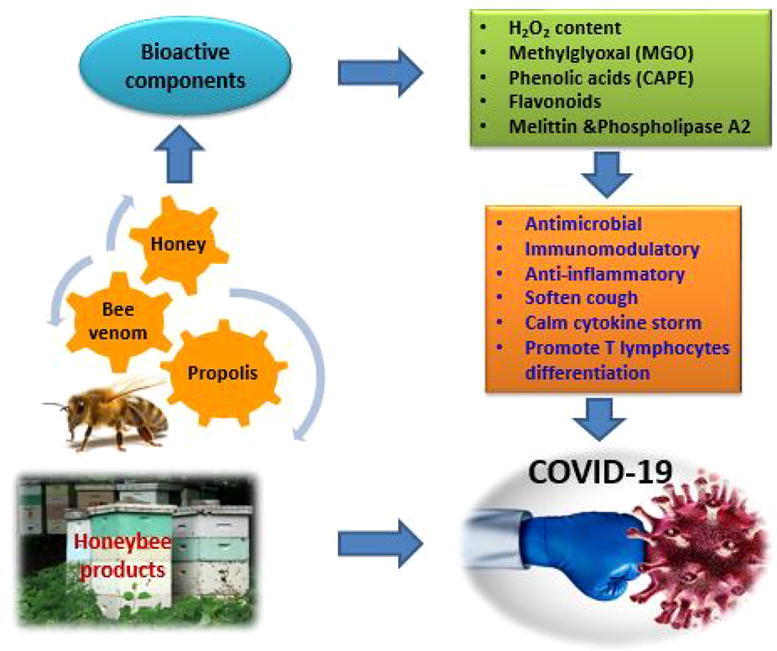
Graphical abstract
Keywords: COVID-19, Honey, Antiviral effect, Alternative medicine, Natural products
Abstract
Coronavirus Disease (COVID-19) has infected people in 210 nations and has been declared a pandemic on March 12, 2020 by the World Health Organization (WHO). In the absence of effective treatment and/or vaccines for COVID-19, natural products of known therapeutic and antiviral activity could offer an inexpensive, effective option for managing the disease. Benefits of products of honey bees such as honey, propolis, and bee venom, against various types of diseases have been observed. Honey bees products are well known for their nutritional and medicinal values, they have been employed for ages for various therapeutic purposes. In this review, promising effects of various bee products against the emerging pandemic COVID-19 are discussed. Products of honey bees that contain mixtures of potentially active chemicals, possess unique properties that might help to protect, fight, and alleviate symptoms of COVID-19 infection.
1. Introduction
Recently, the world has experienced an outbreak of a serious infectious disease; the COVID-19 pandemic caused by severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2). This is the third global outbreak of a Betacoronaviruses (beta-CoVs) after the severe acute respiratory syndrome-coronavirus (SARS-CoV) in 2002 and the Middle East respiratory syndrome-coronavirus (MERS-CoV) in 2012 (Scival, 2018, Thoms et al., 2020). At the beginning of March 2020, COVID-19 was classified as a pandemic by the WHO, and has been creating a global health threat and affecting all aspects of life with more than 81 million reported cases and around 1.8 million death by the end of 2020, which has now surpassing the combined number of cases and deaths of two previously emerging coronaviruses, (WHO, 2020a, WHO, 2020b).
The viruses in this class are enveloped and spherical particles with a single stranded RNA (ssRNA) genome of 26–32 kb, the 5′-terminal two-thirds of their genome ORF1a/b encodes polyproteins, which form the viral replicase transcriptase complex. The other ORFs on the one-third of the genome encode four main structural proteins: spike (S), envelope (E), nucleocapsid (N) and membrane (M) proteins, as well as several accessory proteins (Li et al., 2020). Thus, viruses are quasi alive, in that they can not replicate themselves, but rather need the cellular apparatuses of cells in a host organism to replicate virus particles that can then infect other host individuals.
Coronaviruses were identified in the mid-1960 s and are known to infect humans and a variety of animals, including birds and mammals (Hamre and Procknow, 1966). Since 2002, two coronaviruses infecting animals have mutated and evolved to forms that have caused outbreaks in humans: Severe Acute Respiratory Syndrome SARS-CoV) was identified in southern China in 2003 (Reilley et al., 2003) and Middle East Respiratory Syndrome MERS-CoV) was identified in Saudi Arabia in 2012 (WHO, 2013). Together, they have caused more than 1600 deaths. In January, 2020, the world has experienced the emergence and then rapid dispersion of a new human pathogen that has produced a global pandemic, and a third highly pathogenic beta coronavirus (Cui et al., 2019, Huang et al., 2020, Ksiazek et al., 2003) with clinical presentations resembling viral pneumonia (WHO, 2020b). Deep sequencing analysis of the genome of viruses isolated from the lower respiratory tract has indicated a novel coronavirus, which was named 2019 novel coronavirus (2019-nCoV). SARS-CoV-2 has caused outbreaks of a respiratory illness known as COVID-19.
Scientists are still seeking a successful vaccine to prevent infection or effective treatments once persons are infected. Apitherapy involves use of products of honeybees, such as honey, propolis, bee venom, bee bread, and royal jelly for prolonging, sustaining, and retaining health (Pasupuleti et al., 2017). Because their antiviral potentials were extensively proven in previous studies, such products are considered potential candidates for treatment of COVID-19 in humans (Shahzad and Cohrs, 2012, Zareie, 2011). Phenolic compounds in honey, propolis, and royal jelly, commonly present as flavonoids, contribute to the many functional properties of bee products, including their antioxidant, antimicrobial, antiviral, anti-inflammatory, antifungal, wound healing, and cardioprotective activities (Cornara et al., 2017, Pasupuleti et al., 2017). In this review we present current understanding of three bee products as antiviral defense mechanisms against COVID-19 and suggest important avenues for future investigation.
2. Traditional and medical uses of honeybee products
Honeybees are master chemists and chemical engineers. Their success in the animal kingdom is largely because of the chemistry and the application of their products: honey, beeswax, venom, propolis, pollen, and royal jelly. Three of these products, beeswax, venom, and royal jelly, are chemically synthesized by the bees themselves. The other three are derived from plants and are modified and engineered by the bees for their own use. Evidence from the paintings made during the Stone Age revealed treatment of several diseases with bee products, such as honey and propolis.
2.1. Honey
Honey has been used as a food and medicine since ancient times. Because it has a large proportion of the simple sugar, fructose. Since it is 25% sweeter than table sugar made from sugar cane or sugar beets, which is mostly sucrose, honey from bees has been used as a natural sweetener (Babacan and Rand, 2007, Pataca et al., 2007). In addition, honey is commonly used in drinks (Babacan and Rand, 2007). Ancient scrolls, tablets and books — Sumerian clay tablets (6200 BCE), Egyptian papyri (1900–1250 BCE), Veda (Hindu scripture) 5000 years old, Holy Koran, Jewish and Christian Bible, and Hippocrates (460–357 BCE) demonstrated use of honey to treat several diseases (Ajibola et al., 2012, Mijanur Rahman et al., 2014, Molan, 2001, Newman, 1983). Almost 1000 years ago, Avicenna, the great Iranian scientist and doctor, proposed honey as one of the best remedies for treatment of tuberculosis (Asadi-Pooya et al., 2003). Evidence suggests that honey might have beneficial effects on health including as antioxidants (Ahmed and Othman, 2013), anti-inflammatory (Khalil et al., 2012), antibacterial (Attia et al., 2008), antidiabetic (Estevinho et al., 2008), respiratory, gastrointestinal (Erejuwa et al., 2012), cardiovascular, and nervous system protective effects (Ghosh and Playford, 2003). In 2007, honey received approval from the Food and Drug Administration of the USA (FDA) for use as a topical treatment for wounds because of its potent antimicrobial properties (Pieper, 2009).
