Abstract
The COVID-19 pandemic has raised many issues not the least of which is the reason for its high variability in consequences to the infected person. In this opinion letter, we advocate that the dose and presentation of the infecting virus is a major factor that affects whether the outcome is subclinical, tissue damaging or even lethal following infection. We briefly describe the known effects of virus dose on the course COVID-19 and discuss practical maneuvers as well as largely untested procedures that can raise the threshold dose needed to break through barriers of resistance.
Keywords: Covid-19, Dose, Innate immunity, Galectins
In the annals of infectious diseases, 2020 has been an annus horribilis. It began with the world learning about a new disease caused by an unfamiliar agent called a coronavirus. The virus was named SARS-CoV2 and usually referred to as COVID-19. Since early 2020, COVID-19 has caused a worldwide pandemic, infecting millions and killing more than one and a half million people. Down the millennia, the world has experienced many pandemics two of which, the Black Death and small pox have shaped human history. Conceivably, COVID-19 could join this infamous list given its impact on our lifestyle, economy and our future. Recently, effective vaccines have been produced and these may help resolve the pandemic. However, much still needs to be understood most especially why the consequences of the infection vary so markedly. While the majority of people have inapparent or minimal consequences, others suffer severely and may succumb to the infection. As Shakespeare might proclaim: COVID-19: disease, or no disease, that is the question. To which a famous politician might well answer: It’s the dose stupid!
Whether or not any virus infection has clinical consequences depends on multiple variables that involve the virus, the host genetics, comorbidities, and the circumstances of infection. We and others have discussed this topic in depth [1,2]. With respect to initial infection, the most critical variable is the load and presentation of the virus that infects. This variable has been extensively investigated by experimental virologists, where it is possible to accurately manipulate the number of viral particles given over a unit of time as well as control the nature of the infecting dose. Since none of these maneuvers are feasible with human viral diseases acquired, we must rely on comparing the outcome of natural infection in circumstances where we assume the load of infection would differ. However, the message from virologists who use animal models is clear-cut for most viruses. The outcome includes a spectrum of responses. Minimal infection loads will elicit no clinical or pathologic reactions and the virus would be silently controlled mainly by the innate immune system [2,3]. Most viruses can induce the production of interferons (IFN), which participate in terminating viral infections in a number of ways [4,5]. For example, type I IFN acts primarily, but not exclusively, to protect uninfected cells from being infected.
If the load of infection is raised to moderate levels, clinical disease and tissue pathology becomes more a likely possibility [[1], [2], [3]]. Thus, innate immune defenses are inadequate to stop the virus from causing some overt damage, but under such circumstances adaptive aspects of immunity, which includes antibodies and T cells, are almost invariably elicited to help control the infection although in so doing may cause a tissue-damaging inflammatory reaction [1]. Usually, the combined activity of innate and adaptive immune mechanisms succeed in clearing infection, but this scenario does not always occur and it becomes increasingly unlikely, as the load of infection is further increased. With some viruses, the eventual fate is the host’s demise, although killing its host is not an ideal situation for a virus when viewed from an evolutionary perspective. Fortunately for us few viruses achieve such an outcome [6,7], but the new pathogen COVID-19 is an example where lethality is on the menu. Nevertheless, whereas almost all countries are experiencing the COVID-19 pandemic, the frequency of lethality is very different. We have suggested one explanation for the variable outcome in a recent opinion piece [8]. We advocate that frequent exposure to microbes, as is more likely to occur in less hygienic living conditions, will train innate aspects of immunity to function more effectively and reduce the viral burden infection below levels that are potentially lethal [9].
It seems obvious that wearing masks and staying some distance from potential sources of infection will serve to limit the exposure dose of SARS-CoV2. For example, countries like Taiwan, which has been extraordinarily successful at limiting infection, and especially mortality may provide some guidance [10]. Taiwan successfully implemented mask wearing, social separation and strict quarantine practices backed up by an excellent government health system. In contrast, in countries such as the USA mortality is greater probably because its citizenship is far less compliant with those measures that reduce the magnitude of infection. Curiously, in the early days of the pandemic, mortality rates were far higher in Italy than in Germany, a situation that likely reflected the affectionate greeting customs of the Italians, which would expose them to higher loads of COVID-19 infection [11].
