Abstract
Neurologic complications of COVID-19 infection have been recently described and include dizziness, headache, loss of taste and smell, stroke, and encephalopathy. Brain MRI in these patients have revealed various findings including ischemia, hemorrhage, inflammation, and demyelination. In this article, we report a case of critical illness-associated cerebral microbleeds identified on MRI in a patient with severe COVID-19 infection and discuss the potential etiologies of these neuroimaging findings.
Keywords: Cerebral microbleeds, COVID-19, Neuroimaging
Highlights
-
•
Neurological symptoms are common in patients with COVID-19.
-
•
Critical illness-associated cerebral microbleeds can be seen in the setting of severe COVID-19.
-
•
Critical illness-associated cerebral microbleeds are a neuroimaging finding thought to be related to hypoxemia, ECMO-related complications and/or DIC.
-
•
In patients with severe COVID-19, other potential etiologies of cerebral microbleeds are endotheliopathy, encephalitis and immune response.
1. Introduction
Severe acute respiratory distress syndrome due to novel coronavirus (SARS-CoV-2), which was first diagnosed in Wuhan, China in December 2019, is known for its typical clinical manifestations of fever, cough, and fatigue [1]. However, recent studies have described clinical neurological manifestations of coronavirus disease 2019 (COVID-19), which include dizziness, headache, taste and smell impairment, acute cerebrovascular disease, impaired consciousness, seizure, encephalopathy, confusion, agitation, and corticospinal tract signs [1,2]. Neurological symptoms have been identified more commonly in those with severe or critical infection [1], with a prevalence ranging from 15% to 36.4% [3]. Reported neuroimaging features have included infarcts, hemorrhages, parenchymal (cortical and/or white matter) T2 FLAIR hyperintensity with or without restricted diffusion, cerebral venous thrombosis, acute hemorrhagic necrotizing encephalopathy, leptomeningeal enhancement, and demyelinating lesions [[2], [3], [4], [5], [6]]. In critically ill patients, hypoxemia and respiratory failure have been associated with the presence of cerebral microbleeds [7], which are typically identified as small hypointense foci on T2*-weighted imaging [such as gradient recalled echo (GRE) and susceptibility weighted imaging (SWI)], an MRI sequence that is sensitive to the susceptibility effects of iron within blood products [8]. Of the T2*-weighted sequences, SWI is more sensitive and reliable than GRE for detecting microbleeds, owing to higher signal contrast and spatial resolution [8]. On histopathology, these microbleeds have been shown to contain focal accumulations of hemosiderin-laden macrophages [9]. Herein, we report a case of critical illness-associated cerebral microbleeds in a patient with severe COVID-19 infection and discuss the potential etiologies of these neuroimaging findings.
2. Case report
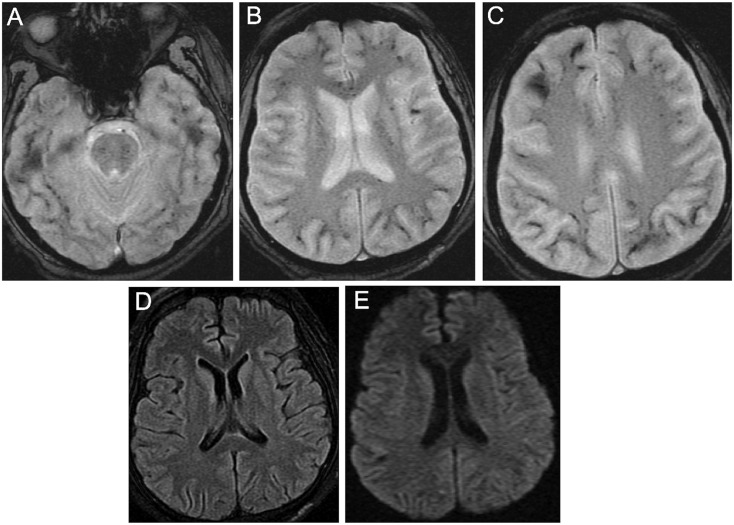
A male in his mid-forties presented to the emergency room with cough, shortness of breath, and intermittent fever for two weeks. He quickly became hypoxic with an oxygen saturation of 84% and was subsequently intubated and admitted to the intensive care unit for higher care. Initial tests for influenza, SARS-CoV-2, and other viruses were negative; however, given high clinical suspicion, he was re-tested for SARS-CoV-2 on day 2 of hospital admission and was confirmed to be positive. The patient developed increasing FIO2 requirements and was placed on extracorporeal membrane oxygenation (ECMO). After continued respiratory and ventilation management, he slowly improved with discontinuation of ECMO and extubation by day 23. However, he remained confused and disoriented, and a brain MRI was obtained. MRI revealed diffuse microhemorrhages in the bilateral subcortical white matter, basal ganglia, corpus callosum, brainstem, and