Abstract
Background
Biological considerations suggest that renin–angiotensin system inhibitors might influence the severity of COVID-19. We aimed to evaluate whether continuing versus discontinuing renin–angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) affects outcomes in patients admitted to hospital with COVID-19.
Methods
The REPLACE COVID trial was a prospective, randomised, open-label trial done at 20 large referral hospitals in seven countries worldwide. Eligible participants were aged 18 years and older who were admitted to hospital with COVID-19 and were receiving a renin–angiotensin system inhibitor before admission. Individuals with contraindications to continuation or discontinuation of renin–angiotensin system inhibitor therapy were excluded. Participants were randomly assigned (1:1) to continuation or discontinuation of their renin–angiotensin system inhibitor using permuted block randomisation, with allocation concealed using a secure web-based randomisation system. The primary outcome was a global rank score in which participants were ranked across four hierarchical tiers incorporating time to death, duration of mechanical ventilation, time on renal replacement or vasopressor therapy, and multiorgan dysfunction during the hospitalisation. Primary analyses were done in the intention-to-treat population. The REPLACE COVID trial is registered with ClinicalTrials.gov, NCT04338009.
Findings
Between March 31 and Aug 20, 2020, 152 participants were enrolled and randomly assigned to either continue or discontinue renin–angiotensin system inhibitor therapy (continuation group n=75; discontinuation group n=77). Mean age of participants was 62 years (SD 12), 68 (45%) were female, mean body-mass index was 33 kg/m2 (SD 8), and 79 (52%) had diabetes. Compared with discontinuation of renin–angiotensin system inhibitors, continuation had no effect on the global rank score (median rank 73 [IQR 40–110] for continuation vs 81 [38–117] for discontinuation; β-coefficient 8 [95% CI −13 to 29]). There were 16 (21%) of 75 participants in the continuation arm versus 14 (18%) of 77 in the discontinuation arm who required intensive care unit admission or invasive mechanical ventilation, and 11 (15%) of 75 participants in the continuation group versus ten (13%) of 77 in the discontinuation group died. 29 (39%) participants in the continuation group and 28 (36%) participants in the discontinuation group had at least one adverse event (χ2 test of adverse events between treatment groups p=0·77). There was no difference in blood pressure, serum potassium, or creatinine during follow-up across the two groups.
Interpretation
Consistent with international society recommendations, renin–angiotensin system inhibitors can be safely continued in patients admitted to hospital with COVID-19.
Funding
REPLACE COVID Investigators, REPLACE COVID Trial Social Fundraising Campaign, and FastGrants.
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a public health crisis. COVID-19 is associated with a high incidence of acute respiratory distress syndrome, multiorgan dysfunction, and mortality, and there are few therapeutic options to reduce adverse outcomes.1, 2 Patients with hypertension, diabetes, or cardiovascular diseases are at the highest risk of admission to hospital and mortality due to COVID-19.3
Angiotensin-converting enzyme 2 (ACE2) has an important counter-regulatory role in the renin–angiotensin system, promoting systemic vasodilatory and anti-inflammatory effects.3 ACE2 also serves as a receptor for SARS-CoV-2, facilitating viral entry into host cells.4 Changes in ACE2 activity and expression might contribute to SARS-CoV-2 infection and COVID-19 severity.4, 5, 6 However, taking into account the pleiotropic effects of ACE2, the expected direction and magnitude of these effects is unclear. Evidence suggests that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), inhibitors of the renin–angiotensin system, might increase ACE2 expression,7, 8, 9, 10 although these findings are not consistent across studies.11
Research in context.
Evidence before this study
We searched PubMed on Nov 24, 2020, for literature published since Jan 1, 2020, using the search terms “COVID-19”, “SARS-CoV-2”, “angiotensin-converting enzyme inhibitors”, and “angiotensin receptor blockers”. We searched for primary research (observational studies and clinical trials) with no language restrictions and found 72 observational studies evaluating the association of angiotensin-converting enyzme inhibitor (ACEI) use, angiotensin receptor blocker (ARB) use, or both, with development or severity of COVID-19, one of which was retracted following publication and several of which had important methodological limitations, such as inadequate adjustment for known confounders. Early evidence of patients admitted to hospital with COVID-19 suggested an association of the use of ACEIs and ARBs, inhibitors of the renin–angiotensin system, with increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19. ACEIs and ARBs are among the most commonly prescribed medications in the world, and a potential link between these medications and COVID-19 has enormous global health implications. Accordingly, there has been substantial interest in the effect of these commonly used medications on COVID-19 severity. Observational studies suggest no association between outpatient ACEI or ARB use and risk of COVID-19-related hospitalisation or mortality, but high-quality randomised trial evidence is lacking.