If, as we advocate, the load of infection received during initial infection is a crucial paradigm what can we do to limit this scenario? The first and most obvious approach is to employ strategies that minimize the magnitude and effective formulation of the virus exposure dose. We know that COVID-19 is mainly a respiratory pathogen, which is transmitted primarily in the form of aerosols or droplets in the expired air of actively infected persons. The levels of virus expelled are greater by 20–30 fold with symptomatic patients as compared to those without symptoms [12]. Infectious virus can also remain for a time on fomites, although this is not thought to be a major source of infection [12]. Infected persons can produce virus-containing droplets ranging in size from 0.1 to 1000 μM, of which the largest settle rapidly and the smallest quickly evaporate, desiccating and soon destroying the virus [[12], [13], [14]]. The most dangerous droplets are those around 0.4 μM since these can access the lower lungs to set the scene for potentially damaging and lethal lesions. Such small droplets may even avoid triggering the innate immune defenses in the upper mucosal microenvironment [15]. Virus containing droplets can be expelled two meters or more with opera singers, trombone players and loud politicians being the long distance champions! Moreover, with COVID-19 infection some persons become so-called super spreaders and can dispel 100,000 virion containing droplets per minute whereas with most symptomatic patients it is closer to 1000 particles per minute [16]. Unfortunately, super spreaders normally are not identified. In consequence, measuring not only the presence, but also the amount (dose) of virus in infected persons would be valuable information to help control the pandemic.
There are simple ways to minimize the exposure dose of SARS-CoV2. These include staying out of the transmission range of potentially infected persons (the practice popularly referred to as social distancing) and/or be equipped with a barrier of some form that will limit, if not entirely prevent, infection i.e., a mask or screen. Actually, there is some evidence that precluding infection entirely may not always be an ideal scenario. Thus, cutting down the infection dose by wearing a mask can change a disease-producing dose to the one that causes asymptomatic or mild infection, but is still capable of inducing adaptive immunity [[17], [18], [19]]. There is good reason to believe that small doses of infection provide minimal danger particularly to young healthy persons. This raises the question whether procedures can be developed that would make high exposure doses less dangerous perhaps even to those with underlying health problems such as diabetes and morbid obesity. Likely, there are ways of achieving this objective, but such therapeutic approaches to control infection have not been adequately explored.
One approach, however, recently became worldwide news when the US President, after acquiring COVID-19 infection, was exposed to an aggressive treatment regimen of high titer (much higher than the general public usually receives) neutralizing monoclonal antibodies along with an intravenously administered antiviral drug. This treatment would succeed in dramatically inhibiting the amount of virus produced and likely preclude it from reaching a disease-producing dose. Conceivably early aggressive antiviral therapy might be valuable to others, but few can receive such medical help in a timely or affordable fashion. We contend that a more generally accessible and less costly strategy could be to use approaches that raise the threshold dose of virus needed to become overtly pathogenic. One approach advocated to mitigate damage caused due to COVID-19 is to use the anti-tuberculosis vaccine Bacillus Calmette–Guérin (BCG). This approach is expected to activate some aspects of innate immunity and may also prime cross-reactive T cell responses to exert protective effects via their release of cytokines and organizing inflammatory reactions [20]. Trials are underway and hope for their success is fueled by reports that the prevalence of lethal COVID-19 is less in communities where BCG is still practiced.
Another approach, we favor that could boost innate immunity and raise the threshold dose required to cause damaging infection is to infuse molecules such as S-type lectins, also known as galectins [21]. Some galectins act to activate some aspects of innate immunity such as NK cell function and as a bonus can potentially reduce viral loads by directly binding to heavily glycosylated viral proteins involved in viral entry into cells [21]. However, using molecules such as galectins to block susceptibility to COVID-19 have yet to be explored or at least reported. Some members of the galectins family may also be useful to switch the balance of T cell reactions to COVID-19 in the lungs from a pattern that is highly damaging to one that favors recovery. This transition can be achieved when the functional type of T cell is changed from a situation where Th1 and Th17 T cells predominate to one where T regulatory cells are more numerous [20]. This occurs when galectins such as galectin 9 are administered during an inflammatory process as was shown in some models of autoimmunity [22] and in some viral-induced inflammatory lesions [21]. The approach could be worth investigating in those suffering severe COVID-19 lung lesions.
