Abstract
Background
Susceptibility to Covid-19 has been found to be associated with the ABO blood group, with O type individuals being at a lower risk. However, the underlying mechanism has not been elucidated. Here, we aimed to test the hypothesis that Covid-19 patients might have lower levels of ABO antibodies than non-infected individuals as they could offer some degree of protection.
Methods
After showing that the viral spike protein harbors the ABO glycan epitopes when produced by cells expressing the relevant glycosyltransferases, like upper respiratory tract epithelial cells, we enrolled 290 patients with Covid-19 and 276 asymptomatic controls to compare their levels of natural ABO blood group antibodies.
Results
We found significantly lower IgM anti-A + anti-B agglutination scores in blood group O patients (76.93 vs 88.29, P-value = 0.034) and lower levels of anti-B (24.93 vs 30.40, P-value = 0.028) and anti-A antibodies (28.56 vs 36.50, P-value = 0.048) in blood group A and blood group B patients, respectively, compared to controls.
Conclusion
In this study, we showed that ABO antibody levels are significantly lower in Covid-19 patients compared to controls. These findings could indicate that patients with low levels of ABO antibodies are at higher risk of being infected.
Keywords: ABO antibodies, ABO blood group, ABO antibody levels, Covid-19, Covid-19 infection risk
Introduction
In 2019, a mass outbreak of a novel coronavirus disease (Covid-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan, China and spread throughout the world (Zhu et al., 2020).
Studies from China and other parts of the world (USA, Turkey, Europe, Middle Eastern countries) have reported a relationship between the ABO blood group and the susceptibility to Covid-19. Despite some discrepancies, they showed a general trend that the risk of developing a SARS-CoV-2 infection was higher for individuals with type A blood and lower for those with type O blood (Abdollahi et al., 2020, Aljanobi et al., 2020, Boudin et al., 2020, Dzik et al., 2020, Ellinghaus et al., 2020, Gallian et al., 2020, Göker et al., 2020, Latz et al., 2020, Leaf et al., 2020, Li et al., 2020, Shelton et al., 2020, Valenti et al., 2020, Wu et al., 2020, Zeng et al., 2020, Zhao et al., 2020, Zietz et al., 2020). A similar lower risk for blood group O individuals had previously been observed in Hong Kong during the first major SARS outbreak, in 2003 (Cheng et al., 2005). However, the mechanism underlying these associations has not been elucidated.
Similar to SARS-CoV-1, SARS-CoV-2 primarily causes respiratory illness and infectious viral particles are mainly synthetized in epithelial cells of the upper respiratory tract (Chen and Subbarao, 2007, Gu and Korteweg, 2007, Lukassen et al., 2020, Sungnak et al., 2020, Wolfel et al., 2020, Ziegler et al., 2020). These cells might express the ABH antigens depending on the host secretory status controlled by polymorphisms in the FUT2 gene (Marionneau et al., 2001, Ravn and Dabelsteen, 2000). Viral particles produced by either A or B secretor individuals are then expected to be tagged with A or B carbohydrate epitopes, respectively. This led to the hypothesis that natural anti-A and/or anti-B isohemagglutinins could play a protective role against SARS-CoV. The hypothesis received preliminary support by the in vitro observation that anti-A antibodies could specifically block the interaction between the SARS-CoV-1 spike (S) glycoprotein and its target, the angiotensin-converting enzyme 2 (ACE2) receptor (Guillon et al., 2008). Since SARS-CoV-1 and SARS-CoV-2 use the same receptor (Ziegler et al., 2020) and exhibit similar glycosylation sites on their envelope S glycoprotein (Kumar et al., 2020), anti-A and possibly, by extension, anti-B antibodies may be expected to have similar effects against SARS-CoV-2, leading to virus attenuation. Alternatively, opsonization of viral particles by anti-A or anti-B antibodies could have immunological consequences, akin to those observed with the B blood group-related anti-αGal antibodies in the case of several other viruses that express the cognate αGal glycan epitope on their envelopes (Galili, 2019). Thus, it has been hypothesized that, when sufficient levels of anti-A and/or anti-B antibody titers are present, they might offer some degree of protection to individuals with O, A, and B blood types against the transmission of SARS-CoV-2 from infected ABO incompatible secretor individuals (Breiman et al., 2020).
Anti-A and anti-B antibody levels remain stable over time (Sprogøe et al., 2017). However, there are large variations in titers between individuals of the same blood type (Berséus et al., 2013). Accordingly, it is expected that individuals with low anti-A and/or anti-B levels would not benefit from the partial protection afforded by these antibodies at the time of infection. Consequently, an over-representation of individuals with low anti-A and/or anti-B antibody titers among patients is expected. The objective of this observational prospective study was to test the hypothesis that natural anti-A and/or anti-B antibody levels might be lower in patients with symptomatic Covid-19 compared to control subjects.
