]. Additionally, five MIAs, Kopsifolines G-K (257–261) were identified from the same plant [118]. Moreover, kopsifoline A (262) was isolated from the aerial parts of an unidentified Kopsia sp. [119]. Compounds 259–261 exhibited cytotoxic effects against dermatoma (HS-1, A431, SCL-1, HS-4), gastric carcinoma (BGC-823), breast cancer (MCF-7), and colon cancer (SW480), with IC50 values in a range between 11.8 and 13.8; between 10.3 and 12.5; between 7.3 and 9.5 μM, respectively (Adriamycin, IC50 < 34 nM). Compound 261 showed a potent cytotoxic effect that may be due to the presence of two hydroxyl groups in the C-14 and C-15 positions, instead of one hydroxyl group at C-15 position in compounds 259 and 260. Compounds 257, 258 and 262 exhibited a weak cytotoxic effect with IC50 values > 20 μM. This may be due to the absence of a hydroxyl group in that position [118]. Compounds 254–256 exhibited weak inhibitory effects on yeast α-glucosidase in vitro with IC50 values > 50 μM [118]. Compounds 259–260 exhibited interesting antifungal and antimicrobial activities toward five pathogen bacteria Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, Shigella dysenteriae and Klebsiella pneumoniae), and also exhibited an antibacterial effect on the oral pathogens Streptococcus viridans and Streptococcus mutans. Netilmicin was used as a reference drug, with MIC values < 0.18 mm. 5-Flucytocine was used as a positive control with MIC values < 0.09 mM. Alkaloid 261 displayed the highest antimicrobial activity toward the tested pathogens, with an MIC value of 0.15–1.14 mM, while compounds 259 and 260 showed significant activities, with MIC values of 0.77–3.09 and 0.72–1.37 mM. Compounds 257, 258 and 262 were inactive. The present of a hydroxyl group at the piperidine ring enhanced the anticancer and antimicrobial activity in this subtype of indoles [118]. The investigation of the aerial parts of K. arborea led to the isolation of three compounds: kopsiarborines A-C (263–265) [120]. Meanwhile, the study of the aerial parts of K. officinalis led to the reporting of three MIAs, kopsiaofficines A–C (266–268) (Figure 22) [121]. Compounds 263 and 264 showed significant cytotoxic activities against H446, H292, A549, H460, ATCC, and 95-D, with IC50 values < 20 μM, (Doxorubicin, IC50 value = 0.06 μM). Compound 264 exhibited a potent activity with IC50 values < 9.5 μM, and compound 265 was inactive [120]. Compound 268 exhibited a potent cytotoxicity against H446, A549, ATCC, 95-D, H460, H292, SPCA-1, and lung cancer cells, with IC50 values < 10 mM, while compound 266 showed some cytotoxic activity with IC50 value < 20 μM (Doxorubicin, IC50 = 13.7–33.7 nM) [121].
Figure 22.
Compounds 254–268.
Kopsiofficines H–L (269–273) [122] (Figure 23), together with fourteen compounds, 164, 208, 239, 241, (+)-O-methyleburnamine (274) [93], (−)-O-methylisoeburnamine (275) [123], 16-isoeburnamine (276) [124], 20-oxoeburnamenine (277) [125], methyl 11, 12-methylenedioxychanofruticosinate (278) [99], methyl N-(decarbomethoxy)-11, 12-(methylenedioxy) chanofruticosinate (279) [126], O-methylleuconolamm (280) [127], leuconodine D (281) [128], oxayohimban-16-carboxylic acid (282) [129], and 19, 20-dihydroisositsirikine (283) [130] (Figure 23), were identified from the stems of K. officinalis plant [122]. Compounds 164, 241, 270, 271, 274, 275, 279, and 281 exhibited significant anti-inflammatory activity towards IL-1β, PGE2 and TNF-α at 5 μg/mL. Deametasona was used as a positive control at 10 μg/mL [122].
Figure 23.
Compounds 269–283.
Table 1 methyl chanofruticosinate-type (164, 173–175), aspidosoermatan-type (169, 199), kopsine-type (179, 185–186), strychnos-type (180, 187), vincamine-type (181, 188, 189), paucidactine-type (204, 205) and eburnane-type (207–210), all these subtypes belongs to the main class aspidospirma, and very few compounds belong to the main class of corynanthe-type indoles (Figure 4). Vincamine and methyl chanofruticosinate derivatives showed interesting biological activity.
4. Rauvolfia
Rauvolfia (family Apocynaceae) contains 60 species. It contains trees or shrubs that are distributed in Africa, America, and Asia [131]. Rauvolfia serpentine is one of the most important medicinal plant that has been considered as a drug lead for a long time [132]. Rauvolfia has been used traditionally for the treatment of several diseases, such as high blood pressure (hypertensive), fever (malaria), arrhythmia, cancer, oxidative stress, microbial problems, intestinal spleen ailments, and various mental disorders [133]. Therapeutically, it is a source of monoterpenoid indoles, including ajmaline (antiarrhythmic), ajmalicine, yohimbine, reserpine (antihypertensive), and serpentine [133].
A review entitled "Rauvolfia serpentina L. Benth. ex Kurz. phytochemical, pharmacological and therapeutic aspects" was published in 2013 and evaluated various bioactive compounds as ajmaline, ajmalicine, deserpidine, reserpine, reserpiline, serpentine, rescinnamine and yohimbine [132]. A review entitled "Chemical and Biological Perspectives of Monoterpene Indole Compounds from Rauwolfia species" mentioned the compounds obtained until 2016 [134]. Another review described the structures and pharmacological potentials of the plant species Rauvolfia tetraphylla L. (Apocynaceae) [135].
Two normonoterpenoid indole compounds were isolated from the aerial parts of Rauvolfia vomitoria, rauvomines A (284) and B (285) [136] along with two known compounds peraksine (286) (Figure 24) [137] and alstoyunine A (287) [42]. Compound 285 displayed significant anti-inflammatory effects against murine macrophages (RAW 264.7), with an IC50 value = 39.6 μM, whereas, compounds 284, 286 and 287 displayed moderate anti-inflammatory effects with IC50 values = 55.5, 65.2, and 75.3 μM, respectively, (Celecoxib, IC50 = 34.3 μM) [136]. Compound 285 showed a potent activity which maybe double the number of connections linking C-20 to C-16 in sarpagine-type indoles, compared with compound 284 [63].
Figure 24.
Compounds 284–303.
Three compounds, 11-hydroxyburnamine (288) and rauvoyunnanines A and B (289–290) were identified from Chinese R. yunnanensis [138]. Additionally, fourteen compounds 135 [139], lochnerine (291) [140], serpentinic acid (292) [141], reserpine (293) [142], (−)-yohimbine (294) [143], ajmaline (295) [143], mauiensine (296) [144], ajmalicine (297) [145], sitsirikine (298) [146], strictosidinic acid (299) [147], caboxine B (300) [148], isocaboxine B (301) [148], spegatrine (302) [149], and 19(S),20(R)-dihydroperaksine (303) [150] (Figure 24) were isolated also from chines R. yunnanensis. Compound 293 displayed a weak cytotoxicity against HT-29 and SW480, with IC50 values = 35.2 and 45.3 μM, respectively. Auranofin was used as a positive control and showed cytotoxicity with IC50 values = 2.5 and 3.9 μM, respectively. Compounds 294 and 299 displayed immunosuppressive activities on human T cell proliferation, with IC50 values = 5.9 and 5.0 μM, respectively. All compounds except 294 and 299 showed weak activities with IC50 values > 50 μM [138]. The metabolites were identified from genus Rauvolfia and were categorized under the corynanthe-type. The compounds were also classified under three subclasses including: sarpagine-type 284–285, picraline-type 288 and ajmaline-type 295–296 and 298 [138].
5. Ervatamia
The genus Ervatamia contains 120 species. It is distributed in Asia and Australian. Of which, fifteen species and five varieties are grown in south China. Ervatamia is a rich source of iboga-type MIAs, which is characterized by structural novelty and biological diversity including neuroprotective, anti-tumor, and anti-addiction activities [151,152,153].
Six Iboga-type compounds: ervataine (304) [151], ibogaine (305) [154], coronaridine (306) [49], heyneanine (307) [155], voacangine hydroxyindolenine (308) [156,157] and coronaridine hydroxyindolenine (309) [158,159] (Figure 25), were obtained from the Chinese Ervatamia yunnanensis [151].
Figure 25.
Compounds 304–318.
Compound 306 exhibited significant protective effects toward MPP+ (1-methyl-4-phenylpyridinium) and induced damage in primary cortical neurons with an IC50 = 12.5 μM. Parkinson’s disease (PD) is caused by MPP+ a toxic agent that interferes with the function of mitochondria, thus causing neuronal damage and death. Brain-derived neurotrophic factor (BDNF) was used as a positive control and showed an inhibitory effect, with an IC50 value = 200 ng/mL [49].
Eight compounds, coronaridine (306) [49], coronaridine hydroxyindolenine (309) [158,159], 10-hydroxycoronaridine (310) [160], voacangine (311) [153], 19(S)-heyneanine (312) [160], 19(R)-heyneanine (313) [161], heyneanine hydroxyindolenine (314) [162], and vobasine (315) [163], were identified from the stems of E. hainanensis. Compounds 306, 309–315 displayed acetylcholinesterase inhibitory activities. Compounds 306 and 311 displayed a potent cholinesterase inhibitory effect, with IC50 values = 8.6 and 4.4 μM, respectively. Galantamine was used as a reference drug, with an IC50 = 3.2 μM, that is used for Alzheimer’s disease [164]. Compound 310 possessed a hydroxyl group at the phenyl moiety, which was replaced by proton in compound 306. This led to a decrease in the inhibitory activity of AChE in 306 compared to 310. The methoxy group at the phenyl moiety in 311, led to an improvement in the activity. This indicated that the electron-donor substituents attached at the phenyl group were important for the improvement of AChE inhibition [164].
Ervachinine E (316) [165] and rutaecarpine (317) [166] were isolated from E. chinensis [165]. It displayed moderate antitumor activities against HL-60, SMMC-7721, A-549, and SW480 cancer cells, with values of IC50 ranging between 6.59 and 14.70 μM. (Cisplatin, IC50 values between 1.00 and 26.75 μM) [165].
The compound Ervahainine A (318), an oxindole derivative that is cyano-substituted, was identified from the twigs and leaves of E. hainanensis [167]. Compound 318 showed growth inhibitory activities toward HepG2 and HepG2/ADM cells with IC50 values of 12.47 ± 0.24 and 17.68 ± 0.31 μM [167].
Seven new iboga-type derivatives: ervaoffines A–D (319–322), (7S)-3-oxoibogaine hydroxyindolenine (323), ibogaine-5,6-dione (324), and 19-epi-5-oxovoacristine (325), along with ten compounds, 305, 307, 311, iboluteine (326) [168], (7S)- ibogaine hydroxyindolenine (327) [157], ibogaline (328) [169], conopharyngine (329) [170], voacristine (330) [171], 19S-hydroxyibogamine (331) [172], and ibogaine N4-oxide (332) [173,174] (Figure 26), were isolated from the twigs and leaves of E. officinalis.
Figure 26.
Compounds 319–332.
Seven compounds, 3-oxo-7R-coronaridine hydroxyindolenine (333), 3S-cyano-7S-coronaridine hydroxyindolenine (334), 3R-hydroxy-7S-coronaridine hydroxyindolenine (335), 3S-(24S-hydroxyethyl)-coronaridine (336), 3S-(24R-hydroxyethyl)-coronaridine (337), 5-oxo-6S-hydroxycoronaridine (338) and 5-oxo-6S-methoxy-coronaridine (339) [175], along with six others, 306, 7S-coronaridine hydroxyindolenine (340) [176], 3-oxo-7S-coronaridine hydroxylindolenine (341) [177], 5-oxocoronaridine (342) [177], 3-oxocoronaridine (343) [178] and pseudoindoxyl coronaridine (344) [177], (Figure 27) from identified from twigs and leaves of E. hainanensis [175].
Figure 27.
Compounds 333–353.
Another study on the twigs and leaves of E. officinalis led to the reporting of three MIAs, ervaoffines E–G (345–347) [179], and six compounds 306, 342, lirofoline A (348), lirofoline B (349) [172], 6-oxo-ibogaine (350) [180], and 8-oxo-ibogaine lactam (351) [179,180,181]. Compound 347 showed a significant neuroprotective effect towards damage induced by oxygen-glucose deprivation (OGD) of the cortical neurons cultured of ischemic stroke in vitro, with an IC50 = 100 μM, Neuroserpin was used as a reference drug, with an IC50 = 20 ng/mL [179]. Two compounds were obtained from the roots of E. chinensis, erchinines A and B (352,353) [63]. Both compounds 352 and 353 displayed a potent significant antibacterial activity toward Bacillus subtilis which was better than that of the antibacterial drugs fibraurtine with an MIC = 25 μM and berberine with an MIC = 12.5 μM that are derived from plant. Additionally, compound 352 displayed an equal antifungal effect against (Trichophyton rubrum) to the reference drug griseofulvin, with an MIC = 6.25 μM.