Added value of this study
In this multicentre, international, randomised controlled trial of participants admitted to hospital with COVID-19 between March 31 and Aug 20, 2020, we observed that continuation compared with discontinuation of renin–angiotensin system inhibitor therapy did not significantly affect the severity or duration of hospitalisation, providing high-quality evidence that adds to the findings of existing observational studies.
Implications of all the available evidence
Consistent with international society recommendations, current evidence supports continuing renin–angiotensin system inhibitor therapy in patients admitted to hospital with COVID-19 unless there is a clear medical contraindication to ongoing therapy.
Renin–angiotensin system inhibitors are among the most commonly prescribed medications12 and the possibility of an effect of these medications on COVID-19 is of substantial importance for public health. However, published data to date have been limited to those from observational cohort studies, which suggest no association between outpatient ACEI or ARB use and risk of COVID-19-related hospital admission or mortality.13, 14, 15, 16 In the absence of a randomised, controlled design, these studies could not address key sources of bias and confounding, and did not evaluate the important question of whether to continue or discontinue these medications in patients admitted to hospital with COVID-19. Continuation of ACEI or ARB therapy could conceivably increase intracellular entry of SARS-CoV-2, resulting in poorer clinical outcomes, or reduce the hyperinflammatory response to SARS-CoV-2, improving COVID-19-related outcomes. The REPLACE COVID trial aimed to evaluate whether continuation of ACEIs and ARBs is beneficial or harmful in patients who are admitted to hospital with COVID-19.
Methods
Study design
The REPLACE COVID trial was a prospective, randomised, open-label trial done at 20 large referral hospitals in the USA, Canada, Mexico, Sweden, Peru, Bolivia, and Argentina. A data coordinating centre at the University of Pennsylvania (Philadelphia, PA, USA) oversaw data management and statistical analyses. The trial design was approved by the ethics committee of each participating centre, or in the USA, via reliance agreements with a central institutional review board (University of Pennsylvania, Philadelphia, PA, USA). An independent data safety monitoring board was assembled to provide independent oversight of the trial. Details of the rationale and trial design have been previously described17 and additional details are included in appendix 2 (pp 2–6).
Participants
Patients aged 18 years or older, who were admitted to hospital with a clinical presentation consistent with COVID-19, and were prescribed ACEI or ARB therapy as an outpatient before the hospital admission, were eligible for enrolment. Participants were recruited upon admission to hospital. Participants were excluded if they had a negative SARS-CoV-2 test or clinical contraindications to continuing or discontinuing ACEI or ARB therapy, including systolic blood pressure less than 100 mm Hg or more than 180 mm Hg (or >160 mm Hg if unable to substitute their ACEI or ARB for another antihypertensive class of drugs); diastolic blood pressure more than 110 mm Hg; heart failure with reduced ejection fraction, or a clinically significant interim event likely to be associated with a reduction in ejection fraction since the last ejection fraction was assessed; serum potassium concentration of more than 5 mmol/L; known pregnancy or breast feeding; acute kidney injury with a 100% or more increase in creatinine concentration (to a creatinine concentration of >177 μmol/L) compared with the most recent creatinine concentration in the past 6 months, if available; severe proteinuria (urine protein-to-creatinine ratio >3 g/g or equivalent); or ongoing treatment with aliskiren or sacubitril–valsartan. Additionally, individuals were excluded if they were imprisoned or incarcerated. All participants provided written or electronic informed consent.
Randomisation and masking
Participants were randomly assigned 1:1 to discontinuation of ACEI or ARB therapy or continuation of therapy at the dose previously prescribed during their routine care, for the duration of their hospitalisation. Randomisation lists were generated by a statistician at the data coordinating centre (University of Pennsylvania, Philadelphia, PA, USA) using a standard random number generator in Stata version 16.1 with permuted block randomisation in randomly varying block sizes of two, four, or six by clinical site, sex, and age. Randomisation was controlled centrally by the data coordinating centre. Allocation was concealed using a secure web-based randomisation system.
Investigators and treating clinicians were aware of the assigned treatment strategy, but outcome adjudicators were not. A clinician panel was appointed to do masked adjudications of the outcome events. Each site used a standardised approach to redacting patient records so that the adjudicators were fully masked to the treatment assignments but were able to assess other important components of the hospitalisations.
