Highlights
-
•
Whether SARS-CoV-2 infection confers immunity to reinfection is uncertain.
-
•
The ‘second wave’ of transmission offered an opportunity to examine this.
-
•
We observed no symptomatic reinfections in a cohort of healthcare workers.
-
•
This apparent immunity to re-infection was maintained for at least 6 months.
-
•
Further studies are required to define immunological mechanism(s) and durability.
Keywords: COVID-19, Immunity, Healthcare workers, Protection, Antibody
Graphical abstract
To the editor,
It has been recently suggested that prior exposure to seasonal coronaviruses might confer cross-protection against SARS-CoV-2.1 Whether SARS-CoV-2 infection itself confers immunity to reinfection has not been established. Immunity is probable, at least in the short term, because reinfections are infrequently reported, despite over 55 million primary infections occurring worldwide since December 2019.2 Protection against short term reinfection has been observed in a non-human primate model of SARS-CoV-2.3 A small clinical study was also suggestive.4 Immunity to seasonal coronaviruses is maintained for up to 12 months.5
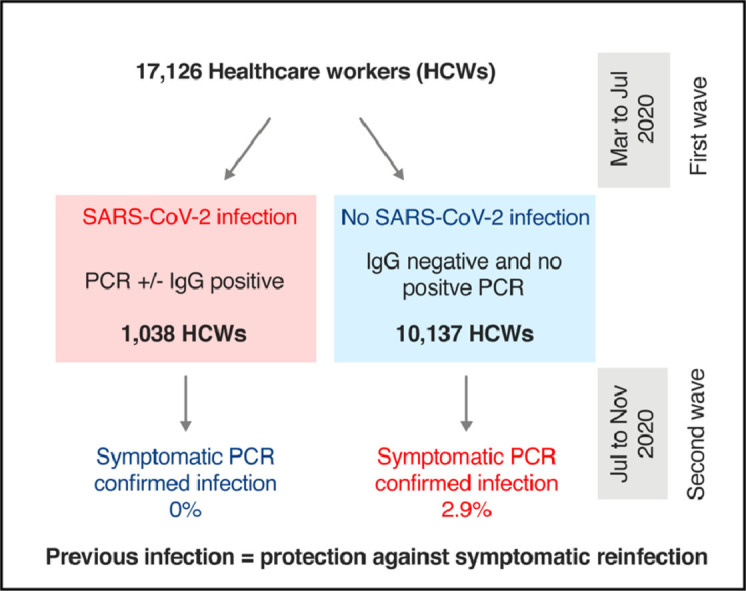
The second wave of SARS-CoV-2 transmission provides an opportunity to detect early signals of immunity. We previously defined a large cohort of over 11,000 healthcare workers (HCWs) with documented evidence of previous infection status during the first wave of the SARS-CoV-2 pandemic, from March to April 2020. A second wave of SARS-CoV-2 transmission occurred in our setting from October to November 2020, approximately 7 months later. We undertook a retrospective analysis of HCW testing data during this second wave, to address the question of whether previous SARS-CoV-2 infection was associated with protection.
This study was granted a waiver by the Newcastle and North Tyne Research Ethics Committee. Access to data was granted under Regulation 3(4) of the Health Service Control of Patient Information Regulations 2002 (March 2020) and approved by the Newcastle-upon-Tyne Hospitals (NUTH) NHS Foundation Trust Caldicott Guardian (7566). NUTH is a large tertiary referral centre employing 17,126 staff. SARS-CoV-2 RT-PCR testing has been provided to all HCWs with symptoms of COVID-19 since early March 2020.6 SARS-CoV-2 PCR testing was undertaken on combined nasopharyngeal and oropharyngeal swabs in viral transport medium, using Public Health England (PHE) approved RT-PCR assays containing two SARS-CoV-2 gene targets. SARS-CoV-2 nucleocapsid IgG antibody testing was undertaken on the Roche Anti-SARS-CoV-2 IgG assay.
We defined two periods for analysis: the first, 10 Mar to 6 July 2020, spanned the first wave of SARS-CoV-2 transmission. The second, 7 July to 20 Nov, included the second wave. Testing data were obtained from a prospectively maintained regional virology diagnostic laboratory internal database. Methods for data collection and cleaning are described separately.7 In the first period, 481/3338 symptomatic HCWs tested positive for SARS-CoV-2 by PCR, while SARS-CoV-2 IgG was detected in 937/11,103 (8.4%) HCWs. Together, these data allowed us to identify 1038 HCWs with evidence of previous infection (positive PCR and/or Ab) and 10,137 without (negative Ab, without positive PCR).
The primary endpoint for analysis was symptomatic SARS-CoV-2 infection, defined as a positive PCR for SARS-CoV-2 from a combined nasopharyngeal/oropharyngeal swab taken as part of the Trust's symptomatic staff testing programme in the period from 7 Jul 2020 to 20 Nov 2020. Analyses were done using GraphPad Prism version 8.4.3 (GraphPad Software LLC, US). Positivity rates between groups were assessed using contingency tables and Chi2 (χ2) hypothesis tests.
We first compared the baseline demographic characteristics of those with or without prior SARS-CoV-2 infection. There were no significant differences in age or gender, but differences in previous positivity rates according to ethnicity and multiple deprivation index were found (Table 1 ). From 7 Jul until 20 Nov 2020, 2243 HCWs underwent PCR testing for symptoms. 128 had previous confirmed SARS-CoV-2 infection, while 2115 had not. In those previously infected, there was a median of 173 (IQR: 162–229) days from the date of first positive PCR/antibody result to the end of the analysis period. Test positivity rates were 0% (0/128 [95% CI: 0–2.9]) in those with previous infection compared to 13.7% (290/2115 [95% CI: 12.3–15.2]) in those without (P<0.0001 χ2 test). Considering the population as a whole, a positive PCR test was returned in 0/1038 (0% [95% CI: 0–0.4) of those with previous infection, compared to 290/10,137 (2.9% [95% CI: 2.6–3.2) of those without (P<0.0001 χ2 test).
