Abstract
The pandemic of novel coronavirus caused COVID-19 had resulted in a high number of hospitalizations and deaths and caused a devastating toll on human and society health. The symptoms of the infected patients vary significantly, from life-threatening to mild or even asymptomatic. This clinical observation led to hypothesize on the critical role of host innate immunity in the disease development and progression. As the first defense barrier against microorganisms, the innate immune reaction determines not only the viral infection rate but also immune-mediated response. Therefore, promote healthy behaviors to enhance innate immunity with functional food and nutritional agents may be a rational strategy for minimizing damages caused by viruses to global health.
Keywords: SARS-CoV-2, Viral infection, Innate immunity, Nutrition, Zinc, COVID-19
COVID-19 pandemic and anti-viral challenges
Humans are continually exposed to multiple endogenous and exogenous viruses. There are estimates that a single human being can generate up to 1012 new viral particles per day [1]. New viruses are discovered continuously; however, only a small subset of the viral population can cause severe diseases. The likelihood of viral infection is significantly increased if the host has compromised immune protection, which results in imbalances between the host proinflammatory responses and anti-viral activities [2].
The global pandemic of coronavirus COVID-19 has swept the world. The virus causes pneumonia of varying severity and vasculitis that has resulted in a high number of hospitalizations and deaths (Case Rate Fatality estimated at 2.1–4.9%) [3]. As the global case numbers keep soaring, health experts are scrambling to find a cure.
Vaccines have been proposed as a solution to this disease. Vaccines work by preparing the body to fight disease without exposing it to disease symptoms. It stimulates the adaptive immune system by exposing the body with inactivated or weakened antigens. The immune system can recognize the antigens as a foreign enemy, produce antibodies in response, and store the information for the future. If the bacteria or virus reappear, the immune system will recognize the antigens immediately and quickly mount a rapid defense by producing massive amounts of antibodies before the pathogen can spread and cause disease [4].
The COVID-19 belongs to the coronavirus family, which includes Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) that had similar symptoms resulting in respiratory illness [5]. While these viruses are not identical, many similarities in the genome may jumpstart in developing a vaccine.
Time is an essential factor in the development of vaccines against emerging viruses, especially those with pandemic potential. The development of vaccines is an expensive, time consuming, and complex process that includes identifying antigens, testing in animal models, and evaluating in humans for safety and efficacy. Despite decades of efforts, there are still no vaccines against viruses that kill millions of people every year, including HIV [6] and respiratory syncytial virus (RSV) [7]. In a recent review of 22 studies and evidence from four systematic reviews [8], the efficacy of the inactivated influenza vaccination is only between 16–59% in healthy adults depending on whether the results are clinically derived or empirically derived. There was no evidence on the impact of the influenza vaccine on overall mortality [9]. There are currently no vaccines approved to prevent other viral infections after years of development work and clinical trials [10,11].
On the other hand, most synthetic anti-viral drugs are expensive, toxic, and ineffective to stop diseases. As of the time of writing this manuscript, there are no clinically effective anti-viral drugs against coronaviruses, like SARS-CoV and MERS-CoV [12]. Anti-viral medications like Remdesivir can stop the virus from replicating, halting the disease state from in vitro and in animal models [13]. Even though the FDA had conditionally approved its use in selective situations, the therapeutic potential of Remdesivir is still controversial and warrants further investigation [[14], [15], [16]]. There are currently four FDA-approved influenza drugs recommended by CDC, but they all carry side effects and are only used in select situations to reduce the duration of illness [17]. Additionally, pathological viruses are constantly changing, and can sometimes mutate into different strains or sequences that could make anti-viral drugs work less effectively or not at all against these viruses. A different approach is urgently needed to combat these viruses.
The curve of COVID -19 urgently needs to be flattened. Identify alternative preventive and therapeutic strategies to contain the viral infection, especially in a scenario where the virus may become endemic and recurrent seasonal.
Clinical features of COVID-19 immune responses
The fact that many people infected with SARS-CoV-2 show only mild or even no symptoms in contrast to the viral-induced damage to more vulnerable populations, including the elderly and patients with chronic conditions. It suggests that the host immune system could be the key to defeat this virus.
The human body is equipped with a complicated and effective immune defense to protect the body against infection and disease. This complex network consists of cells, tissues, and organs that specializes in defending against foreign substances and pathogenic microorganisms, including bacteria, viruses, and fungi. Humans have two kinds of immune systems—innate and adaptive immune systems. The innate immunity involves an immediate, nonspecific defense mechanism that activates almost immediately in response to foreign invaders. It includes barriers, a variety of cells, and molecules to form the first line of the defense, such as airway mucus, anti-microbial soluble proteins (e.g., complement, lysozyme), cells secreting inflammatory mediators (basophils, mast cells, and eosinophils), natural killer (NK) cells, and phagocytic cells (macrophages, neutrophils, dendritic cells). They work together to prevent and control infection. The adaptive immune response, which includes both B cell-based humoral immunity and T cell-based cellular immunity, reacts much more specifically and powerfully to invading pathogens. The development of acquired immunity is a time-dependent, exposure-driven process. T and B lymphocytes 'learn' about the local infectious disease ecology through exposure.