Ervapandine A (354) [182], 3R-hydroxyibogaine (355) [182], and 12-hydroxyakuammicine N4-oxide (356) [182], along with four known ones, 313, 305, 19-epi-voacristine (357) [183], taberdivarine I (358) [184] and 12-hydroxyakuamicine (359) [185], (Figure 28) were identified from the leaves and twigs of Chinese E. pandacaqui [182].
Figure 28.
Compounds 354–369.
Liu et al. (2018) [186] studied the roots of E. divaricate and identified two unprecedented trimeric MIAs, Ervadivamines A (360) and B (361), together with the dimeric compound, 19,20-dihydroervahanine A (362), (Figure 29) and two monomeric ones, ibogaine (305) and Ibogamine (363) [187]. Compound 359 displayed a moderate cytotoxic effect against MCF-7, with an IC50 value = 33.61 μM [182]. Compound 360 showed a significant positive cytotoxicity against MCF-7, A-549, HT-29 and HepG2/ADM and showed potent effect against HepG2/ADM, with an IC50 value = 12.55 ± 0.54 μM (Adriamycin, IC50 = 45.70 ± 2.15 μM) [186].
Figure 29.
Compounds 370–384.
Two pair of MIAs epimers composed of, ervatamine (364), [188] 20-epi-ervatamine (365), [188] dregamine (366), and [188] tabernaemontanine (367) [188] and two compounds, apparicine (368) [189] and isovoacangine (369) [190], were isolated from E. yunnanensis [191].
The Ervatamia genus is known to produce iboga-type indole derivatives, which contain two subclasses, flabelliformide-type (364, 365) and apparicine-type (368) (Figure 28), with compounds belongonging to the main class corynathe. The iboga-type showed an interesting bioactivity in the nervous system.
6. Tabernaemontana
The Genus Tabernaemontana (subfamily Rauvolfioideae) contains 110 species, which are distributed throughout tropical and subtropical regions. Thirty species are grown in Brazil, whereas, 44 species were grown in America and the rest in different places around the world. Traditionally, the plants of this genus have been used for the treatment of hypertension, sore throat, and abdominal pain [6,192]. A review article entitled “Brazilian Tabernaemontana genus: indole compounds and phytochemical activities” activities was published in 2016 [6]. It concerned in the monomeric and dimeric MIAs reported from the genus. A review article entitled: A review on tabernaemontana spp.: Multipotential medicinal plant, shows the MIAs reported from this genus until 2015 [6].
Conodusine A-E (370–374), apocidine A (375) and B (376), conoduzidine A (377), tabernamidine A (378) and B (379) (Figure 29) were isolated from the Malaysian stem-bark of Tabernaemontana corymbose malaysian [193]. Additionally, thirty-two compounds were also identified from the same plant, including 307, 314, 338, (+)-catharanthine (380), tabernamine (381) [194], 19′(S)-hydroxytabernamine (382) [195], and 19′(R)-hydroxytabernamine (383) [195]. 16′-decarbomethoxyvoacamine (384) [180] (Figure 29). The chemical structures were determined based on analysis of the NMR and MS spectral data. However, compounds 370, 372, 374, 375 and 377 were confirmed by X-ray diffraction analyses. 371 and 371 belong to iboga alkaloids and tabernamidine B is an iboga-containing bisindole. Tabernamidine B (379) is notable for the presence of an α-substituted acetyl group at C-20 of the iboga carbon skeleton. The absolute configuration of (+)-conodusine E was based on an analysis of the ECD data in correlation with (−)-heyneanine and X-ray analysis. Compounds 381–384 exhibited growth inhibitory effects against drug-sensitive KB/S, with an IC50 value < 4.7 μM and vincristine-resistant (KB/VJ300) cells with an IC50 value < 4.2 μM. For that type of human oral cancer cell lines, vincristine was used as a reference drug with an IC50 value < 1.8 nM [193].
Two compounds, isoakuammiline (385) and 18-hydroxypseudovincadifformine (386) [196], have been reported from the American fruits of T. litoralis. Additionally, five compounds 3,19-oxidocoronaridine (387) [196], strictosidine (388) [196], 306, heyneanine 307, and tabersonine (17), have been identified from the same species [196]. Strictosidine is the major alkaloid in fruit arils, however in the capsule strictosidine it was converted to mainly iboga and pseudoaspidosperma alkaloids. However, in seeds, strictosidine was converted to both iboga and aspidosperma alkaloids, but the only major iboga alkaloid, coronaridine, was not substituted, whereas in fruit capsule coronaridine was oxidized to form heyneanine and 3,19-oxidocoronaridine.
Tabervarines A (389) and B (390) [197], 311, 369, vobasidine C (391) [198], 311, 368, ervadivaricatine B (392) [187], pedunculine (393) [199], tabernaemontanine (367) [198] and polyervine (394) [200] were published from the twigs and leaves of the Chinese T. divaricate (Figure 30). Compounds 388 and 389 exhibited a weak cytotoxic effect against cancer MCF-7, SMMC-7721, HL-60, A-549, and SW480 cells at a value > 40 μM [197].
Figure 30.
Compounds 385–394.
Four new bisindole compounds, flabellipparicine (395), 19,20-dihydrovobparicine (396), 10′- demethoxy-19,20-dihydrovobatensine D (397) and 3′-(2-oxopropyl)ervahanine A (398) [201], together with ten known compounds, 381, 368, ervahanine A (399) [202], vobparicine (400) [203], 19,20-dihydrotabernamine (401) [204], 19,20-dihydrotabernamine A (402) [205], taberdivarine E (403) [184], tubotaiwine (404) [206], hydroxy-3-(2-oxopropyl)coronaridineindolenine (405) [204], and deoxytubulosine (406) [201] (Figure 31) were identified from the stems of T. divaricate. Compounds 368, 395–403 and 406 exhibited cytotoxic activities against MCF-7 and A-549 with IC50 values < 8.1 μM. Compound 406 exhibited the highest effects against MCF-7 and A-549 with IC50 values of 0.1 and 0.2 nM, respectively. 7-ethyl-10-hydroxycamptothecin (SN38) was employed as a positive control and showed cytotoxic effect, with an IC50 value < 2 nM [201]. The presence of β-carboline benzoquinolizidine nucleus played an important role in increasing the cytotoxicity in 406, whereas, compounds (368 and 395–403) possessed two NH indolic group [201].
Figure 31.
Compounds 395–406.
(3R,7S,14R,19S,20R)-19-hydroxypseudovincadifformine (407) [207], 17-demethoxy-hydroisorhyn chophylline (408) [208], 17-demethoxy-isorhynchophylline (409) [208], voachalotine (410) [171], 12-methoxyl-voaphylline (411) [209], and conophylline (412) [209] (Figure 32) were isolated from the branches and leaves of Chinese T. bufalina. Compound 412 showed potent cytotoxic activities against B16 and MDA-MB-231 cells with IC50 values of 0.13 and 8.9 μM, respectively. Gambogic acid was used as a positive control with IC50 values 22.1 and 13.5 μM, respectively [207].
Figure 32.
Compounds 407–417.
Two compounds, 5,6-dioxo-11-methoxy voacangine (413), and (−)-apparicin-21-one (414), and heyneanine (307), were identified from the fruits of cameroonean T. contorta [210] lipopolysaccharides (LPS)-stimulated RAW 264.7 macrophage cells. BAY 11-7082 was used as positive control with 10 μM [210]. Tabernabovines A–C (415–417) were isolated from T. bovina [211]. Compound 415 displayed potent inhibitory activity of NO production in LPS-stimulated RAW 264.7 macrophages with IC50 value 44.1 value μM. l-NMMA was used as a positive control and showed an inhibitory effect with IC50 value = 48.6 μM [211].
Previous studies have proven that various bisindole compounds have more effect than monomeric indole compounds, including the dimeric indoles such as (euburnane–aspidospermatan, euburnane–ibogan, akuammidine–ibogan, aspidospermatan– aspidospermatan and vobasine–strychnan) type compounds. Interestingly, dimeric indoles showed more cytotoxicity than the monomeric units.
The Tabernaemontana genus produced iboga type indoles, which contained four subclasses, such as vincamine-type, apparicine-type and akuammidine, these compounds which belongs to the main class aspidosperma and corynanthe, respectively.
7. Rhazya
Rhazya comprises two species, Rhazya stricta (R. stricta) and Rhazya orientalis (R. stricta) [212]. R. orientalis grown in western Thrace and northeastern Turkey [213] whereas, R. stricta is grown in South Asia (Afghanistan, Pakistan and India) and on the Arabian Peninsula (Saudi Arabian, Qatar, UEA, Iraq) and Iran. Rhazya is a rich source of indole-containing compounds. Traditionally, it is has been used to cure various diseases, such as fever, rheumatism, inflammation, skin infections, sore throat, diabetes, and stomach disorders. For example, strictanol, sewarine, tetrahydrosecamine vallesiachotamine and tetrahydrosecaminediol exhibit anticancer properties [213,214,215,216,217,218]. A recent study on the aerial parts of R. stricta by Ahmad et al. [215], several MIAs were isolated including, three new, secopleiocarpamine A (418), 16,17-Epoxyisositsirikine (419), and 2-Ethyl-3[2-(3-ethyl-1,2,3,6-tetrahydropyridine)ethyl]-indole (420) [215] (Figure 33), five previously reported compounds from other Apocynaceae genera (126, 127, 133, 298 and 404), and a number of previously isolated MIAs from the same species: 2-ethyl-3[2-(3-ethylpiperidine)ethyl]-indole (421), tetrahydrosecodine (422), 16,17-dihydrosecodine (423) [216], deacetylakuammilin (424) [217], rhazimal (425), strictamine-N-oxide (426) [218], rhazinaline (427) [212], rhazinaline Nb-oxide (428) [219], akuammicine (429) [220], 16R-E-isositsirikine (430) [221], dihydrositsirikine (431) [222], antirhine (432) [129], vincadifformine N(4)-oxide (433) [223], eburenine (434) [93], winchinine B (435), quebrachamine (436) [224] and strictanol (437) (Figure 33) [215,225] were isolated from R. stricta. Furthermore, 16-epi-stemmadenine-N-oxide (438) (Figure 33), stemmadenine-N-methyl (439), and 20-epi-antirhine (440) were reported from R. stricta [226]. Additionally, isopicrinine (441) was isolated from the leaves of R. stricta, collected from Bahra, Saudi Arabia [227]. Abdul-Hameed et al. (2021) [228] identified two new indole alkaloids named, epirhazyaminine (442) and 20-epi-sitsirikine (443), together with five known compounds, 430, 432, 434, 437 and strictamine (444) were obtained from the aerial parts of R. stricta, collected from AL-Madinah city, Saudi Arabia [228]. Compounds 418, 422, 428, 432, 434, and 436 exhibited moderate growth inhibitory activities toward Candida strains (C. guilliermondii, C. albicans, C. krusei, C. lusitaniae and C. glabrata) with MIC values ranging from 3.125 to 50 μg/mL. (Amphotericin B, MIC value < 1 μg/mL) [213]. Compound 438 displayed a cytotoxic effect against HCT-116, PC-3, and HepG2, with IC50 values = 2.20, 2.25, and1.9 μM, respectively, (Cisplatin, IC50 values ≤ 0.90 μM). Furthermore, compound 439 significantly hindered of the cancer cells to migration and preventing the wound healing at 24 and 48 h (from 81 and 77% to 68 and 46%, respectively). It also inhibited proliferation and prevented cell migration of all cancer cell was evaluated, with an IC50 = 70 μM [223]. Compound 441 displayed a potent cytotoxic effect towards MCF-7, with an IC50 value = 240 μM [224]. Compounds 430, 432, 434, 437, and 442–444 displayed weak activities against three cancer cell lines (HCT-116, PC-3, and HepG2), with IC50 in the range of 45.0 ± 0.012 and 85.0 ± 0.068 μM against HCT-116, IC50 in the range 39.0 ± 0.012 and 87.0 ± 0.068 μM against PC-3, and IC50 in the range 72.0 ± 0.164 and 87.0 ± 0.032 μM against HepG-2μM) against HepG-2 [225]. The Rhazya genus contains many MIAs subclasses, such as secodine-type (420–424), akuammiline-type (426), akummicine-type (428) and picrinine-type (441), (Figure 3), with compounds belonging to the main classes aspidosperma and corynanthe.
Figure 33.
Compounds 418–444.
8. Biosynthesis of Monoterpenoid Indole Alkaloids
Monoterpenoidal indoles are obtained from the reaction of tryptamine with secologanin terpenoid. Condensation of tryptamine with Secologanin produces strictosidine by the Mannich-link reaction. The deglycosylation of strictosidine converts it to a hemiacetal. Opening the hemiacetal led to forming an aldehyde group, which then reacts with the (N-4) secondary amine of strictosidine to form 4,21-dehydrocorynanthenine. Allylic isomerization moves the double bond of vinyl to a conjugation with iminium nitrogen that generates dehydrogeissoschizine, which is then cyclized to form cathenamine. The reduction of cathenamine in the presence of NADPH forms ajmalicine (corynanthe-type) [229].
The formation of Preakuammicine occurs from dehydrogeissoschizine. Preakuammicine intermediate (strychnos-type) is the common precursor of the strychnos, aspidosperma and iboga indole alkaloids. Preakuammicine reduced to form stemmadenine, then rearranged to form the acrylic ester dehydrosecodine, which is a common intermediate for iboga and aspidosperma skeletons. Tabersonine (aspidosperma type) and catharanthine (iboga type) are formed the Diels-Alder reaction (Scheme 1) [229].