With regard to boosting the innate immune barrier to withstand higher doses of COVID-19 infection as for most infections interferon response is critical and coronaviruses are adept in blocking IFN response in the host cells [23,24]. Interferons exert a range of activities that include making susceptible uninfected cells resistant to infection and modulating some protective aspects of inflammatory responses serving to facilitate recovery. There are approaches that induce interferon responses to raise the resistance barrier such as poly IC to activate TLR3. Additionally, it would be possible to administer interferon proteins directly into the upper respiratory tract that would succeed in raising resistance to high dose exposure. Such an approach could be used for prophylaxis or in the early stages of COVID-19, but so far this strategy has not been reported.
Finally, once infection has occurred it should be possible to successfully diminish virus replication and therefore diminish the pathological consequences by using humanized neutralizing antibodies in very high doses. The controlled trials are currently ongoing, but the data is not available from such studies. Many groups are pursuing the development of such antibodies or their shorter variants as well as using convalescent plasma to treat infected patients early after infection. However, the success of such procedures so far has been variable. One approach to diminishing the viral yield after infection, so far untried, that we favor is to develop single domain antibodies (sdAbs) of camelid origin [25,26]. Such sdAbs are less immunogenic, can be produced in abundance in usable formats, remain soluble and stable even at elevated temperatures thereby enhancing their durability in functioning [25,26]. Furthermore, such antibodies can be injected via intranasal routes to neutralize the virus limit its infecting dose and will cause no antibody dependent enhancement of infection.
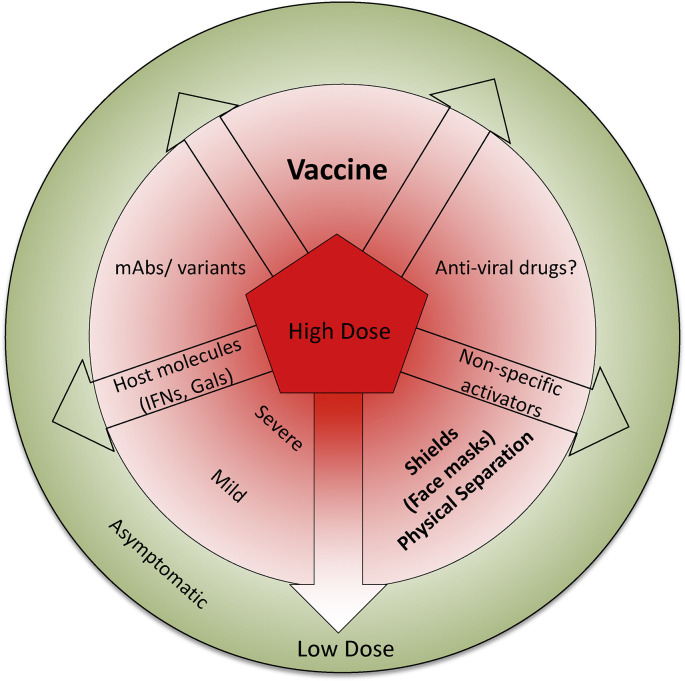
We can conclude that an important variable that affects the outcome of infection with COVID-19 is the dose of exposure to the virus that a person receives. We also advocate that it is possible to raise the threshold dose that would be needed to cause serious infections by treating persons early after exposure with some so far poorly explored maneuvers (Fig. 1). Of course the long time solution to control any virus is with an effective vaccine and these are now available. It is likely that the vaccines against COVID-19 will not provide the levels and duration of protection achieved by measles or poliomyelitis vaccines. In fact, some genetic based vaccines are now being used in the USA and other countries and these appear to afford protection very rapidly. This might be attributed to their inducing a type IFN response [[27], [28]], which could occur by ligating cytosolic innate immune receptors, such as toll like receptors (TLRs), retinoic acid inducible gene-1 like receptor (RLR) to offer a broad based protection against different viruses including against COVID-19. This short-term phase of protection by the genetic vaccines is followed by inducing adaptive immunity which appears to be initiated by 9 days post immunization, but how long and how effective this protection is sustained needs to be established. However, even though COVID-19 vaccines may not provide long term sterile immunity, they should succeed in raising, perhaps substantially, the dose of virus needed to establish infection and cause significant clinical disease.
Fig. 1.