Materials and methods
Experimental in vitro study
Cell lines
Chinese hamster ovary (CHO) stable cell lines were transfected with either pDR2 empty vector (CHO-PDR2) or pDR2 containing cDNA encoding the rat α1,2-Fucosyltransferase B (pDR2-FTB) (CHO-H) or pDR2-FTB and pBK containing cDNA encoding the rat enzyme A (CHO-A) or pDR2-FTB and pBK containing cDNA encoding the rat-enzyme B (CHO-B), as previously described (Farkas et al., 2019, Guillon et al., 2008). HEK-293T cells were obtained from the American Type Culture Collection (ATCC) (CRL-3216).
Surface expression of A, B and H antigens on cell lines by flow cytometry
CHO cells and HEK-293T cells were trypsinized, and resuspended in phosphate-buffered saline (PBS)-0.1% bovine serum albumin (BSA). A total of 250,000 cells were stained with either monoclonal mouse anti-A (Diagast Laboratories, France), anti-B (EFS Rennes, France) or anti-H (FITC-conjugated Ulex europeaus Agglutinin) (Sigma–Aldrich) antibodies for 30–60 min at 4 °C. Finally, goat anti-mouse IgG F(Ab’)2 fragment conjugated to fluorescein isothiocyanate (F(Ab’)2-FITC) (Beckman Coulter) was added at a 1:200 dilution. Analysis was performed on a Celesta flow cytometer using the DIVA software (BD Biosciences).
Detection of A, B and H antigens on the SARS-CoV-2 S protein by enzyme-linked immunosorbent assay (ELISA)
The CHO stable cell lines were transfected with a plasmid encoding the S1 domain of the SARS-CoV-2 S protein fused with the Fc domain of a mouse IgG (obtained from Dr Jianxun Qi, Chinese Academy of Science, Beijing). After 48 h, the supernatant containing the S1-Fc protein was collected and concentrated as previously described (Wang et al., 2020).
ELISA plates (Maxisorp, Nunc, Thermo Fisher Scientific, Roskilde, Denmark) were coated at 4 °C overnight with either group A1-specific Dolichos biflorus Agglutinin (DBA) (50 μg/mL)(Vector Laboratories, Burlingame, CA), or αGal/group B-specific Griffonia simplicifolia Isolectin B4 (GSI-b4) (50 μg/mL) (Vector Laboratories, Burlingame, CA), or α1,2-Fucose-specific lectin UEA-I (10 μg/mL) (Vector Laboratories, Burlingame, CA) or a rabbit polyclonal antibody against SARS-CoV-2 S protein (10 μg/mL) (Genetex, Irvine, CA). The plates were washed 3 times with PBS-0.05% Tween 20 (PBS-T), and unbound sites were blocked with PBS-5% BSA for at least 1 h at 37 °C. The plates were washed 3 times again with PBS-T, and supernatants containing the S1-Fc protein were added to the plates at a 1:5 dilution in PBS-1% BSA for 1 h at 37 °C. After 3 more washes, a goat anti-mouse IgG (H + L)-conjugated horseradish peroxidase (IgG (H + L)-HRP) antibody (Uptima) (Interchim, Montluçon, France) was added at a 1:1000 dilution in PBS-1% BSA for 1 h at 37 °C. Finally, after 3 last washes with PBS-T and one with plain PBS, revelation was performed with 50 μL/well of TMB (Sigma–Aldrich, St Louis, MO) and the reaction was stopped with 50 μL/well of 1 M Phosphorous acid. Optical densities were read at 450 nm with a SPECTROstar Nano spectrophotometer (BMG Labtech, Champigny-sur-Marne, France).
Prospective observational study (CHUB-BDS-Covid19 ClinicalTrials.gov number: NCT04462627).
Study design and patients
This prospective observational study was carried out between March 11 and June 3, 2020 in CHU Brugmann and HUDERF. Every symptomatic patient with a positive real-time reverse transcriptase polymerase-chain-reaction (RT-PCR) test for SARS-CoV-2 on nasal and pharyngeal swab specimens was enrolled (Covid-19 group: n = 290). Residual blood samples (in EDTA) from routine laboratory testing were collected for hospitalized patients. Among ambulatory patients, blood samples (in EDTA) were specifically drawn for the study. ABO typing was performed with standardized tests, and IgM anti-A and/or anti-B agglutination scores were evaluated. The following data were obtained from patient medical records: date of birth, gender, and the date of the positive SARS-CoV-2 RT-PCR test.
A control group (n = 276) was recruited between May 6 and June 16, 2020. This group included asymptomatic ambulatory patients who were not tested for Covid-19 or hospitalized patients with a negative SARS-CoV-2 RT-PCR test. We collected residual blood samples (in EDTA) to determine standard IgM anti-A and/or anti-B agglutination scores.
When available, remaining blood sera from both the Covid-19 group (n = 74) and the control group (n = 88) were tested for anti-sheep erythrocytes and anti-αGal antibodies.
Outcomes
The primary outcomes were the IgM anti-A + anti-B agglutination scores for individuals with blood type O, the IgM anti-B agglutination score for individuals with blood type A, and the IgM anti-A agglutination score for individuals with blood type B. Secondary outcomes were the natural anti-αGal antibody agglutination score and the anti-sheep erythrocyte antibody level.
