Abstract
Objectives
The coronavirus disease 2019 pandemic increased global demand for personal protective equipment (PPE) and resulted in shortages. The study evaluated the re-use of surgical masks and respirators by analysing their performance and safety before and after reprocessing using the following methods: oven, thermal drying, autoclave, and hydrogen peroxide plasma vapour.
Methods
In total, 45 surgical masks and 69 respirators were decontaminated. Visual integrity, air permeability, burst resistance, pressure differential and particulate filtration efficiency of new and decontaminated surgical masks and respirators were evaluated. In addition, 14 used respirators were analysed after work shifts before and after decontamination using reverse transcription polymerase chain reaction (RT-PCR) and viral culturing. Finally, reprocessed respirators were evaluated by users in terms of functionality and comfort.
Results
Oven decontamination (75 °C for 45 min) was found to be the simplest decontamination method. Physical and filtration assays indicated that all reprocessing methods were safe after one cycle. Oven decontamination maintained the characteristics of surgical masks and respirators for at least five reprocessing cycles. Viral RNA was detected by RT-PCR in two of the 14 used respirators. Four respirators submitted to viral culture were PCR-negative and culture-negative. Reprocessed respirators used in work shifts were evaluated positively by users, even after three decontamination cycles.
Conclusion
Oven decontamination is a safe method for reprocessing surgical masks and respirators for at least five cycles, and is feasible in the hospital setting.
Keywords: SARS-CoV-2 pandemic, Mask reuse, COVID-19, Surgical masks, Respirators
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), emerged in Wuhan, China in December 2019, and has since spread worldwide. By 8 December 2020, there had been 66,729,375 cases of infection worldwide (World Health Organization, 2020a). The main modes of transmission and spread are human-to-human contact through droplets (expelled during sneezing, coughing or speaking) and close contact. However, possible airborne transmission is a concern. The World Health Organization (WHO) recommends the use of surgical masks for regular care of patients with COVID-19, and respirators (e.g. N95, FFP2 and FFP3 respirators) when aerosol-generating procedures are performed (World Health Organization, 2020b). Healthcare workers (HCWs) represent the most exposed population in this pandemic. A Chinese study evaluated 138 hospitalized patients with COVID-19, of which 29% were HCWs (Wang et al., 2020). Global demand for personal protective equipment (PPE), such as surgical masks and respirators, has increased substantially due to the pandemic. This has led to a shortage of PPE and the raw materials for its manufacture (Chaib, 2020). Most PPE is certified and recommended by manufacturers for single use. However, due to shortages, alternative measures are required. HCWs are sometimes obliged to use PPE for days or weeks without demonstrated effective and suitable methods of decontamination. As such, there is a need to evaluate the effectiveness of reprocessing, to identify the best method for decontamination, and to determine the maximum number of decontamination cycles that can be performed safely (Cheng et al., 2020, Mackenzie, 2020). This study aimed to compare the performance of surgical masks and respirators after reprocessing using methods available in hospital central sterile service departments.
Methods
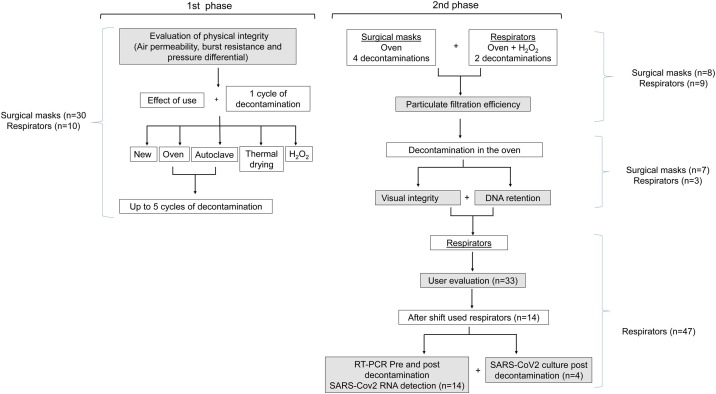
In total, 45 new surgical masks (Descarpack, São Paulo, Brazil) and 69 new respirators (KSN, São Paulo, Brazil) were evaluated (Figure 1 ). Each surgical mask and respirator was used for 4 h (to evaluate the effect of use), 20 min (to evaluate several decontamination cycles) or at least 12 h (work shift), and subjected to decontamination using one of four methods: dry heat in an oven (Fanem 502; Fanem, Guarulhos, São Paulo, Brazil; 75 °C for 45 min, 230-L capacity); thermal drying machine (Getinge, Wayne, NJ, USA; drying cycle: 84 °C for 40 min, 300-L capacity); autoclaving (HS 6620; Getinge; 134 °C for 4 min for decontamination and 10 min for drying; 24 min to reach 134 °C, 960-L capacity), and hydrogen peroxide plasma vapour (Sterrad 100NX; Sterrad, Irvine, CA, USA; standard cycle for 47 min, temperature <55 °C, double tray, 100-cm deep). Up to 30 surgical masks and respirators were tested in each decontamination cycle for each method. After each cycle, the surgical masks and respirators were inspected visually and submitted to another cycle (maximum of 10 cycles). In addition, five different brands of respirators [Deltaplus PFF2 and PFF3, Maskface, Tayco and Proteplus (with headquarters in São Paulo, Brazil)] were analysed after three cycles of oven decontamination. All decontamination methods were performed in the Central Sterile Service Department of Hospital das Clinicas, São Paulo, Brazil. Hospital das Clinicas is a tertiary hospital with 2400 beds and is a referral hospital for patients with COVID-19.