Scheme 1.
Biosynthesis of corynanthe, aspidosperma and iboga indoles.
Polyneuridine aldehyde (sarpagan type) is an intermediate compound of the ajmaline pathway. The possibility of a mechanism where the sarpagan bridge enzyme converts an isomer of 4,21-dehydrogeissoschizine to polyneuridine aldehyde is shown (Scheme 2). Polyneuridine aldehyde methyl ester is hydrolyzed by polyneuridine aldehyde esterase, generating an acid which decarboxylates, to yield epi-vellosamine. Epi-vellosamine transforms to the ajmaline alkaloid vinorine. The hydroxylation of vinorine to vomilene is caused by the vinorine hydroxylase enzyme. After formation of vomilene, two step reduction occurs. First, the indolenine bond is reduced by an NADPH enzyme to yield 1,2-dihydrovomilenene. The second step, reducing the 1,2-dihydrovomilenene to acetylnorajmaline by a 1,2-dihydrovomilenene reductase enzyme. The acetyl linkage of acetylnorajmaline is hydrolyzed by acetylesterase to yield norajmaline. Finally, the production of ajmaline by N-methyl transferase of a methyl group at the indole nitrogen of norajmaline occurs (Scheme 2) [229,230].
Scheme 2.
Biosynthesis of ajmaline indole alkaloids. (SB) Sarpagan bridge enzyme; polyneuridine aldehyde reductase (PNAE), vinorine synthase (VS), vinorine hydroxylase (VH), vomilenine reductase (VR), dihydrovomilenine reductase (DHVR) 17-O-acetyl-ajmalanesterase (AAE), norajmaline-N-methyltransferase (NMT).
It is noteworthy to mention that, sarpagine, ajmaline, and macroline alkaloids are biosynthetically similar or all derived from the same origin. Whereas, sarpagine can be converted into macroline by means of Michael addition [231], on the other hand macroline can be converted into sarpagine by through a retro-Michael reaction [231,232,233]. Similarly, some sarpagine-containing alkaloids can be converted into ajmalines under strong acidic conditions, which refers to the great similarity between them [233].
9. Conclusions and Future Prospectives
Natural products have an unprecedented molecular conformity with a diversity of functionalities. These characteristics enable them to produce biological effects, which validates the initial step for a drug lead. In recent years, the majority of new drugs reported have been natural or originated from natural sources. Alkaloids are an important source of drugs. It is noteworthy that, many alkaloids displaying fascinating molecular structures with diverse physiological and pharmacological effects have been isolated from plant families. The Apocynaceae family has been noted as a unique producer of biologically active natural metabolites such as vincristine, vinblastine, reserpine and yohimbine. This review is interested in discussing the metabolites produced from six genera belong to the family Apocynaceae. These six genera contain 400 species, which represent 20% of the Apocynaceae family. Only 30 species, which represent 7.5% of the total species of the six genera were studied. Chemical investigation of these genera led to the reporting of 444 MIAs, in the period between 2010 until December 2020, which were discussed in this review.
Figure 34 illustrates the number of compounds isolated from the six species; there are 157 (35.4%), 126 (28.4%), 66 (14.9%), 48 (10.8%), 27 (6.1%), and 20 (4.4 %), from Alstonia, Kopsia, Ervatamia, Tabernaemontana, Rhazya and Rauvolfia, respectively. We believe that the six genera are interesting candidate for further investigation. This record coincided with the data illustrated in Figure 35. For example, Alstonia scholaris is a species that belongs to the genus Alstonia that has produced the highest number of MIAs (71 compounds) and represents 45.2 % of the MITs identified from the same genus between 2010 and 2020. The second and third most interesting species are Kopsia officinalis and Kopsia pauciflora which produced 45 and 27 compounds, respectively. These two species represent 35.7% and 21.4% of the total compounds produced from the genus Kopsia. The fourth most interesting species belong to the genus Alstonia (Alstonia mairei), which produced 26 compounds and represents 16.5 % of the MITs identified from the genus Alstonia.
Figure 34.
Number of compounds isolated from the six genera.
Figure 35.
Percentage of reported compounds from the species.
It is interesting that the majority of compounds were isolated from twigs and leaves as illustrated in Figure 36. Additionally, the majority of the examined species belonging to the selected six genera were Chinese species and led to the identification of 360 compounds.
Figure 36.
Number of compounds identified from different organs.
Figure 37 presents the biological activities of the compounds. The prominent activity was cytotoxicity followed by anti-inflammatory and antimicrobial activities. Thus, these compounds could be a source of anticancer drugs.
Figure 37.
Number of compounds versus biological activities.
The family of terpene indole alkaloids has been discovered for over a century. There are numbers of total syntheses studies of these intricate scaffolds have been achieved. Additionally, several reviews and book chapters, as well as the references therein, are interested in the synthetic efforts have been reported.
Acknowledgments
The authors acknowledge with thanks Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, for funding through the Fast-track Research Funding Program.
Author Contributions
Conceptualization, W.M.A., A.A.-L. and Z.H.A.-H.; resources, A.E.M., M.O.A. and N.O.B.; data curation, Z.H.A.-H., W.M.A. and A.A.-L.; writing—original draft prepa-ration, Z.H.A.-H., W.M.A. and A.A.-L.; writing—review and editing, Z.H.A.-H., W.M.A. and A.A.-L.; supervision, T.R.S.; funding acquisition, A.E.M. and M.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah Bint Abdulrahman University through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
| A431 | Dermatoma cancer |
| A-549 | Lung cancer |
| AChE | Acetylcholinesterase |
| B16F10 | Melanogenesis activity |
| BEN-MEN-1 | Meningioma |
| BGC-823 | Human gastric carcinoma |
| CAL-27 | Head and neck squamous cell carcinomas |
| CCF-STTG1 | Astrocytoma |
| CHG-5 | Glioma |
| CI | Confidence intervals |
| Detroit-562 | Head and neck squamous cell carcinomas |
| ED50 | Median effective dose |
| F.sp. | Forma specialis, abbreviated f. sp., is an informal taxonomic grouping allowed by the International Code of Nomenclature for algae, fungi, and plants |
| HCT 116 | Human colorectal carcinoma |
| HeLa | Human Gastric cancer |
| Hep-2 | Head and neck squamous cell carcinomas |
| HepG2 | Human hepatocellular |
| HIF-α | Hypoxia-inducible factor |
| HL-60 | Human myeloid leukemia |
| HS-1 | Dermatona cancer |
| HS-4 | Dermatona cancer |
| HT-29 | Human colorectal carcinoma |
| IC50 | Half maximal inhibitory concentration |
| ID50 | Median infective dose |
| IL-1β | Interleukin 1 beta |
| LNCaP | Human prostate carcinoma |
| M663 | Osteosarcoma cells |
| MCF-7 | Human breast cancer |
| MDA-MB-231 | Human breast adenocarcinoma |
| MG-63 | Osteosarcoma cells |
| MIAs | Terpenoid indole compounds |
| MIAs | Monoterpenoid indole compounds |
| MIC | Minimum inhibitory concentration |
| NF-kB | Nuclear factor k-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| PANC-1 | Pancreatic cancer |
| PC-3 | Human prostate carcinoma |
| PGE2 | Prostaglandin E2 |
| SAOS-2 | Osteosarcoma cell lines |
| SCC-PKU | Head and neck squamous cell carcinomas |
| SCL-1 | Head and neck squamous cell carcinomas |
| SGC-7901 | Gastric cancer |
| SHG-44 | Human glioma cancer |
| SK-BR-3 | Human breast cancer |
| SK-MEL-2 | Human skin cancer |
| SMMC-7721 | Hepatocellular carcinoma |
| SOSP-9607 | Human Osteosarcoma cell lines |
| SW480 | Human Colon cancer |
| TCA-83 | Head and neck squamous cell carcinomas |
| TNF-α | Tumor necrosis factor-α |
| U251 | Human glioma cancer |
| U2-OS | Osteosarcoma cell lines |
| UMSCC-1 | Head and neck squamous cell carcinomas |
References
- 1.Dey A., Mukherjee A., Chaudhury M. Alkaloids from Apocynaceae: Origin, pharmacotherapeutic properties, and structure-activity studies. Stud. Nat. Prod. Chem. 2017;52:373–488. [Google Scholar]
- 2.Mondal A., Gandhi A., Fimognari C., Atanasov A.G., Bishayee A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019;858:172472. doi: 10.1016/j.ejphar.2019.172472. [DOI] [PubMed] [Google Scholar]
- 3.Rosales P.F., Bordin G.S., Gower A.E., Moura S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia. 2020;143:104558. doi: 10.1016/j.fitote.2020.104558. [DOI] [PubMed] [Google Scholar]
- 4.Marcos I.S., Moro R.F., Costales I., Basabe P., David D. Sesquiterpenyl Indoles. Nat. Prod. Rep. 2013;30:1509–1526. doi: 10.1039/c3np70067d. [DOI] [PubMed] [Google Scholar]
- 5.De Luca V., Salim V., Thamm A., Masada S.A., Yu F. Making iridoids/secoiridoids and monoterpenoid indole alkaloids: Progress on pathway elucidation. Curr. Opin. Plant. Biol. 2014;19:35–42. doi: 10.1016/j.pbi.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Marinho F.F., Simões A.O., Barcellos T., Moura S. Brazilian Tabernaemontana genus: Indole alkaloids and phytochemical activities. Fitoterapia. 2016;114:127–137. doi: 10.1016/j.fitote.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang C.H., Wang G.C., Wang Y., Zhang X.Q., Huang X.J., Zhang D.M., Chen M.F., Ye W.C. Cytotoxic dimeric indole alkaloids from Catharanthus roseus. Fitoterapia. 2012;83:765–769. doi: 10.1016/j.fitote.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Hanafy M.S., Matter M.A., Asker M.S., Rady M.R. Production of indole alkaloids in hairy root cultures of Catharanthus roseus L. and their antimicrobial activity. South. African J. Bot. 2016;105:9–18. doi: 10.1016/j.sajb.2016.01.004. [DOI] [Google Scholar]
- 9.Le Men J., Taylor W.I. A uniform numbering system for indole alkaloids. Experientia. 1965;21:508–510. doi: 10.1007/BF02138961. [DOI] [PubMed] [Google Scholar]
- 10.Facchini P.J. Regulation of alkaloid biosynthesis in plants. In the alkaloids. Chem. Biol. 2006;63:1–44. doi: 10.1016/s1099-4831(06)63001-0. [DOI] [PubMed] [Google Scholar]
- 11.El-Sayed M., Verpoorte R. Catharanthus terpenoid indole alkaloids: Biosynthesis and regulation. Phytochem. Rev. 2007;6:277–305. doi: 10.1007/s11101-006-9047-8. [DOI] [Google Scholar]