Assessment of IgM anti-A and/or anti-B agglutination scores
ABO typing was performed with monoclonal reagents and the column-agglutination technique (Bio-Rad, Cressier sur Morat, Switzerland). IgM anti-A and anti-B antibody titers were determined with serial dilutions of patient plasma and the ID-Card NaCl, Enzyme Test and Cold Agglutinins on the IH-500 automated system (Bio-Rad, Cressier sur Morat, Switzerland), according to the manufacturer’s protocol. Titers were expressed as an agglutination score, which was calculated as the sum of scored agglutination reaction results in each dilution, starting with the 1:2 dilution (Funk et al., 2014). Agglutination reaction scores of 12, 10, 8, 5, 2, or 0 indicated 4+, 3+, 2+, 1+, ±, and negative reactions, respectively.
Assessment of other natural antibodies
Anti-αGal antibody levels were assessed by ELISA. ELISA plates (Maxisorp, Nunc, Thermo Fisher Scientific, Roskilde, Denmark) were coated with synthetic αGal trisaccharide (Galα1,3-Galβ1,4-GlcNAcβ-PAA) at 5 μg/mL in 0.1 M Carbonate buffer pH 9.0 overnight at 4 °C. The plates were washed 3 times with PBS-T, and unbound sites were blocked with PBS-5% BSA for 2 h at 37 °C. The plates were washed 3 times again with PBS-T and plasma samples (EDTA) from patients with Covid-19 or controls were added to the plate at a 1:50 dilution in PBS-1% BSA for 1 h at 37 °C. The plates were washed 3 times with PBS-T and Donkey anti-human IgG (H + L)-HRP (Jackson ImmunoResearch Laboratories Inc, Ely, United Kingdom) was added at a 1:5000 dilution in PBS-1% BSA for 1 h at 37 °C. Finally, after 3 last washes with PBS-T and one with plain PBS, revelation was performed with 50 μL/well of TMB (Sigma–Aldrich, St Louis, MO) and the reaction was stopped with 50 μL/well of 1 M Phosphorous acid. Optical densities were read twice at 450 nm with a SPECTROstar Nano spectrophotometer (BMG Labtech, Champigny-sur-Marne, France).
Natural anti-sheep erythrocytes were quantified by measuring agglutination with sheep erythrocytes, similar to the technique used for quantifying natural anti-A and/or anti-B antibodies. Hemagglutination test was performed in V-bottom plates (Thermo-Fisher, Roskilde, Denmark). Plasma samples (EDTA) from patients with Covid-19 or controls were serially diluted in PBS and added to each well (25 μL), then a 1% sheep red blood cell (RBC) suspension (25 μL) was added. A PBS control was performed to check for non-lysis of the RBCs or autoagglutination. The plates were gently mixed by tapping, and left for 45 min at room temperature. The agglutination score was 10, when the agglutinate fell to the bottom of the well, and 5, when the agglutinate formed a veil. For each plasma sample, a final score was computed as the sum of scores for all positive wells.
Animal handling and blood collection were conducted at the Research and Preclinical Investigation Centre at Oniris (Authorization n°44-274), according to the European Community Guidelines for the care and use of laboratory animals (2010/63/UE) and after approval by the national ethical committee and the animal welfare committee of the Oniris College of Veterinary Medicine.
Statistics
Statistical analyses were performed with R version 3.6.2.
Propensity scores were applied on observational data from both the Covid-19 and control groups to reduce the effects of confounding variables. The Covariate Balancing Propensity Score provided in the R package was used to estimate an Average Treatment Effect (ATE). The ATE was estimated by covariate balancing and requesting an exact match. An absolute standardized difference less than 10–15% was considered to support the assumption of balance between the groups. Unlike the P-value, this method was not affected by the sample size, and it could be used to compare the relative balance of variables that were measured in different units (Austin, 2011). Results (obtained after matching) are expressed as the mean and standard deviation (SD) for continuous variables or the percentage for categorical variables.
The survey tool provided in the R package was used to perform linear regressions for continuous outcome variables. These variables included the treatment group effect, the weight resulting from the matching, and variables present in the propensity score. This procedure provided a doubly-robust estimator, which corrected the last remaining possible imbalance between the covariates and produced an unbiased treatment effect (Funk et al., 2010). Adjusted P-values less than 0.05 were considered significant.
Results
Experimental in vitro study
Could infectious SARS-CoV-2 particles carry blood group antigens A or B?
Several structural analyses of the SARS-CoV-2 S protein glycans have been reported, showing the presence of a large fraction of mature N- and O-glycans, alongside immature forms. However, none of these studies described the expression of blood group antigens on the S protein (Gao et al., 2020, Sanda et al., 2020, Shajahan et al., 2020, Sun et al., 2020, Watanabe et al., 2020). Yet, all analyses were performed on recombinant S protein produced in HEK-293T cells which do not express blood group antigens, unlike epithelial cells of the larynx and the bronchial mucosa where infectious viral particles are likely produced.