cerebellum without corresponding signal abnormality on T2 FLAIR or diffusion-weighted imaging (DWI) (Fig. 1 ). Based on his initial and follow-up laboratory tests over the span of five days, he did not meet criteria for overt disseminated intravascular coagulation (DIC) [10], with only slightly increasing D-dimer (0.32 to 3.01 mg/L) and decreasing fibrinogen (847 to 490 mg/dL) but normal prothrombin time and platelets. He also had a normal CRP of 46.7 mg/dL. Lumbar puncture was not performed. Supportive treatment was continued, and the patient's respiratory status and neurological exam improved. He was discharged to a skilled nursing facility on day 41.
Fig. 1.
MRI shows diffuse microbleeds in the bilateral subcortical white matter, basal ganglia, corpus callosum, and brainstem on two-dimensional gradient recalled echo (GRE) (A–C), without any corresponding signal abnormality on T2 FLAIR (D) or diffusion-weighted (E) images.
3. Discussion
Critical illness-associated cerebral microbleeds have been reported in patients with high altitude cerebral edema (HACE), acute respiratory failure, and in those requiring ECMO [7,11,12]. They can extensively involve the juxtacortical white matter and corpus callosum (often the splenium) and less frequently involve the cortex and deep and periventricular white matter [7]. The etiology of these microbleeds is likely related to one or more of the following processes: 1) hypoxia-induced effects on the blood-brain barrier resulting in extravasation of blood, 2) potential side effects of ECMO therapy such as gas embolism or coagulopathy, and 3) disseminated intravascular coagulation [7,11].
Hypoxia is a common factor for the development of microbleeds in patients with acute respiratory failure [7,11,12]. The likely pathogenesis is a hydrostatic- or chemical-related disruption of the blood-brain barrier, which leads to extravasation of red blood cells [7]. The presence of cerebral venous hypertension, which is also suspected to play an important role in HACE, may further contribute to increased capillary stress and permeability [12,13]. HACE is a life-threatening illness due to hypoxemia at high altitudes and is characterized by severe ataxia and altered consciousness; consequently, it can result in death within 24 to 48 hours from vasogenic cerebral edema and brain herniation [12,13]. Neuroimaging in patients with HACE include microhemorrhages in the juxtacortical white matter and corpus callosum, often in a splenial distribution [12,13]. Similarly, brain MRIs in patients without HACE but with severe acute respiratory distress syndrome (including those with morphine intoxication and chronic obstructive pulmonary disease on mechanical ventilation) have shown disseminated microhemorrhages in the same distribution [13]. In the setting of patients with severe or critical COVID-19 infection, small case series have shown a similar pattern of isolated or extensive cerebral microbleeds in the white matter and corpus callosum [5,6].
Supportive measures used in patients with severe hypoxic respiratory failure may also contribute to the development of microhemorrhages. Cerebral gas embolism has been proposed as a cause of microhemorrhages in patients on venoarterial ECMO; this explanation is supported by the presence of microbleeds in the cerebral borderzone or watershed territories, often found in the right hemisphere, a finding suggestive of an embolic phenomenon [14]. Patients requiring ECMO therapy are also at an increased risk of bleeding complications due to heparin coating of the ECMO equipment and systemic anticoagulation usually with unfractionated heparin [13]. Furthermore, ECMO can lead to increased cerebral venous pressures, possibly related to positioning of the cannulae within the internal jugular vein, which can cause increased blood flow to the right atrium of the heart and, consequently, increased central venous pressure [13].
