Human coronaviruses account for up to 30% of infections of the upper respiratory tract and 8.1% of enteritis [1,2]. However, related to the wide distribution of angiotensin-converting enzyme 2 in human tissues, several reports have already hypothesized that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may directly infect multiple organs, including liver, stomach, ileum and colon [3].
Our patient, a man in his 50s with no relevant medical history, had a positive nop-swab result for SARS-CoV-2 RNA 4 days after the start of symptoms. He was treated at home with hydroxychloroquine and azithromycin for 7 days. The patient recovered and had two repeated nop-swabs, both with negative results. After 2 weeks, he was admitted to the emergency department complaining of stomach pain followed by a syncopal episode and intestinal bleeding. Asymptomatic bilateral interstitial pneumonia was documented by computed tomographic scan.
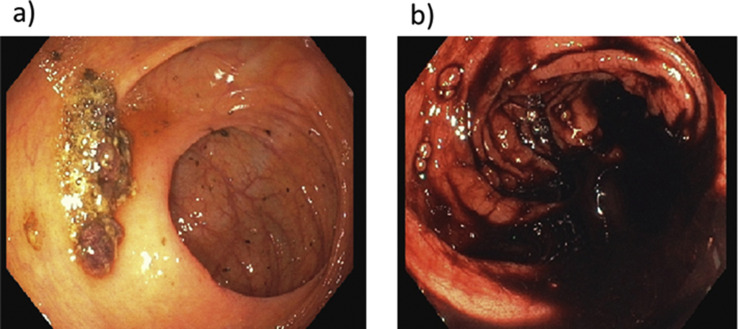
A colonoscopy detected an ileocecal valve proximal ulceration (Supplementary Fig. S1) without active bleeding. The patient underwent emergency surgery due to a hypotensive episode associated with haematic stools. Terminal ileum and cecum were resected as well as Meckel diverticulum.
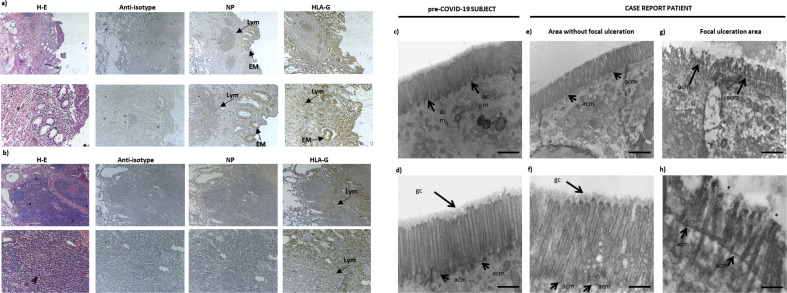
At histologic examination, the terminal ileum wall showed chronic inflammatory infiltrates with prevalence of lymphocytes as well as focal ulceration of both mucosa (Fig. 1 (a)) and submucosa (Fig. 1(b)). Abnormally enlarged and tortuous thick-walled veins were seen in the submucosa. Immunohistochemical analysis with anti–SARS-CoV-2 nucleocapsid protein revealed the presence of viral protein expression in epithelial cell of ulcerated intestinal mucosa (cytoplasmic staining) and in a minority of lymphocytes (Fig. 1(a)), but with no staining in the submucosa (Fig. 1(b)). We also analysed the expression of a nonclassical major histocompatibility complex I molecule, human leukocyte antigen G (HLA-G), which blocks endothelial cell proliferation and vessel formation [4]. We observed HLA-G in epithelial cells of the intestinal mucosa and in some lymphocytes (Fig. 1(a)), in correspondence with SARS-CoV-2–positive sites. In the submucosa, HLA-G expression was detectable only in few lymphocytes (Fig. 1(b)).
Fig. 1.