First, we checked the expression of A and B antigens in the lungs of A, B and O secretor individuals (Appendix, supplementary method). Strong expression of A and B antigens was observed in ciliated and secretory tracheal and bronchial cells of A and B blood group individuals, respectively. These antigens were also expressed in the vascular endothelium and in pneumocytes. However, they could not be detected in the tissue of blood group O individuals, which indicates the specificity of these staining reactions (Appendix Figure 1).
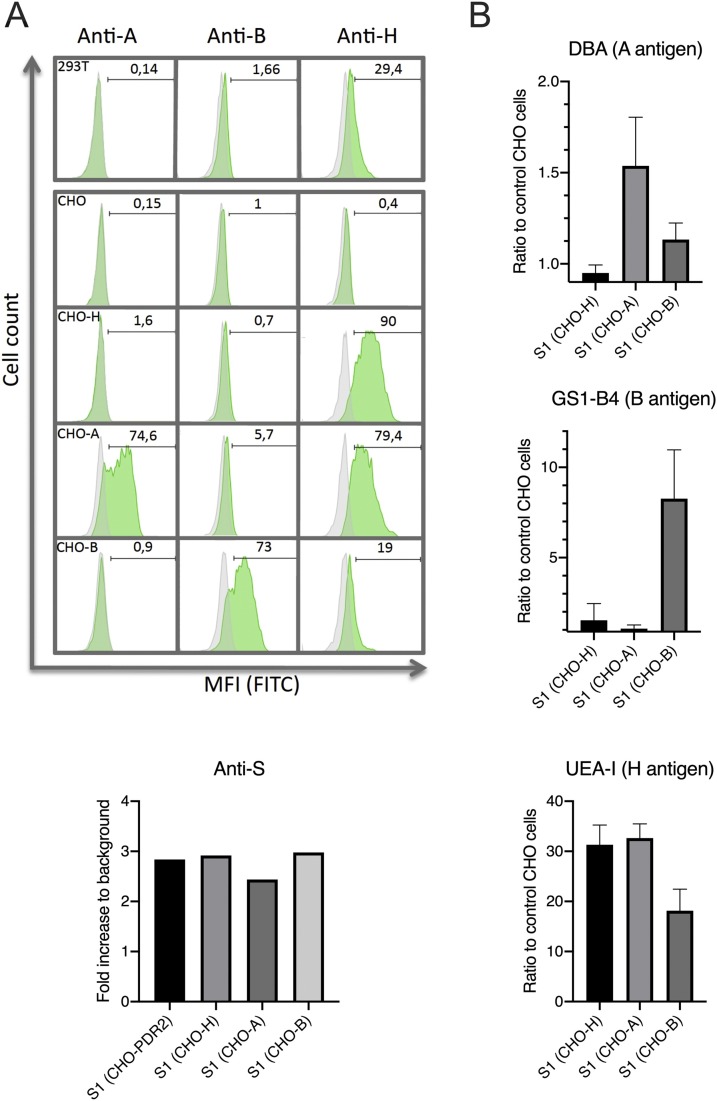
Then, in order to determine whether viral particles originating from cells that do express A and B blood group antigens can potentially be decorated by these antigens, we produced the heavily glycosylated S1 domain of the viral S protein fused with the Fc domain of a mouse IgG (S1-Fc) in CHO cells stably transfected with the FUT2 fucosyltransferase to generate the H antigen precursor (CHO-H) and with cDNA encoding the A or B enzymes to express the A (CHO-A) or B (CHO-B) antigens, respectively (Figure 1A).
Figure 1.
The SARS-CoV-2 S protein can be decorated with blood group antigens. (A) Surface expression of A, B and H antigens on HEK-293T cells, CHO-PDR2 (CHO), CHO-PDR2-FTB (CHO-H), CHO-PDR2-FTB + PBK-enzA (CHO-A) and CHO-PDR2-FTB + PBK-enzB (CHO-B) determined by flow cytometry. Percentage of positive cells is indicated on each histogram (MFI: mean fluorescence intensity). (B) Detection of A, B and H antigens on the S1-Fc protein produced in glycosyltransferases transfected CHO cells lines by ELISA. The S1-Fc protein expression in the 4 CHO cell lines was evaluated by using an anti-S protein. The results are expressed as -fold increase in the OD values relative to the mock transfected cells (background). For detection of A, B or H antigens, results are expressed as the ratio of the OD values obtained with the S1-Fc fusion protein produced in CHO-H, CHO-A and CHO-B cell lines to the OD values obtained with the S1-Fc fusion protein produced in CHO cell lines (controls). Mean and standard deviations of 3 independent experiments are shown.
The presence of the A, B and H antigens on the S1-Fc protein was detected by ELISA using lectins that are specific for these antigens. As shown in Figure 1B, the S1-Fc protein carried the A, B or H epitopes in accordance with the cells’ ability to express the antigen.
These results suggest that the S protein of viral particles produced by respiratory epithelial cells, which can express the ABH antigens, could also be tagged with these antigens.
