Abstract
Tyrosyl DNA phosphodiesterase II (TDP2) is a recently discovered enzyme that specifically repairs DNA damages induced by topoisomerase II (Top2) poisons and causes resistance to these drugs. Inhibiting TDP2 is expected to enhance the efficacy of clinically important Top2-targeting anticancer drugs. However, TDP2 as a therapeutic target remains poorly understood. We report herein the discovery of isoquinoline-1,3-dione as a viable chemotype for selectively inhibiting TDP2. The initial hit compound 43 was identified by screening our in-house collection of synthetic compounds. Further structure–activity relationship (SAR) studies identified numerous analogues inhibiting TDP2 in low micromolar range without appreciable inhibition against the homologous TDP1 at the highest testing concentration (111 μM). The best compound 64 inhibited recombinant TDP2 with an IC50 of 1.9 μM. The discovery of this chemotype may provide a platform toward understanding TDP2 as a drug target.
Graphical Abstract

INTRODUCTION
Many cancer chemotherapies work by inducing excessive DNA damage that causes cell death. The survival of damaged cancer cells hinges on the repair of these DNA lesions through various pathways which ultimately cause therapeutic resistance. Therefore, targeting DNA repair pathways can enhance the efficacy of DNA-damaging anticancer drugs.1 Among DNA damaging drugs are Top2 poisons, such as etoposide (ETP), teniposide, doxorubicin, epirubicin, and idarubicin, which are widely used for treating a broad spectrum of cancers.2 Mechanistically, Top2 (1, Figure 1) relaxes DNA torsional strains by cutting DNA substrate (2), using its tyrosine residue to form the covalent Top2 cleavage complex (4) along with the released 3′ DNA-end (3) (Figure 1). The DNA break is normally resealed when the 3′-end (3) attacks back to resolve Top2 cleavage complex, which allows the continuation of functional DNA transcription and replication. However, the dynamic equilibrium between DNA and Top2 cleavage complex can shift toward the accumulation of abortive Top2 cleavage complex (5) in which DNA breaks fail to rejoin. Clinical Top2 poisons work by this exact mechanism as they bind to and stabilize the Top2 cleavage complex to prevent religation (5, Figure 1). TDP2 is the only known human enzyme that can cleave the unique 5′ phosphotyrosyl covalent bond featured in abortive Top2 cleavage complex,3 demonstrating that TDP2 plays a critical role in the repair of Top2-mediated DNA damage and in resistance development to clinical Top2 poisons. This is supported by observations both in cultured cells and animal models that lack of TDP2 leads to enhanced sensitivity to Top2-induced DNA breaks caused by Top2 poisons.3–7 Importantly, up-regulation of TDP2 transcription through a gain-of-function p53 mutation has indeed been linked to etoposide resistance in human lung cancer.8 These observations provide strong rationales for developing TDP2 inhibitors to sensitize cancer cells toward clinical Top2 poisons.
Figure 1.
TDP2 repairs abortive Top2–DNA cleavage complex: 2, DNA substrate; 4, transient Top2–DNA complex; 5, Top2–DNA complex stabilized by a Top2 poison; cyan, Top2; green, TDP2; yellow, Top2 poison.
In addition, recent studies have also linked the unique function of TDP2 to the replication of picornaviruses9 and particularly hepatitis B virus (HBV).10 HBV chronically infects over 350 million people globally. Current antivirals against HBV can not eradicate the virus to achieve disease cure. This is mainly due to the persistence of viral covalently closed circular DNA (cccDNA)11,12 produced from a precursor relaxed circular DNA (RC-DNA) during a key step of HBV replication. A recent report10 found that this conversion is critically dependent on cellular TDP2 for the cleavage of a protein–DNA adduct similar to the Top2 cleavage complex. Inhibiting cellular TDP2 thus may lead to the depletion of HBV reservoir cccDNA and may provide a path toward viral eradication, although the role of TDP2 in HBV cccDNA formation in vivo remains debatable.13
Since the discovery of TDP2 in 2009,3 efforts in biochemistry and crystallography have generated critical knowledge to allow basic understanding on TDP2 active site structure, mode of substrate recognition, enzyme kinetics, and mechanism of catalysis.14–16 These studies support either a one- or two-metal catalytic mechanism characteristic of the exonuclease-endonuclease-phosphatase (EEP) nuclease superfamily. On the basis of these mechanisms, TDP2 repairs abortive Top2 cleavage complex through a hydrolytic reaction promoted by either one or two Mg2+ ion(s) and a few key residues at the active site. Particularly important to catalysis are residues D262, E152, H351, and N120, which are highly conserved across species. Knowledge on substrate binding and mechanism of catalysis could assist inhibitor discovery, particularly the structural and biochemical kinship of TDP2 to another DNA repair enzyme, human apurinic/apyrimidinic endonuclease 1 (hAPE1),17 could allow ligand-based inhibitor design. However, full-fledged medicinal chemistry efforts targeting TDP2 require good lead compounds and the understanding of their inhibition mechanisms, both of which are still lacking. As a result, TDP2 inhibitors remain largely unexplored. Toxoflavins (6) and deazaflavins (7) (Figure 2a) identified through a high-throughput screening (HTS) are the only known inhibitor scaffolds of TDP2.18 While biochemically inhibiting TDP2, these scaffolds are flawed as drug candidates because 6 poses a redox liability and shows poor in vitro pharmacokinetics (PK) profiles due to its inhibition of metabolic enzyme CYP450, whereas 7 lacks cell permeability.18 Therefore, there is a pressing need to identify more drug-like TDP2 inhibitors that can also be used as tool compounds toward understanding the mechanism of TDP2 inhibition.
Figure 2.
Chemotypes of interest. (a) Reported inhibitor types of TDP2; (b) in-house synthetic chemotypes screened against TDP2.
RESULTS AND DISCUSSION
Hit Identification.
Our efforts began with screening a carefully curated library comprising our in-house synthetic compounds. The screen used a biochemical assay with recombinant human TDP2.14 The library comprises compounds of various chemotypes (Figure 2b), many featuring a metal chelating functionality, such as 8–9, 12–14, 17, and 19b (X = OH). The rationale is that the catalysis of TDP2 requires at least one Mg2+. From this initial screening, one analogue of the isoquinoline-1,3-dione chemotype (19a), namely 6-furanoisoquinoline-1,3-dione (43, Figure 3a) was found to selectively inhibit TDP2 (Figure 3c). Dose–response testing revealed that the inhibitory IC50 for 43 was 10 μM (Figure 3d). This potency was confirmed through a secondary assay using a more robust assay where endogenous TDP2 from whole cell extracts was used (WCE, IC50 = 12 μM, Figure 3b). Remarkably, none of any other screened chemotypes showed significant TDP2 inhibition, including the highly homologous 19b where the only structural difference is the presence of the N-2 OH group. That the 2-hydroxyisoquinoline-1,3-dione (HID) analogue 46 did not inhibit TDP2 at concentrations up to 111 μM was remarkable. It implies that unlike the inhibition of some other Mg2+ dependent DNA processing enzymes, TDP2 inhibition may not require a chelating functionality. On the other hand, counter assays against the homologous TDP1 showed no inhibition by 43 (Figure 3b), strongly suggesting that the observed TDP2 inhibition was highly specific. In addition, the small size (mw = 227) and the lack of apparent PK liabilities render 43 an attractive hit, which is amenable to further SAR.
Figure 3.
SAR and selective TDP2 inhibition of the isoquinolinedione chemotype. (a) Structure of isoquinoline-1,3-dione 43 and HID 46. (b) Inhibition profile: 43 selectively inhibits TDP2 in assays using either recombinant (Rec) or endogenous TDP2 from whole cell extracts (WCE), while 46 was inactive in either condition. (c) Gel images of dose response testing of 43 in Rec TDP2 biochemical assays. Conc (μM): 1.4, 4.1, 12.3, 37, 111. (d) Dose–response curve for 43 in Rec TDP2 assay expressed as mean ± SD from at least three independent experiments.
Chemistry.
The SAR of compound 43 concerns primarily three distinct series of compounds: (1) isoquinoline-1,3-diones with a simple functional group (Br, I, CO2H, CF3, NO2) around the left ring (33–42, Table 1), (2) isoquinoline-1,3-diones with an aromatic ring substituted around the left ring (43–88, Table 1), and (3) scaffold analogues (89–96, Table 1). Synthetically, all isoquinoline-1,3-dione analogues of the first two series (43–88) were accessed by either of the two routes delineated in Schemes 1–2. The first approach (Scheme 1) was adapted based on our previously reported route for the synthesis of HID.19 This approach features key iodo homophthalic acid intermediates (20) which were synthesized using a Hurtley reaction.19 The structural diversity for analogues of series two was subsequently introduced via a Suzuki coupling reaction to afford intermediates 21, which were condensed with urea in 1,2-dichlorobenzene to yield desired isoquinoline-1,3-dione analogues (Scheme 1).20
Table 1.
Biochemical Inhibition of Synthetic Isoquinolinedione Analogues against Recombinant hTDP2
 |
IC50: concentration of a compound producing 50% inhibition, expressed as mean ± standard deviation from at least three independent experiments.
Value from a second independent experiment.
Table 2.
Inhibitory Activity of Selected Compounds against hTDP2 Whole Cell Extract (WCE)
| compd | Rec. TDP2 IC50a (μM) | WCE IC50b (μM) | CC90c(μM) |
|---|---|---|---|
| 36d | 7.6 ± 4.1 | 9.3 | 9.5 |
| 38 | 7.5 ± 3.0 | 2.6 | |
| 40d | 3.0 ± 1.0 | 5.2 | >50 |
| 41 | 7.0 ± 1.7 | 3.5 | |
| 43d | 8.5 | 25 | 2.4 |
| 44 | 13 | 50 | |
| 48 | 23 | >111 | |
| 49 | 16 ± 5.0 | 23 | |
| 51 | 13 ± 3.0 | 35 | |
| 55 | 10 ± 2.0 | 25 | |
| 59 | 42 ± 2.0 | 55 | |
| 61 | 36 ± 3.0 | 40 | |
| 62 | 18 ± 8.0 | 25 | |
| 64 | 1.9 ± 0.90 | 2.2 | |
| 69 | 21 ± 8.0 | 42 | |
| 71 | 5.5 ± 2.0 | 15 | |
| 72 | 5.6 ± 2.6 | 3.3 | |
| 73 | 1.7 ± 0.30 | 3.2 | |
| 74 | 6.4 ± 4.2 | 4.5 | |
| 75 | 8.0 ± 5.1 | 6.5 | |
| 79 | 3.0 ± 2.3 | 4.3 | |
| 80 | 4.1 ± 1.9 | 5.3 | |
| 81 | 4.5 ± 1.5 | 5.5 | |
| 82 | 3.2 ± 2.5 | 2.7 | |
| 84d | 5.1 ± 2.0 | 11 | 12 |
| 85 | 9.3 | >111 |
IC50: concentration of a compound producing 50% inhibition, expressed as mean ± standard deviation from at least three independent experiments.
IC50: concentration of a compound producing 50% inhibition.
CC90: concentration of a compound killing 90% of the cells. Assay was done in the absence of EPT against hTDP2 DT40 cells.6
Synergy was not observed when tested in combination with different concentrations of EPT.6
Scheme 1a.
aReagents and conditions: (a) arylboronic acid, K2CO3, Pd(PPh3)4, EtOH/H2O (1:1), 150 °C, 30 min, MW, 60–85%; (b) urea, 1,2-dichlorobenzene, 170 °C, 45 min, MW, 55–80%.
