Abstract
Aims
Early diagnosis and appropriate treatment are essential in reducing the morbidity and mortality of COVID-19-infected patients. The current study aimed to measure the levels of serum IP-10 and SAA in positive COVID-19 Egyptian patients to explore their clinical values and significance in discrimination between moderate and severe COVID-19 infection and predicting the severity and prognosis of COVID-19 disease.
Main methods
A total of 150 COVID-19 patients and 50 controls were enrolled into our study. Beside the routine lab work of positive COVID-19 patients; IP-10 and SAA were measured using ELISA kit.
Key findings
Our results revealed that the levels of D-dimer (2.64 ± 3.34), ferritin (494.11 ± 260.96), SAA (171.89 ± 51.96), IP-10 (405.0 ± 85.27), WBCs count (14.38 ± 6.06) and neutrophils count (79.26 ± 5.57) were highly significantly increased in severe to critically severe patients when compared with mild to moderate patients; while lymphocytes count (14.21 ± 5.13) was highly significantly decreased when compared to moderate patients. ROC curve analysis results showed that AUC from high to low was IP-10 ˃ SAA ˃ Ferritin ˃ D-dimer ˃ CRP.
Significance
From these results we can conclude that both IP-10 and SAA could be excellent biomarkers in discrimination between moderate and severe COVID-19 infection and predicting the severity and prognosis of COVID-19 disease.
Keywords: Coronavirus, COVID-19, Interferon-γ-induced protein 10 (IP-10), Serum amyloid A (SAA), Cytokine storm syndrome (CSS)
1. Introduction
Coronaviruses are nonsegmented, single-stranded, and positive-sense RNA enveloped viruses that can infect a wide range of hosts including humans [1]. These viruses are the causative agent for several worldwide outbreaks, such as the severe acute respiratory syndrome (SARS) pandemic in 2002–2003 and the Middle East respiratory syndrome (MERS) outbreak in South Korea in 2015 [2]. At the end of 2019, an outbreak of pneumonia of unknown cause appeared in Wuhan city, China. By Jan 7, 2020, a novel coronavirus was isolated from these patients by Chinese scientists. This virus at the beginning known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; or 2019-nCoV), which was later designated coronavirus disease 2019 (COVID-19) in February 2020, by WHO [3].
COVID-19 initial symptoms are fever, fatigue, cough, myalgias, anorexia, and diarrhea [4]. COVID-19 can cause severe acute respiratory syndrome and different system injuries or even death [5]. Pneumonia appears to be the most frequent serious manifestation of COVID-19 infection; which characterized by fever, cough, headaches, sore throat, and rhinorrhea, dyspnea and bilateral infiltrates on chest imaging [6]. Severe COVID-19 could cause acute lung, heart, kidney, and liver injury, in addition to cardiac arrhythmias, rhabdomyolysis, coagulopathy, and shock [4].
Most people who infected with COVID-19 have mild to moderate symptoms and recover after the suitable medication. However, 15–32% develop severe or critical COVID-19 with a mortality rate of 1–15% [7].
Several studies found that COVID-19 patients who admitted to ICUs have high concentrations of cytokines and chemokines such as tumor necrosis factor alpha (TNF-α), IP-10, monocyte chemoattractant protein 1 (MCP-1), and different interleukins (IL-2, IL-6, IL-7, IL-10) [8]. Also different markers of inflammation, such as SAA, C-reactive protein (CRP), procalcitonin (PCT), white blood cells (WBC), lymphocyte (L) and platelet (PLT) have been used in COVID-19 patients as inflammation indicators [5].
Therefore; the aim of the current study was to measure the levels of serum IP-10 and SAA in positive COVID-19 Egyptian patients to explore their clinical values and significance in discrimination between moderate and severe COVID-19 infection and predicting the severity and prognosis of COVID-19 disease.