- 12.Zhu G.Y., Yao X.J., Liu L., Bai L.P., Jiang Z.H. Alistonitrine A, a caged monoterpene indole alkaloid from Alstonia scholaris. Org. Lett. 2014;16:1080–1083. doi: 10.1021/ol403625g. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X., Zeng X., Sun C., Chen S. Biosynthetic pathway of terpenoid indole alkaloids in Catharanthus roseus. Front. Med. 2014;8:285–293. doi: 10.1007/s11684-014-0350-2. [DOI] [PubMed] [Google Scholar]
- 14.Wong S.K., Lim Y.Y., Ling S.K., Wei E., Chan C. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacognosy Res. 2014;6:67–72. doi: 10.4103/0974-8490.122921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endress M.E., Bruyns P.V. A revised classification of the Apocynaceae sl. Botanical Rev. 2000;66:1–56. doi: 10.1007/BF02857781. [DOI] [Google Scholar]
- 16.Góngora-Castillo E., Childs K.L., Fedewa G., Hamilton J.P., Liscombe D.K., Magallanes-Lundback M., Mandadi K.K., Nims E., Runguphan W., Vaillancourt B., et al. Development of transcriptomic resources for interrogating the biosynthesis of monoterpene indole alkaloids in medicinal plant species. PLoS ONE. 2012;7:e52506. doi: 10.1371/journal.pone.0052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaushik N.K., Kaushik N., Attri P., Kumar N., Kim C.H., Verma A.K., Choi E.H. Biomedical importance of indoles. Molecules. 2013;18:6620–6662. doi: 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai X., Du Z., Luo X. Unique monoterpenoid indole alkaloids from Alstonia scholaris. Org. Lett. 2007;9:1817–1820. doi: 10.1021/ol0705301. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa Y., Arai H., Zaima K., Oktarina R., Rahman A., Ekasari W., Widyawaruyanti A., Indrayanto G., Zaini N.C., Morita H. Alstiphyllanines A-D, indole alkaloids from Alstonia macrophylla. J. Nat. Prod. 2009;72:304–307. doi: 10.1021/np8007107. [DOI] [PubMed] [Google Scholar]
- 20.Adotey J.P.K., Adukpo G.E., Opoku B.Y., Armah F.A. A Review of the ethnobotany and pharmacological importance of Alstonia boonei De Wild (Apocynaceae) ISRN Pharmacol. 2012;2012:1–9. doi: 10.5402/2012/587160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai X., Zeng C., Feng T., Li Y., Luo X. Monoterpenoid indole alkaloids from Alstonia mairei. Helv. Chim. Acta. 2010;93:2037–2044. doi: 10.1002/hlca.201000040. [DOI] [Google Scholar]
- 22.Ahond A., Janot M.-M., Langlois N., Lukacs G., Potier P., Rasoanaivo P., Sangare´ M., Neuss N., Plat M., Le Men J., et al. Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances. XXIII. structure of vindolinine. Am. Chem. Soc. 1974;96:633–634. doi: 10.1021/ja00809a078. [DOI] [Google Scholar]
- 23.Dçpke W., Meisel H., Grndemann E. Stereochemie des 16-methoxyminovincinins. Tetrahedron Lett. 1971;12:1287–1290. [Google Scholar]
- 24.Majumder P.L., Basu A. Absolute-configuration at C-19 of 19-hydroxy acyloxy-(+)-vincadifformine type of alkaloids. Indian J. Chem. Sect. B- Org. Chem. Incl. Med. Chem. 1985;24:649–650. [Google Scholar]
- 25.Majumbar P.L., Joardar S., Chanda T.K., Dinda B.N., Banerjee M., Ray A.B., Chatteriee A. Structures and absolute sterochemistry of (-)-echitoveniline, (-)-11-methoxyechitoveniline and (-)-11-methoxyechitovenedine - new indole alkaloids of Alstonia venenata R.Br. Tetrahedron. 1979;35:1151–1157. [Google Scholar]
- 26.Majumder P.L., Chanda T.K., Dinda B.N. Structure of echitovenaldine: A new alkaloid of the leaves of Alstonia veneneta R. Br. Chem. Ind. L. 1973;21:1032–1033. [Google Scholar]
- 27.Das B., Biemann K., Chatterjee A., Ray A.B., Majumder P. The alkaloids of the fruits of Alstonia Venenata R. Br. echitovenidine and (+)-minovincinine. Tetrahedron Lett. 1966;22:2483–2486. doi: 10.1016/S0040-4039(00)75680-2. [DOI] [Google Scholar]
- 28.Majumder P.L., Dinda B.N. Echitoserpidine: A new alkaloid of the fruits of Alstonia venenata. Phytochemistry. 1974;13:645–648. doi: 10.1016/S0031-9422(00)91368-2. [DOI] [Google Scholar]
- 29.Majumder P.L., Joardar S., Dinda B.N., Bandyopadhyay D., Basu A. Structure and absolute stereochemistry of 19-epi-(+)-echitoveniline: A new Indole alkaloid of the leaves of Alstonia venenata r. Br. Tetrahedron. 1981;37:1243–1248. doi: 10.1016/S0040-4020(01)92058-7. [DOI] [Google Scholar]
- 30.Moza B.K., Trojanek J., Bose A.K., Das K.G., Funke P. The structure of lochnericine and lochnerinine. Tetrahedron Lett. 1964;5:2561–2566. [Google Scholar]
- 31.Ulshafer P.R., Bartlett M.F., Dorfman L., Gillen M.A., Schlittler E., Wenker E. Isolation and structure of perakine. Tetrahedron Lett. 1961;2:363–367. doi: 10.1016/S0040-4039(01)91640-5. [DOI] [Google Scholar]
- 32.Abe F., Chen R.F., Yamauchi T., Marubayashi N., Ueda I. Alschomine and isoalschomine, new alkaloids from the leaves of Alstonia scholaris. Chem. Pharm. Bull. 1989;37:887–890. doi: 10.1248/cpb.37.887. [DOI] [Google Scholar]
- 33.Chen W., Yan Y.P., Wang Y.J., Liang X.T. Isolation and identification of three new alkaloids from roots of Alstonia yunnanensis. Acta Pharm. Sin. (Yaoxuexuebao) 1985;20:906–912. [PubMed] [Google Scholar]
- 34.Evans D.A., Joule J.A., Smith G.F. The alkaloids of Rhazya orientalis. Phytochemistry. 1968;7:1429–1431. doi: 10.1016/S0031-9422(00)85654-X. [DOI] [Google Scholar]
- 35.Ahmad Y., Fatima K., Le Quesne P.W., Atta-ur-Rahman Further alkaloidal constituents of the leaves of Rhazya stricta. Phyrochemistry. 1983;22:1017–1019. doi: 10.1016/0031-9422(83)85045-6. [DOI] [Google Scholar]
- 36.Koyama K., Hirasawa Y., Nugroho A.E., Hosoya T., Hoe T.C., Chan K., Morita H. Alsmaphorazines A and B, indole alkaloids from Alstonia pneumatophora. Org. Lett. 2010;12:4188–4191. doi: 10.1021/ol101825f. [DOI] [PubMed] [Google Scholar]
- 37.Cai X., Bao M., Zhang Y., Zeng C., Liu Y., Luo X. A new type of monoterpenoid indole alkaloid precursor from Alstonia rostrata. Org. Lett. 2011;13:3568–3571. doi: 10.1021/ol200996a. [DOI] [PubMed] [Google Scholar]
- 38.Bao M., Zeng C., Qu Y., Kong L., Liu Y., Cai X., Luo X. Monoterpenoid indole alkaloids from Alstonia rostrata. Nat. Prod. Bioprospect. 2012;2:121–125. doi: 10.1007/s13659-012-0019-y. [DOI] [Google Scholar]
- 39.Cao P., Liang Y., Gao X., Li X., Song Z., Liang G. Monoterpenoid indole alkaloids from Alstonia yunnanensis and their cytotoxic and anti-inflammatory activities. Molecules. 2012;17:13631–13641. doi: 10.3390/molecules171113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyama K., Hirasawa Y., Nugroho A.E., Kaneda T., Hoe T.C., Chan K.-L., Morita H. Alsmaphorazines C-E, indole alkaloids from Alstonia pneumatophora. Teonhedron. 2012;68:1502–1506. doi: 10.1016/j.tet.2011.12.014. [DOI] [Google Scholar]
- 41.Wang W., Cheng M.H., Wang X.H. Monoterpenoid indole alkaloids from Alstonia rupestris with cytotoxic, anti-inflammatory and antifungal activities. Molecules. 2013;18:7309–7322. doi: 10.3390/molecules18067309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng T., Cai X.H., Zhao P.J., Du Z.Z., Li W.Q., Luo X.D. Monoterpenoid indole alkaloids from the bark of Alstonia scholaris. Planta Med. 2009;75:1537–1541. doi: 10.1055/s-0029-1185900. [DOI] [PubMed] [Google Scholar]
- 43.Yang X., Qin X., Zhao Y., Lunga P.K., Li X., Jiang S., Cheng G., Liu Y., Luo X. Alstolactines A – C, novel monoterpenoid indole alkaloids from Alstonia scholaris. Tetrahedron Lett. 2014;55:4593–4596. doi: 10.1016/j.tetlet.2014.06.048. [DOI] [Google Scholar]
- 44.Zhang L., Hua Z., Song Y., Feng C. Monoterpenoid indole alkaloids from Alstonia rupestris with cytotoxic, antibacterial and antifungal activities. Fitoterapia. 2014;97:142–147. doi: 10.1016/j.fitote.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Pan L., Terrazas C., Acuña U.M., Ninh T.N., Chai H., Carcache De Blanco E.J., Soejarto D.D., Satoskar A.R., Kinghorn A.D. Bioactive indole alkaloids isolated from Alstonia angustifolia. Phytochem. Lett. 2015;10 doi: 10.1016/j.phytol.2014.06.010. liv–lix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kam T.S., Choo Y.M., Komiyama K. Unusual spirocyclic macroline alkaloids, nitrogenous derivatives, and a cytotoxic bisindole from Alstonia. Tetrahedron. 2004;60:3957–3966. doi: 10.1016/j.tet.2004.03.031. [DOI] [Google Scholar]
- 47.Kam T.S., Iek I.H., Choo Y.M. Alkaloids from the stem-bark of Alstonia macrophylla. Phytochemistry. 1999;51:839–844. doi: 10.1016/S0031-9422(99)00044-8. [DOI] [Google Scholar]
- 48.Bi Y., Zhang L.H., Hamaker L.K., Cook J.M. Enantiospecific synthesis of (-)-alstonerine and (+)-macroline as well as a partial synthesis of (+)-villalstonine. J. Am. Chem. Soc. 1994;116:9027–9041. doi: 10.1021/ja00099a021. [DOI] [Google Scholar]
- 49.Zocoler M.A., De Oliveira A.J.B., Sarragiotto M.H., Grzesiuk V.L., Vidotti G.J. Qualitative Determination of indole alkaloids of Tabernaemontana fuchsiaefolia (Apocynaceae) J. Braz. Chem. Soc. 2005;16:1372–1377. doi: 10.1590/S0103-50532005000800011. [DOI] [Google Scholar]
- 50.Ghedira K., Zeches-Hanrot M., Richard B., Massiot G., Le Men-Olivier L., Sevenet T., Goh S.H. Alkaloids of Alstonia angustifolia. Phytochemistry. 1988;27:3955–3962. doi: 10.1016/0031-9422(88)83053-X. [DOI] [Google Scholar]
- 51.Tan S.J., Lim K.H., Subramaniam G., Kam T.S. Macroline-sarpagine and macroline-pleiocarpamine bisindole alkaloids from Alstonia angustifolia. Phytochemistry. 2013;85:194–202. doi: 10.1016/j.phytochem.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Liu L., Chen Y., Qin X., Wang B., Jin Q., Liu Y., Luo X. Antibacterial monoterpenoid indole alkaloids from Alstonia scholaris cultivated in temperate zone. Fitoterapia. 2015;105:160–164. doi: 10.1016/j.fitote.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Qin X., Zhao Y., Lunga P., Yang X., Song C., Cheng G., Liu L., Chen Y., Liu Y.-P., Luo X. Indole alkaloids with antibacterial activity from aqueous fraction of Alstonia scholaris. Tetrahedron. 2015;71:4372–4378. doi: 10.1016/j.tet.2015.04.046. [DOI] [Google Scholar]
- 54.Qin X.J., Zhao Y.L., Song C.W., Wang B., Chen Y.Y., Liu L., Li Q., Li D., Liu Y.P., Luo X.D. Monoterpenoid indole alkaloids from inadequately dried leaves of Alstonia scholaris. Nat. Products Bioprospect. 2015;5:185–193. doi: 10.1007/s13659-015-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X.W., Luo X.D., Lunga P.K., Zhao Y.L., Qin X.J., Chen Y.Y., Liu L., Li X.N., Liu Y.P. Scholarisines H-O, novel indole alkaloid derivatives from long-term stored Alstonia scholaris. Tetrahedron. 2015;71:3694–3698. doi: 10.1016/j.tet.2014.09.052. [DOI] [Google Scholar]