Procedures
After participants were randomly assigned, their treating clinicians were asked to maintain the participant on the assigned treatment strategy unless an important clinical indication to change that strategy occurred before discharge (such as hypotension, severe uncontrolled hypertension despite treatment with other agents, hyperkalaemia, acute kidney injury, new onset heart failure with reduced ejection fraction). Clinicians were encouraged to temporarily use other antihypertensive classes that do not inhibit the renin–angiotensin system as needed for blood-pressure control among participants who were assigned to discontinuation of therapy.
Demographic and clinical data were collected at baseline from the participant and supplemented by the electronic health record. Clinical data, including the components of the Sequential Organ Failure Assessment (SOFA) score, medication administration, and adverse events, were updated daily by review of the electronic health record throughout the inpatient hospitalisation.
Outcomes
The primary endpoint was a global rank score in which each participant was ranked against all other participants across four hierarchies of clinical outcomes collected over the duration of the hospitalisation: (1) days to death during the hospitalisation (ranked lowest to highest); followed by (2) days on invasive mechanical ventilation or extracorporeal membrane oxygenation (ranked highest to lowest); followed by (3) days on renal replacement therapy or inotropic or vasopressor therapy (ranked highest to lowest); followed by (4) area under the curve (AUC) of a modified SOFA score (appendix 2 pp 12–13). The modified SOFA score incorporated the cardiac, respiratory, coagulation, and renal domains of the SOFA score (which were assessed consistently in all participants and were thought to be most relevant to renin–angiotensin system inhibition), and was weighted by duration of hospitalisation via computation of the AUC (which increases with every day of hospitalisation for any given mean modified SOFA score). Because arterial oxygen saturation (from arterial blood gas analyses) was not consistently available, we used peripheral capillary oxygen saturation (measured from pulse oximetry) instead of arterial oxygen saturation for computation of the modified SOFA score.18 The primary outcome was determined centrally by a statistician at the data coordinating centre after the masked adjudications were completed.
The secondary endpoints were time to all-cause death; length of hospital stay; length of intensive care unit stay, invasive mechanical ventilation, or extracorporeal membrane oxygenation (among those individuals requiring intensive care unit-level care or invasive mechanical ventilation); and AUC of the SOFA score (weighted to account for death and duration of hospitalisation). The exploratory endpoints reported here were time to intensive care unit admission or invasive mechanical ventilation; hypotension requiring vasopressors, inotropes, or mechanical haemodynamic support such as a ventricular assist device or intra-aortic balloon pump; and acute kidney injury during hospitalisation. The exploratory endpoints that will be reported elsewhere were the number of 28-day ventilator-free days (invasive or non-invasive); maximal change in NT-proB-type natriuretic peptide concentration from baseline; change in serum creatinine concentration between baseline and discharge or death; and proteinuria or haematuria (appendix 2 pp 3–4).
Serious adverse events were actively monitored and reported by investigators for the duration of hospitalisation and at 28 days following discharge. Detailed information is in appendix 2 (pp 4–5) regarding adverse event definitions and reporting. Post-hoc analyses were done evaluating several factors monitored for safety and adverse event reporting during hospitalisation (blood pressure, serum potassium concentration, serum creatinine concentration, additional antihypertensive medications given for hypertension management, and off-label and adjuvant treatments for COVID-19).