Prospective observational study
IgM anti-A and/or anti-B agglutination scores
We tested 290 patients with Covid-19 and 276 control subjects for IgM anti-A and/or anti-B titers and agglutination scores. The Covid-19 group included 116, 126, and 48 patients with types O, A and B blood, respectively. The control group included 116, 108, and 52 subjects with types O, A and B blood, respectively. For each blood group, the propensity score nearly equalized the Covid-19 and control groups for age and gender. Therefore, we considered the two groups matched for these parameters (Table 1 ).
Table 1.
Age and gender of patients in Covid-19 and control groups, before and after matching by propensity score, in each blood group.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Controls | Covid-19 | ASD (%) | Controls | Covid-19 | ASD (%) | |
| O blood group | ||||||
| n | 116 | 116 | 116 | 116 | ||
| Age (years), mean ± SD | 49.05 ± 26.42 | 64.98 ± 22.52 | 64.89 | 56.92 ± 26.09 | 56.92 ± 26.15 | 0.00 |
| Male sex (%) | 44.83 | 49.14 | 8.64 | 45.34 | 45.34 | 0.00 |
| A blood group | ||||||
| n | 108 | 126 | 108 | 126 | ||
| Age (years), mean ± SD | 48.82 ± 26.79 | 65.23 ± 20.88 | 68.29 | 58.12 ± 25.54 | 58.12 ± 23.77 | 0.00 |
| Male sex (%) | 40.75 | 47.62 | 13.88 | 43.53 | 43.53 | 0.00 |
| B blood group | ||||||
| n | 52 | 48 | 52 | 48 | ||
| Age (years), mean ± SD | 45.82 ± 24.27 | 64.14 ± 18.58 | 84.73 | 56.75 ± 25.61 | 56.75 ± 20.51 | 0.00 |
| Male sex (%) | 28.85 | 41.67 | 27.08 | 36.56 | 36.56 | 0.00 |
ASD = absolute standardized difference; SD = standard deviation.
For blood group O, we found a significant difference between the Covid-19 and control groups, in terms of IgM anti-A + anti-B agglutination scores. The Covid-19 group had a lower mean agglutination score than the control group (76.93 vs. 88.29, P-value = 0.034). Similarly, for blood groups A and B, the Covid-19 group had lower mean anti-B (24.93 vs. 30.40, P-value = 0.028) and anti-A (28.56 vs. 36.50, P-value = 0.048) agglutination scores, respectively, compared to the control group (Table 2 ).
Table 2.
IgM anti-A and/or anti-B agglutination scores for each blood group, after equalizing the Covid-19 and control groups on age and gender.
| Controls | Covid-19 | Adjusted P-value | |
|---|---|---|---|
| O blood group | |||
| n | 116 | 116 | |
| IgM Anti-A + anti-B (a.u.), mean ± SD | 88.29 ± 33.01 | 76.93 ± 34.93 | 0.034* |
| A blood group | |||
| n | 108 | 126 | |
| IgM Anti-B (a.u.), mean ± SD | 30.40 ± 18.84 | 24.93 ± 18.73 | 0.028* |
| B blood group | |||
| n | 52 | 48 | |
| IgM Anti-A (a.u.), mean ± SD | 36.50 ± 17.41 | 28.56 ± 17.41 | 0.048* |
p < 0.05; a.u. = arbitrary unit; SD = standard deviation.
Anti-αGal and anti-sheep erythrocytes antibody levels
We analyzed smaller groups to evaluate anti-αGal and anti-sheep erythrocytes antibody levels. Again, the control (n = 88) and Covid-19 (n = 74) groups were not equal in age, gender, or blood group (A/B/O) before matching. The propensity score matching nearly equalized the two groups on age, gender, and blood group (A/B/O) (Table 3 ). Therefore, we considered the groups matched on these parameters.
Table 3.
Age, gender, and ABO blood groups of subjects in the Covid-19 and control groups, before and after matching with propensity scores.
| Before Matching |
After Matching |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Controls (n = 88) | Covid-19 (n = 74) | ASD (%) | Controls (n = 88) | Covid-19 (n = 74) | ASD (%) |
| Age (years), mean ± SD | 42.69 ± 25.01 | 67.63 ± 17.87 | 114.72 | 57.85 ± 29.26 | 57.85 ± 27.57 | 0.00 |
| Male sex (%) | 22.73 | 43.24 | 44.71 | 34.58 | 34.58 | 0.00 |
| Blood group A (%) | 36.36 | 37.84 | 3.05 | 40.68 | 40.68 | 0.00 |
| Blood group B (%) | 30.68 | 24.32 | 14.27 | 28.73 | 28.73 | 0.00 |
| Blood group O (%) | 32.95 | 37.84 | 10.23 | 30.59 | 30.59 | 0.00 |
ASD = absolute standardized difference; SD = standard deviation.
We found no difference between these groups in anti-αGal levels and anti-sheep erythrocytes agglutination scores (Table 4 ).
Table 4.