- 56.Kuok C., Zhang J., Fan C., Zhang Q., Fan R., Zhang D., Zhang X., Ye W. Meloslines A and B, Two novel indole alkaloids from Alstonia scholaris. Tetrahedron Lett. 2017;58:2740–2742. doi: 10.1016/j.tetlet.2017.05.094. [DOI] [Google Scholar]
- 57.Edwards P.N., Smith G.F. 287. Akuamma alkaloids. Part I V.The decomposition of akuammicine in methanol. J. Chem. Soc. 1961;152:1458–1462. doi: 10.1039/jr9610001458. [DOI] [Google Scholar]
- 58.Li C., Chen S., Sun C., Zhang L., Shi X., Wu S. Cytotoxic monoterpenoid indole alkaloids from Alstonia yunnanensis Diels. Fitoterapia. 2017;117:79–83. doi: 10.1016/j.fitote.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Yan T., Han D., Hu J., Huang X., Wang H. Monoterpenoid indole alkaloids from Alstonia mairei and their cytotoxicity. J. Asian Nat. Prod. Res. 2017;6020:1–7. doi: 10.1080/10286020.2017.1313242. [DOI] [PubMed] [Google Scholar]
- 60.Koyama K., Hirasawa Y., Zaima K., Hoe T.C., Chan K.L., Morita H. Alstilobanines A-E, new indole alkaloids from Alstonia angustiloba. Bioorganic Med. Chem. 2008;16:6483–6488. doi: 10.1016/j.bmc.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 61.Zhong X.H., Bao M.F., Zeng C.X., Zhang B.J., Wu J., Zhang Y., Cai X.H. Polycyclic monoterpenoid indole alkaloids from Alstonia rostrata and their reticulate derivation. Phytochem. Lett. 2017;20:77–83. doi: 10.1016/j.phytol.2017.04.008. [DOI] [Google Scholar]
- 62.Cai X.H., Tan Q.G., Liu Y.P., Feng T., Du Z.Z., Li W.Q., Luo X.D. A cage-monoterpene indole alkaloid from Alstonia scholaris. Org. Lett. 2008;10:577–580. doi: 10.1021/ol702682h. [DOI] [PubMed] [Google Scholar]
- 63.Yu H.F., Qin X.J., Ding C.F., Wei X., Yang J., Luo J.R., Liu L., Khan A., Zhang L.C., Xia C.F., et al. Nepenthe-like indole alkaloids with antimicrobial activity from Ervatamia chinensis. Org. Lett. 2018;20:4116–4120. doi: 10.1021/acs.orglett.8b01675. [DOI] [PubMed] [Google Scholar]
- 64.Lounasmaa M., Jokela R., Hanhinen P., Miettcnen J., Salo J. The rhazimanine-bhimberine enigma. J. Nat. Prod. 1995;58:131–133. doi: 10.1021/np50115a020. [DOI] [Google Scholar]
- 65.Morita Y., Hesse M., Schmid H., Banerji A., Banerji J., Chatterjee A., Oberhänsli W.E. Alstonia scholaris: Struktur des indolalkaloides Narelin. Helv. Chim. Acta. 1977;60:1419–1434. doi: 10.1002/hlca.19770600434. [DOI] [PubMed] [Google Scholar]
- 66.Goh S.H., Ali A.R.M., Wong W.H. Alkaloids of Leuconotis griffithii and L. eugenifolia (Apocynaceae) Tetrahedron. 1989;45:7899–7920. doi: 10.1016/S0040-4020(01)85802-6. [DOI] [Google Scholar]
- 67.Hu W.L., Zhu J.P., Hesse M. Indole alkaloids from Aistonia angustjfolia. Planta Med. 1989;55:463–466. doi: 10.1055/s-2006-962065. [DOI] [PubMed] [Google Scholar]
- 68.Yamauchi T., Abe F., fu Chen R., Nonaka G.I., Santisuk T., Padolina W.G. Alkaloids from the leaves of Alstonia scholaris in Taiwan, Thailand, Indonesia and the Philippines. Phytochemistry. 1990;29:3547–3552. doi: 10.1016/0031-9422(90)85273-I. [DOI] [Google Scholar]
- 69.Lounasmaa M., Jokela R., Tolvanen A., Kan S.K. A 400 MHz 1H-NMR study of seven sarpagine-type alkaloids. Planta Med. 1985;6:519–521. doi: 10.1055/s-2007-969581. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L., Zhang C.J., Zhang D.B., Wen J., Zhao X.W., Li Y., Gao K. An Unusual indole alkaloid with anti-adenovirus and anti-HSV activities from Alstonia scholaris. Tetrahedron Lett. 2014;55:1815–1817. [Google Scholar]
- 71.Morita H., Ichihara Y., Takeya K., Watanabe K., Itokawa H., Motidome M. A New indole alkaloid glycoside from the leaves of Palicourea marcgravii. Planta Med. 1989;55:288–289. doi: 10.1055/s-2006-962007. [DOI] [PubMed] [Google Scholar]
- 72.Yang J., Fu J., Liu X., Jiang Z., Zhu G. Monoterpenoid indole alkaloids from the leaves of Alstonia scholaris and their NF- κ B inhibitory activity. Fitoterapia. 2018;124:73–79. doi: 10.1016/j.fitote.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Yeap J.S.Y., Navanesan S., Sim K.S., Yong K.T., Gurusamy S., Lim S.H., Low Y.Y., Kam T.S. Ajmaline, oxindole, and cytotoxic macroline-akuammiline bisindole alkaloids from Alstonia penangiana. J. Nat. Prod. 2018;81:1266–1277. doi: 10.1021/acs.jnatprod.8b00170. [DOI] [PubMed] [Google Scholar]
- 74.Yuan Y.X., Guo F., He H.P., Zhang Y., Hao X.J. Two new monoterpenoid indole alkaloids from Alstonia rostrata. Nat. Prod. Res. 2018;32:844–848. doi: 10.1080/14786419.2017.1360886. [DOI] [PubMed] [Google Scholar]
- 75.Ming C.W., Ling Z.P., Ruecker G. Nb-demethylechitamine N-oxide from roots of Winchia calophylla. Planta Med. 1988;54:480–481. doi: 10.1055/s-2006-962521. [DOI] [PubMed] [Google Scholar]
- 76.Keawpradub N., Takayama H., Aimi N., Sakai S.I. Indole alkaloids from Alstonia glaucescens. Phytochemistry. 1994;37:1745–1749. doi: 10.1016/S0031-9422(00)89603-X. [DOI] [Google Scholar]
- 77.Gan L.-S., Yang S.-P., Wu Y., Jian D., Yue J.-M. Terpenoid indole alkaloids from Winchia calophylla. J. Nat. Prod. 2006;69:18–22. doi: 10.1021/np0502701. [DOI] [PubMed] [Google Scholar]
- 78.Hu J., Mao X., Shi X., Jin N., Shi J. Monoterpenoid indole alkaloids from the leaves of Alstonia scholaris. Chem. Nat. Compd. 2018;54:934–937. doi: 10.1007/s10600-018-2516-7. [DOI] [Google Scholar]
- 79.Krishnan P., Lee F.K., Chong K.W., Mai C.W., Muhamad A., Lim S.H., Low Y.Y., Ting K.N., Lim K.H. Alstoscholactine and alstolaxepine, monoterpenoid indole alkaloids with γ-lactone-bridged cycloheptane and oxepane moieties from Alstonia scholaris. Org. Lett. 2018;20:8014–8018. doi: 10.1021/acs.orglett.8b03592. [DOI] [PubMed] [Google Scholar]
- 80.Krishnan P., Mai C., Yong K., Low Y., Lim K. Alstobrogaline, an Unusual pentacyclic monoterpenoid indole alkaloid with aldimine and aldimine- N -oxide moieties from Alstonia scholaris. Tetrahedron Lett. 2019;60:789–791. doi: 10.1016/j.tetlet.2019.02.018. [DOI] [Google Scholar]
- 81.Macabeo A.P.G., Krohn K., Gehle D., Read R.W., Brophy J.J., Cordell G.A., Franzblau S.G., Aguinaldo A.M. Indole alkaloids from the leaves of Philippine Alstonia scholaris. Phytochemistry. 2005;66:1158–1162. doi: 10.1016/j.phytochem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 82.Khyade M.S., Kasote D.M., Vaikos N.P. Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. Ex G. Don: A comparative review on traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2014;153:1–18. doi: 10.1016/j.jep.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 83.Mason J.D., Weinreb S.M. The Alkaloids. Volume 81. Elsevier Inc.; Amsterdam, The Netherlands: 2019. The Alstoscholarisine alkaloids: Isolation , structure determination , biogenesis , biological evaluation , and synthesis; pp. 115–150. [DOI] [PubMed] [Google Scholar]
- 84.Maurya A., Verma S.C., Meena R.A.J., Srivastava A., Shankar M.B., Sharma R.K. Phytochemistry and chromatographic analysis of Alstonia scholaris (L.) R.Br. used as a traditional medicine: A review. World J. Pharm. Res. 2016;5:1503–1519. [Google Scholar]
- 85.Wong S.P., Gan C.Y., Lim K.H., Ting K.N., Low Y.Y., Kam T.S. Arboridinine, a pentacyclic indole alkaloid with a new cage carbon-nitrogen skeleton derived from a pericine precursor. Org. Lett. 2015;17:3628–3631. doi: 10.1021/acs.orglett.5b01757. [DOI] [PubMed] [Google Scholar]
- 86.Cheenprachaa S., Raksata A., Ritthiwigromb T.A., Laphookhieoc S. Monoterpene indole alkaloids from the twigs of Kopsia arborea. Nat. Prod. Commun. 2014;9:1441–1443. doi: 10.1177/1934578X1400901010. [DOI] [PubMed] [Google Scholar]
- 87.Kam T.S., Sim K.M., Koyano T., Komiyama K. Leishmanicidal alkaloids from Kopsia griffithii. Phytochemistry. 1999;50:75–79. doi: 10.1016/S0031-9422(98)00492-0. [DOI] [Google Scholar]
- 88.Lim K.H., Hiraku O., Komiyama K., Koyano T., Hayashi M., Kam T.S. Biologically active indole alkaloids from Kopsia arborea. J. Nat. Prod. 2007;70:1302–1307. doi: 10.1021/np0702234. [DOI] [PubMed] [Google Scholar]
- 89.Tan M.J., Yin C., Tang C.P., Ke C.Q., Lin G., Ye Y. Antitussive indole alkaloids from Kopsia hainanensis. Planta Med. 2011;77:939–944. doi: 10.1055/s-0030-1250631. [DOI] [PubMed] [Google Scholar]
- 90.Gan C., Yoganathan K., Sim K., Low Y., Lim S., Kam T. Corynanthean, eburnan, secoleuconoxine, and pauciflorine alkaloids from Kopsia pauciflora. Phytochemistry. 2014;108:234–242. doi: 10.1016/j.phytochem.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 91.Yap W.S., Gan C.Y., Sim K.S., Lim S.H., Low Y.Y., Kam T.S. Aspidofractinine and eburnane alkaloids from a north Borneo Kopsia. ring-contracted, additional ring-fused, and paucidactine-type aspidofractinine alkaloids from K. pauciflora. J. Nat. Prod. 2015;79:230–239. doi: 10.1021/acs.jnatprod.5b00992. [DOI] [PubMed] [Google Scholar]
- 92.Yap W.S., Gan C.Y., Low Y.Y., Choo Y.M., Etoh T., Hayashi M., Komiyama K., Kam T.S. Grandilodines A - C, biologically active indole alkaloids from Kopsia grandifolia. J. Nat. Prod. 2011;74:1309–1312. doi: 10.1021/np200008g. [DOI] [PubMed] [Google Scholar]
- 93.Wu Y., Kitajima M., Kogure N., Wang Y., Zhang R., Takayama H. Two New Aspidosperma Indole Alkaloids from Yunnan Kopsia Arborea. Chem. Pharm. Bull. 2010;58:961–963. doi: 10.1248/cpb.58.961. [DOI] [PubMed] [Google Scholar]
- 94.Kam T., Tan P., Hoong P.Y., Cheng-Hock C. Methyl Chanofruticosinates from leaves of Kopsia arborea. Phytochemistry. 1993;32:489–491. [Google Scholar]
- 95.Ahmad K., Hirasawa Y., Nugroho A.E., Hadi A.H.A., Takeya K., Thomas N.F., Awang K., Morita H., Ping T.S., Nafiah M.A. New indole alkaloids from Kopsia singapurensis (RIDL.) Open Conf. Proc. J. 2013;4:75–82. doi: 10.2174/2210289201304020075. [DOI] [Google Scholar]
- 96.Chen J., Yang M., Zeng J., Gao K. New broad-spectrum antibacterial and antifungal alkaloids from Kopsia hainanensis. Phytochem. Lett. 2014;7:156–160. doi: 10.1016/j.phytol.2013.11.016. [DOI] [Google Scholar]
- 97.Kitajima M., Anbe M., Kogure N., Wongseripipatana S., Takayama H. Indole alkaloids from Kopsia jasminiflora. Tetrahedron. 2014;70:9099–9106. doi: 10.1016/j.tet.2014.10.002. [DOI] [Google Scholar]
- 98.Feng X., Kan C., Potier P., Kan S., Lounasmaa M. Monomeric indole alkaloids from Kopsia officinalis. Planta Med. 1983;48:280–282. doi: 10.1055/s-2007-969934. [DOI] [PubMed] [Google Scholar]
- 99.Lim K.H., Low Y.Y., Tan G.H., Kam T.S., Lim T.-M. Kopsine and danuphylline alkaloids from Kopsia. biomimetic partial synthesis of danuphylline B. Helv. Chim. Acta. 2008;91:1559–1566. doi: 10.1002/hlca.200890169. [DOI] [Google Scholar]