In COVID-19 patients, two clinical features seem to correlate with disease severity and death, overly active of some innate immune cells, and suppressive of lymphocytes for adaptive immunity.
In particular, the number of both inflammatory macrophages and activated neutrophils is found to increase in the lung [18]. A significantly higher proportion of activated mast cells, neutrophils, and a higher neutrophil-lymphocyte ratio (NLR) were also observed [19]. On the other hand, a marked decrease in the circulating T cell (both CD4+, CD8+), B cells [20] in the COVID-19 patients. In addition, most of the patients with severe COVID-19 displayed significantly increased serum levels of proinflammatory cytokines (e.g. IL-6, IL-1β, IL-2, IL-8, IL-17, G-CSF, GM-CSF, IP-10, MCP-1, CCL3, and TNFα) [21], and inflammation markers C-reactive protein (Hs-CRP) and procalcitonin serum levels. The server COVID-19 patients usually have sudden deterioration around 8–9 days after onset, suggesting that the coronaviruses could hijack innate immune surveillance at the early infectious stage. At later stages, the hyper-inflammatory reaction could be initiated by innate immune cells to create fatally cytokine storms [22]. The COVID-19 clinical feature fits characteristics of the "primary cytokine" storm induced by innate immune cells such as alveolar macrophages, epithelial cells, and endothelial cells, rather than those observed in "secondary cytokine" storm induced by activated T cells in the late stage of viral infection [23]. These precious COVID-19 patients derived data suggest deleterious innate immune function may be the Achille tendon of COVID-19.
Modulate innate immunity may hold the key to defeat COVID-19
The pathogenesis of COVID-19 is complicated and multifactorial, involving both viral and host factors. However, it is well-established nutritional deficiency can impair immune functions and responsible for the increased susceptibility to pathogen infection and disease progression. Deficiencies in certain nutrients may overly activate tissue-resident innate immune cells, impair phagocytic function, and released a wrong set of signals to activate inflammation cascade or recruit wrong types of immune cells at the infection site. Malnutrition at cellular level adversely affects several aspects of adaptive immunity, including cytokine production as well as antibody- and cell-mediated immunities [24,25].
Even though many inflammation-associated diseases result from dysregulation of innate immunity, few pharmaceutical drugs have been developed based on innate immune research. Many nutritional food components, minerals, and herbal medicines have demonstrated their ability to maintain, improve, and modulate the innate immune functions. Therefore, to control the viral infection and their pathological consequences, the application of nutrients and botanicals to regulate multiple players at different phases of the disease should be considered. Their actions may include the prevention of viral attachment and entry, inhibition of viral replication, boosting viral clearance, balancing the hemostasis of immunity, controlling the inflammation, and resolving the repair and remodeling process.
Viral-associated molecules (immunostimulants) triggers two types of innate immune responses, inflammation, and phagocytosis. The inflammatory response involves acute phase proteins and the recruitment of phagocytic cells to the injury or infection sites to defend against a wide range of pathogens [26]. These cells express surface receptors that identify unique recognition patterns on the microorganism surfaces. For example, scavenger receptors, Toll-like receptors, and Nod-like receptors are major representatives within the host receptors groups [27]. Through receptor-ligand binding, signal transduction initiates a complex cascade of cellular reactions, which leads to the production of one or more of a wide array of effector molecules. Important innate effector molecules are oxygen and nitrogen species, antimicrobial peptides, lectins, fibrinogen-related peptides, leucine rich repeats, pentraxins, and complement-related proteins [28]. Recently, more cells have been identified to possess innate immune functions, such as airway epithelial cells [28,29] and stromal cells [30]. For example, airway epithelial cells contribute to innate deference by different mechanisms including the barrier function, mucociliary clearance, as well as the production of antimicrobial peptides, reactive oxygen species (ROS) and nitrogen species (RNS) and range of cytokines [29].
Tissue-resident mast cells are present in the submucosa of the nose, airway, and lungs, playing a pivotal role in the inflammatory response [31]. The pathogens, including viruses, interact with MCs to produce both positive and negative reactions in the host. MCs activation and degranulation could occur through RNA virus-induced Toll-like receptor-3 (TLR3) to produce interferon (IFN) α and β and IL-8 and to recruit NK cells [32]. In this sense, MCs may play a significant anti-viral role in COVID-19 pneumonia. However, in another scenario, the virus could stimulate mucosa MCs to release inflammatory cytokines and mediators, such as TNF-α, IL-1, IL-6, IL-33, IL-18 and proteases, histamine, prostaglandin D2, leukotriene C4, resulting in lung inflammation and bronchoconstriction [32]. Therefore, stabilize MC membrane and reduce mast cell activation may be a target. Along the same line, it is worth noting that recent cell culture-based studies indicate some naturally occurring flavonoids, such as quercetin [33], luteolin, and tetramethoxyluteolin [34], have significant MCs-stabilizing activities.