Endothelial dysfunction and immune response may be another cause of cerebral microhemorrhages. SARS-CoV-2 can directly enter endothelial cells by binding to the Angiotensin Converting Enzyme 2 receptor (ACE2), which is found in arterial and venous endothelial cells in a variety of human tissues including the brain, and can induce endotheliopathy, hypercoagulability, and microvascular leak [[15], [16], [17]]. Patients with severe COVID-19 infection have shown elevated levels of pro-inflammatory cytokines, such as IL-2 and IL-6, which can bind to receptors on endothelial cells and induce capillary leakage and dysfunction, potentially leading to coagulation dysregulation, complement and platelet activation, thrombus formation, and potentially DIC [15]. These mechanisms may help to explain the predisposition of COVID-19 patients to arterial and venous thromboembolism. In fact, acute ischemia was the most common imaging finding in COVID-19 patients who underwent brain imaging in recent retrospective studies from Italy and New York [3,16]. However, despite being the most common COVID-19 related neuroimaging finding, acute stroke was still only found in 1.1% of 3218 hospitalized COVID-19 patients in the New York study [16].
Immune cell activation within the central nervous system can further lead to acute or chronic inflammation and brain damage. Infectious toxic encephalopathy, a reversible brain dysfunction syndrome due to systemic factors from acute infection, has been described in patients with COVID-19 and confirmed in autopsy reports [18]. Recently, Poyiadji et al. described a case of acute hemorrhagic necrotizing encephalopathy occurring in a patient with COVID-19 [4]. While the exact pathomechanism for this entity has not been elucidated, it is thought to be most likely related to the development of an intracranial “cytokine storm”, as a complication of viral infection (rather than direct viral invasion), causing blood-brain barrier breakdown, which can be detected on MRI as areas of edema, hemorrhage, and contrast enhancement [4].
Damage to the central nervous system can be related to direct viral invasion. Viral encephalitis caused by SARS-CoV-2 was recently confirmed with the detection of the virus in the cerebrospinal fluid of patients with COVID-19 by genome sequencing by a research team in China [18]. Furthermore, infection of neuronal pathways by viruses may help to explain the symptoms of anosmia (where there is presumed involvement of the olfactory tract between the nasal epithelium and olfactory bulb) experienced by a subset of patients with COVID-19 infection [17,18].
Given the multiple potential causes of critical illness-associated cerebral microbleeds, the exact etiology for the microbleeds in our patient is not entirely clear, but it is most likely multifactorial and related to hypoxia, ECMO-related complications, and/or a coagulopathy disorder. Although the patient's coagulation labs were not entirely normal, he did not meet the criteria for overt disseminated intravascular coagulation [10]. In addition, the absence of brain edema or acute infarcts on T2 FLAIR and DWI on MRI, respectively, argued against direct viral invasion, encephalitis, or other acute inflammatory, demyelinating, or ischemic processes. Although brain MRI can be normal in viral encephalitides [19], the clinical absence of an acute exacerbation of neurologic symptoms and lack of focal neurologic deficit in our patient did not support an acute infectious or inflammatory process of the brain parenchyma or meninges. Nonetheless, the correlation between cerebral microbleeds and neurocognitive impairment and disability in these patients is unclear [9]. While elevated inflammatory serum markers such as D-dimer levels and the occurrence of acute strokes have been associated with poor prognosis in COVID-19 patients [16], prior reports of patients with acute respiratory distress syndrome treated with ECMO, including those with H1N1 influenza infection, had favorable clinical outcomes [11,20]. At this time, the long-term clinical significance of cerebral microbleeds in patients with severe or critical COVID-19 infection remains to be seen. Therefore, it is important for radiologists and clinicians to recognize this entity in order to diagnosis and manage patients appropriately and to exercise caution when prognosticating patients based on these neuroimaging findings.