- 100.Trigo-Mouriño P., Sifuentes R., Navarro-Vázquez A., Gayathri C., Maruenda H., Gil R.R. Determination of the absolute configuration of 19-OH-(-)-eburnamonine using a combination of residual dipolar couplings, DFT chemical shift predictions, and chiroptics. Nat. Prod. Commun. 2012;7:735–738. doi: 10.1177/1934578X1200700611. [DOI] [PubMed] [Google Scholar]
- 101.Feng T., Cai X.H., Liu Y.P., Li Y., Wang Y.Y., Luo X.D. Melodinines A-G, Monoterpenoid indole alkaloids from Melodinus henryi. J. Nat. Prod. 2010;73:22–26. doi: 10.1021/np900595v. [DOI] [PubMed] [Google Scholar]
- 102.Kam T.S., Subramaniam G., Chen W. Alkaloids from Kopsia dasyrachis. Phytochemistry. 1999;51:159–169. doi: 10.1016/S0031-9422(98)00721-3. [DOI] [Google Scholar]
- 103.Smith G.F., Wahid M.A. 760. The Isolation of (±) - and (+)-vincadifformine and of ( +)- 1,2-dehydroaspidospermidine from Rhazya stricta. J. Chem. Soc. 1963:4002–4004. doi: 10.1039/JR9630004002. [DOI] [Google Scholar]
- 104.Kitajima M., Koyama T., Wu Y., Kogure N., Zhang R., Takayam H. Kopsiyunnanines J1 and J2, new strychnos-type Homo-monoterpenoid indole alkaloids from Kopsia arborea. Nat. Prod. Commun. 2015;10:49–51. doi: 10.1177/1934578X1501000114. [DOI] [PubMed] [Google Scholar]
- 105.Kam T.-S., Arasu L., Yoganathan K. Alkaloids from Koposia pauciflora. Phytochemistry. 1996;43:1385–1387. doi: 10.1016/S0031-9422(96)00496-7. [DOI] [Google Scholar]
- 106.Atta-ur-Rahman, Zaman K., Perveen S., Muzaffar A., Choudhary M.I., Pervin A. Alkaloids from Rhazya Stricta. Phytochemistry. 1991;30:1285–1293. doi: 10.1016/S0031-9422(00)95218-X. [DOI] [Google Scholar]
- 107.Kan-Fan C., Sevenet T., Husson H.P. Kopsia pauciflora, I. structure et configuration absolue de nouveaux alcaloïdes indoliques dimeres de type aspidospermane-eburnane de La serie pleiomutine-kopsoffine. J. Nat. Prod. 1985;48:124–127. doi: 10.1021/np50037a024. [DOI] [Google Scholar]
- 108.Kitajima M., Nakazawa M., Wu Y., Kogure N., Zhang R.P., Takayama H. Kopsiyunnanines L and M, strychnos-related monoterpenoid indole alkaloids from Yunnan Kopsia arborea. Tetrahedron. 2016;72:6692–6696. doi: 10.1016/j.tet.2016.08.082. [DOI] [Google Scholar]
- 109.Wong S., Chong K., Lim K., Lim S., Low Y., Kam T. Arborisidine and arbornamine, two monoterpenoid indole alkaloids with new polycyclic carbon − nitrogen skeletons derived from a common pericine precursor. Org. Lett. 2016;18:1618–1621. doi: 10.1021/acs.orglett.6b00478. [DOI] [PubMed] [Google Scholar]
- 110.Zeng T., Wu X., Yang S., Lai W., Shi S., Zou Q., Liu Y., Li L. Monoterpenoid indole alkaloids from Kopsia officinalis and the immunosuppressive activity of rhazinilam. Nat. Prod. 2017;80:864–871. doi: 10.1021/acs.jnatprod.6b00697. [DOI] [PubMed] [Google Scholar]
- 111.Klyne W., Swan R.J., Gorman A.A., Guggisberg A., Schmid H. Optische rotationsdispersion von indolinalkaloiden mit ketogruppen. Helv. Chim. Acta. 1968;51:1168–1184. doi: 10.1002/hlca.19680510522. [DOI] [PubMed] [Google Scholar]
- 112.Husain K., Jantan I., Kamaruddin N., Said I.M., Aimi N., Takayama H. Methyl chanofruticosinates from leaves of Kopsia flavida Blume. Phytochemistry. 2001;57:603–606. doi: 10.1016/S0031-9422(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 113.Zheng J., Zhou Y., Huang Z. The Isolation and Characterization of indole alkaloids from the fruits of Kopsia officinalis. Acta Chim. Sin. English Ed. 1989;7:168–175. doi: 10.1002/cjoc.19890070211. [DOI] [Google Scholar]
- 114.Thomas D.W., Achenbach H., Biemann K. Revised structures of the pleiocarpa alkaloids pleiocarpoline (pleiocarpine Nb-oxide), pleiocarpolinine (pleiocarpinine Nb-oxide), and kopsinoline (kopsinine Nb-oxide) J. Am. Chem. Soc. 1966;88:3423–3426. doi: 10.1021/ja00966a043. [DOI] [Google Scholar]
- 115.Kitajima M., Ohara S., Kogure N. New Indole alkaloids from Melodinus henryi. heterocycles. Int. J. Rev. Commun. Heterocycl. Chem. 2012;85:1949–1959. [Google Scholar]
- 116.Burnell R.H., Chapelle A., Khalil M.F. Quaternary bases from Hunteria eburnea pichon. Can. J. Chem. 1974;52:2327–2330. doi: 10.1139/v74-335. [DOI] [Google Scholar]
- 117.Wang Z., Shi X., Mu Y., Fang L., Chen Y., Lin Y. Three novel indole alkaloids from Kopsia officinalis. Fitoterapia. 2017;119:8–11. doi: 10.1016/j.fitote.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 118.Long S., Li C., Hu J., Zhao Q., Chen D. Indole alkaloids from the aerial parts of Kopsia fruticosa and their cytotoxic, antimicrobial and antifungal activities. Fitoterapia. 2018;129:145–149. doi: 10.1016/j.fitote.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 119.Kam T.S., Choo Y.M. Kopsifolines A–F: A new structural class of monoterpenoid indole alkaloids from Kopsia. Helv. Chem. Acta. 2004;87:991–998. doi: 10.1002/hlca.200490092. [DOI] [Google Scholar]
- 120.Chen X.D., Hu J., Li J., Chi F. Cytotoxic monoterpenoid indole alkaloids from the aerial part of Kopsia arborea. J. Asian Nat. Prod. Res. 2019;22:1024–1030. doi: 10.1080/10286020.2019.1680646. [DOI] [PubMed] [Google Scholar]
- 121.Liu T., Hu J., Li J., Chen M. Cytotoxic monoterpenoid indole alkaloids from the aerial parts of Kopsia officinalis. J. Asian Nat. Prod. Res. 2020;22:724–731. doi: 10.1080/10286020.2019.1621851. [DOI] [PubMed] [Google Scholar]
- 122.Xie T., Zhao Y., He J., Zhao L., Wei X., Liu Y., Luo X. Monoterpenoid indole alkaloids from the stems of Kopsia officinalis. Fitoterapia. 2020:104547. doi: 10.1016/j.fitote.2020.104547. [DOI] [PubMed] [Google Scholar]
- 123.Bao M.F., Zeng C.X., Liu Y.P., Zhang B.J., Ni L., Luo X.D., Cai X.H. Indole alkaloids from Hunteria zeylanica. J. Nat. Prod. 2017;80:790–797. doi: 10.1021/acs.jnatprod.5b01035. [DOI] [PubMed] [Google Scholar]
- 124.Zhou H., He H.P., Kong N.C., Wang Y.H., Liu X.D., Hao X.J. Three new indole alkaloids from the leaves of Kopsia officinalis. Helv. Chim. Acta. 2006;89:515–519. doi: 10.1002/hlca.200690053. [DOI] [Google Scholar]
- 125.Huang Y.Z., Huang L., Zhu J., Li C., Wu G. Alkaloids of Kopsia officinalis. Acta Chim. Sin. 1984;42:82–83. [Google Scholar]
- 126.Yang C.Q., Ma Y.F., Chen Y.G. Indole alkaloids from the twigs of Kopsia officinalis. Chem. Nat. Compd. 2017;53:595–597. doi: 10.1007/s10600-017-2062-8. [DOI] [Google Scholar]
- 127.Gan C.Y., Low Y.Y., Thomas N.F., Kam T.S. Rhazinilam-Leuconolam-Leuconoxine alkaloids from Leuconotis griffithii. J. Nat. Prod. 2013;76:957–964. doi: 10.1021/np400214y. [DOI] [PubMed] [Google Scholar]
- 128.Cai X.H., Jiang H., Li Y., Cheng G.G., Liu Y.P., Feng T., Luo X.D. Cytotoxic indole alkaloids from Melodinus fusiformis and M. morsei. Chin. J. Nat. Med. 2011;9:259–263. doi: 10.1016/S1875-5364(11)60061-7. [DOI] [Google Scholar]
- 129.Jiang H., Li Y.B., Li Y., Li L., Ma S.G., Qu J., Yu S.S. Analgesic corynanthe-type alkaloids from Strychnos angustiflora. Tetrahedron. 2016;72:1276–1284. doi: 10.1016/j.tet.2015.11.011. [DOI] [Google Scholar]
- 130.Robert G.M.T., Ahond A., Poupat C., Potier P., Jacquemin H., Kan S.K. Aspidosperma de Guyane: Alcaloïdes des Graines de Aspidosperma oblongum. J. Nat. Prod. 1983;46:259–263. doi: 10.1021/np50029a019. [DOI] [Google Scholar]
- 131.Gao Y., Yu A., Li G., Hai P., Li Y., Liu J., Wang F. Hexacyclic monoterpenoid indole alkaloids from Rauvolfia verticillata. Fitoterapia. 2015;107:44–48. doi: 10.1016/j.fitote.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 132.Kumaria R., Rathib B., Ranic A., Bhatnagar S. Rauvolfia serpentina L. Benth. ex Kurz.: Phytochemical, Pharmacological and Therapeutic Aspects. Int. J. Pharm. Sci. Rev. Res. 2013;23:348–355. [Google Scholar]
- 133.Kumar S., Singh A., Bajpai V., Srivastava M., Singh B.P., Ojha S., Kumar B. Simultaneous Determination of Bioactive Monoterpene Indole Alkaloids in Ethanolic Extract of Seven Rauvolfia Species using UHPLC with Hybrid Triple Quadrupole Linear Ion Trap Mass Spectrometry. Phytochem. Anal. 2016:296–303. doi: 10.1002/pca.2631. [DOI] [PubMed] [Google Scholar]
- 134.Boğa M., Bingül M., Özkan E.E., Şahin H. Chemical and Biological Perspectives of Monoterpene Indole Alkaloids from Rauwolfia species. Studies Nat. Prod. Chem. 2019;61:251–299. [Google Scholar]
- 135.Mahalakshmi S.N., Achala H.G., Ramyashree K.R., Kekuda T.R. Rauvolfia tetraphylla L. (Apocynaceae) - A Comprehensive Review on Its Ethnobotanical Uses, Phytochemistry and Pharmacological Activities. Int. J. Pharm. Biol. Sci. 2019;9:664–682. [Google Scholar]
- 136.Zeng J., Zhang D., Zhou P., Zhang Q., Zhao L., Chen J., Gao K. Rauvomines A and B, Two Monoterpenoid Indole Alkaloids from Rauvolfia vomitoria. Org. Lett. 2017;19:3998–4001. doi: 10.1021/acs.orglett.7b01723. [DOI] [PubMed] [Google Scholar]
- 137.Arthur H.R., Johns S.R., Lamberton J.A., Loo S.N. Alkaloids of Rauwolfia verticillata (Lour.) Bail. of Hong Kong. Identification of vellosimine and peraksine, and demonstration from N.M.R. data that peraksine is a mixture of two epimers. Aust. J. Chem. 1968;21:1399–1401. doi: 10.1071/CH9681399. [DOI] [Google Scholar]
- 138.Li L.M., Shi S.D., Liu Y., Zou Q. Bioactivity-Guided Isolation and Identification of New and Immunosuppressive Monoterpenoid Indole Alkaloids from Rauvolfia yunnanensis Tsiang. Molecules. 2019;24:4574. doi: 10.3390/molecules24244574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Z., ElSohly H.N., Jacob M.R., Pasco D.S., Walker L.A.A., Clark A.M. New Indole Alkaloids from the Bark of Nauclea orientalis. J. Nat. Prod. 2001;64:1001–1005. doi: 10.1021/np010042g. [DOI] [PubMed] [Google Scholar]
- 140.Abaul J., Philogéne E., Bourgeois P., Mérault G., Poupat C., Ahond A., Potier P. Alcaloïdes Indoliques De Rauvolfia Biauriculata. J. Nat. Prod. 1986;49:829–832. doi: 10.1021/np50047a011. [DOI] [Google Scholar]
- 141.Schlittler E.A., Schwarz H. Uber das Alkaloid Serpentin aus Rauwolfia serpentina Benth. Helv. Chim. Acta. 1950;33:1463–1477. doi: 10.1002/hlca.19500330609. [DOI] [Google Scholar]
- 142.Geng C.A., Liu X.K. Indole alkaloids from the leaves of Rauvolfia yunnanensis. Chem. J. Chin. U. 2010;31:731–735. doi: 10.1016/j.fitote.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 143.Staerk D., Lemmich E., Christensen J., Kharazmi A., Olsen C.E., Jaroszewski J.W. Leishmanicidal, antiplasmodial and cytotoxic activity of indole alkaloids from Corynanthe pachyceras. Planta Med. 2000;66:531–536. doi: 10.1055/s-2000-8661. [DOI] [PubMed] [Google Scholar]