Statistical analysis
Using Monte Carlo simulations to apply likely distributions of participants across each of the four hierarchies of clinical outcomes based on published data at the initiation of the trial,1, 2 we estimated that the trial would have 80% power to observe a 25% difference in median global rank scores across the treatment groups at a sample size of 152 participants, accounting for α adjustment for interim analyses at 50% of enrolment using an O'Brien-Fleming-type spending function.19, 20
Analyses were done on an intention-to-treat basis using the total number of randomly assigned participants unless otherwise specified. For the primary analyses, we used the non-parametric Wilcoxon rank sum test to compare median global rank scores across treatment groups with two-sided testing. Prespecified secondary analyses of the primary endpoint used linear regression adjusted for age, sex, race or ethnicity, pre-existing heart failure, pre-existing chronic lung disease, and ACEI versus ARB therapy at baseline. Time-to-event outcomes were evaluated using Cox proportional hazards models starting at the time of enrolment and censored at the time of discharge. We assessed for violation of the proportional hazards assumption by statistical evaluation of Schoenfeld residuals and planned to incorporate a time-by-treatment interaction term if the assumption was violated. Kaplan–Meier curves were created for 28-day death and intensive care unit stay or mechanical ventilation, censored at the time of the endpoint or 28 days. Those analyses that did not include death as part of the time-to-event outcome addressed death as a competing risk. Effect modification was assessed in prespecified subgroups (age, sex, race or ethnicity, baseline ACEI vs ARB therapy, chronic kidney disease, diabetes, and body-mass index) for the primary endpoint and length of hospital stay using likelihood ratio testing. Post-hoc analyses of blood pressure and laboratory values (serum potassium concentration and serum creatinine concentration) monitored for the duration of hospitalisation were done using linear mixed-effects modelling with random slope and intercept to account for within-participant random effects, with an independent covariance structure. Multiple imputation with chained equations was used to address missing covariate data. Analyses were done using Stata version 16.1. The REPLACE COVID trial is registered with ClinicalTrials.gov, NCT04338009.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
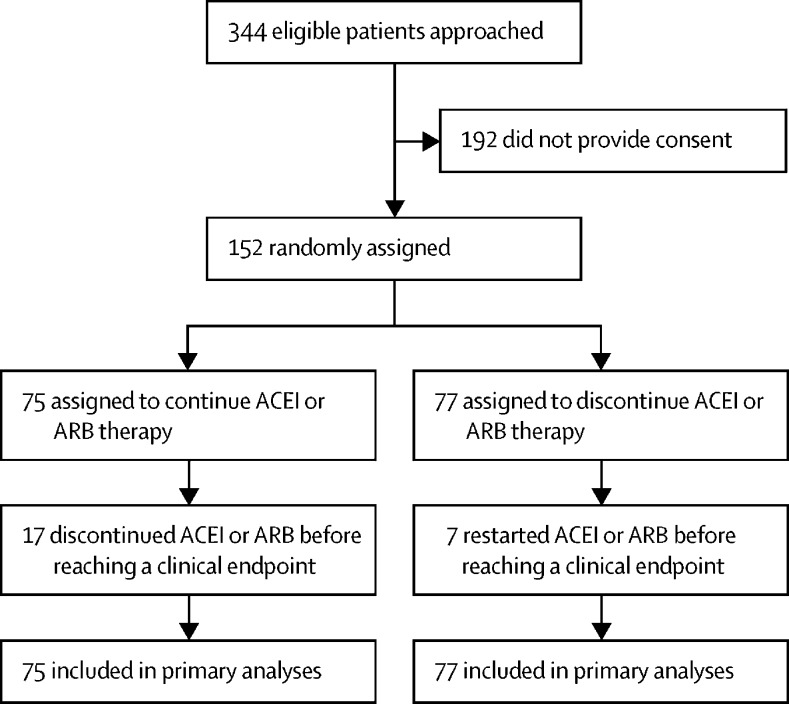
Between March 31 and Aug 20, 2020, 152 participants were enrolled. 75 participants were randomly assigned to continuation of ACEI or ARB therapy and 77 participants were randomly assigned to discontinuation of ACEI or ARB therapy and were included in the intention-to-treat analyses (figure 1 ). The median duration of hospitalisation, and thus randomly assigned treatment allocation, was 5 days (IQR 3–11). No participants were excluded or withdrawn following randomisation or lost to follow-up. Mean age of the 152 participants was 62 years (SD 12), 68 (45%) were female, mean body-mass index was 33 kg/m2 (SD 8), 79 (52%) had diabetes, 24 (16%) had existing cardiac disease, and all participants had a history of hypertension. 90 (59%) participants were enrolled in the USA (appendix 2 p 7). Baseline characteristics were generally similar across the two treatment groups (table 1 ), although ACEI therapy (as opposed to ARB) was slightly more common in the discontinuation group than in the continuation group. All participants were positive for SARS-CoV-2 by real-time PCR testing, except one participant in the continuation group who had a clinical presentation consistent with COVID-19 but there was limited availability of testing upon presentation and they died in the interim.
Figure 1.
Trial profile
ACEI=angiotensin-converting enzyme inhibitor. ARB=angiotensin receptor blocker.
Table 1.