2. Patients and methods
2.1. Study design and participants
The present case control study was conducted on 150 COVID-19 patients and 50 healthy controls. All patients were infected with SARS-CoV-2 and recruited from the isolation hospitals in portsaid; Egypt, during the period from April 2020 and June 2020. Clinical information and laboratory results were collected at the earliest time point after hospital admission and the study was approved by the ethics committees of portsaid Hospital, portsaid University; Egypt (ERN MED (23/04/2020)S.no(5)MED). Informed consents were obtained from all patients with 2019-novel CoV.
At admission time; sputum and throat swab specimens were collected from all patients for testing by qPCR for SARS-Cov-2 RNA within 3 h. Laboratory tests were conducted at admission, including a complete blood count, liver function tests (AST & ALT), kidney function tests (urea & creatinine), C-reactive protein (CRP), D-dimer and ferritin. Then all patients received supportive oxygen therapy, antiviral medication, and other supportive treatments according to Suspected COVID-19 Cases Management in Triage Hospitals by Ministry of Health and Population of Egypt.
2.2. COVID-19 severity classification
Severity of COVID-19 was graded according to Suspected COVID-19 Cases Management in Triage Hospitals by Ministry of Health and Population of Egypt. There are two main groups:
-
1-
Mild to Moderate patients; patients with fever, respiratory manifestations, and radiological findings indicative of pneumonia.
-
2-
Severe and critically ill patients; patients with respiratory distress (respiration rate, ≥30/min), resting oxygen saturation ≤ 93%, arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen ≤300 mm Hg, respiratory failure requiring mechanical ventilation, or failure of other organs requiring ICU admission.
2.3. Measurement of SAA
Concentration of SAA in serum was measured by using Human Serum amyloid A-1 protein, SAA ELISA Kit (cat# E0885h) (EIAab, Inc., China) according to the protocol of manufacturer.
2.4. Measurement of IP-10
Level of serum IP-10 was determined by using Human Interferon Gamma Induced Protein 10 kDa (IP10) ELISA Kit (cat# MBS2087340) (MyBioSource, Inc., USA) according to the protocol of manufacturer.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS software (version 23.0; IBM Corp., Armonk, NY, USA), and data were presented as means ± S.D. The comparison between COVID-19 patients and controls were made by chi-square test for categorical variables, Post Hoc Test (LSD) for normal distributed variables and Post Hoc Test (Dunn's for multiple comparisons test) test for abnormal distributed variables. Spearman correlation coefficient was utilized to measure the degree of correlation of SAA and IP-10 with other variables in this study. The Receiver Operating Characteristic curve (ROC curve) was used to calculate the area under the curve (AUC) of SAA, IP-10, Ferritin, d-dimer and CRP in order to evaluate the sensitivity and specificity of these factors in the prediction of COVID-19 infection and the severity of infection. The criterion for significance was p < 0.05.
3. Results
3.1. Clinicobiological characteristics
The current study included 150 positive COVID-19 patients; 89 male and 61 female; with mean age 49.43 ± 9.10 yrs.; 26 patients died during hospitalization; the clinical and biological data of healthy subjects and COVID-19 patients are summarized in Table 1 . As the study was designed to match the age and gender between cases and controls, no statistically significant difference was observed for these parameters (P ˃ 0.05).
Table 1.
Demographic and clinicopathological characteristics of COVID-19 patients and controls.