Natural antibody levels, after equalizing the Covid-19 and control groups on age, gender, and blood group.
| Controls (n = 88) | Covid-19 (n = 74) | Adjusted P-value | |
|---|---|---|---|
| Anti-αGal (OD450), mean ± SD | 1.07 ± 0.66 | 1.16 ± 0.61 | 0.429 |
| Anti-xenoantigens agglutination score (a.u.), mean ± SD | 25.67 ± 12.32 | 26.53 ± 11.61 | 0.691 |
a.u. = arbitrary unit; SD = standard deviation.
Discussion
In this study, we first showed that, in contrast to the recombinant SARS-CoV-2 S protein originating from HEK-293 cells, the S1-Fc fusion protein produced in cells that express the A, B or H antigens is tagged with the corresponding glycan epitopes. In addition, we confirmed that A and B antigens are expressed in lung epithelial cells, where infectious viral particles are likely produced. These data provide a preliminary validation of the working hypothesis, suggesting that infectious viral particles may be tagged with A or B antigens according to the patient’s ABO phenotype. Glycan structural analyses of native expectorated viral particles will be required for confirmation.
To the best of our knowledge, this study was the first to compare anti-A and/or anti-B levels between patients with Covid-19 and controls. Because isohemagglutinin levels may vary with age and gender, we equalized the Covid-19 and control groups nearly perfectly by applying propensity score matching. We found that patients with Covid-19 had significantly lower IgM anti-A and/or B isoagglutinins compared to controls.
To exclude the possibility that natural antibody levels might have decreased in relation to infection in the Covid-19 group regardless of their carbohydrate specificity, we assessed the levels of other natural antibodies in both groups. Anti-xenoantigen antibodies are naturally occurring antibodies that are produced in response to environmental stimulation, like ABO blood group antibodies, and they are present in all humans. They include, among others, anti-αGal antibodies, natural anti-Forsmann agglutinins, and antibodies directed against the NeuGc form of sialic acids (Altmann and Gagneux, 2019, Santos et al., 2020). Anti-αGal antibodies are directed against the αGal epitope, a unique carbohydrate structure, which is absent in humans, but is abundantly synthesized on glycolipids and glycoproteins of non-primate mammals and New World monkeys (Macher and Galili, 2008). In this study, we tested for both anti-αGal and anti-sheep xenoantigen antibody levels, and we found no difference between the Covid-19 and control groups. These results indicated that there was no general reduction in the level of natural antibodies following a Covid-19 infection. Thus, the lower levels of natural IgM anti-A and/or anti-B antibodies observed in patients with Covid-19 compared to controls were specific to ABO antibodies.
In a previous study, Guillon et al modeled the potential effects of natural anti-A and/or anti-B antibodies on a SARS outbreak. The model predicted that individuals with the AB blood type would be over-represented at the onset of the outbreak, followed by individuals with the A and/or B blood types, and finally, those with blood type O would be the last to be infected (Guillon et al., 2008). Considering that anti-A and anti-B antibodies might have a protective effect against the transmission of ABO incompatible viruses, the results could be understood. Individuals in the AB blood group would lack both anti-A and anti-B antibodies; therefore, they would altogether lack the protective effect. Additionally, the likelihood of neutralizing the virus would be lower for individuals with A and B blood types than for individuals with the O blood type who make both anti-A and anti-B antibodies; thus, individuals with A and B blood types could be expected to become infected more frequently than individuals with the O blood type.
Here, we observed a significant difference in the levels of anti-A and/or anti-B antibodies between patients with Covid-19 and controls, indicating that individuals with low levels of natural anti-A and/or anti-B antibodies would be at high risk of a SARS-CoV-2 infection. Thus, our results strongly suggest that the protective effect predicted for these antibodies by the model described by Guillon et al. could only be achieved when antibody levels were sufficiently high. Another hypothesis according to which ABO antibodies would be adsorbed or consumed by viral particles should also be considered since the level of ABO antibodies before infection was not known. However, we believe that this hypothesis is unlikely and that adsorption/consumption would not be detectable given the high level of ABO antibodies compared to the limited number of viral particles at the very moment of infection. It should be kept in mind that after infection, production of the virus occurs with the blood group of the infected patient.
Most studies that found associations between ABO blood groups and susceptibility to Covid-19, including GWAS studies with large cohorts of patients and controls, reported that individuals with blood group O had a significantly lower risk of SARS-CoV-2 infection (Ellinghaus et al., 2020, Shelton et al., 2020, Valenti et al., 2020). However, some studies failed to observe a significant protective effect of blood group for reasons that are yet to be understood (Boudin et al., 2020, Dzik et al., 2020). More variability in different infection rates across studies occurred for individuals with A, B, and AB blood types. The discrepancies between studies might be explained by the structure of the control group, the variable ABO frequencies in distinct populations, the case definition for Covid-19, an effect of the outbreak kinetics, but also, according to our results, by the levels of ABO antibodies in the studied population. Indeed, based on our results, a high level of ABO antibodies in a given population would be expected to offer strong protection.