Baseline characteristics
| Continuation of ACEI or ARB therapy (n=75) | Discontinuation of ACEI or ARB therapy (n=77) | ||
|---|---|---|---|
| Age, years | 62 (12) | 62 (12) | |
| Sex | |||
| Female | 33 (44%) | 35 (45%) | |
| Male | 42 (56%) | 42 (55%) | |
| Race or ethnicity | |||
| Non-Hispanic Black | 10 (13%) | 12 (16%) | |
| Non-Hispanic White | 12 (16%) | 11 (14%) | |
| Hispanic | 40 (53%) | 42 (55%) | |
| Other | 13 (17%) | 12 (16%) | |
| Hypertension | 75 (100%) | 77 (100%) | |
| ACEI therapy (as opposed to ARB)* | 25 (33%) | 38 (49%) | |
| Lowest recommended ACEI or ARB dose | 14 (18%) | 14 (18%) | |
| Calcium channel blocker therapy | 20 (27%) | 26 (34%) | |
| Diuretic therapy | 25 (33%) | 21 (27%) | |
| β blocker therapy | 11 (15%) | 14 (18%) | |
| Diabetes | 42 (56%) | 37 (48%) | |
| Insulin therapy | 20 (27%) | 16 (21%) | |
| Dyslipidaemia | 34 (45%) | 32 (42%) | |
| Pre-existing cardiac disease | 10 (13%) | 14 (18%) | |
| Ischaemic heart disease | 6 (8%) | 12 (16%) | |
| Heart failure | 3 (4%) | 3 (4%) | |
| Atrial fibrillation | 3 (4%) | 0 (0%) | |
| Previous pulmonary embolism or deep vein thrombosis | 1 (1%) | 3 (4%) | |
| Obstructive sleep apnoea | 7 (9%) | 10 (13%) | |
| Chronic pulmonary disease | 9 (12%) | 17 (22%) | |
| Current smoker | 5 (7%) | 8 (10%) | |
| Illicit drug use | 2 (3%) | 3 (4%) | |
| WHO COVID-19 disease severity on admission | |||
| Mild disease | 38 (51%) | 42 (55%) | |
| Moderate disease | 28 (37%) | 25 (33%) | |
| Severe disease | 9 (12%) | 10 (13%) | |
| Dyspnoea | 66 (88%) | 66 (86%) | |
| Cough | 59 (79%) | 58 (75%) | |
| Multifocal infiltrates on chest x-ray or CT | 48 (64%) | 43 (56%) | |
| Oxygen saturation, % | 92% (8) | 92% (5) | |
| Oxygen supplementation | 63 (84%) | 60 (78%) | |
| Systolic blood pressure, mm Hg | 129 (19) | 133 (22) | |
| Diastolic blood pressure, mm Hg | 75 (13) | 77 (12) | |
| Heart rate, beats per min | 91 (16) | 92 (17) | |
| Body-mass index, kg/m2 | 33 (7) | 33 (9) | |
| eGFR, mL/min/1·73 m2 | 83 (23) | 81 (25) | |
| Serum potassium, mmol/L | 4·0 (0·5) | 4·0 (0·5) | |
| Leukocyte count, 109 cells/L | 9·3 (4·3) | 8·9 (4·5) | |
| Platelets, 103 cells/μL | 239 (109) | 238 (130) | |
| C-reactive protein, mg/dL | 48 (68) | 45 (77) | |
| Days from admission to randomisation | 1·6 (0·9) | 1·5 (0·5) | |
| Days from symptom onset to randomisation | 6·5 (2·3) | 6·8 (2·5) | |
Data are mean (SD) or n (%). ACEI=angiotensin-converting enzyme inhibitor. ARB=angiotensin receptor blocker. eGFR=estimated glomerular filtration rate (calculated using the chronic kidney disease epidemiology consortium equation).