| Variable group | COVID-19 patients |
Control (n = 50) (Mean ± SD) |
||
|---|---|---|---|---|
| Mild & Moderate (n = 98) (Mean ± SD) |
Severe & Critically severe (n = 52) (Mean ± SD) |
Total (n = 150) (Mean ± SD) |
||
| Age (yrs) | 48.36 ± 9.14 | 50.40 ± 9.06 | 49.43 ± 9.10 | 45.80 ± 8.82 |
| Gender (n (%)) Male Female |
56 (57.2%) 42 (42.8%) |
33 (63.5%) 19 (36.5%) |
89 (59.3%) 61 (40.7%) |
32 (64%) 18 (36%) |
| Mortality (n (%)) | 9 (9.2%) | 17 (32.7%) | 26 (17.3%) | – |
| AST (U/L) | 33.15 ± 21.97 | 38.28 ± 37.65⁎, a | 31.12 ± 31.12 | 22.05 ± 9.37 |
| ALT (U/L) | 34.44 ± 23.56⁎, a | 35.0 ± 30.79⁎, a | 34.73 ± 27.42⁎, a | 19.35 ± 7.71 |
| Urea (mg/dl) | 40.71 ± 14.48⁎, a | 41.35 ± 15.20⁎, a | 41.05 ± 14.77⁎, a | 32.05 ± 6.57 |
| Creatinine (mg/dl) | 1.22 ± 0.35⁎, a | 1.18 ± 0.34⁎, a | 1.20 ± 0.34⁎, a | 0.93 ± 0.22 |
| CRP (mg/dl) | 75.04 ± 39.50⁎⁎, a | 78.97 ± 23.31⁎⁎, a | 77.10 ± 31.88 ⁎⁎, a | 4.99 ± 1.57 |
| WBCs (10^3/μl) | 10.03 ± 4.17⁎, a, ⁎⁎, c | 14.38 ± 6.06⁎⁎, a, b | 12.32 ± 5.66⁎⁎, a | 7.47 ± 2.05 |
| Neutrophils (%) | 73.76 ± 7.19⁎⁎, a, c | 79.26 ± 5.57⁎⁎, a, b | 76.65 ± 6.92⁎⁎, a | 65.00 ± 5.06 |
| Lymphocytes (%) | 19.89 ± 6.94⁎⁎, a, c | 14.21 ± 5.13⁎⁎, a, b | 16.91 ± 6.66⁎⁎, a | 29.60 ± 5.30 |
| D-dimer (mg/L) | 0.43 ± 0.35⁎⁎, c | 2.64 ± 3.34⁎⁎, a, b | 1.59 ± 2.66⁎, a | 0.06 ± 0.01 |
| Ferritin (ng/ml) | 213.86 ± 135.24⁎, a, ⁎⁎, c | 494.11 ± 260.96⁎⁎, a, b | 361.0 ± 252.47⁎⁎, a | 104.35 ± 50.85 |
| SAA (ng/ml) | 105.57 ± 17.32⁎⁎, a, c | 171.89 ± 51.96⁎⁎, a, b | 140.39 ± 51.50 ⁎⁎, a | 11.94 ± 8.69 |
| IP-10 (pg/ml) | 260.81 ± 79.02⁎⁎, a, c | 405.0 ± 85.27⁎⁎, a, b | 336.51 ± 109.30⁎⁎, a | 92.63 ± 29.20 |
Significant at p-value < 0.05.
Highly significant at p-value < 0.001.
Significant difference versus Control group.
Significant difference versus Moderate group.
Significant difference versus Severe group.
Our results revealed a significant (p < 0.05) increase in the serum ALT (34.73 ± 27.42), Urea (41.05 ± 14.77), creatinine (1.20 ± 0.34) and D-dimer (1.59 ± 2.66) were observed among the positive COVID-19 patients as compared to controls (19.35 ± 7.71; 32.05 ± 6.57; 0.93 ± 0.22 & 0.06 ± 0.01; respectively); a highly significantly (p < 0.001) increase in the serum CRP (77.10 ± 31.88), ferritin (361.0 ± 252.47), SAA (140.39 ± 51.50) and IP-10 (336.51 ± 109.30) when compared to controls (4.99 ± 1.57; 104.35 ± 50.85; 11.94 ± 8.69 & 92.63 ± 29.20; respectively).
Results of whole blood parameters showed that a highly significantly (p < 0.001) increase in WBCs count (12.32 ± 5.66) and neutrophils count (76.65 ± 6.92) were observed among the positive COVID-19 patients as compared to controls (7.47 ± 2.05 & 65.00 ± 5.06; respectively); while lymphocytes count (16.91 ± 6.66) was highly significantly (p < 0.001) decreased when compared to controls (29.60 ± 5.30).