Figure 1.
Study flowchart.
In addition to visual inspection (cleanliness, nose clip and elastic functionality), surgical masks and respirators were evaluated for air permeability, burst resistance, breathability (pressure differential), particulate filtration efficiency and DNA retention capacity. A pilot study was undertaken to evaluate HCWs’ responses (n = 33) regarding sealing and breathing performance after working a regular shift using a reprocessed respirator.
Furthermore, 14 used respirators from two hospitals underwent SARS-CoV2 reverse transcription polymerase chain reaction (RT-PCR), and four respirators were also submitted for viral culturing. These 14 respirators were collected at different times during the first month of the pandemic; the first four were collected at the beginning of the pandemic and the last five were collected in the last week of the first month. This is important as various changes were implemented during the first month of the pandemic; for example, during the first week, face shields had not been distributed in the hospital, and PPE training of new HCWs was still in progress. By the end of the first month, all frontline HCWs had been trained and had access to full PPE, including face shields.
Evaluation of physical integrity
Physical integrity and particulate filtration of the surgical masks and respirators were evaluated as follows. The results are summarized in Table 1 .
-
•
Air permeability (l m−2 s−1) (following ISO 9237:1995 – Textile – Determination of the permeability of fabrics to air) was tested at five different points in each surgical mask and respirator for each decontamination method. The measurement area was 5 cm2 and the applied pressure drop was 100 Pa.
-
•
Burst resistance (bar) (following ISO 13938.1: 1999 – Textiles – Bursting properties of fabrics) was measured at five different points in each surgical mask and respirator for each decontamination method. The measurement area was 5 cm2.
-
•
Pressure differential ΔP (mmH2O/cm2) (following Annex C of EN 14683:2019 –Medical face masks – Requirements and test methods).
Table 1.
Summary of tests performed on surgical masks and respirators.
| Test | Details | Surgical masks |
Respirators |
||||
|---|---|---|---|---|---|---|---|
| n | Pass | Fail | n | Pass | Fail | ||
| Decontamination method | 75 °C, 45 min | Oven | |||||
| Visual integrity | Cleanliness | 20 | Until 9th cycle | 10th cycle | 14 | All 7 cycles | – |
| Nose clip | 20 | All 10 cycles | – | 14 | Until 2nd cycle | 3rd cycle | |
| Elastic/handle | 20 | Until 5th cycle | 6th cycle | 14 | All 7 cycles | – | |
| Physical integrity | Air permeability | 5 | All 5 cycles | – | 5 | All 5 cycles | – |
| Burst resistance | 5 | All 5 cycles | – | 5 | All 5 cycles | – | |
| Pressure differential | 5 | All 5 cycles | – | 5 | All 5 cycles | – | |
| Particulate filtration efficiency | 20–800-nm particles | 5 | All 4 cycles | – | 3 | All 2 cycles | – |
| DNA retention | DNA spray | 6 | Until 5th cycle | 10th cycle | 3 | All 7 cycles | – |
| SARS-CoV-2 RNA detection | RT-PCR | – | – | – | 14 | 13 | 1 |
| Viral culture | – | – | – | 4 | 4 | – | |
| Decontamination method | 134 °C, 4 min | Autoclave | |||||
| Visual integrity | Cleanliness | 15 | All 5 cycles | – | 15 | All 5 cycles | – |
| Nose clip | 15 | All 5 cycles | – | 15 | 1 cycle | 2nd cycle | |
| Elastic/handle | 15 | All 5 cycles | – | 15 | All 5 cycles | – | |
| Physical integrity | Air permeability | 5 | All 5 cycles | – | 5 | All 5 cycles | – |
| Burst resistance | 5 | Until 2nd cycle | 3rd cycle | 5 | All 5 cycles | – | |
| Pressure differential | 5 | All 5 cycles | – | 5 | All 5 cycles | - | |
| Decontamination method | H2O2 plasma vapour | H2O2 | |||||
| Visual integrity | Cleanliness | 2 | 1 cycle | – | 3 | All 2 cycles | – |
| Nose clip | 2 | 1 cycle | – | 3 | All 2 cycles | – | |
| Elastic/handle | 2 | 1 cycle | – | 3 | All 2 cycles | – | |
| Physical integrity | Air permeability | 2 | 1 cycle | – | 2 | 1 cycle | – |
| Burst resistance | 2 | 1 cycle | – | 2 | 1 cycle | – | |
| Pressure differential | 2 | 1 cycle | – | 2 | 1 cycle | – | |
| Particulate filtration efficiency | 20–800-nm particles | – | – | – | 2 | All 2 cycles | – |
| Decontamination method | 84 °C, 40 min | Thermal drying | |||||
| Visual integrity | Cleanliness | 2 | 1 cycle | – | 2 | 1 cycle | – |
| Nose clip | 2 | 1 cycle | – | 2 | 1 cycle | – | |
| Elastic/handle | 2 | 1 cycle | – | 2 | 1 cycle | – | |
| Physical integrity | Air permeability | 2 | 1 cycle | – | 2 | 1 cycle | – |
| Burst resistance | 2 | 1 cycle | – | 2 | 1 cycle | – | |
| Pressure differential | 2 | 1 cycle | – | 2 | 1 cycle | – | |
SARS-Cov-2, severe acute respiratory syndrome coronavirus-2; RT-PCR, reverse transcription polymerase chain reaction; H2O2, hydrogen peroxide.