Honey coats the inner lining of the throat and destroys potentially harmful microbes while simultaneously smoothing the throat (Gupta and Garg, 2014, Patel and Cichello, 2013). Also, honey is more potent for suppression of cough than dextromethorphan and diphenhydramine (Pasupuleti et al., 2017). Chronic bronchitis and bronchial asthma have been treated using honey, given orally during studies of animal models (Ghosh and Playford, 2003). Additionally, aerosolised honey has been used to effectively treat and manage asthma in rabbits, and has been suggested to be a promising treatment for asthma in humans (Kamaruzaman et al., 2014). In this context, an open randomized primary care, clinical trial has been approved to compare effectiveness of three symptomatic therapies (dextromethorphan, ipratropium or honey) associated with usual care and usual care in acute bronchitis adults (Cots et al., 2019). Some of these potent natural honeys are currently being used under various trade names and brands as medicinal substances such as Medihoney (manuka-based honey), Revamil (blossom-based honey), and Honevo (kanuka-based honey) in dermatological and other medical practices (Jull et al., 2007, Kwakman et al., 2008, Semprini et al., 2016).
2.2. Propolis
Propolis is commonly known as the “bee glue,” a common term that refers to the resinous material that the bees obtain from various plant types (Kusumoto et al., 2001). Propolis works in fixing holes and cracks, and in beehive restoration. This is often used to smooth the beehive's inner surface, maintain internal temperature (35° C) of the hive, avoid weathering and pest invasion. In addition, propolis hards the cell wall and leads to an internal aseptic environment. Propolis becomes generally soft and sticky when heated. Owing to its antiseptic, anti-inflammatory, antioxidant, antibacterial, antimycotic, antifungal, antiulcer, anticancer, and immunomodulatory properties, Propolis and its extracts have multiple applications in treating various diseases. It contains caffeic acid phenethyl ester and artepillin C with antiviral, anti-inflammatory and immunomodulatory effects (Cornara et al., 2017, Kusumoto et al., 2001, Pasupuleti et al., 2017 Al Naggar et al., 2016).
2.3. Bee venom (BV)
Honeybee venom is a salty, colorless liquid containing a combination of peptides and enzymes. The major enzymes are phospholipase A2, phospholipase B, hyaluronidase, phosphatase and α-glucosidase. It also includes peptides such as melittin, apamine, mast cell degranulating peptide, adolapine, tertiapine, secapine, melittin F and cardiopepine (Pak, 2017; Lee et al., 2015) One of the first natural treatments for arthritis were possibly the bee stings. Bee venom was used for apitherapy in ancient China, India, Egypt, Babylon and Greece (Bogdanov, 2016; Shehata et al., 2016). The bee sting therapy was also described in the Huandi Neijing, an ancient Chinese medical book around 500 BCE (YaoChun, 1993). The ancient Greek doctor Hippocrates used venom from bee for medicinal use. He identified it as Arcanum, a mysterious substance of which he did not understand curative properties.
3. Main bioactive components of honeybee products
Recent years have seen wide application of bee products in both traditional and modern medicine. Currently, many studies are targeted toward investigating directed health benefits and pharmacological properties of bee products due to their efficacies, leading to the increasing development of nutraceuticals and functional food from these products (Pasupuleti et al., 2017). The concept of functional food refers to food that can promote better physiological or psychological health compared to traditional remediated and nutritional food (Kaur and Das, 2011).
The main bioactive compounds with antimicrobial activity among honeybee products include polyphenols and vitamins; they occur naturally in food and confer health benefits (Pasupuleti et al., 2017). Phenolic compounds as bioactive molecules are commonly present as flavonoids, they contribute to the antioxidant, antimicrobial, antiviral, anti-inflammatory, antifungal, wound healing, and cardioprotective activities of honeybee products (Pasupuleti et al., 2017; ElSofany et al., 2018). The main flavonoids are pinocembrin, rutin, quercetin, and naringenin, and caffeic acid phenethyl ester (CAPE) as well (Cornara et al., 2017). The main bioactive compounds with antimicrobial activity within honeybee products are summarized (Table 1).
Table 1.
Antimicrobial activity of honey bee products.
| Bioactive compound | Bee product | Medicinal effect | References |
|---|---|---|---|
| Phenolic compound: propolis benzofuran | Propolis | Antifungal | (Pasupuleti et al., 2017;Salatino et al., 2005;Viuda-Martos et al., 2008) |
| Phenolic compound: 2,2-dimethyl-8-prenylchromene | Propolis | Antimicrobial | (Salatino et al., 2005) |
| Phenolic compound: 4-hydroxy-3,5-diprenyl cinnamic acid (artepillin C) | Propolis | Antimicrobial, anti-inflammatory, anticancer | (Cornara et al., 2017, Pasupuleti et al., 2017,Salatino et al., 2005) |
| Phenolic compound: 3-prenyl cinnamic acid allyl ester | Propolis | Antimicrobial | (Cornara et al., 2017, Pasupuleti et al., 2017,Salatino et al., 2005) |
| Terpenoid: isocupressic acid, a labdane diterpenoid | Propolis | Antifungal | (Pasupuleti et al., 2017;Salatino et al., 2005) |
| Terpenoid:13C-symphyoreticulic acid, a clerodane diterpenoid | Propolis | Antitumor | (Pasupuleti et al., 2017;Salatino et al., 2005) |
| Phenolic acid: ellagic acid | Honey | Antioxidant, chemopreventive, antiproliferative | (Cornara et al., 2017, Pasupuleti et al., 2017) |
| Phenolic acid: syringic acid | Honey | Antioxidant, anticancer | (Pasupuleti et al., 2017) |
| Flavonoid: caffeic acid phenethyl ester | Honey and propolis | Antitumor, anticancer, anti inflammatory | (Cornara et al., 2017;Osés et al., 2020;Pasupuleti et al., 2017;Viuda-Martos et al., 2008) |
| Phenolic acid: p-coumaric acid | Honey | Antigenotoxic, neuroprotective | (Cornara et al., 2017;Osés et al., 2020;Pasupuleti et al., 2017) |
| Flavonoid: hesperetin | Honey | Antioxidant, anti-inflammatory | (Pasupuleti et al., 2017) |
| Hydroxyl fatty acid, 3,10-dihydroxydecanoic acid | Royal jelly | anti-inflammatory, antiviral, immunomodulatory | (Cornara et al., 2017) |
4. Antiviral activity of honeybee products
Of all human pathogens, since viruses can remain viable and thus infectious in dry mucus for a long time they are the most common and also among the most difficult pathogens to treat (Madigan et al., 2010). Since viruses can not replicate themselves, they require a host cell, the molecular apparatus of which then can be directed to replicate the virus. In this process when viruses are released, lyse the host cells. Thus, destroying the virus also means killing the host cell. Aside from interventions with drugs that target specific viral proteins, vaccination remains the most effective prophylactic measure of choice to avoid infection by viruses (Madigan et al., 2010, Rémy et al., 2015). In the case of vaccines, antibodies are produced in the vaccinated individuals such that initially when small numbers of a virus enter the host, before they can infect cells and start multiplying, they are recognized and inactivated by the antibodies that have been made to virus-specific antigens. However, it has been proven in many studies that natural products like honey can mitigate microbial diseases (Mandal and Mandal, 2011a).