The use of ACEI therapy was significantly different between the groups (p=0·05).
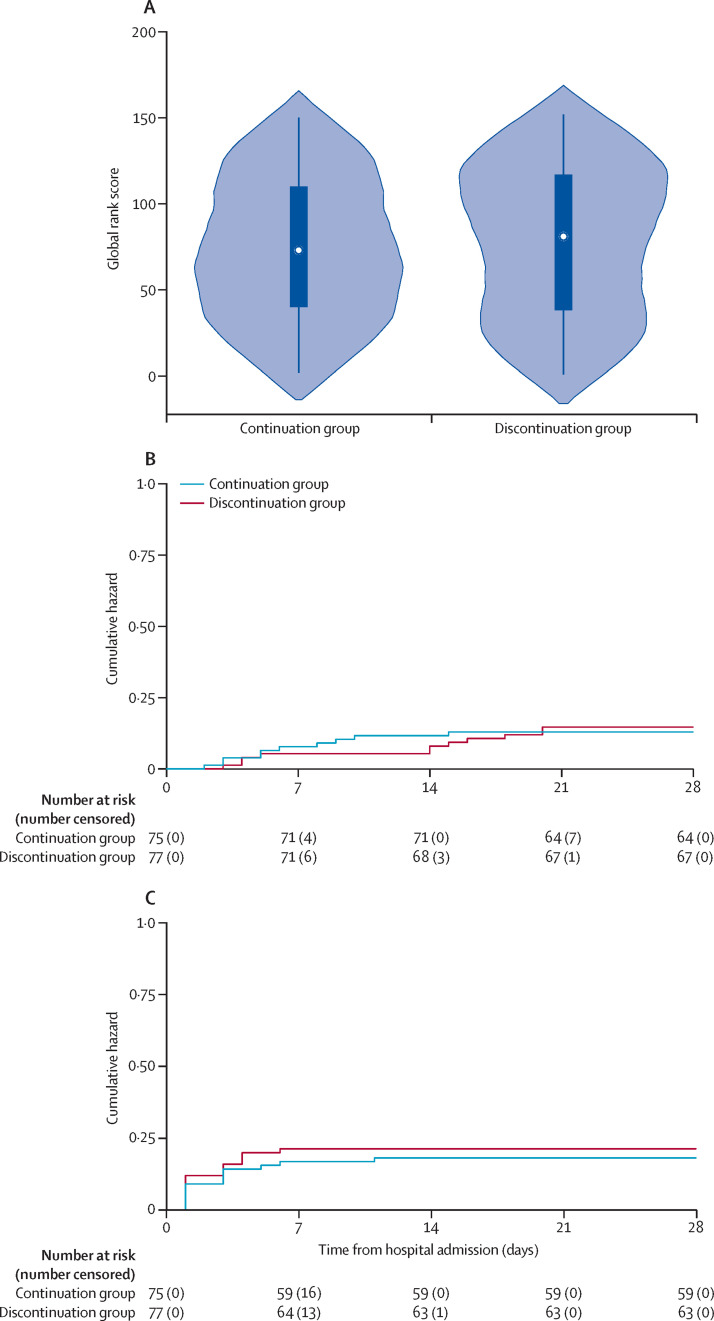
In the primary intention-to-treat analyses, there was no difference in the global rank scores between participants assigned to continuation compared with discontinuation of their ACEI or ARB therapy (a lower rank score signifies more severe COVID-19 hospitalisation; median rank score 73 [IQR 40–110] in the continuation group vs 81 [38–117] in the discontinuation group; p=0·61; table 2 and figure 2A ). The results were corroborated in prespecified analyses adjusted for age, sex, race or ethnicity, pre-existing heart failure, pre-existing chronic lung disease, and ACEI versus ARB therapy at baseline (β-coefficient 8 [95% CI −13 to 29]).
Table 2.
Primary, secondary, and exploratory endpoints
| Continuation of ACEI or ARB therapy (n=75) | Discontinuation of ACEI or ARB therapy (n=77) | Treatment effect*(95% CI) | p value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Global rank score | 73 (40 to 110) | 81 (38 to 117) | 8 (−13 to 29) | 0·61 |
| Secondary endpoints | ||||
| All-cause death | 11 (15%) | 10 (13%) | 1·00 (0·42 to 2·36) | 0·99 |
| Length of hospitalisation, days | 6 (3 to 11) | 5 (3 to 10) | −1 (−4 to 2) | 0·56 |
| Length of intensive care unit stay or invasive mechanical ventilation, days | 13 (6 to 17) | 15 (6 to 27) | 2 (−12 to 178) | 0·59 |
| Area under of the curve of the SOFA score adjusted for death | 12 (3 to 23) | 7 (2 to 20) | −4 (−13 to 5) | 0·38 |
| Exploratory endpoints | ||||
| Intensive care unit admission or invasive mechanical ventilation | 16 (21%) | 14 (18%) | 0·84 (0·43 to 1·66) | 0·61 |
| Hypotension requiring haemodynamic support | 9 (12%) | 8 (10%) | 0·86 (0·34 to 2·17) | 0·74 |
Data are median (IQR) or n (%) unless otherwise specified. ACEI=angiotensin-converting enzyme inhibitor. ARB=angiotensin receptor blocker. SOFA=Sequential Organ Failure Assessment.
For continuous outcomes, the treatment effect is the β-coefficient from unadjusted regression analyses except for the primary endpoint analysis, which was adjusted for age, sex, race or ethnicity, pre-existing heart failure, pre-existing chronic lung disease, and ACEI versus ARB therapy at baseline; for binary outcomes, the treatment effect is the hazard ratio. For binary outcomes other than death, death was addressed as a competing risk. Median length of intensive care unit stay or invasive mechanical ventilation was only calculated among those individuals who were transferred to the intensive care unit or required mechanical ventilation.
Figure 2.