Particulate filtration efficiency
Particulate filtration efficiency was evaluated by measuring the particle size distribution that passed through the surgical masks and respirators as a function of the total amount of NaCl particles generated by an ATM226 aerosol generator (TOPAS, Saxe, Germany) using an electronic particle detection system (Scanning Mobility Particle Sizer 3080; TSI, Shoreview, MN, USA), coupled to a condensation particle counter (CPC Nanoparticle Counter 3771; TSI). The number of particles reaching the detector with and without a surgical mask or respirator was measured from 20 to 800 nm at a distance of 15 cm from the aerosol source. The ratio between the number of aerosol particles reaching the detector after the surgical mask/respirator and the total number of particles indicates the particulate filtration efficiency of the surgical mask or respirator. This experiment was performed with new surgical masks, repeating the procedure up to four times after oven decontamination; and new respirators, repeating the procedure after two cycles of oven decontamination and two cycles of hydrogen peroxide decontamination.
DNA retention capacity after oven decontamination
In order to evaluate the ability of surgical masks and respirators to retain small molecules, DNA of Staphylococcus aureus was extracted using a QiAamp DNA mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s recommendations. A 47-mm/0.45-μm cellulose membrane (Merck Millipore, Burlington, MA, USA) was placed in a Petri dish behind a surgical mask or respirator at a distance of 1 cm. DNA was dosed in NanoDrop 2000c (Thermo Fisher, Waltham, MA, USA) and diluted in water. Water (negative control) or 50 ng DNA was sprayed on to the surface of the surgical mask or respirator four times from a distance of 5 cm. DNA or water sprayed on membranes without a surgical mask or respirator were used as positive and negative controls, respectively. The membranes were transferred to a 1.5-mL Eppendorf tube and 50 μL of water was added and incubated for 10 min at room temperature. Next, 5 μL was used as a template for a PCR reaction spa (Staphylococcus protein A) gene using the following primers: forward, TAAAGACGATCCTTCGGTGAGC; and reverse, CAGCAGTAGTGCCGTTTGCTT. PCR products were applied to agarose (1.2%) gel and a visible band in the gel was considered positive. This test was performed with seven surgical masks (one new surgical mask, three surgical masks that had been decontaminated five times, and three surgical masks that had been decontaminated 10 times) and three respirators (one new respirator and two respirators that had been decontaminated seven times).
User evaluation
Evaluation was undertaken in an intensive care unit with 22 beds used for suspected and confirmed cases of COVID-19. Respirators used during a work shift (12 h) were placed in separate paper envelopes, identified with each HCW’s name and removed each day for oven decontamination. Each respirator was re-used by the same HCW who assessed it before each use. Evaluation involved answering a questionnaire attached to the envelope. Respirators were decontaminated three times, but if a respirator was rejected by the user, he/she was instructed to discard it.
Detection of SARS-CoV-2 RNA in respirators
Fourteen respirators (Deltaplus PFF2) used for three to 28 work shifts were collected from two hospitals: seven from Hospital das Clinicas (HC 1, HC 2, HC 5, HC 6, HC 7, HC 8, HC 9, HC10, HC 11, HC 12, HC 13 and HC 14), a public referral hospital for cases of COVID-19; and two from Hospital São Camilo (SC 3 and SC 4), a private hospital. The respirators were cut, in the region close to the nose and mouth (highest exposure area), generating 16 1-mm punches from each respirator using a micro-punch tool (Harris Uni-Core; Merck, Kenilworth, NJ, USA) with a razor-sharp stainless steel cutting tip (Figure S1, see online Supplementary material). One tool was used per respirator. The procedures were performed in a biosafety level 3 area.
RT-PCR and viral culture were performed to identify the presence of SARS-CoV-2. RNA was extracted from eight respirator fragments in lysis buffer using a QIAamp viral RNA kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. RT-PCR was assessed using the commercial RealStar SARS-CoV2 RT-PCR Kit 1.0 (Altona Diagnostics, Hamburg, Germany) and amplified using the LightCycler 96 System (Roche, Basel, Switzerland). Real-time PCR data were expressed as the cycle threshold (Ct) value, corresponding to the initial amplification cycle. The results were reported as detectable or undetectable.
Viral culture was performed for four respirators (HC 11, HC 12, HC 13 and HC 14) using Vero cells (ATCC CCL-81) as described previously (Lennette and Schmidt, 1979, Ammerman et al., 2008, Harcourt et al., 2020). Cells were cultured in Dulbecco minimal essential medium supplemented with heat-inactivated fetal bovine serum (10%) and antibiotics/antimycotics (Cultilab, Campinas, São Paulo, Brazil). For virus isolation, respirator fragments were inoculated in Vero cell culture in plastic bottles (Jet biofilm, 12.5-cm2 area, 25-mL capacity) immediately after processing. Inoculated cultures were grown at 37 °C in an incubator in an atmosphere of 5% CO2. Cell cultures were maintained for at least 2 weeks and were observed daily for evidence of cytopathic effects (CPEs). At least two subcultures were performed weekly. Presumptive detection of virus in supernatant showing CPEs was investigated using an inverted microscope (Nikon, Chiyoda, Japan) and then confirmed by a specific RT-PCR assay targeting the E gene.
Statistical analysis
Statistical analysis was performed using GraphPad. t-test was used to evaluate new and used surgical masks and respirators. One-way analysis of variance was calculated for each decontamination method, and correlation analysis was undertaken using R 2 for performance and filtration efficiency by decontamination cycle.