In the lung, macrophages are the most abundant immune cells essential for host innate immune defense, surfactant homeostasis, and lung development and repair [35]. Macrophages interact with other cells, such as epithelial cells, dendritic cells and T cells to perform immunosurveillance functions via phagocytosis, secretion of viricidal factors, nitric oxide, tumor necrosis factor (TNF), and interferons [36]. Besides traditional immune cells, airway epithelial cells also exert innate immune functions by producing hydrogen peroxide (H2O2) through an “oxidative extracellular microbicidal system”, consisting of the endogenous airway oxidase (DUOX), and lactoperoxidase (LPO) [37]. Many micronutrients such as zinc, selenium, copper, and iodine modulate the DUOX system to promote oxidative killing power against viruses. In addition, several polyphenols activate mononuclear cells and increase their phagocytic response through influencing MAPK and nuclear factor κB (NF-κB) signaling pathways [38].
Viruses cause infections that are often associated with redox modification's characteristic of oxidative stress. Inflammatory cell infiltration causes damage in the lung through excessive secretion of proteases and reactive oxygen species [39]. Viral infection and replication in airway epithelial cells could cause high levels of virus-linked pyroptosis. The debris and oxidants are highly inflammatory to induce aberrant inflammatory responses through the vicious cycle. Redox changes to an oxidized state also play a critical role in the activation of numerous cell pathways that are hijacked by viruses to assure their replication and suppress the patient's immune defense. Many antioxidative nutritional supplements, such as vitamin C, glutathione, NAC, organic sulfur compounds, and botanicals, can dampen the inflammatory responses.
Nutrients, medicinal phytochemicals, and functional foods have been shown to have beneficial effects on immune function
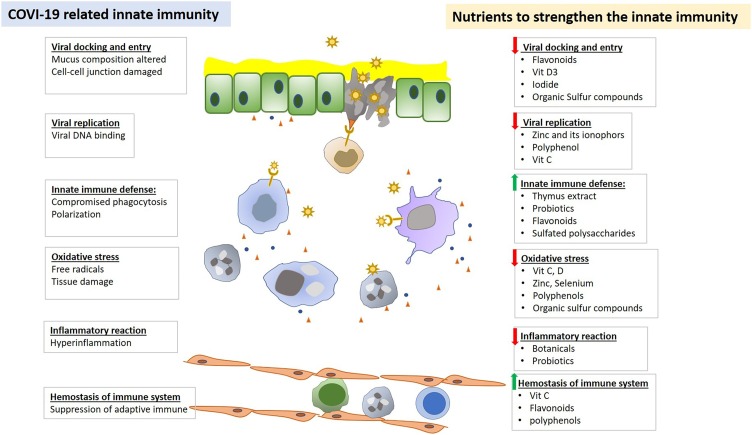
Every step of the viral life cycle is dependent on the host. Given that viral-host interactions are critical for viral propagation, one would imagine that strengthen the host defense by boosting cellular functions could lower the incidence and severity of the infection. “Let food be thy medicine and medicine be thy food.” Many nutrients, medicinal phytochemicals and functional food have been shown to have beneficial effects on host defense, as illustrated in Fig. 1 .
Fig. 1.
Nutrients and botanicals regulate innate immune functions in defecting viral infection.
Vitamin C The health benefits of vitamin C has been well established. It is considered an essential nutrient that cannot be synthesized by humans, having multiple effects related to its ability to donate electrons [40]. Vitamin C is important for things like wound healing, tissue repair, is required for certain enzymes to work, and has immune-modulating effects. Vitamin C is a potent antioxidant, protecting important biomarkers from free radicals and exposure to toxins and pollutants [41]. Additionally, it also boosts the generation of metabolic energy by acting as a cofactor in carnitine biosynthesis, a molecule responsible for the transport of fatty acids into the mitochondria [42]. It is also used as a common adjuvant for the treatment of the common cold, with meta-analysis showing its effectiveness in decreasing the severity, duration [43], and incidence of the common cold [44].