Scheme 2a.
aReagents and conditions: (a) methyl cyanoacetate, NaH, DMSO, 90 °C; (b) DMSO/H2O (9:1), 120 °C, 16 h, 70–85%; (c) arylboronic acid, K2CO3, Pd(PPh3)4, DME/H2O (4:1), 110 °C, 40 min, MW, 55–87%; (d) Pd(OAc)2, Mo(CO)6, K2CO3, anisole (0.2 M), 140 °C, 30 min, MW, 50–62%; (e) conc HCl, 70 °C, 4 h, 60–90%; (f) sulfonyl or acryloyl chloride, pyridine, dioxane, rt; 65–79%; (g) 2-aminothiophenol, T3P (50% in EtOAc), DIPEA, 100 °C, 30 min, MW, 69%.
To limit the usage of 1,2-dichlorobenzene and to produce a range of substituted isoquinoline-1,3-diones, an alternative method was employed (Scheme 2). This method began with the SNAr reaction of substituted-2-fluorobenzonitrile (22) with methyl cyanoacetate at 90 °C to produce the ester intermediate (23), which upon heating in a mixture of DMSO/H2O (9:1) at 120 °C resulted in the smooth decarboxylation to furnish the halogen (I or Br) substituted dinitrile compounds (24). At this stage, structural diversity was introduced via either a Suzuki coupling to afford various aryl substituted dinitrile intermediates (25) or a palladium assisted arylation and carbonyl insertion to produce benzoyl substituted dinitrile compounds (26). Treatment of dinitrile intermediates 24–26 with conc HCl at 70 °C afforded the desired cyclized products (33–88, Scheme 2a). The sulfonamide (62–64) or acrylamide (65–66) analogues were synthesized by derivatizing the aniline analogues (59–61) with sulfonyl or acryloyl chloride (Scheme 2b). The benzothiazole derivative (84) was synthesized using a T3P mediated coupling of 39 with 2-aminothiophenol (Scheme 2c).
The methods for synthesizing scaffold analogues are depicted in Scheme 3. The synthesis of quinazoline-2,4-dione (90) involved a Suzuki coupling reaction of the starting 4-bromomethyl anthranilate 27. The resulting intermediate 28 was then converted to the desired cyclized product 90 via the treatment with potassium cyanate and the subsequent saponification (Scheme 3a).21 The 1,8-naphthalimide derivatives (91–92) were synthesized by the condensation of 1,8-naphthalic anhydrides (29–30) with ammonium hydroxide (Scheme 3b).22 The 5,6-fused heterocyclic core was obtained by treating 2-amino-1-propene-1,1,3-tricarbonitrile (31) with various substituted hydrazine in refluxing ethanol to yield dinitrile compounds (32), which were then cyclized with the aid of acid to furnish compounds 93–95. Compound 95 was acetylated to produce 96 (Scheme 3c).
Scheme 3a.
aReagents and conditions: (a) phenylboronic acid, K2CO3, Pd(PPh3)4, DME/H2O (4:1), 110 °C, 45 min, MW, 50%; (b) (i) KOCN, toluene, rt, (ii) NaOH, EtOH, reflux, 68%; (c) NH4OH, 40 °C, 4 h, 70–90%; (d) 2-(tributylstannyl)furan, Pd(PPh3)4, toluene, 150 °C, 30 min, 72%; (e) R-NHNH2, EtOH, reflux, 70–95%; (f) conc HCl, 70 °C, 4 h, 65–85%; (g) acetyl chloride, AcOH, 70 °C, 62%.
Biology.
All final compounds 33–96 were evaluated biochemically for inhibition of human TDP2. The primary assay used recombinant TDP2, and all compounds were tested in dose–response fashion at concentrations up to 111 μM. A counter assay was also run with each compound against the homologous TDP1, and none of the tested compounds showed significant inhibition at the highest concentration (111 μM). The testing results are summarized in Table 1. The most striking SAR trend was that while the vast majority of the quinolone-1,3-dione analogues (33–89) potently inhibited recombinant human TDP2 in the low micromolar range, none of the scaffold analogues (90–96) showed considerable TDP2 inhibition in the same assay. This observation strongly validates quinolone-1,3-dione as an important TDP2 inhibitor type. In addition, the dramatic OH effect at the N-2 position initially observed with compound 46 was confirmed with an additional pair of analogues (44 and 47). Consistent with the SAR trend, the 2-NH analogue 44 inhibited TDP2 with an IC50 of 13 μM, whereas the 2-NOH analogue 47 was completely inactive (IC50 > 111 μM). This confirmed SAR trend suggests that compounds with a chelating triad, such as the HID chemotype, should not be considered as favorable TDP2 inhibitor types. The third major SAR observation concerns the substitution position on the left ring of the isoquinoline-1,3-dione core. Notably, most of the C6 or C7 substituted analogues potently inhibited recombinant TDP2, whereas C8 analogues (53 and 57) showed substantially reduced inhibitory activity (compared to 51–52 and 55–56), and more dramatically, C5 substituted analogues (34, 45, and 58, Table 1) were all inactive regardless of the nature of the substituent.
To confirm the observed potency with C6 or C7 analogues, selected compounds were also tested in a secondary assay using the whole cell extracts of the TDP2 expressing cells. As shown in Table 2, with the exception of 48 and 85, all compounds tested retained activity in this assay with IC50 values consistent with those obtained from the assays using recombinant TDP2. These data strongly support a genuine and selective TDP2 inhibitory profile of the C6 or C7 substituted isoqunioline-1,3-dione chemotype. In addition, a few compounds (36, 40, 43, and 84) were also tested for sensitivity against DT40 knockout cells complemented with human hTDP2 using a previously reported assay.6 Although synergy to Top2 inhibitor ETP was not observed with any of these compounds, three of them (36, 43, and 84) exhibited potent cytotoxicity at low micromolar concentrations (CC90 = 2.5 – 12 μM). It is likely that further improved TDP2 inhibitors will produce the desired synergism.
Molecular Modeling.
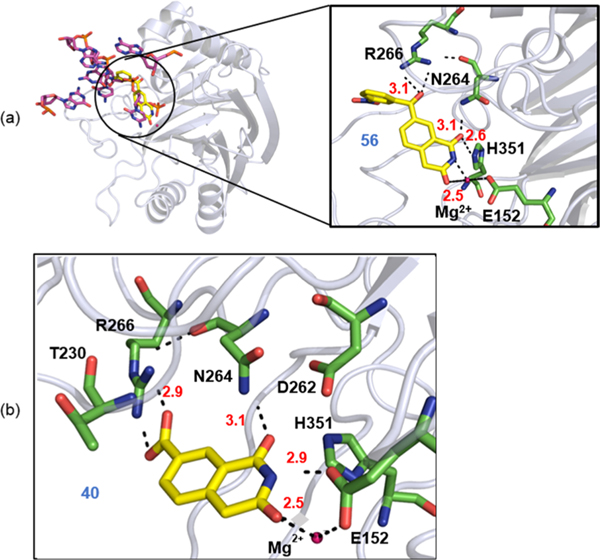
With the lack of a TDP2/inhibitor cocrystal structure, understanding on the inhibitor binding mode and mechanism of inhibition remains elusive. With reported inhibitors 6 and 7, it is unclear exactly how they inhibit TDP2 as molecular modeling revealed little about their binding. To provide a predicted binding mode of our new inhibitors, we have constructed a homology model of hTDP2 based on the available crystal structure of mouse TDP2 (mTDP2; PDB code: 4GZ115), which shares a high degree of sequence identity (65%) with hTDP2. With this model, docking analysis was performed using Glide XP (v.6.4).23,24 The predicted binding mode of compound 56 in the active site of hTDP2 suggests an interaction between the carbonyl at position 1 of isoquinoline-1,3-dione core and the residues, H351 and the backbone NH of N264 of TDP2 (Figure 4a). The NH at position 2 and carbonyl group at position 3 of 56 can coordinate to the magnesium ion (Mg2+). The carbonyl group at position 7 of 56 forms a salt bridge with the R266 within the active site of hTDP2. Whereas in compound 40, the carbonyl group at position 1 forms an H-bond interaction with the backbone NH of N264. The NH at position 2 and carbonyl group at position 3 within 40 coordinate to the E152 and magnesium ion (Mg2+), respectively (Figure 4b).
Figure 4.
Predicted binding mode of (a) 56 and (b) 40 within active site of hTDP2 (numbering was based on hTDP2 sequence). The active site residues are shown as green sticks and metal ions as magenta sphere. The H-bond interactions are depicted as black dotted lines, and the distance between bonded atoms are represented in red. Homology model (hTDP2) was built using the crystal structure of mTDP2 (PDB code: 4GZ115).
CONCLUSIONS
By screening our in-house collection of synthetic small molecules, isoquinoline-1,3-dione was identified as a novel inhibitor type of TDP2. The selective and potent inhibitory profile of this chemotype against TDP2 was further established through the chemical synthesis of 63 functional and scaffold analogues and validated via a secondary assay using WCE as well as a counter assay against TDP1, which shares some substrates with TDP2.25 Analogue synthesis identified compound 64 as the best of the series with an IC50 of 1.9 μM against recombinant TDP2 and 2.2 μM against TDP2 in WCE assay. The main SAR observations also suggest that the C6 or C7 substitution is strongly preferred and that the OH substitution at the N2 position resulting in a CO-NOH-CO chelating triad is undesired. While efforts toward obtaining the TDP2/inhibitor cocrystal structures are still underway, homology modeling did reveal a few specific interactions within the TDP2 active site that may contribute to understanding the molecular mechanism of TDP2 inhibition. Identifying this novel and selective TDP2 inhibitor type may provide an important path toward employing TDP2 as a drug target and developing TDP2 inhibitors as molecular probes.
EXPERIMENTAL SECTION
Chemistry.
General Procedures.
All commercial chemicals were used as supplied unless otherwise indicated. Flash chromatography was performed on a Teledyne Combiflash RF-200 with RediSep columns (silica) and indicated mobile phase. All moisture sensitive reactions were performed under an inert atmosphere of ultrapure argon with oven-dried glassware. 1H and 13C NMR spectra were recorded on a Varian 600 MHz spectrometer. Mass data were acquired on an Agilent TOF II TOS/MS spectrometer capable of ESI and APCI ion sources. Analysis of sample purity was performed on a Varian Prepstar SD-1 HPLC system with a Phenomenex Gemini, 5 μm C18 column (250 mm × 4.6 mm). HPLC conditions: solvent A = H2O, solvent B = MeCN; flow rate =1.0 mL/min; compounds were eluted with a gradient of 10% MeCN/H2O for 0–5 min and to 80% MeCN/H2O from 5 to 30 min followed by 100% MeCN from 30 to 35 min and then 10% MeCN/H2O from 35 to 40 min. Purity was determined by total absorbance at 254 nm. All tested compounds have a purity ≥98%.
General Procedure for Suzuki Coupling (21, 25).
The mixture of compound 20 or 24 (1.0 equiv), aryl boronic acid (1.6 equiv), Pd(PPh3)4 (0.065 equiv), and K2CO3 (3.6 equiv) in EtOH/H2O (1:1) was irradiated at 150 °C for 30 min under microwave conditions. The black residue formed was filtered through Celite and the solvent was concentrated in vacuo. The resulting aqueous solution was acidified (pH = 3) using 2 N HCl. A white precipitate was obtained via filtration, which was washed with water and dried under vacuum to furnish desired compounds (21, 25) as colorless solid.