Results
Visual integrity after use and decontamination
The visual integrity of surgical masks and respirators was analysed after each decontamination cycle using an oven, autoclave, thermal drying or hydrogen peroxide. Figure S2 (see online Supplementary material) shows the visual integrity of surgical masks after up to 10 decontamination cycles and Figure S3 (see online Supplementary material) shows respirators after seven decontamination cycles in an oven. The steel clip of all the respirators came off after three decontamination cycle, the strap of all the surgical masks broke after six decontamination cycles, and the surgical masks were visibly altered after 10 decontamination cycles.
The steel clip of all the respirators came off after two decontamination cycles in an autoclave, and the surgical masks became easy to break by simple handling after three cycles. A single complete thermal drying cycle (including washing with detergent and water) destroyed the surgical masks and respirators so they were submitted to a drying cycle alone. No visual differences were noted in the surgical masks or respirators after two cycles of hydrogen peroxide decontamination.
DNA retention capacity after oven decontamination
DNA filtering capacity was analysed for new surgical masks and respirators, surgical masks re-used six and 10 times, and respirators re-used seven times. Surgical masks were able to filtrate the sprayed DNA-dope aerosol after six decontamination cycles in an oven. New surgical masks and surgical masks that had been re-used six times were able to retain the sprayed DNA. However, after 10 decontamination cycles, DNA was identified in the membrane inside the surgical mask (Figure S4, see online Supplementary material).
Effect of use and decontamination cycles on physical performance
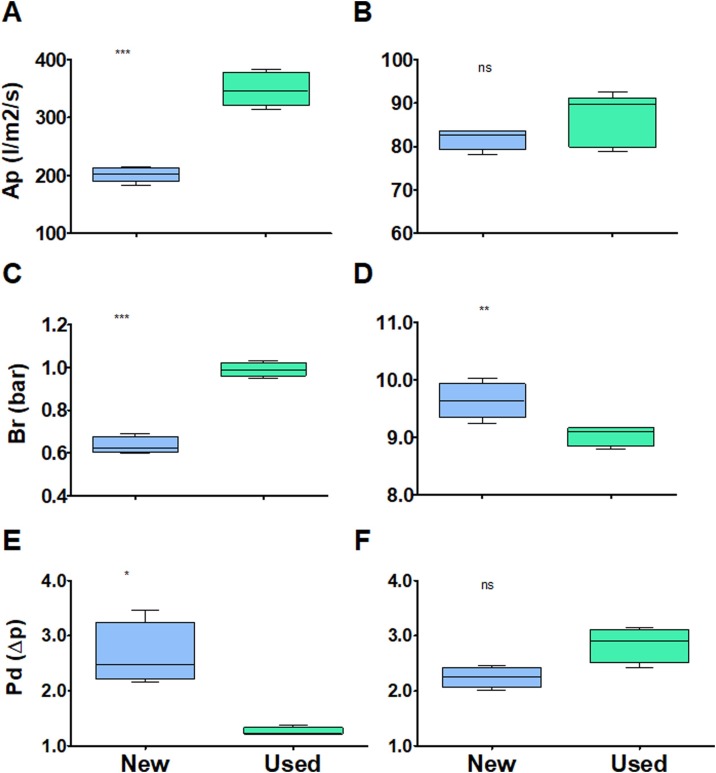
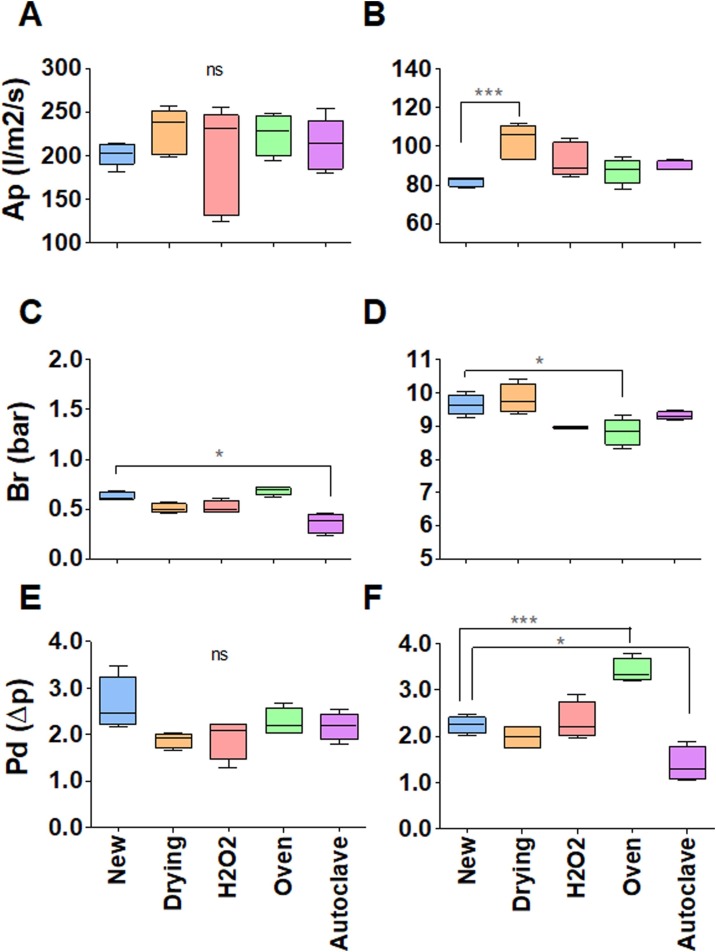
First, the effect of wearing (donning and doffing, humidity from breathing, etc.) a surgical mask or respirator for 4 h on its performance was evaluated. Figure 2 shows that simple wear has a measurable effect on the performance of surgical masks and respirators; however, the effect was much smaller than the differences measured between different brands of respirators, and therefore was of no practical significance. Next, surgical masks and respirators were evaluated after they had been worn for 20 min followed by reprocessing with one of the four decontamination methods. Figure 3 shows that the decontamination method made no practical difference to air permeability (Figure 3A,B), burst resistance (Figure 3C,D) or pressure differential (Figure 3E,F) for surgical masks or respirators. As ovens and autoclaves are readily available in most hospitals, the effect of five decontamination cycles using these methods was assessed.