- 144.Christiane K., Pierre P., Kan S.K., Jokela R., Lounasma M. Indole alkaloids from Rauvolfia media. Phytochemistry. 1986;25:1783–1784. [Google Scholar]
- 145.Wenkert E., Chang C.J., Chawla H.P.S., Cochran D.W., Hagaman E.W., King J.C., Orito K. General Methods of Synthesis of Indole Alkaloids. 14. Short Routes of Construction of Yohimboid and Ajmalicinoid Alkaloid Systems and Their 13C Nuclear Magnetic Resonance Spectral Analysis. J. Am. Chem. Soc. 1976;98:3645–3655. doi: 10.1021/ja00428a044. [DOI] [Google Scholar]
- 146.Nunes D.S., Koike L., Taveira J.J., Reis F.A.M. Indole alkaloids from Aspidosperma pruinosum. Phytochemistry. 1992;31:2507–2511. [Google Scholar]
- 147.Arbain D., Putra D.P., Sargent M.V. The alkaloids of Ophiorrhiza filistipula. Aust. J. Chem. 1993;46:977–985. doi: 10.1071/CH9930977. [DOI] [Google Scholar]
- 148.Reina M., Ruiz-Mesia W., Ruiz-Mesia L., Martínez-Díaz R., González-Coloma A. Indole alkaloids from Aspidosperma rigidum and A. schultesii and their antiparasitic effects. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2011;66:225–234. doi: 10.1515/znc-2011-5-605. [DOI] [PubMed] [Google Scholar]
- 149.Lin M., Yang B.Q., Yu D.Q. Studies on the quaternary alkaloids of Rauvolfia verticillata (lour.) Baill var. Hainanensis Tsiang. Acta Pharm. Sin. 1986;21:114–118. [PubMed] [Google Scholar]
- 150.Sheludko Y., Gerasimenko I., Kolshorn H., Stöckigt J. New alkaloids of the sarpagine group from Rauvolfia serpentina hairy root culture. J. Nat. Prod. 2002;65:1006–1010. doi: 10.1021/np0200919. [DOI] [PubMed] [Google Scholar]
- 151.Jin Y.S., Du J.L., Chen H.S., Jin L., Liang S. A new indole alkaloid from Ervatamia yunnanensis. Fitoterapia. 2010;81:63–65. doi: 10.1016/j.fitote.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 152.Yu D.Q., Yang J.S. Chemical Analytical Manual (the Second Edition), the Seventh Fascicle: NMR Spectra Analysis. Chemical Industry Press; Beijing, China: 2001. pp. 818–836. [Google Scholar]
- 153.Okuyama E., Gao L.H., Yamazaki M. Analgesic Components from Bornean Medicinal Plants, Tabernaemontana pauciflora BLUME and Tabernaemontana pandacaqui POIR. Chem. Pharm. Bull. 1992;40:2075. doi: 10.1248/cpb.40.2075. [DOI] [PubMed] [Google Scholar]
- 154.Bartlett M.F., Dickel D.F., Taylor W.I. The Alkaloids of Tabernanthe iboga. Part 1V. The Structures of Ibogamine, Ibogaine, Tabernanthine and Voacangine. J. Am. Chem. Soc. 1954;80:126–136. [Google Scholar]
- 155.Govindachari T.R., Joshi B.S., Saksena A.K., Sathe S.S., Viswanathan N. The structure of heyneanine. Tetrahedron Lett. 1965;43:3873–3878. doi: 10.1016/S0040-4039(01)89140-1. [DOI] [PubMed] [Google Scholar]
- 156.Thomas D.W., Biemann K. The hydroxyindolenine derivative of voacangine, a new indole alkaloid from Voacanga africana. Tetrahedron. 1968;24:4223–4231. doi: 10.1016/0040-4020(68)88184-0. [DOI] [PubMed] [Google Scholar]
- 157.Madinaveitia A., De La Fuente G., González A. The Absolute Configuration at C(7) of Voacangine Hydroxyindolenine. Helv. Chim. Acta. 1998;81:1645–1653. doi: 10.1002/(SICI)1522-2675(19980909)81:9<1645::AID-HLCA1645>3.0.CO;2-P. [DOI] [Google Scholar]
- 158.Das B.C., Fellion E., Plat M. Alkaloids on Conopharyngia durissima seeds. Isolation of coronaridine, tabersonine, and coronaridine hydroxyindolenine. Comptes Rendus des Seances de l’Academie des Sciences, Serie D: Sciences Naturelles. 1967;264:1765–1767. [Google Scholar]
- 159.Rastogi K., Kapil R.S., Popli S.P. New alkaloids from Tabernaemontana divaricata. Phytochemistry. 1980;19:1209–1212. doi: 10.1016/0031-9422(80)83085-8. [DOI] [Google Scholar]
- 160.Gunasekera S.P., Cordell G., Farnsworth N.R. Anticancer indole alkaloids of Ervatamia heyneana. Phytochemistry. 1980;19:1213–1218. doi: 10.1016/0031-9422(80)83086-X. [DOI] [Google Scholar]
- 161.Matos F.J.A., Fo R.B., Gottlieb O.R., Welbaneide F., Machado L., Iracema M., Madruga L.M. 20-Epiheyneanine, an iboga alkaloid from Peschiera affinis. Phytochemistry. 1976;15:551–553. doi: 10.1016/S0031-9422(00)88971-2. [DOI] [Google Scholar]
- 162.Sharma P., Cordell G.A. Heyneanine hydroxyindolenine, a new indole alkaloid from Ervatamia. J. Nat. Prod. 1988;51:528–531. doi: 10.1021/np50057a012. [DOI] [PubMed] [Google Scholar]
- 163.Liang S., Chen H.S., Jin Y.S., Jin L., Lu J., Du J.L. Study on chemical constituents in rhigome of Ervatamia hainanensis. J. Chin. Mater. Med. 2007;32:1296–1299. [PubMed] [Google Scholar]
- 164.Zhan Z.J., Yu Q., Wang Z.L., Shan W.G. Indole alkaloids from Ervatamia hainanensis with potent acetylcholinesterase inhibition activities. Bioorganic Med. Chem. Lett. 2010;20:6185–6187. doi: 10.1016/j.bmcl.2010.08.123. [DOI] [PubMed] [Google Scholar]
- 165.Guo L.l., Zhang Y., HE H.P., Li Y., Yu J.P., Hao X.J. A new monoterpenoid indole alkaloid from Ervatamia chinensis. Chin. J. Nat. Med. 2012;10:226–229. doi: 10.3724/SP.J.1009.2012.00226. [DOI] [Google Scholar]
- 166.Guoying Z., Hongping H., Bingui W., Xin H., Xiaojiang H. A new indoloquinazoline alkaloid from the fruit of Evodia rutaecarpa. Acta Bot. Yunnan. 2003;25:103–106. [Google Scholar]
- 167.Liu Z.W., Yang T.T., Wang W.J., Li G.Q., Tang B.Q., Zhang Q.W., Fan C.L., Zhang D.M., Zhang X.Q., Ye W.C. Ervahainine A, a new cyano-substituted oxindole alkaloid from Ervatamia hainanensis. Tetrahedron Lett. 2013;54:6498–6500. doi: 10.1016/j.tetlet.2013.09.080. [DOI] [Google Scholar]
- 168.Clivio P., Richard B., Deverre J.R., Sevenet T., Zeches M., Le Men-Oliver L. Alkaloids from leaves and root bark of Ervatamia hirta. Phytochemistry. 1991;30:3785–3792. doi: 10.1016/0031-9422(91)80111-D. [DOI] [Google Scholar]
- 169.Van Beek T.A., Verpoorte R., Svendsen A.B., Fokkens R.J. Antimicrobially Active Alkaloids from Tabernaemontana chippii. J. Nat. Prod. 1985;48:400–423. doi: 10.1021/np50039a008. [DOI] [PubMed] [Google Scholar]
- 170.Thomas J., Starmer A. The isolation and identification of the major alkaloid present in Tabernaemontana pachysiphon stapf var cumminsi (stapf) H. Huber. J. Pharm. Pharmac. 1963;15:487. doi: 10.1111/j.2042-7158.1963.tb12820.x. [DOI] [PubMed] [Google Scholar]
- 171.Pereira P.S., França S.D.C., Oliveira P.V.A.D., Breves C.M.D.S., Pereira S.I.V., Sampaio S.V., Nomizo A., Dias D.A. Chemical Constituents from Tabernaemontana catharinensis Root Bark: A Brief NMR Review of Indole Alkaloids and in vitro Cytotoxicity. Quim. Nova. 2008;31:20–24. doi: 10.1590/S0100-40422008000100004. [DOI] [Google Scholar]
- 172.Kam T., Sim K. Five New Iboga Alkaloids from Tabernaemontana corymbosa. J. Nat. Prod. 2002;65:669–672. doi: 10.1021/np0105432. [DOI] [PubMed] [Google Scholar]
- 173.Low Y.Y., Lim K.H., Choo Y.M., Pang H.S., Etoh T., Hayashi M., Komiyama K., Kam T.S. Structure, biological activity, and a biomimetic partial synthesis of the lirofolines, novel pentacylic indole alkaloids from Tabernaemontana. Tetrahedron Lett. 2010;51:269–272. doi: 10.1016/j.tetlet.2009.10.122. [DOI] [Google Scholar]
- 174.Tang B.Q., Wang W.J., Huang X.J., Li G.Q., Wang L., Jiang R.W., Yang T.T., Shi L., Zhang X.Q., Ye W.C. Iboga-type alkaloids from Ervatamia officinalis. J. Nat. Prod. 2014;77:1839–1846. doi: 10.1021/np500240b. [DOI] [PubMed] [Google Scholar]
- 175.Liu Z.W., Huang X.J., Xiao H.L., Liu G., Zhang J., Shi L., Jiang R.W., Zhang X.Q., Ye W.C. New iboga-type alkaloids from Ervatamia hainanensis. RSC Adv. 2016;6:30277–30284. doi: 10.1039/C6RA00185H. [DOI] [Google Scholar]
- 176.Azoug M., Loukaci A.L.I., Richard B., Nuzillard J., Moreti C., Zèches-Hanrot M., Le Men-Olivier L. Alkaloids from stem bark and leaves of Peschiera buchtieni. Phytochemisty. 1995;39:1223–1228. doi: 10.1016/0031-9422(95)00050-H. [DOI] [Google Scholar]
- 177.Rastogi K., Kapil R.S.A., Popli S.P. Oxidation of Coronaridine. Heterocycles. 1983;20:2001–2009. [Google Scholar]
- 178.De Souza J.J., Mathias L., Braz-Filho R., Vieira I.J.C. Two new indole alkaloids from Tabernaemontana hystrix Steud. (Apocynaceae) Helv. Chim. Acta. 2010;93:422–429. doi: 10.1002/hlca.200900208. [DOI] [Google Scholar]
- 179.Liu Z.W., Tang B.Q., Zhang Q.H., Wang W.J., Huang X.J., Zhang J., Shi L., Zhang X.Q., Ye W.C. Ervaoffines E-G, three iboga-type alkaloids featuring ring C cleavage and rearrangement from Ervatamia officinalis. RSC Adv. 2017;7:21883–21889. doi: 10.1039/C7RA03411C. [DOI] [Google Scholar]
- 180.Lim K.H., Raja V.J., Bradshaw T.D., Lim S.H., Low Y.Y., Kam T.S. Ibogan, tacaman, and cytotoxic bisindole alkaloids from Tabernaemontana. Cononusine, an iboga alkaloid with unusual incorporation of a pyrrolidone moiety. J. Nat. Prod. 2015;78:1129–1138. doi: 10.1021/acs.jnatprod.5b00117. [DOI] [PubMed] [Google Scholar]
- 181.Bartlett M.F., Dickel D.F., Maxfield R.C., Paszek L.E., Smith A.F. The Alkaloids of Tabcrnanthe iboga. Part VIII. J. Am. Chem. Soc. 1959;81:1932–1935. doi: 10.1021/ja01517a037. [DOI] [Google Scholar]
- 182.Zhang J., Ao Y.L., Yao N., Bai W.X., Fan C.L., Ye W.C., Zhang X.Q. Three new monoterpenoid indole alkaloids from Ervatamia pandacaqui. Chem. Biodivers. 2018;15:1800268. doi: 10.1002/cbdv.201800268. [DOI] [PubMed] [Google Scholar]
- 183.Chaturvedula V.S.P., Sprague S., Schilling J.K., Kingston D.G.I. New Cytotoxic Indole Alkaloids from Tabernaemontana calcarea from the Madagascar Rainforest. J. Nat. Prod. 2003;66:528–531. doi: 10.1021/np020548e. [DOI] [PubMed] [Google Scholar]
- 184.Zhang B., Teng X., Bao M., Zhong X., Ni L., Cai X. Cytotoxic indole alkaloids from Tabernaemontana officinalis. Phytochemistry. 2015;120:46–52. doi: 10.1016/j.phytochem.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 185.Tan S., Low Y., Choo Y., Abdullah Z., Etoh T., Hayashi M., Komiyama K., Kam T. Strychnan and Secoangustilobine A Type Alkaloids from Alstonia spatulata. Revision of the C-20 Configuration of Scholaricine. J. Nat. Prod. 2010;73:1891–1897. doi: 10.1021/np100552b. [DOI] [PubMed] [Google Scholar]
- 186.Liu Z.W., Zhang J., Li S.T., Liu M.Q., Huang X.J., Ao Y.L., Fan C.L., Zhang D.M., Zhang Q.W., Ye W.C., et al. Ervadivamines A and B, Two Unusual Trimeric Monoterpenoid Indole Alkaloids from Ervatamia divaricata. J. Org. Chem. 2018;83:10613–10618. doi: 10.1021/acs.joc.8b01371. [DOI] [PubMed] [Google Scholar]
- 187.Zhang H., Wang X.N., Lin L.P., Ding J., Yue J.M.J. Indole Alkaloids from Three Species of the Ervatamia Genus: E. officinalis, E.divaricata, and E. divaricata Gouyahua. J. Nat. Prod. 2007;70:54–59. doi: 10.1021/np060344o. [DOI] [PubMed] [Google Scholar]