General Procedure for the SNAr Reaction (23).
To a suspension of NaH (32 mmol, 2.0 equiv, 60% dispersion in oil) in DMSO (10 mL) at 0 °C was added methyl cyanoacetate (32 mmol, 2.0 equiv) slowly, and the mixture was stirred at rt for 30 min before 2-fluoro-4-iodobenzonitrile (22, 16 mmol, 1.0 equiv) in DMSO (16 mL) was added. The resulting solution was stirred at 90 °C for 8 h and quenched by adding 2 N HCl (20 mL). After extraction with the EtOAc (2 × 30 mL), the combined organics were washed with NaHCO3 (2 × 20 mL), brine (30 mL), dried over Na2SO4, and concentrated in vacuo to leave brown oil. The crude material was used for next step without further purification.
General Procedure for the Synthesis of Dinitriles (24).
A solution of compound 23 in DMSO/H2O (9:1) mixture was stirred at 120 °C for 16 h before being quenched with water. The aqueous solution was extracted using EtOAc (2 × 30 mL). The combined organics were washed with water (2 × 20 mL) and brine (30 mL), dried over Na2SO4, and concentrated in vacuo. The crude mixture was purified using CombiFlash with 0–20% EtOAc in hexane as an eluent to yield the desired products (24) as yellow solid. Yield: 70–85% over two steps.
General Procedure for the Synthesis of Arylketone (26).
A mixture of compound 24 (1.0 equiv), arylboronic acid (2.5 equiv), Pd(OAC)2 (0.1 equiv), Mo(CO)6 (1.5 equiv), and K2CO3 (3.0 equiv) in anisole (0.2M, 5 mL) was irradiated at 140 °C for 30 min under microwave conditions. The black residue formed was filtered through Celite, and the solvent was concentrated in vacuo. The crude mixture was purified using CombiFlash with 0–50% EtOAc in hexane as an eluent to yield the desired product 26 as colorless solid. Yield: 50–62%.
General Procedure for Isoquinoline-1,3-dione (33–88).
Method A: To a suspension of compound 21 (1.0 equiv) in 1,2-dichlorobenzene was added urea (2.0 equiv), and the resulting mixture was irradiated at 175 °C for 45 min under microwave conditions. The solvent was removed in vacuo to produce the crude product as brown solid which was purified using CombiFlash with 0–50% EtOAc in hexane as an eluent to furnish titled compounds (33–88) as yellow solid. Method B: A solution of compound 24/25/26 (0.45 mmol) in conc HCl (2 mL) was stirred at 70 °C for 4 h. After the solvent was removed in vacuo, the residue was purified using CombiFlash with 0–30% EtOAc in hexane as an eluent to yield desired products (33–88) as colorless solid.
Isoquinoline-1,3(2H,4H)-dione (33).
1H NMR (600 MHz, DMSO-d6) δ 11.28 (s, 1H), 8.01 (dd, J = 7.8, 1.4 Hz, 1H), 7.64 (td, J = 7.5, 1.4 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.38 (d, J = 7.6 Hz, 1H), 4.03 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 165.5, 136.8, 133.6, 127.9, 127.7, 127.5, 125.1, 36.1. HRMS-ESI (−) m/z calculated for C9H6NO2, 160.0404 [M – H]−; found, 160.0400.
5-Bromoisoquinoline-1,3(2H,4H)-dione (34).
1H NMR (600 MHz, DMSO-d6) δ 11.48 (s, 1H), 8.06 (d, J = 7.7 Hz, 1H), 7.95 (d, J = 7.8 Hz, 1H), 7.43 (t, J = 7.8 Hz, 1H), 3.87 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 169.2, 164.1, 136.6, 135.7, 128.5, 126.9, 126.7, 122.2, 37.1. HRMS-ESI (−) m/z calculated for C9H5BrNO2, 237.9509 [M – H]−; found, 237.9506.
6-Bromoisoquinoline-1,3(2H,4H)-dione (35).
1H NMR (600 MHz, DMSO-d6) δ 11.37 (s, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.67–7.65 (m, 2H), 4.04 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 174.0, 162.4, 156.9, 137.1, 133.6, 129.9, 128.6, 127.6, 35.9. HRMS-ESI (−) m/z calculated for C9H5BrNO2, 237.9509 [M – H]−; found, 237.9515.
6-Iodoisoquinoline-1,3(2H,4H)-dione (36).
1H NMR (600 MHz, DMSO-d6) δ 11.35 (s, 1H), 7.83 (m, 2H), 7.74 (d, J = 8.7 Hz, 1H), 4.01 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.9, 165.5, 139.1, 136.9, 136.8, 136.4, 125.0, 102.5, 35.9. HRMS-ESI (−) m/z calculated for C9H5INO2, 285.9370 [M – H]−; found, 285.9376.
7-Iodoisoquinoline-1,3(2H,4H)-dione (37).
1H NMR (600 MHz, DMSO-d6) δ 11.38 (s, 1H), 8.25 (d, J = 1.8 Hz, 1H), 7.97 (dd, J = 8.1, 1.8 Hz, 1H), 7.19 (d, J = 8.1 Hz, 1H), 3.96 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.4, 163.9, 141.5, 136.1, 135.3, 129.9, 126.8, 92.1, 35.5. HRMS-ESI (−) m/z calculated for C9H5INO2, 285.9370 [M − H]−; found, 285.9367.
8-Iodoisoquinoline-1,3(2H,4H)-dione (38).
1H NMR (600 MHz, DMSO-d6) δ 11.28 (s, 1H), 8.06 (d, J = 7.7 Hz, 1H), 7.40 (d, J = 7.7 Hz, 1H), 7.26 (t, J = 7.7 Hz, 1H), 4.05 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 169.7, 163.6, 141.8, 134.4, 133.4, 128.2, 124.6, 94.9, 37.5. HRMS-ESI (−) m/z calculated for C9H5INO2, 285.9370 [M − H]−; found, 285.9372.
1,3-Dioxo-1,2,3,4-tetrahydroisoquinoline-6-carboxylic Acid (39).
1H NMR (600 MHz, DMSO-d6) δ 13.37 (s, 1H), 11.40 (s, 1H), 8.08 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.91 (s, 1H), 4.08 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.6, 166.3, 164.6, 136.8, 134.6, 128.6, 128.1, 127.6, 127.4, 35.8. HRMS-ESI (−) m/z calculated for C10H6NO4, 204.0302 [M − H]−; found, 204.0301.
1,3-Dioxo-1,2,3,4-tetrahydroisoquinoline-7-carboxylic Acid (40).
1H NMR (600 MHz, DMSO-d6) δ 13.26 (s, 1H), 11.42 (s, 1H), 8.53 (d, J = 1.8 Hz, 1H), 8.14 (dd, J = 8.1, 1.8 Hz, 1H), 7.51 (d, J = 8.1 Hz, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 175.0, 165.9, 162.8, 135.9, 135.2, 134.1, 130.2, 128.7, 127.2, 36.1. HRMS-ESI (−) m/z calculated for C10H6NO4, 204.0302 [M − H]−; found, 204.0308.
6-(Trifluoromethyl)isoquinoline-1,3(2H,4H)-dione (41).
1H NMR (600 MHz, DMSO-d6) δ 11.50 (s, 1H), 8.19 (d, J = 8.1 Hz, 1H), 7.82 (s, 1H), 7.80 (d, J = 8.3 Hz, 1H), 4.12 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.5, 164.4, 138.0, 132.7, 128.5, 124.9, 124.6, 123.7, 122.7, 36.0. HRMS-ESI (−) m/z calculated for C10H5F3NO2, 228.0278 [M − H]−; found, 228.0285.
6-Nitroisoquinoline-1,3(2H,4H)-dione (42).
1H NMR (600 MHz, DMSO-d6) δ 11.59 (s, 1H), 8.29 (s, 1H), 8.25–8.25 (m, 2H), 4.17 (s, 2H). HRMS-ESI (−) m/z calculated for C9H5N2O4, 205.0255 [M − H]−; found, 205.0252.
6-(Furan-2-yl)isoquinoline-1,3 (2H,4H)-dione (43).
1H NMR (600 MHz, DMSO-d6) δ 11.28 (s, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.86 (s, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.70 (s, 1H), 7.18 (d, J = 3.3 Hz, 1H), 6.67 (dd, J = 3.3, 1.7 Hz, 1H), 4.07 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 175.4, 163.0, 150.9, 145.2, 135.0, 133.2, 129.3, 128.9, 128.0, 120.57, 112.9, 110.1, 39.2. HRMS-ESI (−) m/z calculated for C13H8NO3, 226.0510 [M − H]−; found, 226.0506.
7-(Furan-2-yl)isoquinoline-1,3(2H,4H)-dione (44).
1H NMR (600 MHz, DMSO-d6) δ 11.36 (s, 1H), 8.27 (s, 1H), 7.97 (dd, J = 8.1, 1.7 Hz, 1H), 7.79 (s, 1H), 7.44 (d, J = 8.1 Hz, 1H), 7.09 (d, J = 3.3 Hz, 1H), 6.63 (dd, J = 3.3, 1.7 Hz, 1H), 4.04 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.0, 165.2, 151.9, 143.5, 135.6, 129.5, 128.8, 128.4, 125.6, 121.7, 112.4, 106.8, 35.9. HRMS-ESI (−) m/z calculated for C13H8NO3, 226.0510 [M − H]−; found, 226.0504.
5-(Furan-2-yl)isoquinoline-1,3(2H,4H)-dione (45).
1H NMR (600 MHz, DMSO-d6) δ 11.45 (s, 1H), 8.06 (d, J = 7.7 Hz, 1H), 8.00 (d, J = 7.7 Hz, 1H), 7.88 (d, J = 1.7 Hz, 1H), 7.56 (t, J = 7.8 Hz, 1H), 6.95 (d, J = 3.4 Hz, 1H), 6.69 (dd, J = 3.4, 1.7 Hz, 1H), 4.13 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.5, 165.3, 150.6, 143.6, 132.3, 131.6, 128.8, 127.5, 127.3, 126.1, 112.2, 110.6, 35.8. HRMS-ESI (−) m/z calculated for C13H8NO3, 226.0510 [M − H]−; found, 226.0514.
6-(Furan-2-yl)-2-hydroxyisoquinoline-1,3(2H,4H)-dione (46).
1H NMR (600 MHz, DMSO-d6) δ 10.39 (s, 1H), 8.05 (d, J = 8.3 Hz, 1H), 7.87 (d, J = 0.7 Hz, 1H), 7.80 (d, J = 8.3 Hz, 1H), 7.71 (s, 1H), 7.19 (d, J = 3.4 Hz, 1H), 6.69–6.66 (m, 1H), 4.30 (s, 2H). HRMS-ESI (–) m/z calculated for C13H8NO4, 242.0459 [M − H]−; found, 242.0464.
7-(Furan-2-yl)-2-hydroxyisoquinoline-1,3(2H,4H)-dione (47).