(a, b) Haematoxylin and IHC staining of ulcerated ileum section area. IHC analysis was performed on terminal ileum sections with anti–SARS-CoV-2 NP (Centennial, Novus Biologicals; 1:250 dilution) and anti–HLA-G (MEM-G2, Exbio; 1:200 dilution). Slides were counterstained with haematoxylin. Anti-isotype, anti–SARS-CoV-2 NP and anti–HLA-G were analysed in (a) mucosa and (b) submucosa. Original magnification 4 × (upper line) and 10 × (lower line). (c–f) Representative TEM images of epithelial cell apical surface microvilli in ileum tract. (c, d) Pre–COVID-19 subject. (e, f) Case report patient in an area without focal ulceration. (g, h) Case report patient in a focal ulcerated area. ∗gc corresponding area. For TEM analysis, samples were fixed in 2.5% glutaraldehyde in 0.1 M PBS (pH 7.4) and postfixed in 2% osmium tetroxide, dehydrated in acetone solutions and included in araldite Durcupan ACM, then counterstained with uranyl acetate in saturated solution and lead citrate and observed under Zeiss EM910 TEM at 100 kV. Scale bars = 2 μm (a, c, e), 1 μm (b) and 0.5 μm (d, f). acm, apical cell membrane; COVID-19, coronavirus disease 2019; EM, epithelial cell of intestinal mucosa; gc, glycocalyx; HLA-G, human leukocyte antigen G; IHC, immunohistochemical; Lym, lymphocytes; NP, nucleocapsid protein; PBS, phosphate-buffered saline; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sg, secretion granules; TEM, transmission electron microscope.
Transmission electron microscopy revealed a significantly different morphologic microvilli profile; the intestinal tract of a normal ileum from a subject before appearance of coronavirus disease 2019 (COVID-19) showed well-organized and aligned microvilli, with a regular distribution protruding from the apical cell membrane (acm) and an homogeneous glycocalyx (gc) (Figs. 1(c) and (d)). Tissue examined in the area without focal ulceration showed a morphology similar to control (Figs. 1 (e) and (f)). Regarding the ulcerated area, microvilli appeared shorter than those in the unulcerated area, partially deepened beyond the acm and gc, appearing disorganized and almost absent with a relevant cytopathic effect (Figs. 1(g) and (h)).
We describe the presence of SARS-CoV-2 nucleocapsid proteins and related microvilli morphologic alterations in the small intestinal wall in correspondence with the site of a bleeding ulceration in a patient free of COVID-19 for 4 weeks. The ulceration has two possible explanations: it may have been caused by a systemic inflammation (cytokine storm) [5]; or it may have been caused by an inflammation potentially induced by viral replication in a plaque restricted to the area of ulceration induced by the presence of SARS-CoV-2 [6].
The induction of HLA-G expression, known to be involved in both viral immune-escape mechanism and neoangiogenesis processes, at the site of SARS-CoV-2 infection might be a cause of the COVID-19–dependent bleeding.
We highlight the presence of SARS-CoV-2 nucleocapsid proteins in the gastrointestinal tract. It is conceivable that other tissues may behave as virus storage, thus indicating that nonoropharyngeal swab may not be enough to characterize a COVID-19–free patient. Furthermore, it could be hypothesized that this storage is the basis for SARS-CoV-2 infection's becoming chronic in some subjects.
Acknowledgements
We acknowledge financial support from a COVID-19 grant from the University of Ferrara (PI: Roberta Rizzo and Angelina Passaro). The authors are grateful to Paola Boldrini (LTTA–Electron Microscopy Center, University of Ferrara, Italy) and Cristina Bosi (Department of Translational Medicine, University of Ferrara, Italy) for helpful collaboration.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.01.012.
Transparency declaration
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Gastrointestinal Endoscopic image: a) First colonscopy showing 5 cm ulceration proximally to the ileo-cecal valve. b) Second colonscopy displaying clotted blood within the colon.
References
- 1.Jevšnik M., Steyer A., Pokorn M., Mrvič T., Grosek Š, Strle F. The role of human coronaviruses in children hospitalized for acute bronchiolitis, acute gastroenteritis, and febrile seizures: a 2-year prospective study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu S.S., Hung Chan K., Wing Chu K., Kwan S.W., Guan Y., Man Poon L.L. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mönkemüller K., Fry L.C., Rickes S. COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Rev Esp Enfermedades Dig. 2020;112:383–388. doi: 10.17235/reed.2020.7137/2020. [DOI] [PubMed] [Google Scholar]
- 4.Le Bouteiller P., Fons P., Herault J.P., Bono F., Chabot S., Cartwright J.E. Soluble HLA-G and control of angiogenesis. J Reprod Immunol. 2007;76:17–22. doi: 10.1016/j.jri.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendoza E.J., Manguiat K., Wood H., Drebot M. Two detailed plaque assay protocols for the quantification of infectious SARS-CoV-2. Curr Protoc Microbiol. 2020;57 doi: 10.1002/cpmc.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.