INTRODUCTION
The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has made a staggering global impact and has become one of the most pressing international health and economic crises of recent years. COVID-19 primarily affects the human respiratory system, often progressing to pneumonia and potentially death in susceptible populations (10). A recent analysis of COVID-19 cases and recovery outcomes has identified several predictors of progression severity and mortality, such as age, hypertension and cardiovascular disease, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, cancer, and immunodeficiency (7). SARS-CoV-2 mediates infection via interaction of its spike domain with angiotensin-converting enzyme 2 (ACE2) of host cells (8, 23). Viral transmission and symptom severity are enhanced through the elegant exploitation of host cellular proprotein convertase furin, and cell-surface proteases to facilitate S2 spike domain-mediated membrane fusion (8, 23). Both cardiac and skeletal muscle tissues exhibit robust expression of ACE2 (17, 20), suggestive of potential SARS-CoV-2 infection susceptibility in both tissue types. Notably, skeletal muscle weakness, fatigue, pain, and injury occurrence are common symptoms of COVID-19 manifestation (10, 10a, 12, 25, 29). Thus it is plausible to suggest SARS-CoV-2 may directly impact skeletal muscle (Fig. 1). Here we review the current insights into COVID-19 as it relates to potential skeletal muscle susceptibility. We then aim to describe the importance of skeletal muscle in facilitating respiration, while highlighting our unique position regarding respiratory muscle vulnerability in the context of COVID-19 disease outcomes and skeletal muscle myopathies.
Fig. 1.
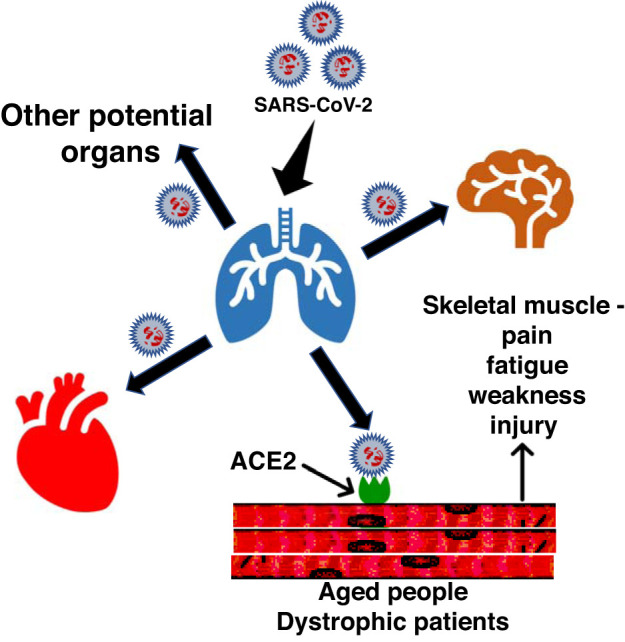
Representative diagram displays the potential spread of SARS-CoV-2 into skeletal muscle and the cause of myopathies.
SARS-COV-2 INFECTION OF NON-PULMONARY TISSUES
Emerging data provide evidence for SARS-CoV-2 migration and infection of several tissue types, including the central nervous system (12) and cardiovascular system (10). A recent review of SARS-CoV-2-related infections highlights several possibilities of viral entry into the CNS, while providing potential evidence for subsequent dysfunction of cardiorespiratory areas of the brainstem (12). Myocardial injury is also of concern, as elevations in cardiac damage markers troponin I (10) and creatine kinase-MB (CK-MB) (28) have been shown in patients with COVID-19.
SARS-CoV-2 relies on host cellular ACE2 expression for S1 spike domain receptor binding, while utilizing host surface proteases [e.g., transmembrane protease serine 2 (TMPRSS2) and lysosomal cathepsins] to facilitate S2 spike domain exposure (8, 23). Hoffman et al. (8) demonstrated that TMPRSS2 expression is crucial for membrane fusion and ultimate cellular entry. However, recent insights indicate that SARS-CoV-2 exploits host furin for S1/S2 preactivation, reducing viral reliance on cellular TMPRSS2 and improving entry into cell types exhibiting lower TMPRSS2 expression (23). In this way, SARS-CoV-2 may: achieve initial infection via nasal and pulmonary tissues, undergo preactivation by host cell furin during viral packaging, enter blood circulation, infect off-target tissues containing ACE2- and TMPRSS2-rich cell-types, as well as those with lower TMPRSS2 expression (23).