1H NMR (600 MHz, CD3OD) δ 8.40 (d, J = 1.8 Hz, 1H), 7.95 (dd, J = 8.1, 1.8 Hz, 1H), 7.61 (d, J = 1.3 Hz, 1H), 7.42 (d, J = 8.1 Hz, 1H), 6.89 (d, J = 3.3 Hz, 1H), 6.55 (dd, J = 3.3, 1.8 Hz, 1H). HRMS-ESI (−) m/z calculated for C13H8NO4, 242.0459 [M − H]−; found, 242.0466.
6-Phenylisoquinoline-1,3(2H,4H)-dione (48).
1H NMR (600 MHz, DMSO-d6) δ 11.31 (s, 1H), 8.08 (d, J = 8.1 Hz, 1H), 7.76–7.74 (m, 3H), 7.71 (s, 1H), 7.52 (t, J = 7.6 Hz, 2H), 7.44 (t, J = 7.3 Hz, 1H), 4.10 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 175.7, 163.1, 157.6, 145.3, 137.8, 132.9, 132.9, 129.5, 129.2, 128.6, 127.2, 124.2, 39.1. HRMS-ESI (−) m/z calculated for C15H10NO2, 236.0717 [M − H]−; found, 236.0712.
6-(Pyridin-3-yl)isoquinoline-1,3(2H,4H)-dione (49).
1H NMR (600 MHz, DMSO-d6) δ 11.40 (s, 1H), 9.18 (s, 1H), 8.83 (d, J = 4.4 Hz, 1H), 8.62 (d, J = 7.8 Hz, 1H), 8.14 (d, J = 8.0 Hz, 1H), 7.96–7.84 (m, 3H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.6, 164.8, 144.7, 143.4, 139.6, 139.5, 137.4, 135.8, 128.2, 126.5, 125.8, 125.8, 125.2, 35.9. HRMS-ESI (−) m/z calculated for C14H9N2O2, 237.0670 [M − H]; found, 237.0672.
7-(Pyridin-3-yl)isoquinoline-1,3(2H,4H)-dione (50).
1H NMR (600 MHz, DMSO-d6) δ 11.44 (s, 1H), 9.29 (s, 1H), 8.87 (d, J = 5.5 Hz, 1H), 8.82 (d, J = 8.1 Hz, 1H), 8.42 (d, J = 2.1 Hz, 1H), 8.15 (dd, J = 8.0, 2.1 Hz, 1H), 8.04 (dd, J = 8.0, 5.5 Hz, 1H), 7.59 (d, J = 8.1 Hz, 1H), 4.13 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.6, 143.5, 142.8, 141.7, 138.5, 137.4, 134.3, 132.8, 129.7, 127.1, 126.7, 126.6, 36.6. HRMS-ESI (−) m/z calculated for C14H9N2O2, 237.0670 [M − H]−; found, 237.0673.
6-(3-Hydroxyphenyl)isoquinoline-1,3(2H,4H)-dione (51).
1H NMR (600 MHz, DMSO-d6) δ 11.30 (s, 1H), 9.64 (s, 1H), 8.07 (d, J = 8.2 Hz, 1H), 7.69 (dd, J = 8.2, 1.7 Hz, 1H), 7.63 (d, J = 0.9 Hz, 1H), 7.31 (t, J = 7.9 Hz, 1H), 7.17–7.13 (m, 1H), 7.09 (t, J = 2.3 Hz, 1H), 6.85 (ddd, J = 8.2, 2.3, 0.9 Hz, 1H), 4.10 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.2, 165.3, 158.1, 145.1, 140.2, 137.4, 130.3, 128.2, 126.0, 125.6, 124.0, 117.9, 115.7, 113.9, 36.3. HRMS-ESI (−) m/z calculated for C15H10NO3, 252.0666 [M − H]−; found, 252.0670.
7-(3-Hydroxyphenyl)isoquinoline-1,3(2H,4H)-dione (52).
1H NMR (600 MHz, DMSO-d6) δ 11.35 (s, 1H), 9.58 (s, 1H), 8.17 (d, J = 1.9 Hz, 1H), 7.90 (dd, J = 7.9, 1.9 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.29 (t, J = 7.9 Hz, 1H), 7.12 (d, J = 7.7 Hz, 1H), 7.07 (s, 1H), 6.87–6.75 (m, 1H), 4.07 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.7, 165.1, 157.8, 139.9, 138.9, 135.5, 131.4, 129.9, 128.4, 125.2, 124.7, 117.1, 114.7, 113.1, 35.5. HRMS-ESI (−) m/z calculated for C15H10NO3, 252.0666 [M − H]−; found, 252.0667.
8-(3-Hydroxyphenyl)isoquinoline-1,3(2H,4H)-dione (53).
1H NMR (600 MHz, DMSO-d6) δ 11.00 (s, 1H), 9.32 (s, 1H), 7.60 (t, J = 7.6 Hz, 1H), 7.37 (d, J = 7.3 Hz, 1H), 7.15 (d, J = 7.5 Hz, 1H), 7.12 (t, J = 7.8 Hz, 1H), 6.71 (dd, J = 8.2, 1.7 Hz, 1H), 6.61 (d, J = 7.5 Hz, 1H), 6.60–6.58 (m, 1H), 4.08 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.9, 164.6, 156.8, 144.3, 143.8, 137.9, 132.4, 130.7, 128.7, 127.6, 123.0, 119.44, 115.6, 113.8, 37.0. HRMS-ESI (−) m/z calculated for C15H10NO3, 252.0666 [M − H]−; found, 252.0670.
7-(4-Hydroxyphenyl)isoquinoline-1,3(2H,4H)-dione (54).
1H NMR (600 MHz, DMSO-d6) δ 11.32 (s, 1H), 9.63 (s, 1H), 8.15 (d, J = 2.0 Hz, 1H), 7.87 (dd, J = 8.0, 2.0 Hz, 1H), 7.54 (d, J = 8.6 Hz, 2H), 7.42 (d, J = 8.1 Hz, 1H), 6.87 (d, J = 8.6 Hz, 2H), 4.04 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.9, 165.2, 157.4, 139.0, 134.4, 130.9, 129.4, 128.4, 127.6, 125.2, 124.0, 115.8, 35.5. HRMS-ESI (−) m/z calculated for C15H10NO3, 252.0666 [M − H]−; found, 252.0669.
6-(3-Nitrophenyl)isoquinoline-1,3(2H,4H)-dione (55).
1H NMR (600 MHz, DMSO-d6) δ 11.36 (s, 1H), 8.53 (t, J = 1.9 Hz, 1H), 8.29 (dd, J = 8.2, 1.9 Hz, 1H), 8.23 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 8.1 Hz, 1H), 7.88 (d, J = 8.2 Hz, 1H), 7.86 (s, 1H), 7.81 (t, J = 8.1 Hz, 1H), 4.12 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.5, 148.9, 142.7, 140.7, 138.0, 134.1, 131.1, 128.9, 126.9, 126.2, 125.3, 123.7, 122.0, 36.6. HRMS-ESI (−) m/z calculated for C15H9N2O4, 281.0568 [M − H]−; found, 281.0561.
7-(3-Nitrophenyl)isoquinoline-1,3(2H,4H)-dione (56).
1H NMR (600 MHz, DMSO-d6) δ 11.41 (s, 1H), 8.49 (s, 1H), 8.33 (s, 1H), 8.26 (d, J = 8.1 Hz, 1H), 8.22 (d, J = 8.1 Hz, 1H), 8.10 (d, J = 9.8 Hz, 1H), 7.79 (t, J = 7.9 Hz, 1H), 7.55 (d, J = 8.1 Hz, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.3, 165.5, 148.9, 140.8, 137.4, 134.2, 133.74, 132.4, 131.2, 129.4, 125.9, 123.1, 122.3, 121.6, 36.3. HRMS-ESI (−) m/z calculated for C15H9N2O4, 281.0568 [M − H]−; found, 281.0570.
8-(3-Nitrophenyl)isoquinoline-1,3(2H,4H)-dione (57).
1H NMR (600 MHz, DMSO-d6) δ 11.12 (s, 1H), 8.23–8.19 (m, 1H), 8.08 (s, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.70–7.64 (m, 2H), 7.48 (d, J = 7.7 Hz, 1H), 7.26 (d, J = 7.5 Hz, 1H), 4.12 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.8, 165.0, 147.4, 144.1, 141.5, 138.5, 135.5, 132.8, 130.9, 130.7, 129.3, 123.6, 123.3, 121.8, 36.9. HRMS-ESI (−) m/z calculated for C15H9N2O4, 281.0568 [M − H]−; found, 281.0575
5-(3-Nitrophenyl)isoquinoline-1,3(2H,4H)-dione (58).
1H NMR (600 MHz, DMSO-d6) δ 11.42 (s, 1H), 8.30 (d, J = 7.8 Hz, 1H), 8.23 (s, 1H), 8.15 (d, J = 7.5 Hz, 1H), 7.89 (d, J = 7.5 Hz, 1H), 7.80 (t, J = 7.8 Hz, 1H), 7.64 (d, J = 7.1 Hz, 1H), 7.60 (t, J = 7.5 Hz, 1H), 3.85 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.1, 165.0, 147.7, 140.1, 138.0, 135.5, 134.4, 133.9, 129.9, 127.6, 127.2, 125.4, 123.5, 122.5, 35.0. HRMS-ESI (−) m/z calculated for C15H9N2O4, 281.0568 [M − H]−; found, 281.0572.
6-(3-Aminophenyl)isoquinoline-1,3(2H,4H)-dione (59).
1H NMR (600 MHz, DMSO-d6) δ 11.34 (s, 1H), 9.84 (s, 1H), 8.11 (d, J = 8.2 Hz, 1H), 7.71 (dd, J = 8.2, 1.7 Hz, 1H), 7.65–7.63 (m, 2H), 7.61 – 7.52 (m, 2H), 7.32 (s, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.5, 144.1, 140.4, 137.9, 130.9, 128.8, 126.4, 125.9, 124.9, 122.2, 120.6, 117.5, 114.2, 36.6. HRMS-ESI (−) m/z calculated for C15H11N2O2, 251.0826 [M − H]−; found, 251.0829.
7-(3-Aminophenyl)isoquinoline-1,3(2H,4H)-dione (60).
1H NMR (600 MHz, DMSO-d6) δ 11.39 (s, 1H), 10.19 (s, 2H), 8.23 (d, J = 2.0 Hz, 1H), 7.95 (dd, J = 8.0, 2.0 Hz, 1H), 7.69 (d, J = 7.9 Hz, 1H), 7.67 (s, 1H), 7.57 (t, J = 7.9 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.38–7.34 (m, 1H), 4.09 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.3, 165.6, 140.5, 138.3, 136.9, 134.9, 131.9, 130.9, 129.4, 126.1, 125.5, 122.3, 120.9, 36.3. HRMS-ESI (−) m/z calculated for C15H11N2O2, 251.0826 [M − H]−; found, 251.0829.
6-(4-Aminophenyl)isoquinoline-1,3(2H,4H)-dione (61).
1H NMR (600 MHz, DMSO-d6) δ 11.30 (s, 1H), 9.59 (s, 2H), 8.06 (d, J = 8.2 Hz, 1H), 7.81–7.77 (m, 2H), 7.74 (dd, J = 8.2, 1.7 Hz, 1H), 7.69 (d, J = 1.7 Hz, 1H), 7.35 (d, J = 8.4 Hz, 2H), 4.08 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.6, 145.7, 144.3, 137.8, 133.5, 128.6, 126.1, 125.7, 124.3, 122.4, 119.2, 36.6. HRMS-ESI (−) m/z calculated for C15H11N2O2, 251.0826 [M − H]−; found, 251.0830.