Figure 2.
Effect of 4 h of use on the performance of surgical masks (left) and respirators (right). Performance was measured by air permeability (Ap) (l/m2/s) (A, B); burst resistance (Br) (bar) (C, D); and pressure differential (Pd) (Δp) (E, F). *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
Figure 3.
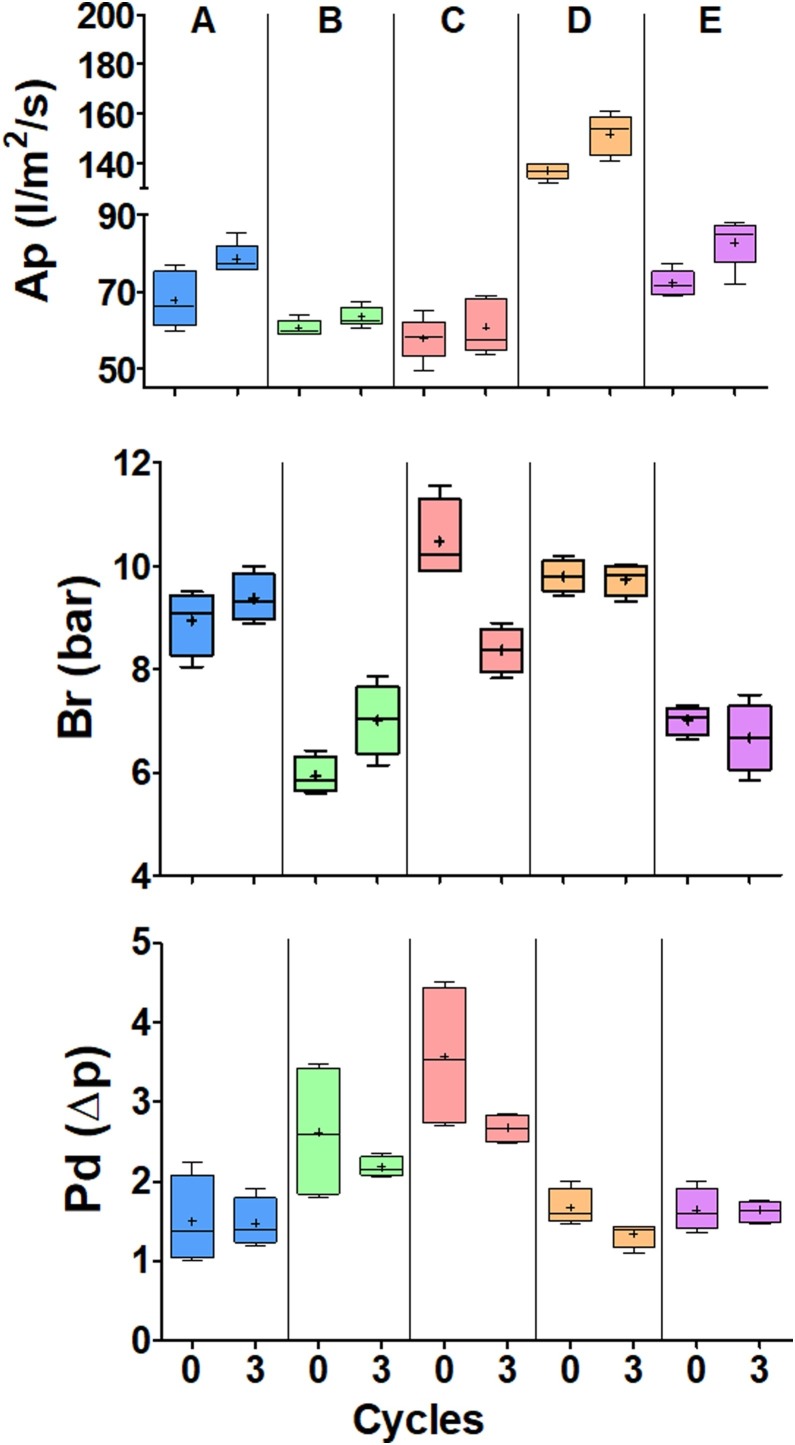
Effect of 20 min of use followed by one decontamination cycle using different methods – thermal drying (orange), hydrogen peroxide (H2O2) (pink), oven (green) or autoclave (purple) – on the performance of surgical masks (left) and respirators (right). New surgical masks and respirators are represented in blue. Performance was measured by air permeability (Ap) (l/m2/s) (A, B); burst resistance (Br) (bar) (C, D); and pressure differential (Pd) (Δp) (E, F). The graphics do not have the same scale. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
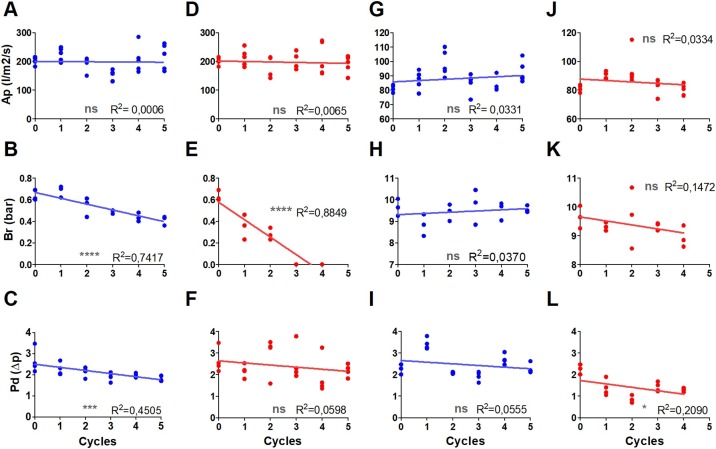
After three cycles of autoclave decontamination, the mechanical resistance (burst) of surgical masks was affected, rendering it impossible to attach them to the testing machine without damage. The air permeability of surgical masks was not affected by oven or autoclave decontamination (Figure 4 A,D). Burst resistance decreased linearly with the number of decontamination cycles, and the effect of oven decontamination was much lower than that of autoclave decontamination (Figure 4B,E). The pressure differential reduced slightly following oven decontamination (Figure 4C), but the autoclave results presented higher dispersal and no clear tendency (Figure 4F). Except for a slight improvement in pressure differential due to autoclave decontamination (Figure 4L), no significant effect was observed in respirators (Figure 4G,H,I,J,K,L).