Natural honey showed profound antiviral activity against rubella virus (Zeina et al., 1996) and varicella-zoster virus (VZV) (Shahzad and Cohrs, 2012). Potencies of various honeys against influenza and HIV have also been reported (Ratcliffe et al., 2011; Watanabe et al., 2014). Antiviral efficacies of honey have been checked against the respiratory syncytial virus. A series of tests were developed using cell cultures to assess efficacy of honey against respiratory syncytial virus. The results indicated that honey inhibited replication of viruses (Zareie, 2011). Additionally, the anti-influenza activity of honey from various sources were investigated (Watanabe et al., 2014). Honey in general, and particularly Manuka honey, had potent inhibitory effects against the influenza virus (Watanabe et al., 2014). Profound, in vitro antiviral activity of a mixture of natural honey, ginger and garlic extracts against various strains of influenza virus was observed, moreover they showed in their study that this mixture promotes the proliferation of human lymphocytes (Vahed & Batool Jafri 2016).
Hydrogen peroxide, phenols and bioflavonoids, found honey bee products are the major classes of bioactive compounds responsible for their antiviral activity against various viral infections (Charyasriwong et al., 2015). Flavonoids are major constituents of honey and play an important role for this activity. Galangin, a flavonoid has been proven to be effective against HSV and coxsackie B virus, whereas quercetin and rutin show antiviral activity against HSV, syncytial virus, poliovirus, and Sindbis virus (Viuda-Martos et al., 2008). The flavonoids, alangin, quercetin and rutin that occur in honey might be directly or indirectly linked to antiviral properties of honey (Ferreres et al., 1994, Hadjmohammadi and Nazari, 2010, Pimentel et al., 2013). Antiviral activity of honeybee products is summarized in (Table. 2).
Table 2.
Antiviral activity of natural honey.
| Types of Honey/compound | Target virus | References |
|---|---|---|
| Manuka and Clover honey | Varicella Zoster virus (VZV, the cause of chicken pox and shingles) | (Shahzad and Cohrs, 2012; P. Zareie, 2011) |
| Honey and thyme extract | Respiratory Syncytial Virus | (Feás and Estevinho, 2011) |
| Manuka, buckwheat, honeydew and acacia honeys | Influenza virus | (Watanabe et al., 2014b) |
| Manuka, Honeydew and Rewarewa honeys | Rubella virus | (Littlejohn, 2009) |
| adenovirus and herpes simplex virus | ||
| Multi-floral honey from UAE | Herpes simplex virus and rubella virus | (Al-Waili, 2004) |
| Honey and Royal Jelly | Herpes simplex virus type 1 (HSV-1) | (Hashemipour et al., 2014) |
| Mono-floral Iranian honey | HIV-1 | (Behbahani, 2014) |
| Type 1 herpes simplex virus | (Ghapanchi et al., 2011) | |
| New Zealand medical grade kanuka honey | Herpes simplex virus | (Semprini et al., 2019) |
| Honey, Ginger and Garlic Decoction | Influenza Virus | (Vahed and Batool Jafri, 2016) |
| MGO (methylglyoxal) a major component of Manuka Honey | Foot and mouth disease virus influenza B viruses; | (Ghizatullina, 1976)(Charyasriwong et al., 2016) |
| Tualang honey (Malaysian multi-floral jungle honey) | Common cold | (Sobhanian et al., 2014) |
| Reduction of virus load in AIDS patients | (Wan Yusuf et al., 2019) | |
| Phenols and bioflavonoids found in honey | Virus families such as retroviridae, hepadnaviridae, hespervirides, HIV virus, influenza virus, herpes simplex virus, dengue virus, polio virus etc | (Kamboj, 2012) |
The virucidal activity of bee venom against DNA and RNA viruses; including Herpes simplex virus type-1(HSV-1), Adenovirus type −7(adeno-7), human immunodeficiency virus (HIV) and West Nile Virus was clearly documented (Ramadan , et al., 2009).
Potential anti COVID-19 effects of honeybees and other bee products while require further investigations, many studies suggest promising effects of bee pharmacy against COVID-19 either by direct antiviral effects of their bioactive peroxides, flavonoids and phenolics or indirect effects due to their immunomodulatory effect on the host immune system and interfering with host inflammatory response aroused by COVID-19 infection, the two effects are going to be discussed in more details below.
5. Pathogenesis of COVID-19
SARS-CoV2 binds to ACE2 with high affinity as a virus receptor to infect humans and does so through spike glycoproteins on the surface of its membrane. With the presence of the S glycoproteins on the surface of the virus, coronavirus is able to penetrate the alveolar cells, such as type II pneumocytes, transferring the viral genome for replication within the cell host. Upon the replication of the viral material, it is released from type II pneumocytes and causes a cascade of cytokines to be released. The cytokine storm triggers symptoms such as dyspnea, chest tightness, etc. The initial stages of COVID-19 manifestations present with symptoms that often are confused with that of the common cold such as a fever, myalgias, sneezing, stuffy nose, etc are reviewed (Moore and June, 2020, Sun et al., 2020).
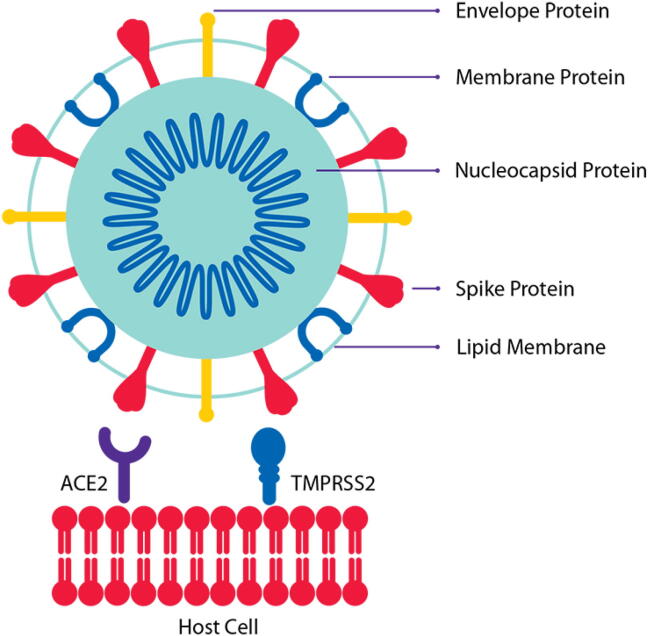
Patients infected by SARS-CoV-2 develop varying symptoms like dry cough, fever, headache, fatigue, shortness of breath, and diarrhea. In 5% of cases, the disease progresses and results in acute injury to the lung (ALI) that is manifested as acute respiratory distress syndrome (ARDS), respiratory failure, heart failure, sepsis, and sudden cardiac arrest within a few days (Huang et al., 2020, Preskorn, 2020). SARS-CoV-2 attaches to the host cells through binding of its spike (S) protein to Angiotensin converting enzyme-2 (ACE2) receptors, such receptors are widely expressed in cardiopulmonary tissues and in some hematopoietic cells, including monocytes and macrophages (Moore and June 2020). However, to achieve efficient fusion, S protein requires priming by a host cellular transmembrane serine protease TMPRSS (Preskorn, 2020, Zhou et al., 2020). Upon viral entry, the spike proteins of both SARS-CoV and SARS-CoV-2 cause the internalization and degradation of ACE2 (Fig. 1). Once, SARS-CoV-2 nucleic acid is introduced and translated by host cell machinery, the viral protein products are processed by viral specific proteinases. Meanwhile, the nucleic acid of the virus is processed to prepare template for further replication to synthesis new copies of the viral nucleic acid, then the synthesized genomic RNA packaged within nucleocapsid protein in the cytoplasm to assemble viral nucleocapsids, which bud into the lumen of the endoplasmic reticulum—Golgi inter-mediate compartment ready to be released from the cell through exocytosis to infect other cells. Studies conducted in animal models revealed that SARS-CoV-2 can infect not only type II alveolar cells but also other cells that can express ACE2 e.g. myocardial cells, proximal tubule cells of kidney, bladder urothelial, ileum, colon, esophagus epithelial cells, and oral mucosa cells (Du et al., 2009, Hoffmann et al., 2020, Xu et al., 2020, Zou et al., 2020). This might, in part, explain the multiple systemic presentations of COVID-19.