General Procedure for the Synthesis of Methanesulfonamide Derivative (62–64).
To a solution of 59/60/61 (0.39 mmol, 1.0 equiv) in dioxane (5 mL) was added pyridine (0.99 mmol, 2.5 equiv) and methanesulfonyl chloride (0.79 mmol, 2.0 equiv), and the resulting solution was stirred at rt for 1 h before being quenched with water. After extraction with EtOAc (2 × 20 mL), the combined organic layers were washed with 1 N HCl (2 × 20 mL) and brine (20 mL), dried over Na2SO4, and concentrated in vacuo. The crude mixture was purified using CombiFlash with 0–5% methanol in DCM as an eluent to yield the desired product as colorless solid. Yield: 65–77%.
N-(3-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)phenyl)-methanesulfonamide (62).
1H NMR (600 MHz, DMSO-d6) δ 11.32 (s, 1H), 9.89 (s, 1H), 8.10 (d, J = 8.2 Hz, 1H), 7.69 (d, J = 8.5 Hz, 1H), 7.64 (s, 1H), 7.53 (s, 1H), 7.50–7.45 (m, 2H), 7.28 (d, J = 7.0 Hz, 1H), 3.05 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.6, 144.9, 140.4, 139.6, 137.9, 130.6, 128.7, 126.5, 126.0, 124.7, 123.1, 120.2, 118.6, 39.9, 36.6. HRMS-ESI (−) m/z calculated for C16H13N2O4S, 329.0602 [M − H]−; found, 329.0595.
N-(3-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-7-yl)phenyl)-methanesulfonamide (63).
1H NMR (600 MHz, DMSO-d6) δ 11.37 (s, 1H), 9.84 (s, 1H), 8.20 (d, J = 2.0 Hz, 1H), 7.91 (dd, J = 8.1, 2.0 Hz, 1H), 7.52 (s, 1H), 7.49 (d, J = 8.1 Hz, 1H), 7.47–7.44 (m, 2H), 7.36–7.18 (m, 1H), 4.07 (s, 2H), 3.04 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.7, 140.4, 139.6, 139.1, 136.5, 132.1, 130.6, 129.2, 126.0, 125.4, 122.6, 119.5, 118.2, 39.8, 36.2. HRMS-ESI (−) m/z calculated for C16H13N2O4S, 329.0602 [M − H]−; found, 329.0598.
N-(4-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)phenyl)-methanesulfonamide (64).
1H NMR (600 MHz, DMSO-d6) δ 11.29 (s, 1H), 9.97 (s, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.76–7.72 (m, 3H), 7.67 (s, 1H), 7.33 (d, J = 8.6 Hz, 2H), 4.08 (s, 2H), 3.05 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 175.5, 163.1, 157.6, 144.7, 139.5, 132.9, 132.7, 132.2, 129.2, 128.3, 123.6, 119.6, 40.2, 36.5. HRMS-ESI (−) m/z calculated for C16H13N2O4S, 329.0602 [M − H]−; found, 329.0602.
General Procedure for the Synthesis of Acrylamide Derivative (65–66).
To a solution of 60/61 (0.39 mmol, 1.0 equiv) in dioxane (5 mL) was added pyridine (0.99 mmol, 2.5 equiv) and acryloyl chloride (0.79 mmol, 2.0 equiv), and the resulting solution was stirred at rt for 1 h before being quenched with water. After extraction with EtOAc (2 × 20 mL), the combined organic layers were washed with 1 N HCl (2 × 20 mL) and brine (20 mL), dried over Na2SO4, and concentrated in vacuo. The crude mixture was purified using CombiFlash with 0–10% methanol in DCM as an eluent to yield the desired product as colorless solid. Yield: 70–79%.
N-(4-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)phenyl)-acrylamide (65).
1H NMR (600 MHz, DMSO-d6) δ 11.28 (s, 1H), 10.30 (s, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 8.7 Hz, 2H), 7.75 (dd, J = 8.7, 2.2 Hz, 3H), 7.69 (s, 1H), 6.46 (dd, J = 17.0, 10.2 Hz, 1H), 6.28 (dd, J = 17.0, 1.8 Hz, 1H), 5.78 (dd, J = 10.2, 1.8 Hz, 1H), 4.08 (s, 2H). HRMS-ESI (−) m/z calculated for C18H13N2O3, 305.0932 [M − H]−; found, 305.0928.
N-(3-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-7-yl)phenyl)-acrylamide (66).
1H NMR (600 MHz, DMSO-d6) δ 11.38 (s, 1H), 10.29 (s, 1H), 8.24 (s, 1H), 8.07 (s, 1H), 7.94 (d, J = 7.9 Hz, 1H), 7.72 (d, J = 6.6 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.45 (d, J = 7.4 Hz, 2H), 6.46 (dd, J = 16.8, 10.2 Hz, 1H), 6.29 (d, J = 16.8 Hz, 1H), 5.79 (d, J = 10.2 Hz, 1H), 4.08 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 165.4, 163.5, 139.9, 139.41, 138.9, 136.1, 131.9, 131.7, 129.8, 128.9, 127.3, 125.7, 125.0, 121.9, 118.9, 117.5, 35.9. HRMS-ESI (−) m/z calculated for C18H13N2O3, 305.0932 [M − H]−; found, 305.0936.
2-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)benzoic Acid (67).
1H NMR (600 MHz, DMSO-d6) δ 12.90 (s, 1H), 11.31 (s, 1H), 8.02 (d, J = 7.8 Hz, 1H), 7.82 (d, J = 7.5 Hz, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.43–7.36 (m, 3H), 4.06 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 169.1, 165.3, 146.3, 140.2, 136.6, 131.9, 131.4, 130.6, 129.7, 128.3, 127.8, 127.6, 127.2, 123.9, 36.2. HRMS-ESI (−) m/z calculated for C16H10NO4, 280.0615 [M − H]−; found, 280.0608.
4-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)benzoic Acid (68).
1H NMR (600 MHz, DMSO-d6) δ 13.05 (s, 1H), 11.34 (s, 1H), 8.11 (d, J = 8.2 Hz, 1H), 8.06 (d, J = 8.2 Hz, 2H), 7.88 (d, J = 8.2 Hz, 2H), 7.83 (d, J = 8.3 Hz, 1H), 7.78 (s, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.8, 166.8, 164.9, 143.4, 142.6, 137.3, 130.4, 129.9, 128.1, 127.1, 126.2, 125.6, 124.5, 35.9. HRMS-ESI (−) m/z calculated for C16H10NO4, 280.0615 [M − H]−; found, 280.0620.
3-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)benzoic Acid (69).
1H NMR (600 MHz, DMSO-d6) δ 13.16 (s, 1H), 11.32 (s, 1H), 8.26 (t, J = 1.6 Hz, 1H), 8.10 (d, J = 8.2 Hz, 1H), 8.00 (dd, J = 7.8, 1.8 Hz, 2H), 7.80 (dd, J = 8.2, 1.6 Hz, 1H), 7.76 (s, 1H), 7.65 (t, J = 7.8 Hz, 1H), 4.12 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 167.2, 165.2, 143.9, 139.1, 137.7, 131.8, 131.5, 129.7, 129.3, 128.4, 127.7, 126.3, 125.8, 124.5, 36.2. HRMS-ESI (−) m/z calculated for C16H10NO4, 280.0615 [M − H]−; found, 280.0616.
3-(1,3-Dioxo-1,2,3,4-tetrahydroisoquinolin-6-yl)benzamide (70).
1H NMR (600 MHz, DMSO-d6) δ 11.33 (s, 1H), 8.25 (s, 1H), 8.17 (s, 1H), 8.10 (d, J = 8.2 Hz, 1H), 7.93 (d, J = 7.7 Hz, 1H), 7.90 (d, J = 8.0 Hz, 1H), 7.84 (d, J = 8.2 Hz, 1H), 7.79 (s, 1H), 7.59 (t, J = 7.7 Hz, 1H), 7.48 (s, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 175.7, 167.7, 163.3, 157.8, 144.9, 137.9, 135.5, 133.2, 130.1, 129.7, 129.3, 129.1, 128.5, 126.2, 124.7, 37.8. HRMS-ESI (−) m/z calculated for C16H11N2O3, 279.0775 [M − H]−; found, 279.0778.
6-(3-Acetylphenyl)isoquinoline-1,3(2H,4H)-dione (71).
1H NMR (600 MHz, DMSO-d6) δ 11.16 (s, 1H), 8.09 (s, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.87–7.83 (m, 2H), 7.67 (d, J = 8.1 Hz, 1H), 7.63 (s, 1H), 7.50 (t, J = 7.7 Hz, 1H), 3.95 (s, 2H), 2.33 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 198.3, 171.4, 165.6, 144.3, 139.5, 138.0, 137.9, 132.0, 130.1, 128.7, 127.0, 126.7, 126.2, 125.6, 124.8, 36.6, 27.4. HRMS-ESI (−) m/z calculated for C17H12NO3, 278.0823 [M − H]−; found, 278.0816.
6-(3-Chlorophenyl)isoquinoline-1,3(2H,4H)-dione (72).
1H NMR (600 MHz, DMSO-d6) δ 11.33 (s, 1H), 8.08 (d, J = 8.2 Hz, 1H), 7.82 (t, J = 1.8 Hz, 1H), 7.80 (d, J = 8.2 Hz, 1H), 7.76 (s, 1H), 7.73 (d, J = 7.7 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.51 (d, J = 8.5 Hz, 1H), 4.09 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 165.2, 143.2, 140.9, 137.5, 134.1, 131.1, 128.5, 128.3, 126.9, 126.4, 125.8, 125.8, 124.6, 36.2. HRMS-ESI (−) m/z calculated for C15H9ClNO2, 270.0327 [M − H]−; found, 270.0330.
7-(3-Chlorophenyl)isoquinoline-1,3(2H,4H)-dione (73).
1H NMR (600 MHz, DMSO-d6) δ 11.38 (s, 1H), 8.24 (d, J = 1.8 Hz, 1H), 8.00 (dd, J = 8.1, 1.8 Hz, 1H), 7.78 (d, J = 1.8 Hz, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.52 (t, J = 7.8 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 8.8 Hz, 1H), 4.08 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 165.4, 141.3, 137.8, 136.8, 134.2, 132.1, 131.2, 129.1, 128.1, 126.7, 125.9, 125.7, 125.6, 36.1. HRMS-ESI (−) m/z calculated for C15H9ClNO2, 270.0327 [M − H]−; found, 270.0330.
6-(4-Chlorophenyl)isoquinoline-1,3(2H,4H)-dione (74).
1H NMR (600 MHz, DMSO-d6) δ 11.33 (s, 1H), 8.08 (d, J = 8.2 Hz, 1H), 7.79–7.75 (m, 3H), 7.72 (s, 1H), 7.58 (d, J = 8.5 Hz, 2H), 4.09 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.2, 165.4, 143.7, 137.7, 133.8, 129.4, 129.1, 128.5, 126.2, 125.7, 124.5, 123.3, 36.4. HRMS-ESI (−) m/z calculated for C15H9BrNO2, 313.9822 [M − H]−; found, 313.9826.
6-(3-Bromophenyl)isoquinoline-1,3(2H,4H)-dione (75).