Table 1.
Baseline demographic data (where available) and reinfection rates by prior infection category. IMD = index of multiple deprivation. BAME = Black, Asian and Minority Ethnic groups. NS = non-significant (P > 0.05).
| Factor | Prior infection (N = 1038) | No prior infection (N = 10,137) | P value |
| Baseline characteristics | |||
| Age median | 39.5 (30–49) | 40 (30–50) | n.s. |
| Female | 798/967 (82.5%) | 7792/9674 (80.5%) | n.s. |
| BAME | 107/949 (11.2%) | 796/9497 (8.4%) | 0.0025 |
| Lowest quartile IMD | 326/958 (34.0%) | 2620/9594 (27.3%) | <0.0001 |
| Outcome data | |||
| PCR tested | 128/1038 (12.3%) | 2115/10,137 (20.8%) | <0.0001 |
| Per test positivity | 0/128 (0%) | 290/2115 (13.7%) | <0.0001 |
| Proportion PCR positive | 0/1038 (0%) | 290/10,137 (2.9%) | <0.0001 |
Fewer HCWs in the previous infection group presented for symptomatic testing. 128/1038 (12.3% [95% CI: 10.5–14.5]) of those with evidence of prior infection had a test due to symptoms in the second period compared to 2115/10,137 (20.8% [95% CI: 20.1–21.6]) in the group without previous infection (P<0.0001 χ2 test). Asymptomatic PCR screening was undertaken on a pilot basis in an additional 481 HCWs, 106 with past infection and 375 without. These HCWs were distinct from the population who underwent symptomatic testing. There were similarly no positive results in the group with previous infection 0/106 (0% [95% CI: 0–3.5]), compared to 22/375 (5.9% [95% CI: 3.9–8.7], P = 0.011) positive PCR results in the group without previous infection, consistent with results of symptomatic testing.
Few published studies have addressed the question of whether prior SARS-CoV-2 infection leads to immunity.8 Recent unpublished studies in HCWs reported no evidence of symptomatic reinfection, suggesting that immunity is maintained for at least 6 months.9 , 10 In these studies there was a relatively low event rate (123 and 20 symptomatic infections respectively). It is possible that the protective immunity observed may have been explained in part by the relatively low incidence of SARS-CoV-2 infection. Our data confirms and extends these findings. Despite 290 symptomatic infections in 10,137 non-immune HCWs, there were no symptomatic reinfections in over 1000 HCWs with past infection. We conclude that SARS-CoV-2 infection appears to result in protection against symptomatic infection in working age adults, at least in the short term. This is consistent with the low rates of reinfection reported in the literature2 and with the reported ability of vaccines to protect with high efficacy against symptomatic SARS-CoV-2. Whether this is true of asymptomatic infection remains uncertain. It was not possible to investigate a correlation between baseline antibody titre and protection, since no infections occurred in those with detectable IgG.
We acknowledge several limitations to this study. It employed a retrospective design. Individuals were aware of their infection status, which may have influenced testing behaviour, introducing potential ascertainment bias. However, we consider this unlikely to have a major impact on the conclusions, since occupational health advice to HCWs on self-isolation and PCR testing was applied uniformly, without consideration of prior infection status, and similar results were observed in preliminary asymptomatic screening. Investigation of the nature of immunity was beyond the scope of this analysis. Future longitudinal studies will focus on (i) the durability of immunity, (ii) immune correlates of protection, and (iii) the generalisability of these findings to other at-risk populations such as the elderly, those with comorbidities and the immunocompromised.
Acknowledgments
Funding
This work was supported by the Wellcome Trust (CJAD: 211153/Z/18/Z; BAIP: 109975/Z/15/Z, 203105/Z/16/Z); the British Medical Association (ATH); and the Barbour Foundation (CJAD). This work was partly funded by United Kingdom Research & Innovation (UKRI) / National Institute for Health Research (NIHR) through the UK Coronavirus Immunology Consortium (UK-CIC). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The funders had no role in study design, data collection or decision to publish.
Acknowledgement
We acknowledge the enormous contribution of the great many nursing, laboratory, medical, administrative, IT and managerial colleagues within NUTH who did the work to generate these data. Specific thanks go to colleagues in Occupational Health, the Medicine Directorate, and Integrated Laboratory Medicine for their dedication in implementing the testing programme. We are especially grateful to the staff of NUTH who came forward for testing.
References
- 1.Aran D., Beachler D.C., Lanes S., Overhage J.M. Prior presumed coronavirus infection reduces COVID-19 risk: a cohort study. J Infect. 2020;81:923–930. doi: 10.1016/j.jinf.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng W. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addetia A. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter E. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395:e77–e78. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanrath A.T. SARS-CoV-2 testing of 11,884 healthcare workers at an acute NHS hospital trust in England: a retrospective analysis. MedRxiv. 2020 doi: 10.3389/fmed.2021.636160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens D.S., McElrath M.J. COVID-19 and the Path to Immunity. JAMA. 2020;324:1279–1281. doi: 10.1001/jama.2020.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumley S.F. Antibodies to SARS-CoV-2 are associated with protection against reinfection. New Engl J Med. 2020 doi: 10.1056/NEJMoa2034545. [DOI] [Google Scholar]
- 10.Wylie D. SARS-CoV-2 responsive T cell numbers are associated with protection from COVID-19: a prospective cohort study in keyworkers. MedRxiv. 2020 [Google Scholar]