Fig. 1.
Host cell and SARS-CoV-2 viral envelope (E), membrane (M), nucleocapsid (N), and spike (S) proteins (Source; https://www.sigmaaldrich.com/technical-documents/protocols/biology/ncov-coronavirus-proteins.html).
In response to infection with coronaviruses, due to activation of the immune system, a cocktail of inflammatory mediators including cytokines and chemokines including interleukin-1 β (IL-1β), IL-2, IL-6, IL-8, granulocyte–macrophage colony-stimulating factor (GM-CSF), both IFN-α/β, vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF), macrophage inflammatory protein (MIP1), MIP1A, MIP1B, platelet derived growth factor (PDGF), and interferon-inducible protein 10 (IP-10), are produced as reported from accumulating studies and clinical evaluation of COVID-19 patients . Excessive production of these mediators contributes to manifestation of the disease and exacerbation of pathogenesis of COVID-19, where intensified inflammatory response and uncontrolled production of mediators and cytokine storm; maladaptive cytokine release in response to infection and other stimuli; manifested as loss of regulatory control of proinflammatory cytokine production at the local and systemic levels (Ye et al., 2020), and favor massive accumulation of inflammatory monocytes, macrophages and neutrophils in the lung alveoli (Channappanavar et al., 2016, Clark and Vissel, 2017, Huang et al., 2020, Mehta et al., 2020b) leading to cellular necrosis.
6. Potential protective role of honeybee products against COVID-19
6.1. Honey
Honey's general antimicrobial activity and antiviral activity are likely due, in part, to hydrogen peroxide (H2O2) and the bee-derived antibacterial peptide defensin-1 (Def-1) (Bucekova et al., 2019, Weston, 2000). Based on several in vivo and in vitro studies, other phytochemical non-peroxide components such as methylglyoxal (MGO) in some kinds of honey e.g. Manuka honey, exhibit similarly unusual enhanced antimicrobial activity (Mavric et al., 2008). Amounts of H2O2 can vary among types of honey. More specifically, concentrations of H2O2 affect generation of hydroxyl radicals from degraded H2O2 through a Fenton reaction. Oxidative stress, which formed hydroxyl radical has been shown to be the primary mechanism by which H2O2 in honey inhibited bacterial cells (Brudzynski and Lannigan, (2012). In addition, hydrolysis of H2O2 can also generate oxygen, which can accelerate auto-oxidation of polyphenols in honey, which, when they become pro-oxidant agents, generate more H2O2 and, in the presence of transition metals, drive production of hydroxyl radicals from H2O2 (Brudzynski and Lannigan, 2012). In 40% honey solutions (pH 7.0), the content of H2O2 in blossom honey was determined immediately after homogeneous solutions were prepared, varying from 2.4 to 47.2 μg / g honey (Bucekova et al., 2019).
Like other respiratory viruses, including various strains of influenza, COVID-19 spreads through tiny droplets released from an infected person's nose and mouth during cough (Okada et al., 2020). One question, which has not yet been answered is exactly how long SARA-CoV-2 (virus which causes the COVID-19 disease) will survive outside the human body. Recent research on related coronaviruses, including SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome), found that they can stay viable up to nine days on metal, glass and plastic unless properly disinfected (Kampf et al., 2020). Coronaviruses can be rapidly inactivated after disinfecting carrier surfaces with 70% alcohol, 0.5% hydrogen peroxide, or other household bleach containing 0.1% sodium hypochlorite (Kampf et al., 2020).
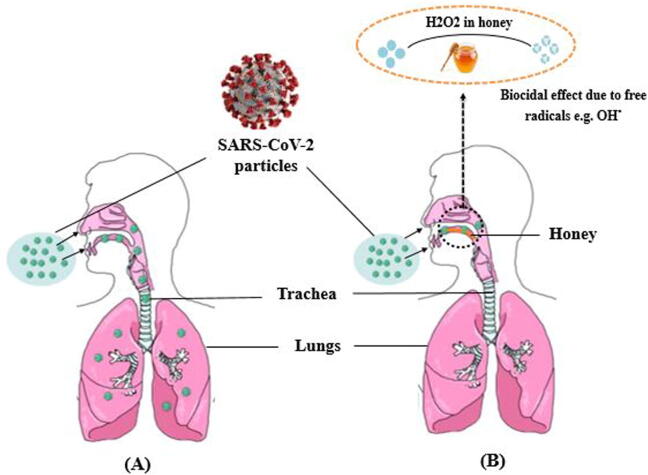
Honey naturally contains hydrogen peroxide, meaning that routine daily honey intake might provide a protective measure because of the biocidal effect of hydrogen peroxide in honey could help to clean the throat from any virus particles. Besides H2O2, antimicrobial potency of honey is strongly correlated with its natural physicochemical properties such as osmolarity, pH, viscosity and thickness. Honey is characteristically acidic with a pH from 3.5 to 4.5 and this acidic pH maximizes it’s antimicrobial activity (Mandal and Mandal, 2011, Rao et al., 2010). The first barriers against any microbial infection are body secretions like sweat, saliva, mucus and gastric acid. Body secretions belong to the innate immune system and form chemical barriers to microbial invasion by infectious agents adhering to epithelial surfaces (Chaplin, 2010). Therefore, honey could imitate body secretions in developing an environment that is not conducive for microbes 'survival, in addition to its unusual antimicrobial activity (Olaitan et al., 2007).Taken all together. The chemical biocidal properties as well as the physical properties of honey might help to disinfect or trap Covid-19 virus particles before passing to the lungs (Fig. 2).
Fig. 2.
Diagram indicating potential protective effects of honey against SARS-CoV-2, which is the virus that causes Covid-19. SARS-CoV-2 is transmitted through tiny droplets released from an infected person to another person then the virus particles either pass to the lungs and progress as in A or trapped by honey then destroyed by the biocidal activity of honey or pass to the gastric acid of stomach as we propose in B.