Figure 4.
Correlation of the effect of 20 min of use followed by up to five decontamination cycles in an oven (75 °C for 45 min; blue) or autoclave (red) on the performance of surgical masks (A, B, C, D, E, F) and respirators (G, H, I, J, K, L). Performance was measured by air permeability (Ap) (l/m2/s) (A, D, G, J); burst resistance (Br) (bar) (B, E, H, K); and pressure differential (Pd) (Δp) (C, F, I, L). *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
To test if the effects were reproducible in other brands of respirators, five different brands were subjected to three decontamination cycles in an oven (Figure 5 ). The effect of decontamination was significant but was much smaller than the differences found between the new respirators of various brands.
Figure 5.
Evaluation of the physical integrity of respirators of five different brands when new and after undergoing three decontamination cycles in an oven. (A) Deltaplus PFF2. (B) Deltaplus PFF3. (C) MaskFace. (D) Tayco. (E) Proteplus. Performance was measured by air permeability (Ap) (l/m2/s), burst resistance (Br) (bar) and pressure differential (Pd) (Δp). The graphics do not have the same scale.
Particulate filtration efficiency
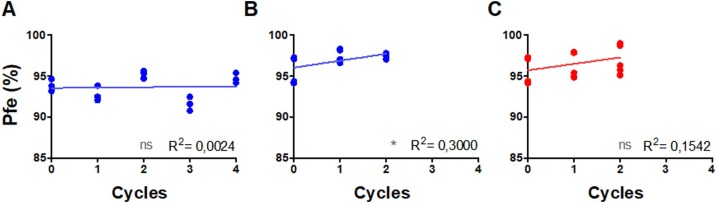
The number of decontamination cycles did not affect particulate filtration efficiency. Differences observed for surgical masks and respirators are most probably due to variability between surgical masks and respirators and experimental error. The particulate filtration efficiency indicates that filtration capacity remained >92% and >96% for surgical masks and respirators, respectively, after decontamination (Figure 6 ).
Figure 6.
Correlation between the percentage of particulate filtration efficiency (Pfe) and decontamination cycles of surgical masks (A) and respirators (B, C) after oven decontamination (A, B; blue) and decontamination with hydrogen peroxide plasma vapour (H2O2; C) (red). *P < 0.05. ns, not significant.
Detection of SARS-CoV-2 RNA and viral culture
Among the 14 respirators (HC 1, HC 2, SC 3, SC 4, HC 5-14) used by HCWs during work shifts, RNA was detected in two respirators before decontamination, collected in the first week of the pandemic: HC 1 and SC3. One of them (SC 3, 7%) remained positive after oven decontamination. HC 1 had a Ct value of 36 before oven decontamination and undetectable after decontamination. SC3 had a Ct value of 33 before decontamination and 32 after oven decontamination. HCWs were notified of the positive results but did not report any symptoms. Four respirators (HC 11, HC 12, HC 13 and HC 14) were subjected to viral culture; none of them showed a cytopathic effect and RT-PCR results were negative.
User evaluation
Visual and functional evaluation of respirators following oven decontamination are shown in Table 2 . It is important to note that the respirators were not handled by the persons involved in the decontamination process. The respirators were decontaminated without removal from the paper envelopes in which they were placed by the HCWs. Dirt on the respirators was mainly the user’s make-up. Clip adhesion and elasticity of straps are likely to be the problems limiting re-use.
Table 2.