SKELETAL MUSCLE, ACE2, AND UNIQUE COVID-19 CONSEQUENCES
Skeletal muscle is a complex tissue displaying remarkable plasticity (9). The importance of skeletal muscle function may be best exemplified by the process of respiratory ventilation. The diaphragm and parasternal intercostal muscles play an essential role in respiration, working together as a respiratory pump during ventilation (6). In this regard, infections and pathologies which negatively affect skeletal muscle function can disrupt pulmonary ventilation via abatement of respiratory muscle strength (13, 18). Skeletal muscle myofibers are multinucleated and display enhanced regenerative capability (24). Similar to cardiac tissue, skeletal muscle expression of ACE2 is well-documented (17, 20). The skeletal muscle environment contains various cell types beyond myofibers, such as satellite cells, leukocytes, fibroblasts, and endothelial cells (4, 9). Single-cell sequencing data from cardiac tissue indicate that ACE2 expression is highly observed in cardiomyocytes, as well as to a lesser extent in leukocytes and endothelial cells (19). The Tabula Muris single-cell transcriptome compendium indicates that ACE2 expression is detectable in a select number of skeletal muscle satellite cells, mesenchymal stem cells, endothelial cells, and lymphocytes derived from mouse limb muscle (22). Further, Giordani et al. (5) performed single-cell RNA sequencing on mouse limb muscle, which revealed detectable ACE2 expression in select skeletal muscle stem cells, Twist2-positive progenitor cells, and smooth muscle mesenchymal stem cells (5).
It is plausible that various skeletal muscle cell types may independently and/or collectively exhibit susceptibility to SARS-CoV-2 via ACE2. Resident immune cells may be involved in SARS-CoV-2 through ACE2 interaction, as SARS-CoV-2 infection in lung activates various leukocytes to release a cascade of cytokines, including interleukin-6 (IL-6) (26). Importantly, systemic elevations of IL-6 can disrupt muscle metabolic homeostasis and exacerbate muscle loss (27). Thus, we posit that skeletal muscle may be impacted by SARS-CoV-2 through direct infection of resident ACE2-rich cell types via circulating SARS-CoV-2, and/or indirectly through systemic cytokine release and subsequent homeostatic perturbation. The potential susceptibility of skeletal muscle is partially supported by clinical evidence indicating skeletal muscle pain, weakness, and injury as common symptoms of COVID-19 affliction (10, 12, 25, 29). A recent clinical case demonstrates that skeletal muscle complications may prove to be drastic in late-stage COVID-19 infection, manifesting as severe rhabdomyolysis (10a). The combined evidence of skeletal muscle ACE2 expression (17, 20), infection potential of ACE2-rich cell-types (8, 12, 23), COVID-19-mediated systemic inflammatory response (26), and clinical accounts of muscle symptoms (10, 12, 25, 29) are illustrative of potential skeletal muscle susceptibility to COVID-19. These data raise unique outcome concerns, considering the importance of skeletal muscle function in maintaining healthy respiration.
COVID-19, SKELETAL MUSCLE MYOPATHIES, AND RESPIRATORY CONSEQUENCE
Skeletal muscle myopathies are widespread (1, 3, 15, 21), and associated with populations that are known to be at-risk for COVID-19. The aging population, who are of particular risk to COVID-19 (7, 30), are also commonly afflicted with age-related skeletal muscle wasting and loss of function (i.e., sarcopenia) (1). Perhaps equally pertinent are dystrophic populations, who have received significantly less attention regarding COVID-19 infection susceptibility. Duchenne muscular dystrophy (DMD) is a critical type of muscular dystrophy resulting in system-wide skeletal muscle function loss, and diminished lifespan (21). Severe cases of DMD target the respiratory muscles, ultimately leading to respiratory failure (21).
As COVID-19 primarily affects the pulmonary system (10, 28–30), and DMD patients exhibit compromised respiratory muscle function, we posit that DMD patients infected with SARS-CoV-2 may suffer more severe symptoms and worsened mortality outcomes. Additional evidence suggests that DMD augments skeletal muscle ACE2 expression (20), which may increase SARS-CoV-2 infection of dystrophic muscle, contributing to worsened DMD muscle-related symptoms. Factoring in the observed COVID-19 systemic inflammatory response may further contribute to this milieu. These data suggest that COVID-19 and DMD may amplify symptom severity of both diseases, concurrently. This may be of specific importance to diaphragm muscle failure, which often marks ultimate respiratory failure in DMD (14). Diaphragm muscle function is similarly impacted during aging due to sarcopenia (11), while the severity of sarcopenic muscle loss correlates with markers of systemic inflammation in chronic obstructive pulmonary disease (COPD) patients (2). Thus skeletal muscle susceptibility to COVID-19 may extend to several myopathic conditions associated with aging and comorbidities.