Funding
None.
Declaration of competing interest
Dr. Iv is a consultant for Octave Bioscience, Inc. which is unrelated to the submitted work. Dr. Gupta and Dr. Lien have no interests to declare.
Footnotes
Name and street address of the institution from which the work originated: Regional Medical Center, 225 N Jackson Ave, San Jose, CA 95116.
References
- 1.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahammedi A., Saba L., Vagal A., Leali M., Rossi A., Gaskill M. Imaging in neurological disease of hospitalized COVID-19 patients: an Italian multicenter retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020201933. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M. COVID-19-associated diffuse Leukoencephalopathy and microhemorrhages. Radiology. 2020:202040. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer S., Lersy F., de Seze J., Ferre J.C., Maamar A., Carsin-Nicol B. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020:202222. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanou E.M., Coutinho J.M., Shannon P., Kiehl T.R., Levi M.M., Wilcox M.E. Critical illness-associated cerebral microbleeds. Stroke. 2017;48(4):1085–1087. doi: 10.1161/STROKEAHA.116.016289. [DOI] [PubMed] [Google Scholar]
- 8.Cheng A.L., Batool S., McCreary C.R., Lauzon M.L., Frayne R., Goyal M. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. 2013;44(10):2782–2786. doi: 10.1161/STROKEAHA.113.002267. [DOI] [PubMed] [Google Scholar]
- 9.Haller S., Vernooij M.W., Kuijer J.P.A., Larsson E.M., Jager H.R., Barkhof F. Cerebral microbleeds: imaging and clinical significance. Radiology. 2018;287(1):11–28. doi: 10.1148/radiol.2018170803. [DOI] [PubMed] [Google Scholar]
- 10.Levi M., Toh C.H., Thachil J., Watson H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 11.Chow F.C., Edlow B.L., Frosch M.P., Copen W.A., Greer D.M. Outcome in patients with H1N1 influenza and cerebrovascular injury treated with extracorporeal membrane oxygenation. Neurocrit Care. 2011;15(1):156–160. doi: 10.1007/s12028-011-9534-7. [DOI] [PubMed] [Google Scholar]
- 12.Kallenberg K., Dehnert C., Dorfler A., Schellinger P.D., Bailey D.M., Knauth M. Microhemorrhages in nonfatal high-altitude cerebral edema. J Cereb Blood Flow Metab. 2008;28(9):1635–1642. doi: 10.1038/jcbfm.2008.55. [DOI] [PubMed] [Google Scholar]
- 13.Riech S., Kallenberg K., Moerer O., Hellen P., Bartsch P., Quintel M. The pattern of brain microhemorrhages after severe lung failure resembles the one seen in high-altitude cerebral edema. Crit Care Med. 2015;43(9):e386–e389. doi: 10.1097/CCM.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 14.Liebeskind D.S., Sanossian N., Sapo M.L., Saver J.L. Cerebral microbleeds after use of extracorporeal membrane oxygenation in children. J Neuroimaging. 2013;23(1):75–78. doi: 10.1111/j.1552-6569.2012.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain R., Young M., Dogra S., Kennedy H., Nguyen V., Jones S. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414 doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mankad K., Perry M.D., Mirsky D.M., Rossi A. COVID-19: a primer for Neuroradiologists. Neuroradiology. 2020;62(6):647–648. doi: 10.1007/s00234-020-02437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilden D.H. Brain imaging abnormalities in CNS virus infections. Neurology. 2008;70(1):84. doi: 10.1212/01.wnl.0000286937.09760.e4. [DOI] [PubMed] [Google Scholar]
- 20.Le Guennec L., Bertrand A., Laurent C., Roze H., Chastre J., Combes A. Diffuse cerebral microbleeds after extracorporeal membrane oxygenation support. Am J Respir Crit Care Med. 2015;191(5):594–596. doi: 10.1164/rccm.201411-2118LE. [DOI] [PubMed] [Google Scholar]