1H NMR (600 MHz, DMSO-d6) δ 11.32 (s, 1H), 8.09–8.01 (m, 2H), 7.91–7.78 (m, 3H), 7.62–7.47 (m, 2H), 4.12 (s, 2H). HRMS-ESI (−) m/z calculated for C15H9ClNO2, 270.0327 [M − H]−; found, 270.0329.
7-(4-Bromophenyl)isoquinoline-1,3(2H, 4H)-dione (76).
1H NMR (600 MHz, DMSO-d6) δ 11.38 (s, 1H), 8.23 (s, 1H), 7.96–7.82 (m, 2H), 7.69–7.64 (m, 4H), 4.07 (s, 2H). HRMS-ESI (−) m/z calculated for C15H9ClNO2, 270.0327 [M − H]−; found, 270.0326.
6-(2,6-Dimethoxyphenyl)isoquinoline-1,3(2H,4H)-dione (77).
1H NMR (600 MHz, DMSO-d6) δ 11.23 (s, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.35 (t, J = 8.4 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.21 (s, 1H), 6.77 (d, J = 8.4 Hz, 2H), 4.02 (s, 2H), 3.66 (s, 6H). 13C NMR (150 MHz, DMSO-d6) δ 171.7, 165.9, 157.6, 140.6, 136.5, 130.6, 130.4, 127.3, 124.0, 118.1, 117.8, 105.0, 56.4, 36.6. HRMS-ESI (−) m/z calculated for C17H14NO4, 296.0928 [M − H]−; found, 296.0931.
6-(2,5-Dimethoxyphenyl)isoquinoline-1,3(2H,4H)-dione (78).
1H NMR (600 MHz, DMSO-d6) δ 11.27 (s, 1H), 8.01 (d, J = 8.1 Hz, 1H), 7.57 (dd, J = 8.1, 1.6 Hz, 1H), 7.48 (d, J = 1.6 Hz, 1H), 7.08 (d, J = 9.0 Hz, 1H), 6.96 (dd, J = 9.0, 3.1 Hz, 1H), 6.90 (d, J = 3.1 Hz, 1H), 4.05 (s, 2H), 3.75 (s, 3H), 3.71 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 171.2, 165.3, 153.5, 150.4, 143.3, 136.5, 129.3, 128.6, 128.4, 127.2, 123.7, 116.1, 114.6, 113.4, 56.3, 55.7, 36.2. HRMS-ESI (−) m/z calculated for C17H14NO4, 296.0928 [M − H]−; found, 296.0931.
6-(3-Fluoro-4-methoxyphenyl)isoquinoline-1,3(2H,4H)-dione (79).
1H NMR (600 MHz, DMSO-d6) δ 11.29 (s, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.75 (dd, J = 8.2, 1.7 Hz, 1H), 7.70 (s, 1H), 7.66 (dd, J = 12.9, 2.2 Hz, 1H), 7.63–7.52 (m, 1H), 7.29 (t, J = 8.8 Hz, 1H), 4.06 (s, 2H), 3.89 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.6, 152.9, 151.4, 148.0, 147.9, 143.6, 137.7, 131.7, 128.5, 125.9, 124.1, 123.7, 114.9, 56.6, 36.6. HRMS-ESI (−) m/z calculated for C16H11FNO3, 284.0728 [M − H]−; found, 284.0726.
6-(3-Chloro-4-fluorophenyl)isoquinoline-1,3(2H,4H)-dione (80).
1H NMR (600 MHz, DMSO-d6) δ 11.35 (s, 1H), 8.14–7.88 (m, 2H), 7.87–7.64 (m, 3H), 7.58 (s, 1H), 4.10 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.5, 142.7, 137.8, 136.9, 133.9, 129.5, 128.6, 128.2, 128.2, 126.6, 126.0, 124.8, 117.8, 36.5. HRMS-ESI (−) m/z calculated for C15H8ClFNO2, 288.0233 [M − H]−; found, 288.0237.
6-(2,4-Difluorophenyl)isoquinoline-1,3(2H,4H)-dione (81).
1H NMR (600 MHz, DMSO-d6) δ 11.34 (s, 1H), 8.09 (d, J = 8.1 Hz, 1H), 7.65 (dd, J = 12.1, 5.4 Hz, 1H), 7.63–7.60 (m, 1H), 7.56 (s, 1H), 7.47–7.39 (m, 1H), 7.25 (td, J = 8.4, 2.3 Hz, 1H), 4.09 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.7, 164.8, 158.1, 138.9, 136.9, 131.8, 127.9, 127.6, 127.4, 124.2, 112.2, 112.1, 104.7, 104.5, 35.8. HRMS-ESI (−) m/z calculated for C15H8F2NO2, 272.0529 [M − H]−; found, 272.0528.
6-(4-(Trifluoromethyl)phenyl)isoquinoline-1,3(2H,4H)-dione (82).
1H NMR (600 MHz, DMSO-d6) δ 11.36 (s, 1H), 8.12 (d, J = 8.2 Hz, 1H), 7.97 (d, J = 8.2 Hz, 2H), 7.87 (d, J = 8.2 Hz, 2H), 7.83 (dd, J = 8.2, 1.7 Hz, 1H), 7.79 (s, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.2, 165.3, 143.4, 142.9, 137.8, 128.5, 128.4, 128.2, 126.8, 126.2, 126.2, 125.1, 124.9, 36.4. HRMS-ESI (−) m/z calculated for C16H9F3NO2, 304.0591 [M − H]−; found, 304.0586.
6-([1,1′-Biphenyl]-3-yl)isoquinoline-1,3(2H, 4H)-dione (83).
1H NMR (600 MHz, DMSO-d6) δ 11.32 (s, 1H), 8.10 (d, J = 8.3 Hz, 1H), 7.99 (s, 1H), 7.88 (d, J = 7.8 Hz, 1H), 7.84 (s, 1H), 7.78 (d, J = 7.6 Hz, 2H), 7.74 (t, J = 7.2 Hz, 2H), 7.61 (t, J = 7.8 Hz, 1H), 7.51 (t, J = 7.6 Hz, 2H), 7.42 (d, J = 7.2 Hz, 1H), 4.11 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 175.8, 163.6, 158.1, 145.7, 141.8, 140.1, 139.0, 133.3, 130.4, 129.3, 129.1, 127.6, 127.4, 126.1, 125.9, 124.8, 124.6, 110.0, 35.8. HRMS-ESI (−) m/z calculated for C21H14NO2, 312.1030 [M − H]−; found, 312.1020.
Synthesis of Benzothiazole (84).
To the mixture of o-phenylenedi-amine (1 mmol) was added carboxylic acid 39 (1.0 mmol), N,N-diisopropylethylamine (1.5 mmol), and propylphosphonic anhydride (1.0 mmol, 50% w/w in EtOAc), and the resulting solution was irradiated at 100 °C for 30 min under microwave conditions before it was diluted with H2O. The precipitate was collected via filtration and washed thoroughly with H2O. Recrystallization of the crude sample in a mixture of EtOH/H2O furnished the title compound as pale-brown solid. 1H NMR (600 MHz, DMSO-d6) δ 11.43 (s, 1H), 8.22 (d, J = 7.9 Hz, 1H), 8.18 (d, J = 8.1 Hz, 1H), 8.17–8.10 (m, 3H), 7.60 (t, J = 7.6 Hz, 1H), 7.52 (t, J = 7.6 Hz, 1H), 4.18 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.6, 165.6, 164.5, 153.2, 137.7, 136.5, 134.6, 128.4, 126.8, 126.8, 126.1, 125.9, 125.5, 123.0, 122.4, 35.8. HRMS-ESI (−) m/z calculated for C16H9N2O2S, 293.0390 [M − H]−; found, 293.0384.
6-(Quinolin-3-yl)isoquinoline-1,3(2H,4H)-dione (85).
1H NMR (600 MHz, DMSO-d6) δ 11.37 (s, 1H), 9.32 (d, J = 2.2 Hz, 1H), 8.78 (d, J = 2.2 Hz, 1H), 8.16 (d, J = 8.3 Hz, 1H), 8.11–8.06 (m, 2H), 8.00 (d, J = 8.3 Hz, 1H), 7.97 (s, 1H), 7.82 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 4.14 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 175.3, 163.0, 157.6, 148.9, 146.9, 142.2, 134.8, 133.2, 133.1, 130.7, 130.6, 129.2, 129.2, 128.9, 128.36, 127.6, 124.8, 39.1. HRMS-ESI (−) m/z calculated for C18H11N2O2, 287.0826 [M − H]−; found, 287.0826.
6-Benzylisoquinoline-1,3(2H,4H)-dione (86).
1H NMR (600 MHz, DMSO-d6) δ 11.20 (s, 1H), 7.92 (d, J = 8.0 Hz, 1H), 7.33–7.28 (m, 3H), 7.25 (d, J = 6.2 Hz, 3H), 7.19 (t, J = 7.2 Hz, 1H), 4.00 (s, 2H), 3.97 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 165.6, 147.8, 135.6, 129.3, 129.3, 129.1, 129.0, 128.3, 128.2, 126.9, 126.7, 41.4, 36.4. HRMS-ESI (−) m/z calculated for C16H12NO2, 250.0874 [M − H]−; found, 250.0868.
6-(3-Nitrobenzoyl)isoquinoline-1,3(2H,4H)-dione (87).
1H NMR (600 MHz, DMSO-d6) δ 11.49 (s, 1H), 8.54 (dd, J = 7.9, 1.8 Hz, 1H), 8.47–8.44 (m, 1H), 8.20 (s, 1H), 8.18 (s, 1H), 7.89 (t, J = 8.0 Hz, 1H), 7.83–7.79 (m, 2H), 4.13 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 193.8, 171.1, 165.1, 148.2, 140.2, 138.1, 137.7, 136.2, 131.0, 129.5, 128.9, 128.4, 128.3, 127.8, 124.6, 36.5. HRMS-ESI (−) m/z calculated for C16H9N2O5, 309.0517 [M − H]−; found, 309.0510.
7-(3-Nitrobenzoyl)isoquinoline-1,3(2H,4H)-dione (88).
1H NMR (600 MHz, DMSO-d6) δ 11.49 (s, 1H), 8.53 (d, J = 8.5 Hz, 1H), 8.45 (s, 1H), 8.32 (s, 1H), 8.17 (d, J = 7.6 Hz, 1H), 8.06 (d, J = 7.9 Hz, 1H), 7.89 (t, J = 8.0 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 4.17 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 192.7, 170.3, 164.5, 147.6, 141.8, 137.9, 135.4, 134.8, 133.8, 130.4, 128.7, 128.5, 126.9, 125.3, 123.8, 36.2. HRMS-ESI (−) m/z calculated for C16H9N2O5, 309.0517 [M − H]−; found, 309.0523.
Ethyl-6-bromo-1,3-dioxo-1,2,3,4-tetrahydroisoquinoline-4-car-boxylate (89).
1H NMR (600 MHz, DMSO-d6) δ 12.63 (s, 1H), 11.37 (s, 1H, enol isomer), 8.63 (s, 1H), 8.04 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 8.4 Hz, 1H, enol isomer), 7.66–7.61 (m, 2H, enol isomer), 7.51 (d, J = 8.0 Hz, 1H), 4.47 (q, J = 6.9 Hz, 2H), 4.03 (s, 1H), 1.42 (t, J = 6.9 Hz, 3H). HRMS-ESI (−) m/z calculated for C12H9BrNO4, 309.9720 [M − H]−; found, 309.9722.