Vitamin D (VitD) is one of the oldest evolutionary hormones. In addition to the regulation of minerals metabolism, it has been recognized to play a role in the regulation of inflammation and protection from viral infection [45,46]. Studies have confirmed that low VitD levels correlate with the incidence and risk of several viral infections and flu [47,48]. Vit D3 receptor located on all immune cells. It is known VitD regulates monocyte and macrophage activation that is important for not only kill the virus but also clean up the debris that collects in the lung air sac during infection. VitD enhances regulatory T cell function, and VitD also inhibits proinflammatory cytokine expression, which could be potentially relevant in infection and could counteract the cytokine storm. However, VitD deficiency is a global health problem. It occurs in 20 %–60 % in Europe and up to 80 % in Middle East countries. Severe deficiency is found in >10 % of Europeans [49]. VitD deficiency showed a significant relationship to the development of asthma attacks [50]; it is a risk factor for infection, sepsis, and mortality in ICU patients [51]. Recent studies found there is a significant association between low serum concentrations of zinc and VitD [52,53]. VitD produced in the skin during exposure to the sun, and humans have the lowest levels of VitD in winter and spring [54]. Available evidence from systematic review suggests that high dose vitamin D may prevent asthma exacerbation [55]. Therefore, Vitamin D supplementation may be required for many individuals, especially in winter to prevent and alleviate symptoms from COVID-19
Zinc is an essential nutrient that people need to stay healthy. Zinc is found throughout the body, in the cells, and is a factor for enzymes to work. It necessary for the immune system to function normally. In fact, when zinc is deficient, one of the significant clinical symptoms is depressed immunity, which leads to increased infections and frequency of disease [11]. Studies have shown that this micronutrient is crucial for maintaining homeostasis of both innate and adaptive immune systems, and its deficiency is correlated with compromised immune cell development and functions. The use of zinc has been proven effective against infectious diseases in the human population. Double-blinded placebo-controlled trials have shown that daily zinc supplementation can reduce the incidence and duration of chronic diarrhea by 25–30 % [56], lower rates of acute respiratory infection up to 45 % [57], and can even decrease the duration of the common cold [58].
Iodine is recognized as a necessary nutrient for proper immune function by the Institute of Medicine and as well as the United Nations Nutritional Policy Board [59,60]. There have been many historical reports of immune deficiencies among populations of iodine-deficient people [61]. This is because leukocyte myeloperoxidase enzyme uses iodine in cell-mediated immunity and is an essential component of many immune cells [62]. Additionally, iodides also have many other biologic effects, including regulating inflammation, improves phagocytosis of bacteria by immune cells, and boosting the innate immune system [63]. Because iodine molecules tend to sublimate, instability is its biggest drawback. Lack of iodine is widespread in modern dietary and lifestyle. High dietary perchlorate, glucosinolate, thiocyanate, calcium nitrate, cobalt and rubidium interfere with iodine metabolism and may increase iodine requirements [64,65]. Household hygiene products such as chlorine containing beach and fluoride in water and toothpaste further depletes iodine in our body.
Andrographis paniculata is a medicinal plant whose underground stem and leaf are used to make medicine. Andrographis has a history of use in both Ayurvedic and traditional Chinese and Vietnamese medicine for thousands of years to prevent and treat the common cold and flu (influenza). It contains several bitter components that seem to have both immune-stimulating, anti-viral, antibacterial, and anti-inflammatory activities. Randomized double-blind studies have found evidence that Andrographis may reduce the severity of symptoms in individuals suffering from the common cold [[66], [67], [68], [69]]. Several well-designed clinical studies have shown the robust clinical efficacy of the herb for viral infections, showing statistically significant efficacy of Andrographis compared to control [70,71].
Echinacea purpurea is an immune stimulant and is effective in the prevention and treatment of colds and influenza. Research suggests that Echinacea stimulates the immune system by activating immune cells such as lymphocytes and macrophages [72]. Additionally, Echinacea appears to increase the production of interferon, a group of signal proteins released in response to viruses, speeding up the immune response of viral infections [73]. Several double-blind, clinical studies have confirmed Echinacea's effectiveness in treating colds and flu [[74], [75], [76], [77], [78], [79]].
Garlic has been used for centuries as both a food ingredient and a medicine. Eating garlic provides a wide variety of health benefits including enhanced immune function [[80], [81], [82]]. Garlic contains powerful anti-viral phytochemicals such as ajoene and allicin that help the immune system to fight pathogens. These compounds boost the disease-fighting response of white blood cells in the body when they encounter viruses, including ones that cause the common cold or flu [83,84]. Other studies have shown that the ingestion of garlic reduces the risk of viral infection in the first place, shortens the disease duration, and relieves the severity of symptoms [83,85].
Astragalus radix (Hwang-Qi) is most commonly used in traditional Chinese medicine and holistic medicine. It contains many functional components to strengthen the host defense system described as tonic medicine in "Chinese pharmacopeia" [86]. There are preclinical studies that have suggested some immune-stimulating effects, including stimulating murine macrophages to produce more interleukin-6 and tumor necrosis factor, two cytokines involved in the inflammatory process, and in the in vitro antitumor activity [87]. Chemical components of Astragalus include polysaccharides mannose, d-glucose, d-galactose, xylose, and l-arabinose, which are used as immunomodulating agents in mixed herbal decoctions to treat common colds, diarrhea, fatigue, and anorexia [88,89].