Positive COVID-19 patients conducted in this study were classified according to their clinical data and healthy state into two groups; mild to moderate patients and severe to critically severe patients. As shown in Table 1; the results of severe to critically severe patients indicating that there are no statistically changes in the levels of serum AST (38.28 ± 37.65), ALT (35.0 ± 30.79), urea (41.35 ± 15.20), creatinine (1.18 ± 0.34) and CPR (78.97 ± 23.31) when compared to mild to moderate patients (33.15 ± 21.97; 34.44 ± 23.56; 40.71 ± 14.48; 1.22 ± 0.35 & 75.04 ± 39.50; respectively).
Levels of serum D-dimer (2.64 ± 3.34), ferritin (494.11 ± 260.96), SAA (171.89 ± 51.96) and IP-10 (405.0 ± 85.27) were highly significantly increased (p < 0.001) in severe to critically severe patients when compared to mild to moderate patients (0.43 ± 0.35; 213.86 ± 135.24; 105.57 ± 17.32 & 260.81 ± 79.02; respectively).
Also results of the current study showing a highly significant (p < 0.001) increase in the levels of whole blood WBCs count (14.38 ± 6.06) and neutrophils count (79.26 ± 5.57) when compared with mild to moderate patients (10.03 ± 4.17 & 73.76 ± 7.19; respectively); while lymphocytes count (14.21 ± 5.13) was highly significantly (p < 0.001) decreased when compared to moderate patients (19.89 ± 6.94).
3.2. Correlation of SAA and IP-10
Data recorded in Table 2 showed the correlation matrix of SAA and IP-10 with the different measured parameters in this study, results revealed that there is a significant positive correlation between SAA and AST & Urea; and highly significant positive correlation with Mortality, CRP, WBCs count, neutrophiles count, D-dimer, ferritin & IP-10; while there is a highly significant negative correlation between SAA and lymphocytes count.
Table 2.
Correlations of SAA and IP-10 and different parameters among COVID-19 patients.
| Variable | SAA(ng/ml) |
IP-10(pg/ml) |
||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Age (yrs) | −0.05 | 0.59 | −0.035 | 0.73 |
| Mortality (n (%)) | 0.79 | 0.000⁎⁎ | 0.46 | 0.000⁎⁎ |
| AST (U/L) | 0.21 | 0.04⁎ | 0.28 | 0.005⁎ |
| ALT (U/L) | 0.16 | 0.11 | 0.22 | 0.03⁎ |
| Urea (mg/dl) | 0.19 | 0.05⁎ | 0.20 | 0.04⁎ |
| Creatinine (mg/dl) | 0.19 | 0.06 | 0.17 | 0.09 |
| CRP (mg/dl) | 0.44 | 0.000⁎⁎ | 0.46 | 0.000⁎⁎ |
| WBCs (10^3/μl) | 0.41 | 0.000⁎⁎ | 0.44 | 0.000⁎⁎ |
| Neutrophils (%) | 0.59 | 0.000⁎⁎ | 0.50 | 0.000⁎⁎ |
| Lymphocytes (%) | −0.64 | 0.000⁎⁎ | −0.48 | 0.000⁎⁎ |
| D-dimer (mg/L) | 0.71 | 0.000⁎⁎ | 0.66 | 0.000⁎⁎ |
| Ferritin (ng/ml) | 0.64 | 0.000⁎⁎ | 0.54 | 0.000⁎⁎ |
| SAA (ng/ml) | – | – | 0.74 | 0.000⁎⁎ |
| IP-10 (pg/ml) | 0.74 | 0.000⁎⁎ | – | – |
Significant at p-value < 0.05.
Highly significant at p-value < 0.001.
While for IP-10; it was found that it has a significant positive correlation with AST, ALT& Urea; and highly significant positive correlation with Mortality, CRP, WBCs count, neutrophiles count, D-dimer, ferritin & SAA; also there is a highly significant negative correlation between IP-10 and lymphocytes count.