Synthesis of Compound 90.
The mixture of methyl-4-bromo-2-aminobenzoate (27,1.0 equiv), phenylboronic acid (1.6 equiv), Pd(PPh3)4 (0.065 equiv), and K2CO3 (3.6 equiv) in DME/H2O (4:1) was irradiated at 110 °C for 45 min under microwave conditions. The black residue formed was filtered through Celite, and the solvent was concentrated in vacuo. The crude mixture was purified using CombiFlash with 0–50% EtOAc in hexane as an eluent to yield the desired product (28) as colorless solid. Yield: 50%.
To a solution of potassium isocyanate (1 mmol) in 1 mL of dry toluene was added compound 28, and the resulting mixture was stirred at rt for 3 h before water was added. The aqueous solution was extracted with DCM (2 × 10 mL), and the combined organics were washed with NaHCO3 (2 × 10 mL) and brine (15 mL), dried over Na2SO4, and concentrated in vacuo to leave colorless oil. To the resulting oil in EtOH (2 mL) was added 1N NaOH (2 mL), and the reaction mixture was stirred under reflux for 1 h before it was acidified (pH = 3) using 1 N HCl. The precipitate was collected via filtration, washed with water, and then dried to obtain the titled compound (90) as colorless solid. Yield: 68%. 1H NMR (600 MHz, DMSO-d6) δ 11.30 (s, 1H), 11.20 (s, 1H), 7.96 (d, J = 8.2 Hz, 1H), 7.67 (s, 1H), 7.66 (s, 1H), 7.53 (t, J = 7.6 Hz, 2H), 7.49–7.44 (m, 2H), 7.38 (d, J = 1.3 Hz, 1H). 13C NMR (150 MHz, DMSO-d6) δ 162.8, 150.5, 146.7, 141.5, 138.9, 129.3, 127.9, 127.8, 127.1, 113.5, 113.3, 113.0, 39.2. HRMS-ESI (−) m/z calculated for C14H9N2O2, 237.0670 [M − H]−; found, 237.0672.
Synthesis of Naphthalimides 91–92.
A mixture of 4-bromo-1,8-naphthalic anhydride (29, 1.0 equiv), 2-(tributylstannyl)furan (1.2 equiv), and Pd(PPh3)4 (0.03 equiv) in toluene (5.0 mL) was stirred at 150 °C for 30 min under microwave conditions. The black residue formed was filtered through Celite, and the solvent was concentrated in vacuo. The crude mixture was purified using CombiFlash with 0–30% EtOAc in hexane as an eluent to yield 4-furylnaphthalic anhydride (30) as colorless solid. Yield: 70%.
A mixture of 29/30 (0.72 mmol) in ammonium hydroxide (15 mL) was stirred at 40 °C for 4 h. The resulting solution was extracted with EtOAc (2 × 20 mL), and the combined organic extracts were washed with H2O (2 × 20 mL) and brine (20 mL), dried over Na2SO4, and concentrated in vacuo. Recrystallization of the crude sample from EtOH produced desired compounds (91–92) as colorless solid. Yield: 70–90%.
6-Bromo-1H-benzo[d]isoquinoline-1,3(2H)-dione (91).
1H NMR (600 MHz, DMSO-d6) δ 11.84 (s, 1H), 8.53–8.48 (m, 2H), 8.25 (d, J = 7.8 Hz, 1H), 8.18 (d, J = 7.8 Hz, 1H), 7.96 (dd, J = 8.3, 7.4 Hz, 1H). 13C NMR (150 MHz, DMSO-d6) δ 163.7, 163.6, 132.7, 131.4, 130.9, 130.4, 130.2, 129.7, 129.2, 128.8, 123.4, 122.6. HRMS-ESI (−) m/z calculated for C12H5BrNO2, 273.9509 [M − H]−; found, 273.9504.
6-(Furan-2-yl)-1H-benzo[d]isoquinoline-1,3(2H)-dione (92).
1H NMR (600 MHz, DMSO-d6) δ 11.80 (s, 1H), 8.93 (d, J = 8.6 Hz, 1H), 8.52 (d, J = 7.1 Hz, 1H), 8.48 (d, J = 7.7 Hz, 1H), 8.12 (d, J = 7.7 Hz, 1H), 8.07 (d, J = 1.8 Hz, 1H), 7.98–7.87 (m, 1H), 7.30 (d, J = 3.4 Hz, 1H), 6.83 (dd, J = 3.4, 1.8 Hz, 1H). 13C NMR (150 MHz, DMSO-d6) δ 164.5, 164.1, 151.2, 145.5, 133.7, 132.3, 130.6, 130.1, 129.8, 128.1, 127.8, 125.9, 123.1, 113.3, 112.9, 39.1. HRMS-ESI (−) m/z calculated for C16H8NO3, 262.0510 [M − H]−; found, 262.0512.
Synthesis of 93–95.
To a suspension of 2-amino-1-propene-1,1,3-tricarbonitrile (31, 7.6 mmol, 1.0 equiv) in EtOH (10 mL) was added arylhydrazine (8.4 mmol, 1.1 equiv) slowly and the solution was heated at reflux for 30 min before it was cooled. The solvent was removed in vacuo to leave pale-brown solid. Recrystallization of the crude mixture from EtOH furnished intermediates 32 as brown needles. Yield: 70–95%.
A solution of compound 32 (0.45 mmol) in conc HCl (2 mL) was stirred at 70 °C for 4 h. After the solvent was removed in vacuo, the residue was purified using CombiFlash with 0–100% EtOAc in hexane as an eluent to yield desired products (93–95) as colorless solid. Yield: 65–85%.
3-Amino-2-(4-fluorophenyl)-2H-pyrazolo[4,3-c]pyridine-4,6-(5H,7H)dione (93).
1H NMR (600 MHz, DMSO-d6) δ 10.60 (s, 1H), 7.55 (dd, J = 8.9, 4.9 Hz, 2H), 7.36 (t, J = 8.8 Hz, 2H), 6.48 (s, 2H), 3.75 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.4, 162.5, 160.5, 152.9, 148.4, 148.2, 134.2, 126.6, 126.5, 116.7, 116.5, 92.9, 32.2. HRMS-ESI (−) m/z calculated for C12H8FN4O2, 259.0637 [M − H]−; found, 259.0636.
3-Amino-2-(pyridin-2-yl)-2H-pyrazolo[4,3-c]pyridine-4,6(5H,7H)-dione (94).
1H NMR (600 MHz, DMSO-d6) δ 10.64 (s, 1H), 8.51–8.43 (m, 1H), 8.02–7.96 (m, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.77 (s, 2H), 7.35–7.29 (m, 1H), 3.80 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 171.1, 162.2, 153.8, 149.7, 149.6, 147.5, 140.0, 121.1, 113.2, 92.8, 32.2. HRMS-ESI (+) m/z calculated for C11H10N5O2, 244.0829 [M + H]−; found, 244.0832.
3-Amino-2-phenyl-2H-pyrazolo[4,3-c]pyridine-4,6(5H,7H)dione (95).
1H NMR (600 MHz, DMSO-d6) δ 10.60 (s, 1H), 7.76–7.45 (m, 4H), 7.41 (dd, J = 5.6, 3.0 Hz, 1H), 6.45 (s, 2H), 3.76 (s, 2H). 13C NMR (150 MHz, DMSO-d6) δ 170.8, 161.9, 147.8, 147.4, 137.3, 129.2, 123.2, 92.5, 31.7. HRMS-ESI (+) m/z calculated for C12H11N4O2, 243.0882 [M + H]−; found, 243.0890.
N-(4,6-Dioxo-2-phenyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]-pyridine-3-yl)acetamide (96).
To a solution of compound 95 (1.0 equiv) in AcOH (5.0 mL) was added acetic anhydride (1.2 equiv) and stirred at 70 °C for 5 h. The solvent was evaporated in vacuo to leave pale solid. Recrystallization of crude sample from MeOH produced the title compound (96) as colorless solid. Yield: 62%. 1H NMR (600 MHz, DMSO-d6) δ 10.94 (s, 1H), 10.32 (s, 1H), 7.64–7.36 (m, 5H), 3.95 (s, 2H), 1.97 (s, 3H). HRMS-ESI (−) m/z calculated for C14H11N4O3, 283.0837 [M − H]−; found, 283.0831.
Biology.
Recombinant TDP1 Assay.
A 5′-[32P]-labeled single-stranded DNA oligonucleotide containing a 3′-phosphotyrosine (N14Y)26 was incubated at 1 nM with 10 pM recombinant TDP1 in the absence or presence of inhibitor for 15 min at room temperature in WCE buffer. When indicated, parallel reactions were performed in the LMP1 assay buffer containing 50 mM Tris HCl, pH 7.5, 80 mM KCl, 2 mM EDTA, 1 mM DTT, 40 μg/mL BSA, and 0.01% Tween-20.27 Reactions were terminated by the addition of 1 volume of gel loading buffer [99.5% (v/v) formamide, 5 mM EDTA, 0.01% (w/v) xylene cyanol, and 0.01% (w/v) bromophenol blue]. Samples were subjected to a 16% denaturing PAGE with multiple loadings at 12 min intervals. Gels were dried and exposed to a PhosphorImager screen (GE Healthcare). Gel images were scanned using a Typhoon 8600 (GE Healthcare), and densitometry analyses were performed using the ImageQuant software (GE Healthcare).
Whole Cell Extract TDP1 Assay.
DT40 knockout cells (1 × 107) for TDP1 (TDP1−/−) complemented with human TDP1 (hTDP1) were collected, washed, and centrifuged. Cell pellets were then resuspended in 100 μL of CellLytic M cell lysis reagent (SIGMA-Aldrich C2978). After 15 min on ice, lysates were centrifuged at 12000g for 10 min, and supernatants were transferred to a new tube. Protein concentrations were determined using a Nanodrop spectrophotometer (Invitrogen), and whole cell extracts were stored at −80 °C. The N14Y DNA substrate was incubated at 1 nM with 5 μg/mL of whole cell extracts in the absence or presence of inhibitor for 15 min at room temperature in the LMP1 assay buffer. Samples were then analyzed similarly to the recombinant TDP1 assay (see above).
Recombinant TDP2 Assay.
TDP2 reactions were carried out as described previously14 with the following modifications. The 18-mer single-stranded oligonucleotide DNA substrate (TY18, α32P-cordyce-pin-3′-labeled) was incubated at 1 nM with 25 pM recombinant human TDP2 in the absence or presence of inhibitor for 15 min at room temperature in the LMP2 assay buffer containing 50 mM Tris-HCl, pH 7.5, 80 mM KCl, 5 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 40 μg/mL BSA, and 0.01% Tween 20. Reactions were terminated and treated similarly to WCE and recombinant TDP1 reactions (see above).
Whole Cell Extract TDP2 Assay.
DT40 knockout cells (1 × 107) for TDP1 (TDP2−/−) complemented with human TDP2 (hTDP2) were collected, washed, and centrifuged. hTDP2 whole cell extracts were prepared and stored similarly to hTDP1 whole cell extracts. The TY18 DNA substrate was incubated at 1 nM with 5 μg/mL of whole cell extracts in the absence or presence of inhibitor for 15 min at room temperature in the LMP2 assay buffer. Reactions were terminated and treated similarly to WCE and recombinant TDP1 reactions (see above).