Natural honeys in general and Manuka honey in particular are rich in phenolics and flavonoids (see Table 2), recently several studies that predict binding affinity of naturally occurring polyphenolic compounds to SARS-CoV-2 viral proteins are conducted in the context of in silico and molecular docking studies, most of these studies propose an expected medicinal value of polyphenols based on their predicted binding to SARS-CoV-2 main protease (Mpro) (Heba, 2020, Mustafa et al., 2020). Clinical investigations have to be performed to examine the predicted medicinal potential of these compounds. Polyphenols are existing among the bioactive compounds of natural honey are currently under phase 3 of clinical investigation as a treatment of COVID-19 patients (Tantawy, 2020)
6.2. Propolis
Propolis is a potent modulator of immune responses (Orsi et al., 2000), and such effects may explain its antimicrobial and antiviral activities to some degree. Several studies have also documented propolis anti-inflammatory properties, likely related to the presence of phenolic acids. Caffeic acid phenethyl ester (CAPE) is considered to be a specially strong anti-inflammatory component, capable of targeting NF-κB signaling specifically (Armutcu et al., 2015). This compound has been also found to modulate ERK MAPK signaling in T cells and mastocytes (Cho et al., 2014), and to regulate PI3K/Akt pathway in different human cell lines (Li et al., 2017). Additionally, CAPE was found to inhibit HIV-1 infection by acting on viral integrase (Costi et al., 2004) and to suppress hepatitis C virus replication in vitro (Zhang, 2003). On the other hand, very few studies investigated the potential effects of propolis on coronaviruses (Bachevski et al., 2020a, Debiaggi et al., 1990). Propolis is rich in phenolics and flavonoids (see Table 2), several in vitro and in silico studies confirmed anti‐coronavirus activity of propolis flavonoids like chrysin and kaempferol that were found to inhibit virus replication in vitro (Debiaggi et al., 1990) and quercetin and its derivatives that inhibit the SARS‐CoV‐1 and MERS‐CoV main protease in vitro besides their aminopeptidase activity and their ability to modulate the unfolded protein response (UPR) utilized by the virus to complete the replication cycle (Bachevski et al., 2020b, Berretta et al., 2020, Polansky and Lori, 2020, Sahlan et al., 2020, Scorza et al., 2020, Shaldam, n.d., Syed and Saleem, 2004). Taken together and based on recent and previous findings about propolis immunomodulator and antiviral activity, it could be used as adjuvant therapy to control the severe inflammatory reaction especially the cytokine storm associated with COVID-19 infection aside from the protective effects of propolis phenolics and flavonoids against COVID-19.
6.3. Bee venom
Bee venom and its two main components (melittin and PLA2) are well known to have antimicrobial activity and can thus be used as complementary antibacterial agents (Perumal Samy et al., 2007, Socarras et al., 2017, Zolfagharian et al., 2016). Such compounds exert their effects on bacteria by inducing pores through their membranes which lead to cleavage and then lysis (Leandro et al., 2015). Nevertheless, studies investigating BV's antiviral activity are still scarce. Bee venom can arouse the immune system (Cherniack and Govorushko, 2018) and promote the differentiation of human regulatory T cells (Caramalho et al., 2015), which play a crucial role in the eradication of SARS-CoV and limiting its pathogenesis (Chen et al., 2010). A recent study investigated BV antiviral potential and came out with interesting findings both in vivo and in vitro. This study showed that BV and melittin have significant antiviral effects against numerous enveloped viruses (vesicular stomatitis virus, influenza A virus, herpes simplex virus, etc.) and nonenveloped viruses (enterovirus-71 and coxsackie virus) in vitro (Uddin et al., 2016). The study also showed that melittin protected mice that were exposed to lethal doses of influenza A H1N1 virus. Bee venom and its constituent melittin have been shown to induce immunity through substantial upregulation of Th1 cytokines (IFN-γ and IL-12) and several forms of immune cells, including CD3 + CD8+, CD4 + CD8 + and γδ T cells, which not only reduced the viral load but also reduced the incidence of interstitial pneumonia in pigs infected with PRRSV (Lee et al., 2015). However, this effect, which could be very significant for SARS-CoV-2 related pneumonia, was only achieved when bee venom was administered through the nasal or rectal pathway (Lee et al., 2015).
A survey of 5115 beekeepers and 121 bee venom-treated patients conducted by the Hubei Province apitherapy clinic, the COVID-19 epicenter in China found that none of the beekeepers had symptoms associated with COVID-19, the current and devastating pandemic (Yang, 2020). Moreover, his team followed 121 patients of who had received bee apitherapy for two months, without any other protective measures. None of the apitherapists, nor the investigated patients were infected by SARS-CoV-2, although they had close contact with immediate family members with confirmed SARS-CoV-2 Infection. These people have one thing in common: they develop a tolerance to bee sting (Yang, 2020). Therefore, bee venom might potentiate the immune system and reduce the susceptibility to SARS-CoV infection (Yang, 2020).
7. Conclusions and recommendations
Bee products and bee venom are well known of their nutritional and medicinal values, they have been employed since ages for different therapeutic purposes. In this review, we comprehensively discussed the promising effects of different bee products against the emerging pandemic COVID-19, bee products possess unique criteria and harbor a magic cocktail of phytomedicines that help to protect, to fight, and to alleviate COVID-19 infection.
Honey has been recommended by the National Institute for Health and Care Excellence (NICE) and Public Health England (PHE) as a first line treatment for cough due to upper respiratory tract infection, which is the main well identified COVID-19 symptom (Wölfel et al., 2020), on the other hand variable concentrations of Manuka honey surprisingly found to modulate the release of cytokines, chemokines and matrix-degrading enzymes that regulate inflammatory and immune responses (Minden-Birkenmaieret al., 2019), currently drugs that quiet cytokine storms and soften the hyperinflammation are greatly considered to protect from acute respiratory distress syndrome (ARDS) the major cause of death due to serious COVID-19 infection (Mehta et al., 2020a). Therefore, we recommend honey as a potential compatible antiseptic prophylaxis to help protect against the virus. Honey might safely disinfect the throat and trap virus particles, beside a major advantage that it has no side effects and of great nutritional value. Furthermore, research into the active ingredients that impart antiviral potency to honey and greater understanding of how those chemicals cause their effects on viruses might help direct development of effective antiviral drugs with potentially fewer side effects. We may even consider diluted solution of natural honey as a home-made antiseptic for hands, skin and mucous membranes or as a mouth gargle since honey is completely safe and widely used as sweetener in several pharmaceutical preparations.
Propolis contains a concentrated dosage of therapeutic flavonoids and phenolic compounds that interfere with maturation and replication machinery of the virus in one hand and mitigate the exaggerated inflammatory response of COVID-19 on the other hand. Propolis belongs to the safest ecological therapies, investigational studies and confirmed anti-corona effects of chemical ingredients of propolis highlight the necessity for further investigations covering the prophylactic effect of propolis in high‐risk groups, especially individuals in close contact with COVID-19 patients, and validating the anti-corona effects of propolis.
As a powerful immune modulator, bee venom should be taken in consideration, it enhance the differentiation of T regulatory immune cells, it works like a protective vaccine that puts the immune system in a standby state to interfere with the virus. Finally, we reconsider bee product in general as a treasure trove to fight COVID-19.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to confirm that there are no known conflicts of interest associated with this publication. The authors thank Dr. Sahar Hagras for their comments and help. YA & GY are grateful to Alexander von Humboldt (AvH) foundation for post-doc fellowships. This research has been funded by Cooperative association for development of bees industry in Riyadh (NAHHAL), Saudi Arabia. This work has been supported by King Saud University, Deanship of Scientific Research, College of Science Research Center.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed S., Othman N.H. Honey as a Potential Natural Anticancer Agent: A Review of Its Mechanisms. Evidence-Based Complement. Altern. Med. 2013;2013:1–7. doi: 10.1155/2013/829070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajibola A., Chamunorwa J.P., Erlwanger K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. (Lond). 2012;9:61. doi: 10.1186/1743-7075-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Naggar Y., Sun J., Robertson A., Giesy J.P., Wiseman S. Chemical characterization and antioxidant properties of Canadian propolis. Journal of apicultural research. 2016;55(4):305–314. [Google Scholar]
- Al-Waili N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004;10:MT94–8. [PubMed] [Google Scholar]
- Armutcu F., Akyol S., Ustunsoy S., Turan F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review) Exp. Ther. Med. 2015;9:1582–1588. doi: 10.3892/etm.2015.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya A.A., Pnjehshahin M.R., Beheshti S. The antimycobacterial effect of honey: an in vitro study. Riv. Biol. 2003;96:491–495. [PubMed] [Google Scholar]
- Attia W.Y., Gabry M.S., El-Shaikh K.A., Othman G.A. The anti-tumor effect of bee honey in Ehrlich ascite tumor model of mice is coincided with stimulation of the immune cells. Egypt. J. Immunol. 2008;15:169–183. doi:20306700. [PubMed] [Google Scholar]
- Babacan S., Rand A.G. Characterization of Honey Amylase. J. Food Sci. 2007;72:C050–C55. doi: 10.1111/j.1750-3841.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- Bachevski, D., Damevska, K., Simeonovski, V., Dimova, M., 2020a. Back to the basics: Propolis and COVID ‐19. Dermatol. Ther. doi:10.1111/dth.13780 [DOI] [PMC free article] [PubMed]
- Bachevski D., Damevska K., Simeonovski V., Dimova M. Back to the basics: Propolis and COVID -19. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbahani M. Anti-HIV-1 Activity of Eight Monofloral Iranian Honey Types. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta A.A., Silveira M.A.D., Cóndor Capcha J.M., De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov, S., 2016. Biological and therapeutic properties of bee venom, in: The Bee Venom Book,. pp. 1–23.