Probiotics influence many functions in the body, including promoting a healthy gut, enhancing the immune system, and reducing the risk of infection in children and adults. The last decade has shown the close connection between gut health and immune function. Microbiomes or colonies of bacteria that live in the gut, directly communicate with the cells of the immune system [90]. The body has a symbiotic relationship with the gut microbiome since these bacteria are responsible for supporting the production of stomach acid and saliva, which can boost the first line of immune defense. When the microbiome is happy and healthy, the immune system responds quickly and effectively to viral infection [[91], [92], [93], [94]]. In addition to immune health, researchers have found evidence that supplementation of probiotics affecting body weight, energy level, and brain function [95,96]. Gou et al.{Gou, 2020 #477}recently reported that the disruption of gut microbiome features by the host and environmental factors might predispose healthy individuals to the abnormal inflammatory response observed in COVID-19.
Thymus extracts are usually derived from thymus glands of young calves. The thymus is responsible for the production of T lymphocytes, immune cells critical for body function. Two double-blind placebo-controlled trials enrolled children with frequent respiratory infections, such as colds, and found that treatment with thymus extract reduced the rate of infection [97,98]. In another double-blind study revealed thymus extract, when given orally to children with recurrent in respiratory infections (RRI), was able to reduce the number of RRIs when compared to placebo controls or to previous year infections in the same child. Continued use prevented relapses of infections and produced an increase in phagocytic responses of alveolar macrophages and serum immunoglobulins [99]. Another placebo-controlled trial compared calf thymus extract to pharmaceutical immunomodulator levamisole to treat children suffering from chronic bronchitis. Both treatment groups (thymus extract and levamisole) showed statistically significant decreases in the number, severity, and duration of episodes, requiring less antibiotic therapy, and greater normalization of the number and function of T lymphocytes [100].
Immune psychology plays a significant role in the prevention and disease courses. Stress is a significant contributing factor in immune function. A meta-analysis of 300 empirical studies found that certain types of stress (both psychological and physical) altered different aspects of the immune system. Brief stressors tend to suppress cellular immunity, lowing defense against viruses while preserving humoral immunity. Chronic stressors tend to contain both types of immunity. Research has shown that people in stressful situations have measurably slower wound healing, a higher incidence of infection, or worse prognosis for infection [101,102].
Conclusion
The quality and magnitude of immune defense dictate how viruses interact with hosts in causing symptoms, severity, duration, infectivity, and outcomes of the disease. Human has multiple defense mechanisms that can protect the body against viruses, bacteria, and fungus infection. Exploring the application of non-toxic pharmaceutical drugs, nutritional agents, and maintaining proper psychosocial environmental may prove key in enhancing the early innate immune and later specific adaptive immune (humoral and cell-mediated) responses. These approaches might prove to be effective and economical strategies to prevent the spread and improve the treatment efficacy against the COVID-19 pandemic.
Funding
No funding sources.
Declaration of Competing Interest
No conflict of interest to declare.
Acknowlegement
None.
References
- 1.Virgin H.W., Wherry E.J., Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Sumbria D., Berber E., Rouse B.T. Factors affecting the tissue damaging consequences of viral infections. Front Microbiol. 2019;10:2314. doi: 10.3389/fmicb.2019.02314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegrist C.-A. Vaccine immunology. Vaccines. 2008;5(1):17–36. [Google Scholar]
- 5.Channappanavar R., Perlman S. Seminars in immunopathology. Springer; 2017. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. Science, American Association for the Advancement of Science; 2020. Another HIV vaccine strategy fails in large-scale study. [Google Scholar]
- 7.Besteman S.B., Bont L.J. American Thoracic Society; 2019. Fail-fast in respiratory syncytial virus vaccine development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demicheli V., Jefferson T., Ferroni E., Rivetti A., Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018;(2):12–28. doi: 10.1002/14651858.CD001269.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel R., Miller J. Is the influenza vaccine effective in decreasing infection, hospitalization, pneumonia, and mortality in healthy adults? J Okla State Med Assoc. 2019;112(3):86. [PMC free article] [PubMed] [Google Scholar]