ABO blood group antibodies appear after birth, and the levels can change with changes in diet and with bacterial colonization in the intestinal tract (Cooling, 2015, Gampa et al., 2017, Vlasova et al., 2019). ABO antibodies are synthetized by immune stimulation from gastrointestinal microbiota (Cooling, 2015), and both antibody production and microbiota abundance vary according to an individual’s age and nutritional status (Yatsunenko et al., 2012). The promotion of ABO-antibody production by dedicated gut microbiota might increase the levels of these natural antibodies sufficiently to enhance this natural mechanism of protection against SARS-CoV-2, and probably other coronaviruses.
This prospective study had some limitations. First, its observational design had inherent limitations. However, we partially countered this limitation by applying propensity scoring to equalize the groups. Yet, we did not take into account co-morbidity factors and severity scores. In addition, since residual samples from routine laboratory testing were used, we were limited by the amount of plasma and could not evaluate anti-αGal and anti-xenoantigen antibody levels in all patients. For the same reason, we only evaluated the level of IgM ABO antibodies in Covid-19 patients and controls. The level of “total” ABO antibodies (IgG + IgM that react at 37 °C) by using an anti-human globulin with incubation at 37 °C, as well as the level of IgG ABO antibodies by using the same method but with dithiotreitol treated plasma would have been interesting to evaluate and compare. Matched control studies will be needed to either confirm or refute the link between the level of ABO antibodies and the susceptibility to viral infection.
In conclusion, our results suggest that natural ABO antibodies could contribute to reduce the transmission of SARS-CoV-2 virus, provided that their levels are sufficiently high.
Conflict of interest
The authors declare no conflict of interest related to this work.
Funding
The authors declare they did not receive any funding for this work.
Ethical approval
The study was approved by the Ethics Committees of the Centre Hospitalier Universitaire Brugmann (CHU Brugmann) and Hôpital Universitaire Des Enfants Reine Fabiola (HUDERF, Brussels, Belgium). Written informed consent was obtained from ambulatory patients that provided blood samples specifically for the study. When only residual material was used, written informed consent was waived.
The study was performed in accordance with the principles of the Declaration of Helsinki. The authors assume responsibility for the accuracy and completeness of the data and analyses.
Acknowledgements
We would like to thank Bio-Rad for reagents supply and Dr Jianxun Qi for his kind gift of the S1-Fc fusion protein expression vector.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.12.025.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abdollahi A., Mahmoudi-Aliabadi M., Mehrtash V., Jafarzadeh B., Salehi M. The novel coronavirus SARS-CoV-2 vulnerabiloty association with ABO/Rh blood types. Iran J Pathol. 2020;15:156–160. doi: 10.30699/ijp.2020.125135.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljanobi G., Alhajjaj A., Alkhabbaz F., Al-Jishi J. The relationship between ABO blood group type and the Covid-19 susceptibility in Qatif Central Hospital, Eastern Province, Saudi Arabia: a retrospective cohort study. Open J Intern Med. 2020;10:232–238. [Google Scholar]
- Altmann M.O., Gagneux P. Absence of Neu5Gc and presence of anti-Neu5Gc antibodies in humans—an evolutionary perspective. Frontiers Immunol. 2019;10:789. doi: 10.3389/fimmu.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berséus O.J., Boman K., Nessen S.C., Westerberg L.A. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53:114S–123S. doi: 10.1111/trf.12045. [DOI] [PubMed] [Google Scholar]
- Boudin L., Janvier F., Bylicki O., Dutasta F. ABO blood groups are not associated with risk of acquiring the SARS-CoV-2 infection in young adults. Haematologica. 2020;105(12):2841–2843. doi: 10.3324/haematol.2020.265066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman A., Ruvoën-Clouet N., Le Pendu J. Harnessing the natural anti-glycan immune response to limit the transmission of enveloped viruses such as SARS-CoV-2. PLoS Pathogens. 2020;16 doi: 10.1371/journal.ppat.1008556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Subbarao K. The immunobiology of SARS. Ann Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Cheng G., Chui C.H., Lau F.Y. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzik S., Eliason K., Morris E.B., Kaufman R.M., North C.M. COVID-19 and ABO blood groups. Transfusion. 2020;60(8):1883–1884. doi: 10.1111/trf.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T., Yang K., Le Pendu J., Baines J.D., Gardin R.D. The coxsackievirus and adenovirus receptor, a required host factor for recovirus infection, is a putative enteric calicivirus receptor. J Virol. 2019;93 doi: 10.1128/JVI.00869-19. e00869-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk M.J., Westreich D., Wiesen C., Sturmer T., Brookhart M.A., Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2010;173:761–767. doi: 10.1093/aje/kwq439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk M.K., Grossman B.J., Hillyer C.D., Westhoff C.M. 18th ed. AABB; Bethesda, MD: 2014. Technical Manual. [Google Scholar]
- Galili U. Evolution in primates by “Catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anticarbohydrate antibodies. Am J Phys Anthropol. 2019;168:352–363. doi: 10.1002/ajpa.23745. [DOI] [PubMed] [Google Scholar]