- Brudzynski K., Lannigan R. Mechanism of Honey Bacteriostatic Action Against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucekova M., Jardekova L., Juricova V., Bugarova V., Di Marco G., Gismondi A., Leonardi D., Farkasovska J., Godocikova J., Laho M., Klaudiny J., Majtan V., Canini A., Majtan J. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules. 2019;24:1573. doi: 10.3390/molecules24081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramalho Í., Nunes-Cabaço H., Foxall R.B., Sousa A.E. Regulatory T-Cell Development in the Human Thymus. Front. Immunol. 2015;6:395. doi: 10.3389/fimmu.2015.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charyasriwong S., Haruyama T., Kobayashi N. In vitro evaluation of the antiviral activity of methylglyoxal against influenza B virus infection. Drug Discov. Ther. 2016;10:201–210. doi: 10.5582/ddt.2016.01045. [DOI] [PubMed] [Google Scholar]
- Charyasriwong S., Watanabe K., Rahmasari R., Matsunaga A., Haruyama T., Kobayashi N. In Vitro Evaluation of Synergistic Inhibitory Effects of Neuraminidase Inhibitors and Methylglyoxal Against Influenza Virus Infection. Arch. Med. Res. 2015;46:8–16. doi: 10.1016/j.arcmed.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Chen Y.Y., Jensen M.C., Smolke C.D. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc. Natl. Acad. Sci. 2010;107:8531–8536. doi: 10.1073/pnas.1001721107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack E.P., Govorushko S. To bee or not to bee: The potential efficacy and safety of bee venom acupuncture in humans. Toxicon. 2018;154:74–78. doi: 10.1016/j.toxicon.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Cho M.S., Park W.S., Jung W.-K., Qian Z., Lee D.-S., Choi J.-S., Lee D.-Y., Park S.-G., Seo S.-K., Kim H.-J., Won J.Y., Yu B.C., Choi I.-W. Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm. Biol. 2014;52:926–932. doi: 10.3109/13880209.2013.865243. [DOI] [PubMed] [Google Scholar]
- Clark I.A., Vissel B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin. Immunopathol. 2017;39:505–516. doi: 10.1007/s00281-017-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornara, L., Biagi, M., Xiao, J., Burlando, B., Gardens, R.B., Università, S., 2017. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products 8, 1–20. doi:10.3389/fphar.2017.00412 [DOI] [PMC free article] [PubMed]
- Costi R., Santo R. Di, Artico M., Massa S., Ragno R., Loddo R., La Colla M., Tramontano E., La Colla P., Pani A. 2,6-Bis(3,4,5-trihydroxybenzylydene) derivatives of cyclohexanone. Bioorg. Med. Chem. 2004;12:199–215. doi: 10.1016/j.bmc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cots J.M., Moragas A., García-Sangenís A., Morros R., Gomez-Lumbreras A., Ouchi D., Monfà R., Pera H., Pujol J., Bayona C., de la Poza-Abad M., Llor C. Effectiveness of antitussives, anticholinergics or honey versus usual care in adults with uncomplicated acute bronchitis: a study protocol of an open randomised clinical trial in primary care. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiaggi M., Tateo F., Pagani L., Luini M., Romero E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica. 1990;13:207–213. [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSofany A., Naggar Y., Naiem E., Seif A. Characterization of Apis mellifera Honey of Different Botanical and Geographical Origins in Egypt. Egypt. J. Exp. Biol. 2018;14:75. doi: 10.5455/egysebz.20180523104927. [DOI] [Google Scholar]
- Erejuwa O.O., Sulaiman S.A., Wahab M.S.A. Honey - A Novel Antidiabetic Agent. Int. J. Biol. Sci. 2012;8:913–934. doi: 10.7150/ijbs.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevinho L., Pereira A.P., Moreira L., Dias L.G., Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008;46:3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Feás X., Estevinho M.L. A Survey of the In Vitro Antifungal Activity of Heather ( Erica Sp.) Organic Honey. J. Med. Food. 2011;14:1284–1288. doi: 10.1089/jmf.2010.0211. [DOI] [PubMed] [Google Scholar]
- Ferreres F., Andrade P., Tomás-Barberán F.A. Flavonoids from Portuguese heather honey. Zeitschrift für Leb. und -forsch. 1994;199:32–37. doi: 10.1007/BF01192949. [DOI] [Google Scholar]
- Ghapanchi J., Moattari A., Tadbir A.A., Talatof Z., Shahidi S.P., AndEbrahimi H. The In Vitro Anti-Viral Activity of Honey on Type 1 Herpes Simplex Virus. Aust. J. Basic Appl. Sci. 2011;5:849–852. [Google Scholar]
- Ghizatullina N.K. Effect of methyl glyoxal on infectivity and antigenicity of foot-and-mouth disease virus. Acta Virol. 1976;20:380–386. [PubMed] [Google Scholar]
- Ghosh S., Playford R.J. Bioactive natural compounds for the treatment of gastrointestinal disorders. Clin. Sci. 2003;104:547–556. doi: 10.1042/CS20030067. [DOI] [PubMed] [Google Scholar]
- Gupta S.P., Garg G. The Huntington procedure: still a reasonable option for large tibial defects in paediatric patients. J. Child. Orthop. 2014;8:413–421. doi: 10.1007/s11832-014-0618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjmohammadi M.R., Nazari S.S.S.J. Separation optimization of quercetin, hesperetin and chrysin in honey by micellar liquid chromatography and experimental design. J. Sep. Sci. 2010;33:3144–3151. doi: 10.1002/jssc.201000326. [DOI] [PubMed] [Google Scholar]
- Hamre D., Procknow J.J. A New Virus Isolated from the Human Respiratory Tract. Exp. Biol. Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- Heba, H., 2020. IN Silico Approach of Some Selected Honey Constituents as SARS-CoV-2 Main Protease (COVID-19) Inhibitors. chemRxiv. doi:10.26434/chemrxiv.12115359.v1
- Hashemipour M.A., Tavakolineghad Z., Arabzadeh S.A.M., Iranmanesh Z., Nassab S.A.H.G. Antiviral Activities of Honey, Royal Jelly, and Acyclovir Against HSV-1. Wounds a Compend. Clin. Res. Pract. 2014;26:47–54. [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jull A., Walker N., Parag V., Molan P., Rodgers A. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br. J. Surg. 2007;95:175–182. doi: 10.1002/bjs.6059. [DOI] [PubMed] [Google Scholar]
- Kamaruzaman N.A., Sulaiman S.A., Kaur G., Yahaya B. Inhalation of honey reduces airway inflammation and histopathological changes in a rabbit model of ovalbumin-induced chronic asthma. BMC Complement. Altern. Med. 2014;14:176. doi: 10.1186/1472-6882-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj A. Antiviral activity of plant polyphenols. J. Pharm. Res. 2012;5:2402–2412. [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Das M. Functional foods: An overview. Food Sci. Biotechnol. 2011;20:861–875. doi: 10.1007/s10068-011-0121-7. [DOI] [Google Scholar]
- Khalil M.I., Moniruzzaman M., Boukraâ L., Benhanifia M., Islam M.A., Islam M.N., Sulaiman S.A., Gan S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules. 2012;17:11199–11215. doi: 10.3390/molecules170911199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.-E., Humphrey C.D., Shieh W.-J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.-Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kusumoto T., Miyamoto T., Higuchi R., Doi S., Sugimoto H., Yamada H. Isolation and structures of two new compounds from the essential oil of Brazilian propolis. Chem. Pharm. Bull. 2001;49:1207–1209. doi: 10.1248/cpb.49.1207. [DOI] [PubMed] [Google Scholar]