Molecular Modeling.
Molecular modeling was performed using the Schrödinger small molecule drug discovery suite 2014–3. Homology models generated from templates having a higher degree of sequence identity with the target are found to be reliable and accurate for the utilization in structure-based drug design. Because of the high sequence homology between hTDP2 and mTDP2 (65%), an available crystal structure of mTDP2 (PDB code: 4GZ115) was used to build a homology model of hTDP2. The sequence of hTDP2 was imported into the program Prime28,29 (Schrödinger Inc.), and the model was built using mTDP2 (PDB code: 4GZ115) as a template. This model was subjected to the loop refinement and energy minimization to improve accuracy and quality of the model. This model was subjected to protein preparation wizard30,31 (Schrodinger Inc.) in which missing hydrogens were added and zero-order bonds to metals were created followed by the generation of metal binding states. The structure of protein was minimized using OPLS 2005 force field32 to optimize hydrogen bonding network and converge heavy atoms to the RMSD of 0.3 Å.
The receptor grid generation tool in Maestro (Schrödinger Inc.) was used to define an active site around the metal (Mg2+) to cover all the residues within 12 Å from it. Compound 40 and 56 were drawn using Maestro and subjected to Lig Prep33 to generate conformers, possible protonation at pH of 7 ± 3, and metal binding states which serves as an input for docking process. All the dockings were performed using Glide XP23,24 (Glide, version 6.4) mode. The van der Waals radii of nonpolar atoms for each of the ligands were scaled by a factor of 0.8. The predicted favorable binding mode of compounds 40 and 56 features the critical interaction between the ligands and E152, T230, D262, N264, R266, H351 (residues are labeled according to hTDP2 sequence), and the metal ion within the active site of hTDP2. All the ligands docked were further refined post docking by minimizing under implicit solvent to account for the local protein flexibility.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Faculty Research Development Grant (to H.A. and Z.W.) of the Academic Health Center, University of Minnesota, and in part by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute (Z01 BC 006161-17), and the Minnesota Supercomputing Institute (MSI) for computational resources involving modeling.
ABBREVIATIONS USED
- TDP2
tyrosyl DNA phosphodiesterase 2
- TDP1
tyrosyl DNA phosphodiesterase 1
- Top2
topoisomerase II
- SAR
structure–activity relationship
- ETP
etoposide
- HBV
hepatitis B virus
- cccDNA
covalently closed circular DNA
- RC-DNA
relaxed circular DNA
- EEP
the exonuclease–endonuclease phosphatase
- hAPE1
human apurinic/apyrimidinic endonuclease 1
- HTS
high-throughput screening
- PK
pharmacokinetics
- WCE
whole cell extract
- HID
2-hydroxyisoquinoline-1,3-dione
- MW
microwave irradiation
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmed-chem.5b01973.
Characterization data, including 1H NMR data of intermediate chemotypes 21, 24, 25, and 32 (PDF) Molecular formula strings (CSV)
The authors declare no competing financial interest.
REFERENCES
- (1).Helleday T; Petermann E; Lundin C; Hodgson B; Sharma RA DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [DOI] [PubMed] [Google Scholar]
- (2).Nitiss JL Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ledesma FC; El Khamisy SF; Zuma MC; Osborn K; Caldecott KW A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 2009, 461, 674–U125. [DOI] [PubMed] [Google Scholar]
- (4).Gomez-Herreros F; Romero-Granados R; Zeng Z; Alvarez-Quilon A; Quintero C; Ju L; Umans L; Vermeire L; Huylebroeck D; Caldecott KW; Cortes-Ledesma F. TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 2013, 9, e1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gomez-Herreros F; Schuurs-Hoeijmakers JHM; McCormack M; Greally MT; Rulten S; Romero-Granados R; Counihan TJ; Chaila E; Conroy J; Ennis S; Delanty N; Cortes-Ledesma F; de Brouwer APM; Cavalleri GL; El-Khamisy SF; de Vries BBA; Caldecott KW TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nat. Genet 2014, 46, 516–521. [DOI] [PubMed] [Google Scholar]
- (6).Maede Y; Shimizu H; Fukushima T; Kogame T; Nakamura T; Miki T; Takeda S; Pommier Y; Murai J. Differential and common DNA repair pathways for topoisomerase I- and II-targeted drugs in a genetic DT40 repair cell screen panel. Mol. Cancer Ther 2014, 13, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zeng ZH; Cortes-Ledesma F; El Khamisy SF; Caldecott KW TDP2/TTRAP is the major 5 ′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J. Biol. Chem 2011, 286, 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Do PM; Varanasi L; Fan S; Li C; Kubacka I; Newman V; Chauhan K; Daniels SR; Boccetta M; Garrett MR; Li R; Martinez LA Mutant p53 cooperates with ETS2 to promote etoposide resistance. Genes Dev. 2012, 26, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Virgen-Slane R; Rozovics JM; Fitzgerald KD; Ngo T; Chou W; van der Heden van Noort GJV; Filippov DV; Gershon PD; Semler BL An RNA virus hijacks an incognito function of a DNA repair enzyme. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 14634–14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Koniger C; Wingert I; Marsmann M; Rosler C; Beck J; Nassal M. Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc. Natl. Acad. Sci. U. S. A 2014, 111, E4244–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Beck J; Nassal M. Hepatitis B virus replication. World J. Gastroenterol 2007, 13, 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [DOI] [PubMed] [Google Scholar]
- (13).Cui XJ.; McAllister R.; Boregowda R.; Sohn JA.; Ledesma FC.; Caldecott KW.; Seeger C.; Hu JM. Does tyrosyl DNA phosphodiesterase-2 play a role in hepatitis B virus genome repair? PLoS One 2015, 10, e0128401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gao R; Huang SYN; Marchand C; Pommier Y. Biochemical characterization of human tyrosyl-DNA phosphodiesterase 2 (TDP2/TTRAP) a Mg2+/Mn2+-dependent phosphodiesterase specific for the repair of topoisomerase cleavage complexes. J. Biol. Chem 2012, 287, 30842–30852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schellenberg MJ; Appel CD; Adhikari S; Robertson PD; Ramsden DA; Williams RS Mechanism of repair of 5′-topoisomerase II-DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2. Nat. Struct. Mol. Biol 2012, 19, 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Shi K; Kurahashi K; Gao R; Tsutakawa SE; Tainer JA; Pommier Y; Aihara H. Structural basis for recognition of 5′-phosphotyrosine adducts by Tdp2. Nat. Struct. Mol. Biol 2012, 19, 1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Garcin ED; Hosfield DJ; Desai SA; Haas BJ; Bjoras M; Cunningham RP; Tainer JA DNA apurinic-apyrimidinic site binding and excision by endonuclease IV. Nat. Struct. Mol. Biol 2008, 15, 515–522. [DOI] [PubMed] [Google Scholar]
- (18).Raoof A; Depledge P; Hamilton NM; Hamilton NS; Hitchin JR; Hopkins GV; Jordan AM; Maguire LA; McGonagle AE; Mould DP; Rushbrooke M; Small HF; Smith KM; Thomson GJ; Turlais F; Waddell ID; Waszkowycz B; Watson AJ; Ogilvie DJ Toxoflavins and deazaflavins as the first reported selective small molecule inhibitors of tyrosyl-DNA phosphodiesterase II. J. Med. Chem 2013, 56, 6352–6370. [DOI] [PubMed] [Google Scholar]
- (19).Chen YL; Tang J; Kesler MJ; Sham YY; Vince R; Geraghty RJ; Wang ZQ The design, synthesis and biological evaluations of C-6 or C-7 substituted 2-hydroxyisoquinoline-1,3-diones as inhibitors of hepatitis C virus. Bioorg. Med. Chem 2012, 20, 467–479. [DOI] [PubMed] [Google Scholar]
- (20).Tsou HR; Otteng M; Tran T; Floyd MB; Reich M; Birnberg G; Kutterer K; Ayral-Kaloustian S; Ravi M; Nilakantan R; Grillo M; McGinnis JP; Rabindran SK 4-(Phenylaminomethylene)isoquinoline-1,3(2H,4H)-diones as potent and selective inhibitors of the cyclin-dependent kinase 4 (CDK4). J. Med. Chem 2008, 51, 3507–3525. [DOI] [PubMed] [Google Scholar]
- (21).Castro A; Jerez MJ; Gil C; Calderon F; Domenech T; Nueda A; Martinez A. CODES, a novel procedure for ligand-based virtual screening: PDE7 inhibitors as an application example. Eur. J. Med. Chem 2008, 43, 1349–1359. [DOI] [PubMed] [Google Scholar]
- (22).Jin Z; Zhang XB; Xie DX; Gong YJ; Zhang J; Chen X; Shen GL; Yu RQ Clicking fluoroionophores onto mesoporous silicas: a universal strategy toward efficient fluorescent surface sensors for metal ions. Anal. Chem 2010, 82, 6343–6346. [DOI] [PubMed] [Google Scholar]
- (23).Ali JA; Jackson AP; Howells AJ; Maxwell A. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry 1993, 32, 2717–2724. [DOI] [PubMed] [Google Scholar]
- (24).Friesner RA; Murphy RB; Repasky MP; Frye LL; Greenwood JR; Halgren TA; Sanschagrin PC; Mainz DT Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem 2006, 49, 6177–6196. [DOI] [PubMed] [Google Scholar]
- (25).Pommier Y; Huang SY; Gao R; Das BB; Murai J; Marchand C. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2). DNA Repair 2014, 19, 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Marchand C; Lea WA; Jadhav A; Dexheimer TS; Austin CP; Inglese J; Pommier Y; Simeonov A. Identification of phosphotyrosine mimetic inhibitors of human tyrosyl-DNA phosphodiesterase I by a novel alphaScreen high-throughput assay. Mol. Cancer Ther 2009, 8, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Nguyen TX; Morrell A; Conda-Sheridan M; Marchand C; Agama K; Bermingam A; Stephen AG; Chergui A; Naumova A; Fisher R; O’Keefe BR; Pommier Y; Cushman M. Synthesis and biological evaluation of the first dual tyrosyl-DNA phosphodiesterase I (Tdp1)-topoisomerase I (Top1) inhibitors. J. Med. Chem 2012, 55, 4457–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).McRobb FM; Capuano B; Crosby IT; Chalmers DK; Yuriev E. Homology modeling and docking evaluation of aminergic G protein-coupled receptors. J. Chem. Inf. Model 2010, 50, 626–637. [DOI] [PubMed] [Google Scholar]
- (29).Goldfeld DA; Zhu K; Beuming T; Friesner RA Successful prediction of the intra and extracellular loops of four G-protein-coupled receptors. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 8275–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Madhavi Sastry G; Adzhigirey M; Day T; Annabhimoju R; Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des 2013, 27, 221–234. [DOI] [PubMed] [Google Scholar]
- (31).Schrödinger Release 2014–3: Schrödinger Suite 2014–3. (a) Protein Preparation Wizard. (b) Epik, version 2.9; Schrödinger, LLC: New York, 2014. (c) Impact, version 6.4; Schrödinger, LLC: New York, 2014. (d) Prime, version 3.7; Schrödinger, LLC: New York, 2014.
- (32).Jorgensen WL; Maxwell DS; TiradoRives J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc 1996, 118, 11225–11236. [Google Scholar]
- (33).Schrödinger Release 2014–3: LigPrep, version 3.1; Schrödinger, LLC: New York, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.