- 10.Castilow E.M., Olson M.R., Varga S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol Res. 2007;39(1–3):225–239. doi: 10.1007/s12026-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 11.Anderson L., Dormitzer P., Nokes D., Rappuoli R., Roca A., Graham B. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31:B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020:1–3. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y.-c., Deng Q.-x., Dai S.-x. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis. 2020;35(101647):1–6. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw M.L. The next wave of influenza drugs. ACS Infect Dis. 2017;3(10):691–694. doi: 10.1021/acsinfecdis.7b00142. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKechnie J.L., Blish C.A. The innate immune system: fighting on the front lines or fanning the flames of COVID-19? Cell Host Microbe. 2020;27(6):863–869. doi: 10.1016/j.chom.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395(10229):1033. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi-zhi J.G., Thomas P.G. Seminars in immunopathology. Springer; 2017. New fronts emerge in the influenza cytokine storm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erickson K.L., Medina E.A., Hubbard N.E. Micronutrients and innate immunity. J Infect Dis. 2000;182(Supplement_1):S5–S10. doi: 10.1086/315922. [DOI] [PubMed] [Google Scholar]
- 25.Lesourd B. Nutrition: a major factor influencing immunity in the elderly. J Nutr Health Aging. 2004;8(1):28–37. [PubMed] [Google Scholar]
- 26.Ulevitch R.J. Therapeutics targeting the innate immune system. Nat Rev Immunol. 2004;4(7):512–520. doi: 10.1038/nri1396. [DOI] [PubMed] [Google Scholar]
- 27.Kollmann T.R., Levy O., Montgomery R.R., Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37(5):771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bals R., Hiemstra P. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23(2):327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 29.Hiemstra P.S., McCray P.B., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45(4):1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens B.M. Inflammation, innate immunity, and the intestinal stromal cell niche: opportunities and challenges. Front Immunol. 2015;6:319. doi: 10.3389/fimmu.2015.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-de-Olano D., Alvarez-Twose I. Mast cells as key players in allergy and inflammation. J Investig Allergol Clin Immunol. 2018;28(6):365–378. doi: 10.18176/jiaci.0327. [DOI] [PubMed] [Google Scholar]
- 32.Kritas S., Ronconi G., Caraffa A., Gallenga C., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020;34(1) doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 33.Weng Z., Zhang B., Asadi S., Sismanopoulos N., Butcher A., Fu X., et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng Z., Patel A.B., Panagiotidou S., Theoharides T.C. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. 2015;135(4) doi: 10.1016/j.jaci.2014.10.032. 1044–1052.e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locati M., Mantovani A., Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. Elsevier. [DOI] [PubMed] [Google Scholar]
- 36.O’reilly P., Hickman-Davis J.M., McArdle P., Young K.R., Matalon S. The role of nitric oxide in lung innate immunity: modulation by surfactant protein-A. Mol Cell Biochem. 2002;234(1):39–48. [PubMed] [Google Scholar]
- 37.Rada B., Leto T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Trends Innate Immun. 2008;15:164–187. doi: 10.1159/000136357. Karger Publishers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C., Yu R., Owuor E.D., Kong A.-N.T. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23(6):605. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 39.Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr A., Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? Faseb J. 1999;13(9):1007–1024. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 42.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta (BBA) Rev Cancer. 2012;1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douglas R., Hemila H., Chalker E., Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD000980.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Johnston C.S., Barkyoumb G.M., Schumacher S.S. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin C status: a randomized controlled trial. Nutrients. 2014;6(7):2572–2583. doi: 10.3390/nu6072572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulie T., Groff A., Redmer J., Hounshell J., Schrager S. Vitamin D: an evidence-based review. J Am Board Fam Med. 2009;22(6):698–706. doi: 10.3122/jabfm.2009.06.090037. [DOI] [PubMed] [Google Scholar]
- 46.Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laaksi I., Ruohola J.-P., Tuohimaa P., Auvinen A., Haataja R., Pihlajamäki H., et al. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 48.McNally J.D., Leis K., Matheson L.A., Karuananyake C., Sankaran K., Rosenberg A.M. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44(10):981–988. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 49.Lips P., Cashman K.D., Lamberg-Allardt C., Bischoff-Ferrari H.A., Obermayer-Pietsch B., Bianchi M.L., et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 50.Arikoglu T., Kuyucu S., Karaismailoglu E., Batmaz S.B., Balci S. The association of vitamin D, cathelicidin, and vitamin D binding protein with acute asthma attacks in children. Allergy Asthma Proc. 2015;36(4):51–58. doi: 10.2500/aap.2015.36.3848. [DOI] [PubMed] [Google Scholar]