CONCLUDING REMARKS
COVID-19 may have the ability to infect multiple tissue-types, with a unique potential to target skeletal muscle. Of particular importance may be the susceptibility of muscles associated with the respiratory pump, such as the diaphragm and intercostal muscles. Myopathies, such as DMD, reduce respiratory muscle function and augment ACE2 expression, potentially increasing the susceptibility and severity of COVID-19 infection. Several of the previously identified COVID-19 at-risk populations also tend to be independently afflicted with myopathic conditions (e.g., sarcopenia, diabetes-associated muscle dysfunction), which may pose unique complications related to COVID-19 infection. Factoring in the systemic SARS-CoV-2-mediated inflammatory response may further exacerbate myopathies. We speculate that skeletal muscle may be uniquely affected by SARS-CoV-2, while myopathies may increase both infection susceptibility, and outcome severity. Specifically, respiratory muscles may be of particular relevance, as any decrement in function would result in compromised ventilation, therefore exacerbating any pulmonary complications as a result of COVID-19 infection.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.F. and J.S.M. drafted manuscript; P.J.F., S.E.A., and J.S.M. edited and revised manuscript; P.J.F., S.E.A., and J.S.M. approved final version of manuscript; J.S.M. prepared figures.
REFERENCES
- 1.Alway SE, Myers MJ, Mohamed JS. Regulation of satellite cell function in sarcopenia. Front Aging Neurosci 6: 246, 2014. doi: 10.3389/fnagi.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis 12: 669–675, 2017. doi: 10.2147/COPD.S130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza DM, Al-Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol 4: 379, 2013. doi: 10.3389/fphys.2013.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deyhle MR, Hyldahl RD. The role of T lymphocytes in skeletal muscle repair from traumatic and contraction-induced injury. Front Physiol 9: 768, 2018. doi: 10.3389/fphys.2018.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordani L, He GJ, Negroni E, Sakai H, Law JYC, Siu MM, Wan R, Corneau A, Tajbakhsh S, Cheung TH, Le Grand F. High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations. Mol Cell 74: 609–621.e6, 2019. doi: 10.1016/j.molcel.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Gransee HM, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Compr Physiol 2: 1441–1462, 2012. doi: 10.1002/cphy.c110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19 . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55: 2000547, 2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppeler H. Molecular networks in skeletal muscle plasticity. J Exp Biol 219: 205–213, 2016. doi: 10.1242/jeb.128207. [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 26: 1618–1620, 2020. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley RC, Ferreira LF. Diaphragm abnormalities in heart failure and aging: mechanisms and integration of cardiovascular and respiratory pathophysiology. Heart Fail Rev 22: 191–207, 2017. doi: 10.1007/s10741-016-9549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J Med Virol 92: 552–555, 2020. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindqvist J, Cheng AJ, Renaud G, Hardeman EC, Ochala J. Distinct underlying mechanisms of limb and respiratory muscle fiber weaknesses in nemaline myopathy. J Neuropathol Exp Neurol 72: 472–481, 2013. doi: 10.1097/NEN.0b013e318293b1cc. [DOI] [PubMed] [Google Scholar]
- 14.Lo Mauro A, Aliverti A. Physiology of respiratory disturbances in muscular dystrophies. Breathe (Sheff) 12: 318–327, 2016. doi: 10.1183/20734735.012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maheshwari J, Kolaitis N, Anderson M, Benvenuto L, Gao Y, Katz P, Wolters P, Golden J, Kukreja J, Hays S, Greenland JR, Shah R, Leard LE, Trinh B, Oyster M, Covinsky K, Calabrese D, Venado A, Patel P, Huang C, Glidden D, Kleinhenz M, Sutter N, Tietje-Ulrich G, Brown M, Arcasoy S, Christie JD, Diamond J, Singer JP. Sarcopenia is associated with frailty in lung transplant candidates. J Heart Lung Transplant 39: S391, 2020. doi: 10.1016/j.healun.2020.01.514. [DOI] [Google Scholar]