3.3. Receiver operating characteristic (ROC) curves analysis
ROC curve analysis was used to calculate the area under the curve (AUC) of SAA, IP-10, Ferritin, d-dimer and CRP in order to evaluate the sensitivity and specificity of these factors in the prediction of COVID-19 infection and the severity of infection. In discrimination of COVID-19 infection; results of the current study showed that AUC for SAA, D-dimer and CRP equal to 1; while for IP-10 is 0.997 and for Ferritin is 0.893; as shown in Table 3 & Fig. 1 . While in discrimination of severe COVID-19 infection; our results revealed that AUC for IP-10 (0.90) ˃ SAA (0.89) ˃ Ferritin (0.85) ˃ D-dimer (0.78) ˃ CRP (0.54); as shown in Table 4 & Fig. 2 .
Table 3.
Area Under the Curve (AUC) & Cut-off value of SAA, IP-10 and different parameters in discrimination of COVID-19 patients.
| D-dimer | Ferritin | SAA | IP-10 | CPR | |
|---|---|---|---|---|---|
| Area Under the Curve | 1 | 0.893 | 1 | 0.997 | 1 |
| Cut-off value | 0.09 | 134.8 | 3.09 | 49.8 | 9.1 |
| Asymptotic sig. | 0.000⁎⁎ | 0.000⁎⁎ | 0.000⁎⁎ | 0.000⁎⁎ | 0.000⁎⁎ |
⁎ Significant at p-value < 0.05.
Highly significant at p-value < 0.001.
Fig. 1.
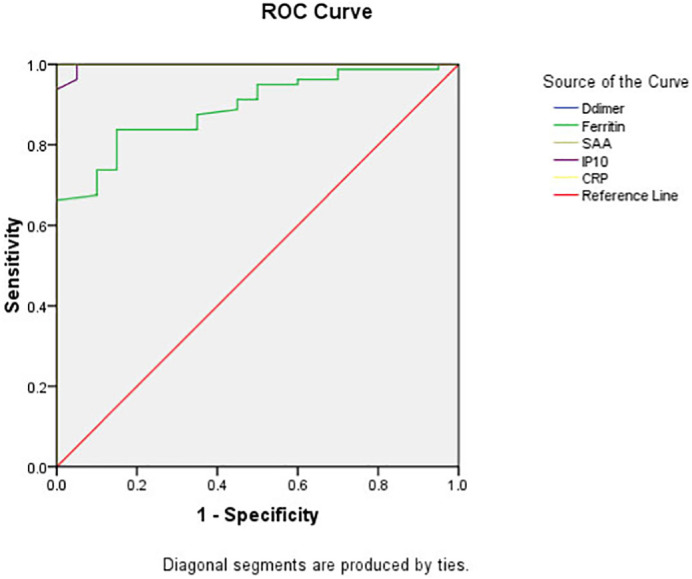
ROC curve of SAA, IP-10 and different parameters in discrimination of COVID-19 patients.
Table 4.
Area Under the Curve (AUC) & Cut-off value of SAA, IP-10 and different parameters in discrimination of severe COVID-19 patients.
| D-dimer | Ferritin | SAA | IP-10 | CPR | |
|---|---|---|---|---|---|
| Area Under the Curve | 0.78 | 0.85 | 0.89 | 0.90 | 0.54 |
| Cut off value | 0.65 | 286.5 | 60.2 | 149.7 | 71.8 |
| Asymptotic Sig. | 0.000⁎⁎ | 0.000⁎⁎ | 0.000⁎⁎ | 0.000⁎⁎ | 0.45 |
⁎ Significant at p-value < 0.05.
Highly significant at p-value < 0.001.
Fig. 2.