- 51.de Haan K., Groeneveld A.J., de Geus H.R., Egal M., Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. 2014;18(6):660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shams B., Afshari E., Tajadini M., Keikha M., Qorbani M., Heshmat R., et al. The relationship of serum vitamin D and Zinc in a nationally representative sample of Iranian children and adolescents: the CASPIAN-III study. Med J Islam Repub Iran. 2016;30:430. [PMC free article] [PubMed] [Google Scholar]
- 53.Ziaei S., Norrozi M., Faghihzadeh S., Jafarbegloo E. A randomised placebo‐controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥13.2 g/dl. Bjog Int J Obstet Gynaecol. 2007;114(6):684–688. doi: 10.1111/j.1471-0528.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 54.Coussens A.K. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16(3):314–338. doi: 10.1039/c6pp00355a. [DOI] [PubMed] [Google Scholar]
- 55.Pojsupap S., Iliriani K., Sampaio T.Z.A.L., O’Hearn K., Kovesi T., Menon K., et al. Efficacy of high-dose vitamin D in pediatric asthma: a systematic review and meta-analysis. J Asthma. 2015;52(4):382–390. doi: 10.3109/02770903.2014.980509. [DOI] [PubMed] [Google Scholar]
- 56.Yazar A.S., Güven Ş., Dinleyici E.Ç. Effects of zinc or synbiotic on the duration of diarrhea in children with acute infectious diarrhea. Turk J Gastroenterol. 2016;27(6):537–540. doi: 10.5152/tjg.2016.16396. [DOI] [PubMed] [Google Scholar]
- 57.Rerksuppaphol S., Rerksuppaphol L. A randomized controlled trial of zinc supplementation in the treatment of acute respiratory tract infection in Thai children. Pediatr Rep. 2019;11(2) doi: 10.4081/pr.2019.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst Rev. 2013;(6):12–20. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 59.Hetzel B.S. 1988. The prevention and control of iodine deficiency disorders. Nutrition Policy Discussion Paper (UN/ACC) [DOI] [PubMed] [Google Scholar]
- 60.Trumbo P., Yates A.A., Schlicker S., Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Acad Nutr Diet. 2001;101(3):294. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 61.Black R. SciELO Public Health; 2003. Micronutrient deficiency: an underlying cause of morbidity and mortality. [PMC free article] [PubMed] [Google Scholar]
- 62.Klebanoff S.J., Kettle A.J., Rosen H., Winterbourn C.C., Nauseef W.M. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93(2):185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bilal M., Dambaeva S., Kwak-Kim J., Gilman-Sachs A., Beaman K. A role for iodide and thyroglobulin in modulating the function of human immune cells. Front Immunol. 2017;2017(8):1573. doi: 10.3389/fimmu.2017.01573. Epub 2017/12/01. PMID: 29187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman M. Thyroid autoimmune disease. J Restor Med. 2013;2(1):70–81. [Google Scholar]
- 65.Rogan W., Paulson J., Baum C., Brock-Utne A., Brumberg H., Campbell C., et al. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics. 2014;133(6):1163–1166. doi: 10.1542/peds.2014-0900. [DOI] [PubMed] [Google Scholar]
- 66.Chantrakul C., Punkrut W., Boontaeng N., Petcharoen S., Riewpaiboon W. Efficacy of Andrographis paniculata, Nees for pharyngotonsillitis in adults. J Med Assoc Thai. 1991;74(10):437–442. [PubMed] [Google Scholar]
- 67.Hancke J., Burgos R., Caceres D., Wikman G. A double-blind study with a new monodrug Kan Jang: decrease of symptoms and improvement in the recovery from common colds. Phytother Res. 1995;9(8):559–562. [Google Scholar]
- 68.Melchior J., Palm S., Wikman G. Controlled clinical study of standardized Andrographis paniculata extract in common cold—a pilot trial. Phytomedicine. 1997;3(4):315–318. doi: 10.1016/S0944-7113(97)80002-5. [DOI] [PubMed] [Google Scholar]
- 69.Caceres D., Hancke J., Burgos R., Sandberg F., Wikman G. Use of visual analogue scale measurements (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomedicine. 1999;6(4):217–223. doi: 10.1016/S0944-7113(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 70.Saxena R., Singh R., Kumar P., Yadav S., Negi M., Saxena V., et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmColdTM) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17(3–4):178–185. doi: 10.1016/j.phymed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Jayakumar T., Hsieh C.-Y., Lee J.-J., Sheu J.-R. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.See D.M., Broumand N., Sahl L., Tilles J.G. In vitro effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology. 1997;35(3):229–235. doi: 10.1016/s0162-3109(96)00125-7. [DOI] [PubMed] [Google Scholar]
- 73.Luettig B., Steinmüller C., Gifford G., Wagner H., Lohmann-Matthes M.-L. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. JNCI J Natl Cancer Inst. 1989;81(9):669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 74.Melchart D., Linde K., Worku F., Bauer R., Wagner H. Immunomodulation with Echinacea—a systematic review of controlled clinical trials. Phytomedicine. 1994;1(3):245–254. doi: 10.1016/S0944-7113(11)80072-3. [DOI] [PubMed] [Google Scholar]
- 75.Dorn M., Knick E., Lewith G. Placebo-controlled, double-blind study of Echinaceae pallidae radix in upper respiratory tract infections. Complement Ther Med. 1997;5(1):40–42. [Google Scholar]
- 76.Brown D. Echinacea shortens the course of the common cold. Q Rev Nutr. 1998;3–4 [Google Scholar]