A cartoon demonstrating the influence of dose of infection on COVID-19 disease and some potential maneuvers to limit the infecting dose. Depending on the infecting dose the spectrum of COVID-19 disease ranges from asymptomatic to mild and severe disease. While the mild infections resolve with favorable outcome, severe disease invariably requires intensive care and some individuals eventually succumb to the infection. Interventions such as shielding devices and social behavior, vaccines, promotion of innate immune function, infusion of host derived or recombinant molecules such as type I IFNs, galectins, high affinity monoclonal antibodies or their variants as well as effective anti-viral drugs could reduce the initial dose of virus infection. The question mark represents uncertain efficacy of currently used anti-viral drugs. It is to be noted that the newly emerging variants such as the B.1.4.3 (N501Y) which appear to be transmitted more efficiently than parent strains and bind the cellular entry receptors with higher affinity. It is conceivable these variants might induce a patent infection at lower doses of infection than the parent strain.
Funding
This work was supported by the NIH 2020 R21 AI (number: 5R21AI142862-02) and NIH 2020 R01 (number: EY5R01EY005093-35).
Declaration of competing interest
The authors declare no conflict of financial interest.
References
- 1.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh S., McCaffery J.M., Eichelberger M.C. Dose-dependent changes in influenza virus-infected dendritic cells result in increased allogeneic T-cell proliferation at low, but not high, doses of virus. J Virol. 2000;74:5460–5469. doi: 10.1128/jvi.74.12.5460-5469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merois I., Cloutier A., Garneau E., Richter M.E. Initial infectious dose dictates the innate, adaptive and memory responses to influenza in the respiratory tract. J Leukoc Biol. 2012;92:107–121. doi: 10.1189/jlb.1011490. [DOI] [PubMed] [Google Scholar]
- 4.Park A., Iwasaki A. Type I and type III interferon-Induction signaling, evasion and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smits S.L., de-Lang A., van den Brand J.M.A., Leijten L.M., IJcken W.F., Eijkemans M.J.C. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLos Pathog. 2010;6:e1000756. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virgin H.W., Wherry J.E., Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Sehrawat S., Kumar D., Rouse B.T. Herpesviruses: harmonious pathogens but relevant cofactors in other diseases? Front Cell Infect Microbiol. 2018;8:177. doi: 10.3389/fcimb.2018.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehrawat S., Rouse B.T. Does hygiene hypothesis apply to COVID-19 susceptibility? Microb Infect. 2020;22:400–402. doi: 10.1016/j.micinf.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea M.G., Joosten L.A.B., Latz E., Mills K.H.G., Natoli G., Stunnberg H.G. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J.C., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan big data analytics, new technology, and proactive testing. J Am Med Assoc. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 11.https://www.nytimes.com/2020/04/04/world/europe/germany-coronavirus-death-rate.html
- 12.Stadnytskyi V., Bax C.E., Bax A., Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci Unit States Am USA. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 14.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368:1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- 16.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi M., Beyrer C., Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063–3066. doi: 10.1007/s11606-020-06067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi M., Rutherford G.W. Perspective facial masking for Covid-19 potential for “Variolation” as we await a vaccine. N Engl J Med. 2020;383:e101. doi: 10.1056/NEJMp2026913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Eggo R.M., Kucharski A.J. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet. 2020;395:10227. doi: 10.1016/S0140-6736(20)30462-1. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea M.G., Giamarellos-Bourboulis E.J., Jorge Domínguez-Andrés J., Curtis N., van Crevel R., van de Veerdonk F.L. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sehrawat S., Suryawanshi A., Rouse B.T. Role of TIM-3/galectin-9 inhibitory interaction in a virus induced immunoinflammatory lesion: shifting the balance towards Tregs. J Immunol. 2009;182:3191. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seki M., Oomizu S., Sakata K.M., Sakata A., Arikawa T., Watnabe K. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.S., Park S., Jeong H.W., Ahn J.Y., Choi S.J., Lee H. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanke L., Vidakovics Perez L., Sheward D.J., Das H., Schulte T., Moliner-Morro A. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat Commun. 2020;11:4420. doi: 10.1038/s41467-020-18174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Custódio T.F., Das H., Sheward D.J., Hanke L., Pazicky S., Pieprzyk J. Selection, biophysical and structural analysis of synthetic nanobodies that effectively neutralize SARS-CoV-2. Nat Commun. 2020;11:5588. doi: 10.1038/s41467-020-19204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2020 doi: 10.1016/S0140-6736(20)32661-1. S0140-6736: 32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahase E. Covid-19: pfizer and BioNTech submit vaccine for US authorization. BMJ. 2020;371 doi: 10.1136/bmj.m4552. m4552. [DOI] [PubMed] [Google Scholar]