- 17.Motta-Santos D, Dos Santos RAS, Oliveira M, Qadri F, Poglitsch M, Mosienko V, Kappes Becker L, Campagnole-Santos MJ, M Penninger J, Alenina N, Bader M. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 39: 506–512, 2016. doi: 10.1038/hr.2016.28. [DOI] [PubMed] [Google Scholar]
- 18.Naddaf E, Milone M. Hereditary myopathies with early respiratory insufficiency in adults. Muscle Nerve 56: 881–886, 2017. doi: 10.1002/mus.25602. [DOI] [PubMed] [Google Scholar]
- 19.Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 41: 1804–1806, 2020. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riquelme C, Acuña MJ, Torrejón J, Rebolledo D, Cabrera D, Santos RA, Brandan E. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS One 9: e93449–e93449, 2014. doi: 10.1371/journal.pone.0093449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M, Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis 12: 79, 2017. doi: 10.1186/s13023-017-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaum N, Karkanias J, Neff NF, May AP, Quake SR, Wyss-Coray T, Darmanis S, Batson J, Botvinnik O, Chen MB, Chen S, Green F, Jones RC, Maynard A, Penland L, Pisco AO, Sit RV, Stanley GM, Webber JT, Zanini F, Baghel AS, Bakerman I, Bansal I, Berdnik D, Bilen B, Brownfield D, Cain C, Chen MB, Chen S, Cho M, Cirolia G, Conley SD, Darmanis S, Demers A, Demir K, de Morree A, Divita T, du Bois H, Dulgeroff LBT, Ebadi H, Espinoza FH, Fish M, Gan Q, George BM, Gillich A, Green F, Genetiano G, Gu X, Gulati GS, Hang Y, Hosseinzadeh S, Huang A, Iram T, Isobe T, Ives F, Jones RC, Kao KS, Karnam G, Kershner AM, Kiss BM, Kong W, Kumar ME, Lam JY, Lee DP, Lee SE, Li G, Li Q, Liu L, Lo A, Lu W-J, Manjunath A, May AP, May KL, May OL, Maynard A, McKay M, Metzger RJ, Mignardi M, Min D, Nabhan AN, Neff NF, Ng KM, Noh J, Patkar R, Peng WC, Penland L, Puccinelli R, Rulifson EJ, Schaum N, Sikandar SS, Sinha R, Sit RV, Szade K, Tan W, Tato C, Tellez K, Travaglini KJ, Tropini C, Waldburger L, van Weele LJ, Wosczyna MN, Xiang J, Xue S, Youngyunpipatkul J, Zanini F, Zardeneta ME, Zhang F, Zhou L, Bansal I, Chen S, Cho M, Cirolia G, Darmanis S, Demers A, Divita T, Ebadi H, Genetiano G, Green F, Hosseinzadeh S, Ives F, Lo A, May AP, Maynard A, McKay M, Neff NF, Penland L, Sit RV, Tan W, Waldburger L, Youngyunpipatkul J, Batson J, Botvinnik O, Castro P, Croote D, Darmanis S, DeRisi JL, Karkanias J, Pisco AO, Stanley GM, Webber JT, Zanini F, Baghel AS, Bakerman I, Batson J, Bilen B, Botvinnik O, Brownfield D, Chen MB, Darmanis S, Demir K, de Morree A, Ebadi H, Espinoza FH, Fish M, Gan Q, George BM, Gillich A, Gu X, Gulati GS, Hang Y, Huang A, Iram T, Isobe T, Karnam G, Kershner AM, Kiss BM, Kong W, Kuo CS, Lam JY, Lehallier B, Li G, Li Q, Liu L, Lu W-J, Min D, Nabhan AN, Ng KM, Nguyen PK, Patkar R, Peng WC, Penland L, Rulifson EJ, Schaum N, Sikandar SS, Sinha R, Szade K, Tan SY, Tellez K, Travaglini KJ, Tropini C, van Weele LJ, Wang BM, Wosczyna MN, Xiang J, Yousef H, Zhou L, Batson J, Botvinnik O, Chen S, Darmanis S, Green F, May AP, Maynard A, Pisco AO, Quake SR, Schaum N, Stanley GM, Webber JT, Wyss-Coray T, Zanini F, Beachy PA, Chan CKF, de Morree A, George BM, Gulati GS, Hang Y, Huang KC, Iram T, Isobe T, Kershner AM, Kiss BM, Kong W, Li G, Li Q, Liu L, Lu W-J, Nabhan AN, Ng KM, Nguyen PK, Peng WC, Rulifson EJ, Schaum N, Sikandar SS, Sinha R, Szade K, Travaglini KJ, Tropini C, Wang BM, Weinberg K, Wosczyna MN, Wu SM, Yousef H, Barres BA, Beachy PA, Chan CKF, Clarke MF, Darmanis S, Huang KC, Karkanias J, Kim SK, Krasnow MA, Kumar ME, Kuo CS, May AP, Metzger RJ, Neff NF, Nusse R, Nguyen PK, Rando TA, Sonnenburg J, Wang BM, Weinberg K, Weissman IL, Wu SM, Quake SR, Wyss-Coray T; Tabula Muris Consortium; Supplemental Text Writing Group . Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562: 367–372, 2018. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA 117: 11727–11734, 2020. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ, Parise G. Satellite cells in human skeletal muscle plasticity. Front Physiol 6: 283, 2015. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol 92: 548–551, 2020. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderVeen BN, Fix DK, Montalvo RN, Counts BR, Smuder AJ, Murphy EA, Koh HJ, Carson JA. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin-6. Exp Physiol 104: 385–397, 2019. doi: 10.1113/EP087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]