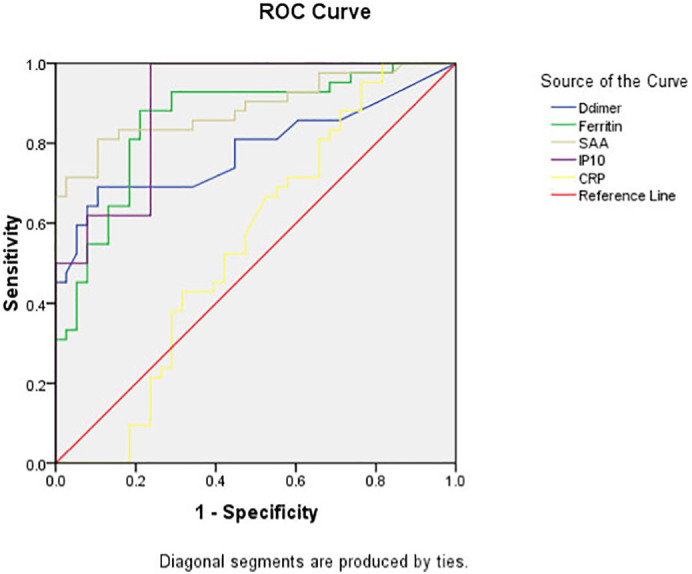
ROC curve of SAA, IP-10 and different parameters in discrimination of Severe COVID-19 patients.
4. Discussion
The severity and prognosis of COVID-19 are complicated by the diversity of symptoms, imaging manifestations, and the degree of disease progression. Therefore, early diagnosis and appropriate treatment are essential in reducing the morbidity and mortality of COVID-19-infected patients [5]. Many studies suggest that some patients with severe COVID-19 might have a cytokine storm syndrome (CSS); which occurs when large numbers of WBCs activated then release inflammatory cytokines, which in turn activate yet more WBCs [9].
Therefore; the aim of the current study was to measure the levels of serum IP-10 and SAA in positive COVID-19 Egyptian patients to explore their clinical values and significance in discrimination between moderate and severe COVID-19 infection and predicting the severity and prognosis of COVID-19.
Interferon (IFN)-γ inducible protein (CXCL10 or IP-10) is one of the CXC chemokine family with pro-inflammatory and anti-angiogenic properties; which has been proposed to be a key link between inflammation and angiogenesis [10]. It is encoded by the CXCL10 gene on chromosome 4 at band q21, and is secreted by fibroblasts, monocytes, endothelial cells and adipocytes. IP-10 has a role in apoptosis, chemotaxis, inhibition of angiogenesis and regulation of growth and proliferation [11].
As consistent with previous studies; our results revealed that the level of serum IP-10 is highly significantly increased in positive COVID-19 patients when compared to controls [12,13]. Also it was found that IP-10 was highly significantly increased in severe to critically severe COVID-19 patients when compared to mild to moderate COVID-19 patients; which suggesting that IP-10 could serve as excellent biomarker for the discrimination between severe and moderate COVID-19 patients and the prediction of disease progression. This confirmed by results of ROC curve which showed that AUC for IP-10 is 0.997 in discrimination of COVID-19 infection; while in discrimination of severe COVID-19 infection; it is the highest one; which consistent with previous study [13].
Serum amyloid A (SAA) is an acute-phase plasma protein that produced by hepatocytes in response to the inflammatory cytokines such as TNF-α, IL-1β and IL-6 [14]. In humans, SAA is considered to be diagnostically superior to CRP in pathological conditions such as viral diseases and transplant rejection [15].
Results of the current study showed that the level of SAA is highly significantly increased in positive COVID-19 patients when compared to controls; and this consistent with previous studies [5,16].
Previous study reported that patients suffer from severe acute respiratory syndrome (ARS) had significantly increased level of SAA; suggesting that SAA could be used as a biomarker to monitor the progression of respiratory diseases [17].
As IP-10; SAA was highly significantly increased in severe COVID-19 patients when compared to moderate COVID-19 patients; which suggesting that SAA also could serve as excellent biomarker for the discrimination between severe and moderate COVID-19 patients and the prediction of disease progression; which is confirmed by results of ROC curve which showed that AUC for SAA is one in discrimination of COVID-19 infection; while in discrimination of severe COVID-19 infection; it is the highest one after IP-10.