Outcomes for the primary endpoint, all-cause death, and intensive care unit admission or invasive mechanical ventilation
(A) The distribution of the primary endpoint (hierarchical rank score) in the continuation and discontinuation groups. The x-axis (and shaded area) shows the frequency density of rank distributions in each treatment group, the white dots show the median global rank score, the solid boxes show the IQR, and the vertical lines show the upper-adjacent and lower-adjacent values. (B) The cumulative hazard for all-cause death. (C) The cumulative hazard for intensive care unit admission or invasive mechanical ventilation.
21 patients died during the study (table 2; figure 2B). Causes of death are summarised in appendix 2 (p 8). Compared with discontinuing ACEI or ARB therapy, participants who continued therapy had a similar length of hospital stay, length of intensive care unit stay or invasive mechanical ventilation, and AUC of the SOFA score.
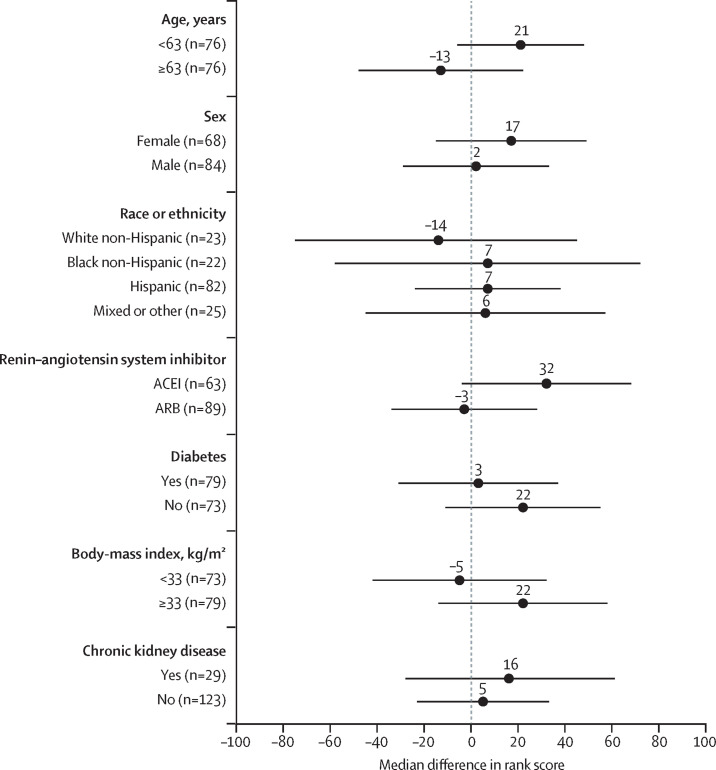
There was no effect modification by age, sex, race, baseline ACEI versus ARB therapy, chronic kidney disease, diabetes, or body-mass index for the primary endpoint or length of hospitalisation (figure 3 ).
Figure 3.
Forest plot of the differences in rank scores across subgroups
Positive values indicate better outcomes in the discontinuation group. The p value for all likelihood ratio tests for effect modification were >0·05. The dots represent the differences in median rank scores between participants in the continuation group versus the discontinuation group in each subgroup. The bars show the 95% CIs. For the figure, continuous variables (age and body-mass index) were stratified at the median value for the study population. ACEI=angiotensin-converting enzyme inhibitor. ARB=angiotensin receptor blocker.
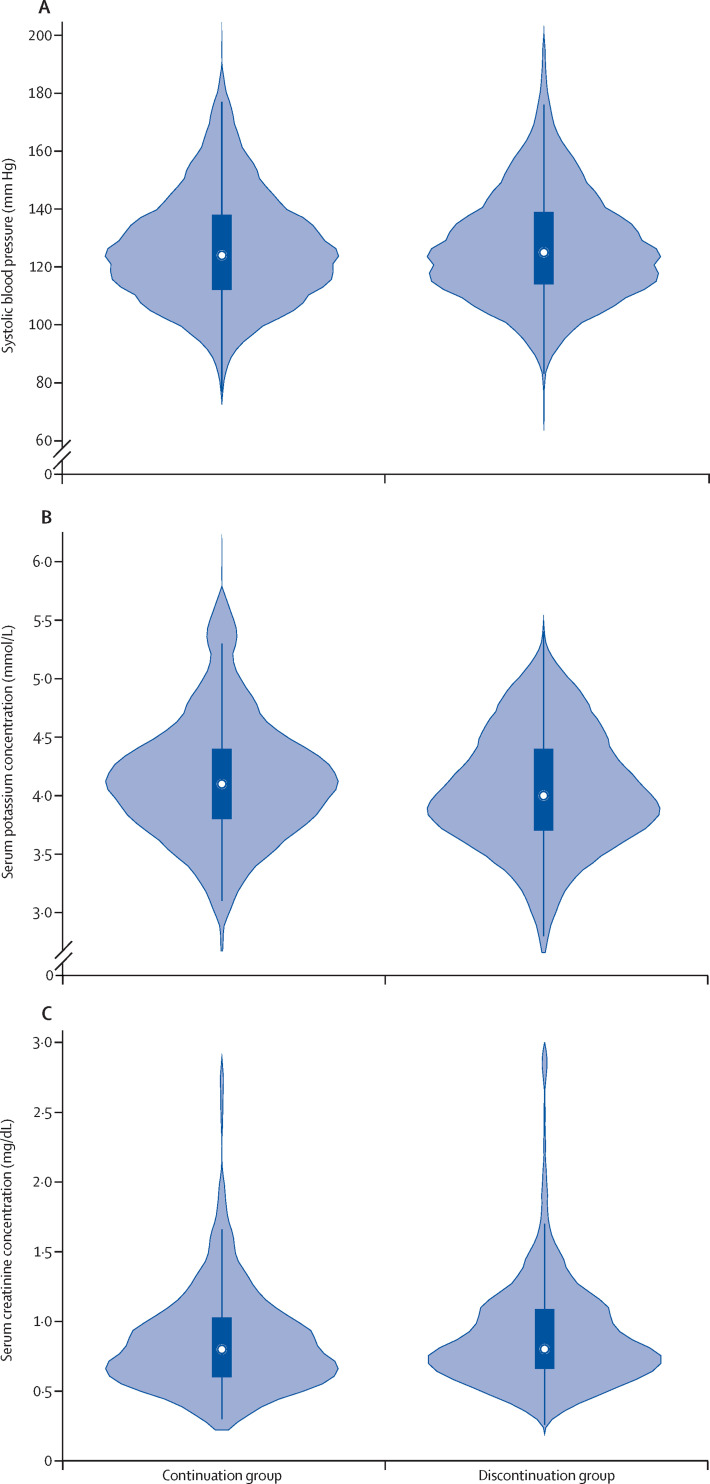
30 participants were admitted to an intensive care unit or had invasive mechanical ventilation and the risk was not significantly different between the groups (table 2; figure 2C). 