- Kwakman P.H.S., Van den Akker J.P.C., Güçlü A., Aslami H., Binnekade J.M., de Boer L., Boszhard L., Paulus F., Middelhoek P., te Velde A.A., Vandenbroucke-Grauls C.M.J.E., Schultz M.J., Zaat S.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria In Vitro and Eradicates Skin Colonization. Clin. Infect. Dis. 2008;46:1677–1682. doi: 10.1086/587892. [DOI] [PubMed] [Google Scholar]
- Leandro L.F., Mendes C.A., Casemiro L.A., Vinholis A.H.C., Cunha W.R., de Almeida R., Martins C.H.G. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An. Acad. Bras. Cienc. 2015;87:147–155. doi: 10.1590/0001-3765201520130511. [DOI] [PubMed] [Google Scholar]
- Li L., Sun W., Wu T., Lu R., Shi B. Caffeic acid phenethyl ester attenuates lipopolysaccharide-stimulated proinflammatory responses in human gingival fibroblasts via NF-κB and PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017;794:61–68. doi: 10.1016/j.ejphar.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Lee W.R., Pak S.C., Park K.K. The protective effect of bee venom on fibrosis causing inflammatory diseases. Toxins. 2015;7(11):4758–4772. doi: 10.3390/toxins7114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M.T., Martinko J.M., Bender K.S., Buckly D.H., Stahl D.A. 14th ed. Artmed; Porto Alegre: 2010. Brock Biology of Microorganisms. [Google Scholar]
- Mandal M.D., Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011;1:154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavric E., Wittmann S., Barth G., Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium)honeys from New Zealand. Mol. Nutr. Food Res. 2008;52:483–489. doi: 10.1002/mnfr.200700282. [DOI] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration U. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijanur Rahman M., Gan S.H., Khalil M.I. Neurological Effects of Honey: Current and Future Prospects. Evidence-Based Complement. Altern. Med. 2014;2014:1–13. doi: 10.1155/2014/958721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden-Birkenmaier B.A., Meadows M.B., Cherukuri K., Smeltzer M.P., Smith R.A., Radic M.Z., Bowlin G.L. The Effect of Manuka Honey on dHL-60 Cytokine, Chemokine, and Matrix-Degrading Enzyme Release under Inflammatory Conditions. Med One. 2019 doi: 10.20900/mo.20190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molan P.C. The potential of honey to promote oral wellness. Gen. Dent. 2001;49:584–589. doi:12024746. [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science (80-.) 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Mustafa M.Z., Shamsuddin S.H., Sulaiman S.A., Abdullah J.M. Anti-inflammatory Properties of Stingless Bee Honey May Reduce the Severity of Pulmonary Manifestations in COVID-19 Infections. Malaysian J. Med. Sci. 2020;27:165–169. doi: 10.21315/mjms2020.27.2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, T.G., 1983. Honey Almanac, Il. ed. Chicago.
- Okada P., Buathong R., Phuygun S., Thanadachakul T., Parnmen S., Wongboot W., Waicharoen S., Wacharapluesadee S., Uttayamakul S., Vachiraphan A., Chittaganpitch M., Mekha N., Janejai N., Iamsirithaworn S., Lee R.T., Maurer-Stroh S. Early transmission patterns of coronavirus disease 2019 (COVID-19) in travellers from Wuhan to Thailand, January 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.8.2000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaitan P.B., Adeleke O.E., Ola I.O. Honey: a reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007;7:159–165. doi: 10.5555/afhs.2007.7.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi R.O., Funari S.R.C., Soares A.M.V.C., Calvi S.A., Oliveira S.L., Sforcin J.M., Bankova V. Immunomodulatory action of propolis on macrophage activation. J. Venom. Anim. Toxins. 2000;6:205–219. doi: 10.1590/S0104-79302000000200006. [DOI] [Google Scholar]
- Osés S.M., Marcos P., Azofra P., de Pabl A., Fernández-Muíño M.Á., Sancho M.T. Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: Needs for analytical harmonization. Antioxidants. 2020;9:20–35. doi: 10.3390/antiox9010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak S.C. Bee Products - Chemical and Biological Properties. Springer International Publishing; Cham: 2017. Chemical Composition of Bee Venom; pp. 279–285. [DOI] [Google Scholar]
- Pasupuleti V.R., Sammugam L., Ramesh N., Gan S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataca L., Neto W., Marcucci M., Poppi R. Determination of apparent reducing sugars, moisture and acidity in honey by attenuated total reflectance-Fourier transform infrared spectrometry. Talanta. 2007;71:1926–1931. doi: 10.1016/j.talanta.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Patel S., Cichello S. Manuka honey: an emerging natural food with medicinal use. Nat. Products Bioprospect. 2013;3:121–128. doi: 10.1007/s13659-013-0018-7. [DOI] [Google Scholar]
- Perumal Samy R., Gopalakrishnakone P., Thwin M.M., Chow T.K.V., Bow H., Yap E.H., Thong T.W.J. Antibacterial activity of snake, scorpion and bee venoms: a comparison with purified venom phospholipase A 2 enzymes. J. Appl. Microbiol. 2007;102:650–659. doi: 10.1111/j.1365-2672.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- Pieper B. Honey-Based Dressings and Wound Care. J. Wound. Ostomy Cont. Nurs. 2009;36:60–66. doi: 10.1097/01.WON.0000345177.58740.7d. [DOI] [PubMed] [Google Scholar]
- Pimentel R.B. de Q., da Costa C.A., Albuquerque P.M., Junior S.D. Antimicrobial activity and rutin identification of honey produced by the stingless bee Melipona compressipes manaosensis and commercial honey. BMC Complement. Altern. Med. 2013;13:151. doi: 10.1186/1472-6882-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky H., Lori G. Coronavirus disease 2019 (COVID-19): first indication of efficacy of Gene-Eden-VIR/Novirin in SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn S.H. The 5% of the Population at High Risk for Severe COVID-19 Infection Is Identifiable and Needs to Be Taken Into Account When Reopening the Economy. J. Psychiatr. Pract. 2020;26:219–227. doi: 10.1097/PRA.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A.S., Reddy S.G., Babu P.P., Reddy A.R. The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran. BMC Complement. Altern. Med. 2010;10:4. doi: 10.1186/1472-6882-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe N.A., Mello C.B., Garcia E.S., Butt T.M., Azambuja P. Insect natural products and processes: new treatments for human disease. Insect Biochem. Mol. Biol. 2011;41:747–769. doi: 10.1016/j.ibmb.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Reilley B., Van Herp M., Sermand D., Dentico N. SARS and Carlo Urbani. N. Engl. J. Med. 2003;348:1951–1952. doi: 10.1056/NEJMp030080. [DOI] [PubMed] [Google Scholar]
- Rémy V., Zöllner Y., Heckmann U. Vaccination: the cornerstone of an efficient healthcare system. J. Mark. access Heal. policy. 2015;3 doi: 10.3402/jmahp.v3.27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlan M., Irdiani R., Flamandita D., Aditama R., Alfarraj S., Ansari M.J., Cahya Khayrani A., Kartika Pratami D., Lischer K. Molecular Interaction Analysis of Sulawesi Propolis Compounds with SARS-CoV-2 Main Protease as Preliminary Study for COVID-19 Drug Discovery. J. King Saud Univ. - Sci. 2020:101234. doi: 10.1016/j.jksus.2020.101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scival, D., 2018. Infectious Disease Outbreaks Research: Insights and Trends.