- 77.Melchart D., Walther E., Linde K., Brandmaier R., Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Arch Fam Med. 1998;7(6):541. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 78.Grimm W., Müller H.-H. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. Am J Med. 1999;106(2):138–143. doi: 10.1016/s0002-9343(98)00406-9. [DOI] [PubMed] [Google Scholar]
- 79.Percival S.S. Use of Echinacea in medicine. Biochem Pharmacol. 2000;60(2):155–158. doi: 10.1016/s0006-2952(99)00413-x. [DOI] [PubMed] [Google Scholar]
- 80.Kyo E., Uda N., Kasuga S., Itakura Y. Immunomodulatory effects of aged garlic extract. J Nutr. 2001;131(3):1075S–1079S. doi: 10.1093/jn/131.3.1075S. [DOI] [PubMed] [Google Scholar]
- 81.Majewski M. Allium sativum: facts and myths regarding human health. Roczniki Państwowego Zakładu Higieny. 2014;65(1) [PubMed] [Google Scholar]
- 82.Varshney R., Budoff M.J. Garlic and heart disease. J Nutr. 2016;146(2):416S–421S. doi: 10.3945/jn.114.202333. [DOI] [PubMed] [Google Scholar]
- 83.Nantz M.P., Rowe C.A., Muller C.E., Creasy R.A., Stanilka J.M., Percival S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: a randomized, double-blind, placebo-controlled nutrition intervention. Clin Nutr. 2012;31(3):337–344. doi: 10.1016/j.clnu.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Arreola R., Quintero-Fabián S., López-Roa R.I., Flores-Gutiérrez E.O., Reyes-Grajeda J.P., Carrera-Quintanar L., et al. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol Res. 2015;2015 doi: 10.1155/2015/401630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Josling P. Preventing the common cold with a garlic supplement: a double-blind, placebo-controlled survey. Adv Ther. 2001;18(4):189–193. doi: 10.1007/BF02850113. [DOI] [PubMed] [Google Scholar]
- 86.Grover J., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida Y., Wang M., Liu J., Shan B., Yamashita U. Immunomodulating activity of Chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol. 1997;19(7):359–370. doi: 10.1016/s0192-0561(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 88.Yin X., Chen L., Liu Y., Yang J., Ma C., Yao Z., et al. Enhancement of the innate immune response of bladder epithelial cells by Astragalus polysaccharides through upregulation of TLR4 expression. Biochem Biophys Res Commun. 2010;397(2):232–238. doi: 10.1016/j.bbrc.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 89.Li J., Zhong Y., Li H., Zhang N., Ma W., Cheng G., et al. Enhancement of Astragalus polysaccharide on the immune responses in pigs inoculated with foot-and-mouth disease virus vaccine. Int J Biol Macromol. 2011;49(3):362–368. doi: 10.1016/j.ijbiomac.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Ashraf R., Shah N.P. Immune system stimulation by probiotic microorganisms. Crit Rev Food Sci Nutr. 2014;54(7):938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- 91.Caricilli A.M., Castoldi A., Câmara N.O.S. Intestinal barrier: a gentlemen’s agreement between microbiota and immunity. World J Gastrointest Pathophysiol. 2014;5(1):18. doi: 10.4291/wjgp.v5.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.King S., Glanville J., Sanders M.E., Fitzgerald A., Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54. doi: 10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei Y.M.K., Nair L., Alegre M.-L. The interplay between the intestinal microbiota and the immune system. Clin Res Hepatol Gastroenterol. 2015;39(1):9–19. doi: 10.1016/j.clinre.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ozen M., Kocabas Sandal G., Dinleyici E.C. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. 2015;15(1):9–20. doi: 10.1517/14712598.2015.980233. [DOI] [PubMed] [Google Scholar]
- 95.Cerdó T., García-Santos J.A., Bermúdez M.G., Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. 2019;11(3):635. doi: 10.3390/nu11030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jäger R., Mohr A.E., Carpenter K.C., Kerksick C.M., Purpura M., Moussa A., et al. International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr. 2019;16(1):62. doi: 10.1186/s12970-019-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fiocchi A., Borella E., Riva E., Arensi D., Travaglini P., Cazzola P., et al. A double-blind clinical trial for the evaluation of the therapeutical effectiveness of a calf thymus derivative (Thymomodulin) in children with recurrent respiratory infections. Thymus. 1986;8(6):331–339. [PubMed] [Google Scholar]
- 98.Longo F., Lepore L., Agosti E., Panizon F. Evaluation of the effectiveness of thymomodulin in children with recurrent respiratory infections. La Pediatria Medica e Chirurgica: Med Surg Pediatr. 1988;10(6):603–607. [PubMed] [Google Scholar]
- 99.Kouttab N., Prada M., Cazzola P. Thymomodulin: biological properties and clinical applications. Med Oncol Tumor Pharmacother. 1989;6(1):5–9. doi: 10.1007/BF02985217. [DOI] [PubMed] [Google Scholar]
- 100.Wilson J.L. Foundation for Immunology and Nutrition, Development, Education nd Research; 1999. Thymus extracts: an international literature review of clinical studies; pp. 100–129. [Google Scholar]
- 101.Segerstrom S.C., Miller G.E. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gouin J.-P., Kiecolt-Glaser J.K. The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am. 2011;31(1):81–93. doi: 10.1016/j.iac.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]