Healthcare workers’ assessment of respirators decontaminated in an oven. Each decontamination cycle was performed after the respirator had been used for a 12-h work shift in a ward dedicated to patients with coronavirus disease 2019 with 22 intensive care beds.
| Assessment | First decontamination cycle |
Second decontamination cycle |
Third decontamination cycle |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | n | Yes | No | n | Yes | No | n | ||||
| Is the respirator dirty? | 8 (24%) | 25 (76%) | 33 | 6 (33%) | 12 (67%) | 18 | 4 (44%) | 5 (56%) | 9 | |||
| Is the elastic functional? | 32 (91%) | 3 (9%) | 35 | 15 (83%) | 3 (17%) | 18 | 10 (91%) | 1 (9%) | 11 | |||
| Is the metal nose clip functional? | 21 (84%) | 4 (16%) | 25 | 9 (100%) | 0 | 9 | 3 (75%) | 1 (25%) | 4 | |||
| Functionality assessment | Better | Worse | Same | n | Better | Worse | Same | n | Better | Worse | Same | n |
| Does the respirator maintain a proper seal? | 3 (9%) | 7 (22%) | 22 (69%) | 32 | 1 (7%) | 4 (26%) | 10 (67%) | 15 | 1 (10%) | 4 (40%) | 5 (50%) | 10 |
| How does breathing compare with previous use? | 1 (3%) | 7 (24%) | 21 (73%) | 29 | 0 | 2 (67%) | 12 (86%) | 14 | 0 | 3 (30%) | 7 (70%) | 10 |
Discussion
After evaluating four decontamination methods for surgical masks and respirators, the simplest and most useful method appears to be oven decontamination (75 °C for 45 min). Oven decontamination did not affect the physical characteristics or filtering capacity of surgical masks or respirators for at least five reprocessing cycles. Reprocessed respirators used in 12-h work shifts in a COVID-19-dedicated intensive care unit were evaluated positively by users even after three decontamination cycles.
In the context of pandemics and global PPE shortages, alternatives such as re-use and adaption of existing technologies are necessary. This study evaluated the possibility of re-using surgical masks and respirators following decontamination in an oven, a thermal drying machine, an autoclave and with hydrogen peroxide, which were available at the hospital. All methods showed acceptable performance after one decontamination cycle, so oven and autoclave decontamination were investigated further as these methods are available in most hospitals. Oven decontamination was found to be the best method for surgical masks, as autoclave decontamination reduced the burst resistance drastically. Wearing a surgical mask augmented its air permeability and burst resistance. Interestingly, this effect was restored after heat decontamination, making the surgical masks better after decontamination. This phenomenon may be explained by thermal shrinkage of the material. No relevant changes were found regarding respirators, as the differences found after decontamination cycles were smaller than the differences found between brands. Air permeability and burst resistance are tests used for general textiles that were included in this study to expand the analysis. According to the Brazilian standard for non-woven articles for medical and hospital use (ABNT NBR 15052), the pressure differential ΔP must be equal to or less than 4 mmH2O, which means that all decontamination methods passed the test. The factor found to prevent re-use of respirators more than twice was detachment of the nasal clip due to failure of the glue. This suggests that innovation in the manufacturing process could increase the number of times that a respirator could be re-used.
Respirators that had been used by HCWs during work shifts were also evaluated. The viral cultures of respirators were negative after oven decontamination, suggesting that this is safe. Finally, HCWs were asked to evaluate their reprocessed respirators, after use during a 12-h work shift, and reported that they were adequate.
The concept of re-using PPE has been considered in previous epidemics (Institute of Medicine, 2006, Pillai et al., 2015, Lin et al., 2017, Mills et al., 2018). Most studies on PPE re-use have evaluated respirators, and data are scarce for surgical masks. Among the processes, some tests on respirators have found that oven reprocessing is safe with respect to integrity, decontamination and filtration (Viscusi et al., 2009). Previous studies have examined different methods for respirator decontamination such as hydrogen peroxide vapour (Schwartz et al., 2020) and ultraviolet irradiation (Mills et al., 2018). In vitro, Darnell et al. (2004) showed that SARS-CoV-1 is inactivated by heat decontamination for 45 min at 75 °C. As such, this method was used in this study, along with other methods that were available in the Central Sterile Service Department at the study hospital. Heat treatment is a simple and accessible method to decontaminate respirators, as viruses are not stable to heat (Abraham et al., 2020, Chin et al., 2020, Loh et al., 2020).
Particulate filtration efficiency was measured using particles ranging from 0.08 to 0.14 μm, and surgical masks and respirators maintained high efficiency (>92% and 96%, respectively) after decontamination. The size of SARS-CoV-2 particles is estimated to be 0.06–0.14 μm (Zhu et al., 2020), which indicates that decontamination methods may be considered appropriate. According to the Brazilian standard, particulate filtration efficiency must be ≥98%. Our particulate efficiency was 92% for surgical masks and 96% for respirators. This difference could be explained because in our analysis we used smaller particules (0.08 μm for example) than those used by the Brazilian standard (0.105 μm). The WHO PPE specifications indicate particulate filtration efficiency ≥95% as level 1 and ≥98% as levels 2 and 3. It is important to note that particulate filtration efficiency increased (slightly for surgical masks and considerably for respirators) with the number of decontamination cycles.
Real-time RT-PCR showed that SARS-CoV-2 RNA could be detected in two (14%) respirators used by HCWs after a work shift. One respirator remained positive after decontamination, which probably means that RNA was present but the virus was inactivated. It was not possible to perform a virus culture of this respirator. It is important to note that of the 14 HCWs, four (including the two HCWs with positive RT-PCR results) did not use face shields because it was the first week of the pandemic and all safety measures had not yet been implemented. Other factors may also influence contamination, such as incorrect doffing. Respirators submitted to viral culture were negative, suggesting that no viable virus was present after heat decontamination.
Due to the shortage of PPE in the pandemic, this study only tested a small sample of surgical masks and respirators. However, the study was driven by the urgent need to find alternatives so that HCWs could work safely. These results may be applicable to future emergencies.
These results show that surgical masks and respirators can be decontaminated in an oven (75 °C for 45 min) at least five times in order to protect HCWs during periods of PPE shortages, such as the SARS-CoV-2 pandemic. Oven decontamination can be performed easily in a hospital’s central sterile services department.