- Salatino A., Teixeira É.W., Negri G., Message D. Origin and chemical variation of Brazilian propolis. Evidence-based Complement. Altern. Med. 2005;2:33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza C.A., Gonçalves V.C., Scorza F.A., Fiorini A.C., de Almeida A.-C.G., Fonseca M.C.M., Finsterer J. Propolis and coronavirus disease 2019 (COVID-19): Lessons from nature. Complement. Ther. Clin. Pract. 2020;41 doi: 10.1016/j.ctcp.2020.101227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semprini A., Braithwaite I., Corin A., Sheahan D., Tofield C., Helm C., Montgomery B., Fingleton J., Weatherall M., Beasley R. Randomised controlled trial of topical kanuka honey for the treatment of acne. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semprini A., Singer J., Braithwaite I., Shortt N., Thayabaran D., McConnell M., Weatherall M., Beasley R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: a randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad A., Cohrs R.J. In vitro antiviral activity of honey against varicella zoster virus (VZV): A translational medicine study for potential remedy for shingles. Transl. Biomed. 2012;3 doi: 10.3823/434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaldam, Moataz A., Yahya, G., Mohamed, N.H., Abdel-Daim, M.M., Al Naggar, Y., n.d. In Silico Screening of Potent Bioactive Compounds from Honey Bee Products Against COVID-19 Target Enzymes. ChemRxiv. Prepr. doi:10.26434/chemrxiv.12644102.v1 [DOI] [PMC free article] [PubMed]
- Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect , the company ’ s public news and information , 2020.
- Socarras, K.M., Theophilus, P.A.S., Torres, J.P., Gupta, K., Sapi, E., 2017. Antimicrobial Activity of Bee Venom and Melittin against Borrelia burgdorferi. Antibiot. (Basel, Switzerland) 6. doi:10.3390/antibiotics6040031 [DOI] [PMC free article] [PubMed]
- Shehata A., Eldorf A., Hussien N., Naeem N., Al Naggar Y., Giesy J. Bee Stings at Sites of Acupuncture as a Potential Therapy for Idiopathic Premature Ovarian Failure: A Pilot Study. Women’s Heal. 2016;2 doi: 10.15406/mojwh.2016.02.00038. [DOI] [Google Scholar]
- Sobhanian S., Pourahmad M., Jafarzadeh A., Mohammad S., Tadayon K., Zabetian H. The prophylactic effect of honey on common cold. Quran Med. 2014;1 [Google Scholar]
- Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J., Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S., Saleem A. Severe Acute Respiratory Syndrome Epidemiology and Control. Lab. Med. 2004;35:112–116. doi: 10.1309/3YHRVBB7CB3LUFL6. [DOI] [Google Scholar]
- Tantawy, M., n.d. Efficacy of Natural Honey Treatment in Patients With Novel Coronavirus [WWW Document]. 2020. URL https://clinicaltrials.gov/ct2/show/NCT04323345 (accessed 5.13.20).
- Thoms, M., Buschauer, R., Ameismeier, M., Koepke, L., Denk, T., Hirschenberger, M., Kratzat, H., Hayn, M., Mackens-Kiani, T., Cheng, J., Stuerzel, C.M., Froehlich, T., Berninghausen, O., Becker, T., Kirchhoff, F., Sparrer, K.M.J., Beckmann, R., 2020. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. bioRxiv 8665, 2020.05.18.102467. doi:10.1101/2020.05.18.102467 [DOI] [PMC free article] [PubMed]
- Uddin M.B., Lee B.-H., Nikapitiya C., Kim J.-H., Kim T.-H., Lee H.-C., Kim C.G., Lee J.-S., Kim C.-J. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. 2016;54:853–866. doi: 10.1007/s12275-016-6376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahed H., Batool Jafri S. Propagation of Influenza Virus in Lymphocytes Determine by Antiviral Effects of Honey, Ginger and Garlic Decoction. J. Antivir. Antiretrovir. 2016;08 doi: 10.4172/jaa.1000129. [DOI] [Google Scholar]
- Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008;73:R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Wan Yusuf W.N., Wan Mohammad W.M.Z., Gan S.H., Mustafa M., Abd Aziz C.B., Sulaiman S.A. Tualang honey ameliorates viral load, CD4 counts and improves quality of life in asymptomatic human immunodeficiency virus infected patients. J. Tradit. Complement. Med. 2019;9:249–256. doi: 10.1016/j.jtcme.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Rahmasari R., Matsunaga A., Haruyama T., Kobayashi N. Anti-influenza viral effects of honey in vitro: potent high activity of manuka honey. Arch. Med. Res. 2014;45:359–365. doi: 10.1016/j.arcmed.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Weston R.J. The contribution of catalase and other natural products to the antibacterial activity of honey: a review. Food Chem. 2000;71:235–239. doi: 10.1016/S0308-8146(00)00162-X. [DOI] [Google Scholar]
- WHO, 2020a. Coronavirus disease (COVID-2019) situation reports.
- WHO, 2020b. Novel Coronavirus – China [WWW Document]. URL https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ (accessed 3.23.20).
- WHO, 2013. Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update.
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. International Journal of Infectious Diseases. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YaoChun C. Agricultural Publishing House; 1993. Apiculture in China. [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’’ in COVID-19’. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie P. University of Waikato; Tauranga: 2011. Honey as an antiviral agent against respiratory syncytial virus. [Google Scholar]
- Zeina B., Othman O., Al-Assad S. Effect of Honey versus Thyme on Rubella Virus Survival in Vitro. J. Altern. Complement. Med. 1996;2:345–348. doi: 10.1089/acm.1996.2.345. [DOI] [PubMed] [Google Scholar]
- Zhang T. Alcohol potentiates hepatitis C virus replicon expression. Hepatology. 2003;38:57–65. doi: 10.1053/jhep.2003.50295. [DOI] [PubMed] [Google Scholar]
- Zhou G., Chen S., Chen Z. Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front. Med. 2020;14:117–125. doi: 10.1007/s11684-020-0773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfagharian H., Mohajeri M., Babaie M. Bee Venom (Apis Mellifera) an Effective Potential Alternative to Gentamicin for Specific Bacteria Strains. J. Pharmacopuncture. 2016;19:225–230. doi: 10.3831/KPI.2016.19.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn, E. S. V. (2009). The Sensitivity of Adenovirus and Herpes simplex virus to Honey (Thesis, Master of Science (MSc)). The University of Waikato, Hamilton, New Zealand. Retrieved from https://hdl.handle.net/10289/2804.