- 188.Knox J.R., Slobbe J. Indole alkaloids from Ervatamia orientalis. II. The constitution of the ervatamine group. Aust. J. Chem. 1975;28:1825–1841. doi: 10.1071/CH9751825. [DOI] [Google Scholar]
- 189.Joule J.A., Monteiro H., Durham L.J., Gilbert B., Djerassi C. Alkaloid studies. 48. The structure of apparicine, a novel aspidosperma alkaloid. Chem. Soc. (Resumed) 1965:4773–4780. doi: 10.1039/jr9650004773. [DOI] [PubMed] [Google Scholar]
- 190.Watts F., Collera O., Sandoval A. Alkaloids from Stemmadenia species. I. Alkaloids of S. donnellsmithii and S. galleottiana. Tetrahedron. 1958;2:173–182. [Google Scholar]
- 191.Zhou J., Du S.Y., Dong H.J., Fang L., Feng J.H. Preparative separation of monoterpenoid indole alkaloid epimers from ervatamia yunnanensis tsiang by ph-zone-refining counter-current chromatography combined with preparative high-performance liquid chromatography. Molecules. 2019;24:1316. doi: 10.3390/molecules24071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Athipornchai A. A review on tabernaemontana spp.: Multipotential medicinal plant. Asian J. Pharm. Clin. Res. 2018;11:45–53. doi: 10.22159/ajpcr.2018.v11i5.11478. [DOI] [Google Scholar]
- 193.Nge C.E., Chong K.W., Thomas N.F., Lim S.H., Low Y.Y., Kam T.S. Ibogan, Aspidosperman, Vincamine, and Bisindole Alkaloids from a Malayan Tabernaemontana corymbosa: Iboga Alkaloids with C-20α Substitution. J. Nat. Prod. 2016;79:1388–1399. doi: 10.1021/acs.jnatprod.6b00129. [DOI] [PubMed] [Google Scholar]
- 194.Kingston D.G., Gerhart B.B., Ionescu F., Mangino M.M., Sami S.M. Plant Anticancer Agents V: New Bisindole Alkaloids from Tabernaemontana johnstonii Stem Bark. Pharm. Sci. 1978;67:249–251. doi: 10.1002/jps.2600670232. [DOI] [PubMed] [Google Scholar]
- 195.Kam T.S., Sim K.M. New Tabernamine Derivatives from Tabernaemontana. Heterocycles. 2002;57:2137–2143. doi: 10.3987/COM-02-9573. [DOI] [Google Scholar]
- 196.Qu Y., Simonescu R., De Luca V. Monoterpene Indole Alkaloids from the Fruit of Tabernaemontana litoralis and Differential Alkaloid Composition in Various Fruit Components. J. Nat. Prod. 2016;79:3143–3147. doi: 10.1021/acs.jnatprod.6b00405. [DOI] [PubMed] [Google Scholar]
- 197.Yuwen H., Yuan Y., Hao X., He H., Zhang Y. Two new monoterpenoid indole alkaloids from Tabernaemontana divaricata. Nat. Prod. Res. 2019;33:2139–2144. doi: 10.1080/14786419.2018.1488707. [DOI] [PubMed] [Google Scholar]
- 198.Sim D.S.Y., Chong K.W., Nge C.E., Low Y.Y., Sim K.S., Kam T.S. Cytotoxic Vobasine, Tacaman, and Corynanthe-Tryptamine Bisindole Alkaloids from Tabernaemontana and Structure Revision of Tronoharine. J. Nat. Prod. 2014;77:2504–2512. doi: 10.1021/np500589u. [DOI] [PubMed] [Google Scholar]
- 199.Zèches-Hanrot M., Nuzillard J.M., Richard B., Schaller H., Hadi H.A., Sévenet T., Le Men-Olivier L. Alkaloids from leaves and stem bark of Ervatamia peduncularis. Phytochemistry. 1995;40:587–591. doi: 10.1016/0031-9422(95)00152-W. [DOI] [Google Scholar]
- 200.Clivio P., Guillaume D., Vercauteren J., Richard B., Nuzillard J.M., Zèches-Hanrot M., Le Men-Olivier L. Two bis-indole alkaloids from leaves of Ervatamia polyneura. Phytochemistry. 1995;40:953–959. doi: 10.1016/0031-9422(95)00392-K. [DOI] [Google Scholar]
- 201.Cai Y., Sarotti A.M., Zhou T., Huang R., Qiu G., Tian C., Cao S., Mándi A., Kurtán T., Yang S., et al. Flabellipparicine, a Flabelliformide-Apparicine-Type Bisindole Alkaloid from Tabernaemontana divaricata. Nat. Prod. 2018;81:1976–1983. doi: 10.1021/acs.jnatprod.8b00191. [DOI] [PubMed] [Google Scholar]
- 202.Feng X.Z., Kan C., Husson H.P., Potier P., Kan S.K., Lounasmaa M. Nouveaux alcaloïdes indoliques dimeres de type voacamine extraits d’Ervatamia hainanensis. J. Nat. Prod. 1981;44:670–675. doi: 10.1021/np50018a008. [DOI] [Google Scholar]
- 203.Van Beek T.A., Verpoorte R., Svendsen A.B. Isolation and synthesis of vobparicine, a novel type dimeric indole alkaloid. Tetrahedron Lett. 1984;25:2057–2060. doi: 10.1016/S0040-4039(01)90112-1. [DOI] [Google Scholar]
- 204.Ingkaninan K., Changwijit K., Suwanborirux K. Vobasinyl-iboga bisindole alkaloids, potent acetylcholinesterase inhibitors from Tabernaemontana divaricata root. J. Pharm. Pharmacol. 2006;58:847–852. doi: 10.1211/jpp.58.6.0015. [DOI] [PubMed] [Google Scholar]
- 205.Henriques A.T., Melo A.A., Moreno P.R.H., Ene L.L., Henriques J.A.P., Schapoval E.E.S. Ervatamia coronaria: Chemical constituents and some pharmacological activities. J. Ethnopharmacol. 1996;50:19–25. doi: 10.1016/0378-8741(95)01328-8. [DOI] [PubMed] [Google Scholar]
- 206.Yamauchi T., Abe F., Padolina W.G., Dayrit F.M. Alkaloids from leaves and bark of Alstonia scholaris in the Philippines. Phytochemistry. 1990;29:3321–3325. doi: 10.1016/0031-9422(90)80208-X. [DOI] [Google Scholar]
- 207.Xu J., Qu W., Cao W.Y., Wang Y., Zheng K.J., Luo S.Z., Wu M.-Y., Liu W.-Y., Feng F., Zhang J. Chemical Constituents from Tabernaemontana bufalina Lour. Chem. Biodivers. 2019;16:1800491. doi: 10.1002/cbdv.201800491. [DOI] [PubMed] [Google Scholar]
- 208.Yu Y., Gao J.M., Liu J.K. Two New Oxindole Alkaloids from Ervatamia yunnanensis. Chin. Chem. Lett. 1999;10:575–578. [Google Scholar]
- 209.Perera P., Samuelsson G., van Beek T.A., Verpoorte R. Tertiary Indole Alkaloids from Fruits of Tabernaemontana dichotoma. Planta Med. 1984;50:148–150. doi: 10.1055/s-2007-969691. [DOI] [PubMed] [Google Scholar]
- 210.Melacheu G.L.F., Njoya E.M., Jouda J.B., Kweka B.N.W., Mbazoa C.D., Wang F., Wandji J. Two new indole alkaloids from Tabernaemontana contorta Stapf. Phytochem. Lett. 2019;30:116–119. doi: 10.1016/j.phytol.2019.01.028. [DOI] [Google Scholar]
- 211.Yu Y., Bao M.F., Wu J., Chen J., Yang Y.R., Schinnerl J., Cai X.H. Tabernabovines A-C: Three Monoterpenoid Indole Alkaloids from the Leaves of Tabernaemontana bovina. Org. Lett. 2019;21:5938–5942. doi: 10.1021/acs.orglett.9b02060. [DOI] [PubMed] [Google Scholar]
- 212.Chatterjee A., Banerji A., Majumder P., Majumder R. Alkaloids of Rhazya stricta Decaisne Studies on Rhazinaline and Geissoschizine. Bull. Chem. Soc. Jpn. 1976;49:2000–2004. doi: 10.1246/bcsj.49.2000. [DOI] [Google Scholar]
- 213.Mukhopadhyay S., Handy G.A., Funayama S., Cordell G.A. Anticancer indole alkaloids of Rhazya stricta. J. Nat. Prod. 1981;44:696–700. doi: 10.1021/np50018a014. [DOI] [PubMed] [Google Scholar]
- 214.Baeshen M.N., Khan R. Therapeutic Potential of the Folkloric Medicinal Plant Rhazya stricta. Biol. Syst. 2015;5:1–5. doi: 10.4172/2329-6577.1000151. [DOI] [Google Scholar]
- 215.Ahmed A., Li W., Chen F., Zhang J., Tang Y., Chen L., Tang G. Monoterpene indole alkaloids from Rhazya stricta. Fitoterapia. 2018;128:1–6. doi: 10.1016/j.fitote.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 216.Gelling A., Jeffery J.C., Poveycand C., Went M.J., Chemistry I., Its B.B.S. The Isolation of Monomeric Secodine-type Alkaloids from Rhazya Species. Chem. Sci. 1991;346:349–351. [Google Scholar]
- 217.Kan C., Deverre J.R., Sevenet T., Quirion J.C., Husson H.P. Indole Alkaloids from Kopsia Deverrei. Nat. Prod. Lett. 1995;7:275–281. doi: 10.1080/10575639508043222. [DOI] [Google Scholar]
- 218.Khanum S. Strictamine-N-oxide from Rhazya stricta. Phytochemistry. 1984;23:709–710. [Google Scholar]
- 219.Abe F., Yamauchi T. Indole alkaloids from leaves and stems of Leuconotis eugenifolius. Phytochemistry. 1993;35:169–171. doi: 10.1016/S0031-9422(00)90527-2. [DOI] [Google Scholar]
- 220.Proksa B., Uhrín D., Grossmann E., Votický Z. New Quaternary Alkaloids from Vinca minor. Planta Med. 1989;55:188–190. doi: 10.1055/s-2006-961921. [DOI] [PubMed] [Google Scholar]
- 221.Lounasmaa M., Hanhinen P. Conformational study of geissoschizine isomers and their model compounds. Heterocycles. 1999;51:649–670. doi: 10.3987/REV-98-512. [DOI] [Google Scholar]
- 222.Kutney J.P., Brown R.T. The structural elucidation of sitsirikine, dihydrositsirikine and isositsirikine: Three new alkaloids from vinca rosea linn. Tetrahedron. 1966;22:321–336. doi: 10.1016/0040-4020(66)80133-3. [DOI] [PubMed] [Google Scholar]
- 223.Danieli B., Lesma G., Palmisano G., Riva R., Tollari S. Absolute Configuration of (-)-Vincatine, the Unique 2,16-Seco Aspidosperma Alkaloid. J. Org. Chem. 1984:547–551. doi: 10.1021/jo00177a033. [DOI] [Google Scholar]
- 224.Jewers K., Pusey D.F.G., Sharma S.R., Ahmad Y. 13C-NMR Spectral Analysis of Rhazine, Quebrachamine and Rhazinilam. Planta Med. 1980;38:359–362. doi: 10.1055/s-2008-1074890. [DOI] [Google Scholar]
- 225.Atta-ur-Rahman, Habib-ur-Rehman, Ahmad Y., Fatima K., Badar Y. Studies on the Alkaloids of Rhazya stricta. Planta Med. 1987;53:256–259. doi: 10.1055/s-2006-962696. [DOI] [PubMed] [Google Scholar]
- 226.Abdul-Hameed Z.H., Alarif W.M., Omer A., El Omri A., Ayyad S.-E.N., Badria F.A., Neamatallah T., Bawakid N.O. Selective Anti-proliferative Activity of Indole Alkaloids from Rhazya stricta Decne Leaves. Lett. Org. Chem. 2019;16:941–947. doi: 10.2174/1570178616666190101095417. [DOI] [Google Scholar]
- 227.Hajrah N.H., Abdul W.M., Abdul-hameed Z.H., Alarif W.M., Al-abbas N.S.A., Ayyad S.E.N., Omer A.M.S., Mutawakil M.Z., Hall N., Obaid A.Y., et al. Gene Expression Profiling to Delineate the Anticancer Potential of a New Alkaloid Isopicrinine From Rhazya stricta. Integr. Cancer Ther. 2020;19:1–13. doi: 10.1177/1534735420920711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Abdul-Hameed Z.H., Alarif W.M., Sobhi T.R., Abdel-Lateff A., Ayyad S.E.N., Badria F.A., Saber J. New cytotoxic indole-type alkaloids obtained from Rhazya stricta leaves. South. Afr. J. Botany. 2021;137:298–302. doi: 10.1016/j.sajb.2020.10.020. [DOI] [Google Scholar]
- 229.Dewick P.M. Medicinal Natural Products; a Biosynthetic Approach. 3rd ed. John Wiley & Sons; Hoboken, NJ, USA: 2009. [Google Scholar]
- 230.O’Connor S.E., Maresh J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006;23:532–547. doi: 10.1039/b512615k. [DOI] [PubMed] [Google Scholar]
- 231.Bi Y., Hamaker L.K., Cook J.M. The Synthesis of Macroline Related Alkaloids. Stud. Nat. Pro. Chem. 1993;13:383–432. [Google Scholar]
- 232.Esmond R.W., LeQuesne P.W. Biomimitic synthesis of macroline. J. Am. Chem. Soc. 1980;102:7116–7117. doi: 10.1021/ja00543a045. [DOI] [Google Scholar]
- 233.Namjoshi O.A., Cook J.M. Sarpagine and related-alkaloids. Alkaloids Chem. Biol. 2016;76:63–169. doi: 10.1016/bs.alkal.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]