Lymphopenia is highly associated with COVID-19 severity; as patients who have died from COVID-19 have had significantly lower lymphocyte counts than survivors [18]. In this study it was found that lymphocytes count was highly significantly decreased among the positive COVID-19 patients as compared to controls and among severe patients when compared to moderate patients. Also there are highly significant negative correlations between both IP-10 and SAA and lymphocytes count.
Laboratory findings of severe COVID-19 patients showed elevation of inflammatory markers, including ferritin, which has been associated with critical and life-threatening illness [19,20]. Consistent with these studies, our results showed that the serum ferritin level was highly significantly increased among COVID-19 patients as compared to controls and among severe patients when compared to moderate patients.
D-Dimer (DD) is the smallest fibrinolysis-specific degradation product found in the circulation and it is the most sensitive test used to confirm hypercoagulation [21]. As reported in several studies; the level of D-Dimer was highly significantly increased among COVID-19 patients as compared to controls and among severe patients when compared to moderate patients [3,22,23].
C-reactive protein (CRP) produced by the liver and is elevated in response to inflammation [24]. As reported in several studies; CRP is associated the severity of COVID-19 disease [24,25]. In the current study; our results reveled CRP is highly significantly increased among COVID-19 patients when compared to controls; while it is slightly increased among severe patients when compared to moderate patients, therefore; CRP could be a useful biomarker in the early stages of infection with COVID-19. This is consistent with previous studies; in which the level of CRP was elevated in most cases infected with COVID-19 [26].
Consistent with other studies, our results revealed that COVID-19 patients had higher WBC count, neutrophilia, lymphocytopenia [12,[27], [28], [29]]. Chen et al. [30] reported that leukocytosis was found in 24% and neutrophilia in 38% of COVID-19 patients. Several studies showed that severe patients with COVID-19 show higher neutrophil count and lower lymphocyte count during the period of disease [[31], [32], [33]]. Therefore, it is possible that neutrophilia and lymphocytopenia are related, at least in part, to the development of critical illness, with a high mortality rate in the confirmed COVID-19 patients.
To compare the sensitivity of IP-10 and SAA in predicting the severity of COVID-19 disease with the other used inflammatory biomarker (CRP, D-dimer and ferritin); which used as a routine work for COVID-19 patients, the authors used ROC curve analysis to calculate AUC, regarding mild to moderate type as negative while severe to critical severe type as positive. The results showed that AUC from high to low was IP-10 (0.90) ˃ SAA (0.89) ˃ Ferritin (0.85) ˃ D-dimer (0.78) ˃ CRP (0.54).
Finally, from these results we can conclude that both IP-10 and SAA could be excellent biomarkers in discrimination between moderate and severe COVID-19 infection and predicting the severity and prognosis of COVID-19 disease.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of competing interest
The authors declare that they have no competing interests.