Conflict of interests
None declared.
Funding
This study was performed with funding from Hospital das Clínicas and Universidade de São Paulo. Fundação de Amparo à Pesquisa do Estado de São Paulo funded one postdoctorate scholarship.
Ethical approval
Not required.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.12.056.
Appendix A. Supplementary data
The following are Supplementary data to this article:
Schematic of severe acute respiratory syndrome coronavirus-2 detection and culture process. RT-PCR, reverse transcription polymerase chain reaction.
Visual appearance of surgical masks after decontamination in an oven (75 °C for 45 min). The number of lines on each strap represents the number of decontamination cycles.
Visual aspect of respirators after decontamination in an oven (75 °C for 45 min). The number of lines on each strap represents the number of decontamination cycles.
(A) Representation of the experiment to assess the ability of surgical masks to retain DNA. A membrane is placed 1 cm over the mask and a solution containing water or DNA is sprayed over the mask. (B) Agarose gel 1.2% of the membranes following polymerase chain reaction. 1, new surgical mask sprayed with DNA; 2, membrane without mask sprayed with DNA; 3, new surgical mask sprayed with water; 4, membrane without mask sprayed with water; 5, surgical mask re-used five times sprayed with DNA; 6, surgical mask re-used five times sprayed with water; 7, surgical mask re-used 10 times sprayed with 50 ng/μL DNA; 8, surgical mask re-used 10 times sprayed with water; 9, 100-bp DNA ladder; 10, respirator used seven times sprayed with water; 11, respirator used seven times sprayed with DNA; 12, negative control of the polymerase chain reaction. This experiment was repeated three times.
References
- Abraham J.P., Plourde B.D., Cheng L. Using heat to kill SARS-CoV-2. Rev Med Virol. 2020;30:e2115. doi: 10.1002/rmv.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammerman N., Beier-Sexton M., Azad A. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008;11:A.4E.1–7. doi: 10.1002/9780471729259.mca04es11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaib F. WHO; Geneva: 2020. Shortage of personal protective equipment endangering health workers worldwide. Available at: https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide. (Last accessed 13 January 2021) [Google Scholar]
- Cheng V.C.C., Wong S.C., Kwan G.S.W., Hui W.T., Yuen K.Y. Disinfection of N95 respirators by ionized hydrogen peroxide during pandemic coronavirus disease 2019 (COVID-19) due to SARS-CoV-2. J Hosp Infect. 2020;105:358–359. doi: 10.1016/j.jhin.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;5247 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J., Tamin A., Lu X., Kamili S., Sakthivel S.K., Murray J. Isolation and characterization of SARS-CoV-2 from the first US COVID-19 patient. bioRxiv. 2020 doi: 10.1101/2020.03.02.972935. [DOI] [Google Scholar]
- Institute of Medicine . The National Academies Press; Washington, DC: 2006. 2006. Reusability of facemasks during an influenza pandemic: facing the flu. [Google Scholar]
- Lennette E.H., Schmidt N.J. American Public Health Association; Washington, DC: 1979. Diagnostic procedures for viral, rickettsial and chlamydial infections. Chapter 3. [Google Scholar]
- Lin T.H., Chen C.C., Huang S.H., Kuo C.W., Lai C.Y., Lin W.Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: effects of five decontamination methods. PLoS One. 2017;12:1–15. doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh M., Clark R., Cherrie J.W. Heat treatment for reuse of disposable respirators during Covid-19 pandemic: is filtration and fit adversely affected? medRxiv. 2020 04.22.20074989. [Google Scholar]
- Mackenzie D. Reuse of N95 masks. Engineering. 2020;6:593–596. doi: 10.1016/j.eng.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D., Harnish D.A., Lawrence C., Sandoval-Powers M., Heimbuch B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S.K., Beekmann S.E., Babcock H.M., Pavia A.T., Koonin L.M., Polgreen P.M. Clinician beliefs and attitudes regarding use of respiratory protective devices and surgical masks for influenza. Health Secur. 2015;13:274–280. doi: 10.1089/hs.2015.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Stiegel M., Greeson N., Vogel A., Thomann W., Brown M. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl Biosaf. 2020;25:67–70. doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Coronavirus disease 2019. [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schematic of severe acute respiratory syndrome coronavirus-2 detection and culture process. RT-PCR, reverse transcription polymerase chain reaction.
Visual appearance of surgical masks after decontamination in an oven (75 °C for 45 min). The number of lines on each strap represents the number of decontamination cycles.
Visual aspect of respirators after decontamination in an oven (75 °C for 45 min). The number of lines on each strap represents the number of decontamination cycles.
(A) Representation of the experiment to assess the ability of surgical masks to retain DNA. A membrane is placed 1 cm over the mask and a solution containing water or DNA is sprayed over the mask. (B) Agarose gel 1.2% of the membranes following polymerase chain reaction. 1, new surgical mask sprayed with DNA; 2, membrane without mask sprayed with DNA; 3, new surgical mask sprayed with water; 4, membrane without mask sprayed with water; 5, surgical mask re-used five times sprayed with DNA; 6, surgical mask re-used five times sprayed with water; 7, surgical mask re-used 10 times sprayed with 50 ng/μL DNA; 8, surgical mask re-used 10 times sprayed with water; 9, 100-bp DNA ladder; 10, respirator used seven times sprayed with water; 11, respirator used seven times sprayed with DNA; 12, negative control of the polymerase chain reaction. This experiment was repeated three times.