- Gallian P., Pastorino B., Morel P., Chiaroni J., Ninove L., Lamballerie X. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res. 2020;181:104880. doi: 10.1016/j.antiviral.2020.104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampa A., Engen P.A., Shobar R., Mutlu E.A. Relationships between gastrointestinal microbiota and blood group antigens. Physiol Genomics. 2017;49:473–483. doi: 10.1152/physiolgenomics.00043.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Zeng J., Jia N., Stavenhagen K., Matsumoto Y., Zhang H. SARS-CoV-2 spike protein interacts with multiple innate immune receptors. bioRxiv. 2020 doi: 10.1101/2020.07.29.227462. [DOI] [Google Scholar]
- Göker H., Aladağ K.E., Demiroğlu H., Ayaz Ceylan Ç.M., Büyükaşik Y., Inkaya A.Ç. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci. 2020;50:679–683. doi: 10.3906/sag-2005-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon P., Clément M., Sébille V., Rivain J.-G., Chou C.-F., Ruvoën-Clouet N. Inhibition of the interaction beteen the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Maurya V.K., Prasad A.K., Bhatt M.L.B. Structural, glycosylation and antigenic variation between 2019 novel coronavirus (2019-nCoV) and SARS coronavirus (SARS-CoV) VirusDis. 2020;31:13–21. doi: 10.1007/s13337-020-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz C.A., DeCarlo C., Boitano L., Png C.Y.M., Patell R., Conrad M.F. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99(9):2113–2118. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf R.K., Al-Samkari H., Brenner S.K., Gupta S., Leaf D.E. ABO phenotype and death in critically ill patients with COVID-19. Br J Haematol. 2020;190:e204–e208. doi: 10.1111/bjh.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X., Chen J., Cai Y., Deng A., Yang M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol. 2020;190:24–27. doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10) doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher B.A., Galili U. The Galα1,3Galβ1,4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochem Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marionneau S., Cailleau-Thomas A., Rocher J., Le Moullac-Vaidye B., Ruvoën-clouet N., Clément M. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–573. doi: 10.1016/s0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- Ravn V., Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000;108:1–28. doi: 10.1034/j.1600-0463.2000.d01-1.x. [DOI] [PubMed] [Google Scholar]
- Sanda M., Morrison L., Goldman R. N and O glycosylation of the SARS-CoV-2 spike protein. bioRxiv. 2020 doi: 10.1101/2020.07.05.187344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos L., Jin C., Mourato C., Mendes F., Hesse C., Teneberg S. Characterization of sheep erythrocyte glycosphingolipids recognized by human anti-Forssman antibodies. Glycobiology. 2020;30(11):881–894. doi: 10.1093/glycob/cwaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan A., Supekar N.T., Gleinich A.S., Azadi P. Deducing the N- and O- glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology. 2020;30(12):981–988. doi: 10.1093/glycob/cwaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J.F., Shastri A.J., Ye C., Weldon C.H., Filshtein-Somnez T., Coker D. Trans-ethnic analysis reveals genetic and non-genetic associations with COVID-19 susceptibility and severity. medRxiv. 2020 doi: 10.1101/2020.09.04.20188318. [DOI] [PubMed] [Google Scholar]
- Sprogøe U., Yazer M., Rasmussen M.H., Antonsen B., Bistrup C., Assing K. Minimal variation in anti-A and -B titers among healthy volunteers over time: Implications for the use of out-of-group blood components. Trauma Acute Care Surg. 2017;82:S87–S90. doi: 10.1097/TA.0000000000001432. [DOI] [PubMed] [Google Scholar]
- Sun Z., Ren K., Zhang X., Chen J., Jiang Z., Jiang J. Mass spectrometry analysis of newly emerging coronavirus HCoV-19 spike protein and human ACE2 reveals camouflaging glycans and unique post-translational modifications. Engineering. 2020 doi: 10.1016/j.eng.2020.07.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti L., Villa S., Baselli G., Temporiti R., Bandera A., Scudeller L. Association of ABO blood groups and secretor phenotype with severe COVID-19. Transfusion. 2020;60(12):3067–3070. doi: 10.1111/trf.16130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A.N., Takanashi S., Miyazaki A., Rajashekara G., Saif L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr Opin Virol. 2019;37:16–25. doi: 10.1016/j.coviro.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4) doi: 10.1016/j.cell.2020.03.045. 894-904 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(6501):330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu Y., Feng Z., Li P., Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin Chim Acta. 2020;509:220–223. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Fan H., Lu D., Meng F., Zhuo L., Tang M. Association between ABO blood groups and clinical outcome of coronavirus disease 2019: evidence from two cohorts. medRxiv. 2020 doi: 10.1101/2020.04.15.20063107. [DOI] [Google Scholar]
- Zhao J., Yang Y., Huang H.-P., Li D., Gu D.-F., Lu X.-F. Relationship between the ABO Blood Group and the COVID-19 Susceptibility. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1150. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5) doi: 10.1016/j.cell.2020.04.035. 1016-35 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietz M., Zucker J., Tatonetti N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. Nat Commun. 2020;11(1):5761. doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.