References
- 1.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9:1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unhale S.S., Ansar Q.B., Sanap S., Thakhre S., Wadatkar S., Bairagi R., Sagrule S., Biyani K.R. A review on corona virus (COVID-19) WJPLS. 2020;6(4):109–115. [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum amyloid A is a biomarker of severe coronavirus disease and poor prognosis. J. Inf. Secur. 2020;80:646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N., Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Public Health. 2020;17:2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q., Cao Y., Chen L., Wu D., Yu J., Wang H., He W., Chen L., Dong F., Chen W., Chen W., Li L., Ran Q., Liu Q., Ren W., Gao F., Chen Z., Gale R.P., Hu Y. Hematological features of persons with COVID-19. Leukemia. 2020;34:2163–2172. doi: 10.1038/s41375-020-0910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velavan T.P., Meyer C.G. Mild versus severe COVID-19: laboratory markers. Int. J. Infect. Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petric D. Cytokine Storm in COVID-19. 2020. http://www.academia.edu/download/62849089/Cytokine_storm_in_COVID20200406-87877-1f8g9xx.pdf (Available from:)
- 10.Gotsch F., Romero R., Friel L., Kusanovic J.P., Espinoza J., Erez O., Than N.G., Mittal P., Edwin S., Yoon B.H., Kim C.J., Mazaki-Tovi S., Chaiworapongsa T., Hassan S.S. CXCL10/IP-10: a missing link between inflammation and anti-angiogenesis in preeclampsia? J. Matern. Fetal Neonatal Med. 2007;20(11):777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayney M.S., Henriquez K.M., Barnet J.H., Ewers T., Champion H.M., Flannery S., Barrett B. Serum IFN-_-induced protein 10 (IP-10) as a biomarker for severity of acute respiratory infection in healthy adults. J. Clin. Virol. 2017;90:32–37. doi: 10.1016/j.jcv.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y., Shen C., Li J., Yuan J., Wei J., Huang F., Wang F., Li G., Li Y., Xing L., Peng L., Yang M., Cao M., Zheng H., Wu W., Zou R., Li D., Xu Z., Wang H., Zhang M., Zhang Z., Gao G.F., Jiang C., Liu L., Liu Y. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen L.E., Whitehead A.S. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem. J. 1998;334:489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuki M., Aoyama R., Nakagawa M., Hirano T., Naitoh E., Kainuma D. A clinical investigation on serum amyloid a concentration in client-owned healthy and diseased cats in a primary care animal hospital. Vet. Sci. 2020;7:45. doi: 10.3390/vetsci7020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo X., Su Z., Lei C., Chen D., Peng H., Chen R., Sang L., Wu H., Li S. Serum amyloid A is a predictor for prognosis of COVID-19. Respirology. 2020;25:764–765. doi: 10.1111/resp.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip T., Chan J., Cho W., Yip T., Wang Z., Kwan T., Law S., Tsang D., Chan J., Lee K., Cheng W., Ma V., Yip C., Lim C., Ngan R., Au J., Chan A., Lim W. Protein chip array profiling analysis in patients with severe acute respiratory syndrome identified serum amyloid a protein as a biomarker potentially useful in monitoring the extent of pneumonia. Clin. Chem. 2005;51(1):47–55. doi: 10.1373/clinchem.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry B.M., Santos de Oliveira M.H., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vargas-Vargas M., Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44 doi: 10.26633/RPSP.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micco P.D., Lodigiani C. D-dimer testing in clinical practice. J Blood Disord Symptoms Treat. 2018;2(2):1–4. [Google Scholar]
- 22.Han H., Yang L., Liu R., Liu F., Wu K., Li J., Liu X., Zhu C. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G., Wu C., Zhang Q., Wu F., Yu B., Lv J., Li Y., Li T., Zhang S., Wu C., Wu G., Zhong Y. C-Reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect. Dis. 2020;7(5) doi: 10.1093/ofid/ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., Zheng K.I., Liu S., Yan Z., Xu C., Qiao Z. Plasma CRP level is positively associated with the severity of COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020;19:18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao K., Li R., Wu X., Zhao Y., Wang T., Zheng Z., Zeng S., Ding X., Nie H. Clinical features in 52 patients with COVID-19 who have increased leukocyte count: a retrospective analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2020;10:1–9. doi: 10.1007/s10096-020-03976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duerr G.D., Heine A., Hamiko M., Zimmer S., Luetkens J.A., Nattermann J., Rieke G., Isaak A., Jehle J., Held S., Wasmuth J.C., Wittmann M., Strassburg C.P., Brossart P., Coburn M., Treede H., Nickenig G., Kurts C., Velten M. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020 Nov 1;260 doi: 10.1016/j.lfs.2020.118400. (Epub 2020 Sep 9. PMID: 32918975; PMCID: PMC7480277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y., Nie H., Hu K., Wu X., Zhang Y., Wang M., Wang T., Zheng Z., Li X., Zeng S. Abnormal immunity of non-survivors with COVID-19: predictors for mortality. Infect Dis Poverty. 2020;9:108. doi: 10.1186/s40249-020-00723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;7;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]