17 individuals had hypotension requiring haemodynamic support (table 2). There was no difference in systolic blood pressure (mean systolic blood pressure 126·1 mm Hg in the continuation group vs 128·6 mm Hg in the discontinuation group; mean difference 2·5 mm Hg [95% CI −1·2 to 6·2]; figure 4A ), serum potassium concentration (mean potassium concentration 4·1 mmol/L in the continuation group vs 4·0 mmol/L in the discontinuation group; mean difference −0·1 mmol/L [–0·3 to 0·1]; figure 4B), or serum creatinine concentration (mean creatinine concentration 0·8 mg/dL in the continuation group vs 0·9 mg/dL in the discontinuation group; mean difference 0·1 mg/dL [–0·1 to 0·1]; figure 4C) across the two groups during follow-up, with similar results after censoring at the time of crossover (mean difference between the groups in systolic blood pressure 1·9 mm Hg [–1·9 to 5·8]; serum potassium −0·1 mmol/L [–0·2 to 0·1]; serum creatinine 0·1 mg/dL [–0·1 to 0·2]). 12 (16%) of 77 participants in the discontinuation group and no participants in the continuation group were given a calcium channel blocker, a β blocker, or both at least once during their hospitalisation for additional antihypertensive management. Three (4%) of 75 participants in the continuation group and three (4%) of 77 participants in the discontinuation group had acute kidney injury (defined as an increase of >2 times in creatinine concentration from admission; χ2 test p=0·97). There was no difference in the use of off-label and adjuvant treatments for COVID-19, such as high-dose corticosteroids and convalescent plasma, between the two groups during follow-up (appendix 2 p 11).
Figure 4.
Blood pressure, serum potassium concentration, and serum creatinine concentration during follow-up
(A) The distribution of systolic blood pressure values during follow-up. (B) The distribution of serum potassium concentrations during follow-up. (C) The distribution of serum creatinine concentrations during follow-up. For all three panels, the x-axis (and shaded area) represents the frequency density of the parameter in each treatment group, the white dot shows the median value of the parameter, the solid box shows the IQR, and the vertical lines show the upper-adjacent and lower-adjacent values.
29 (39%) participants in the continuation group and 28 (36%) participants in the discontinuation group had at least one adverse event (χ2 test of adverse events between treatment groups p=0·77). Adverse events are summarised in appendix 2 (p 9).
24 participants crossed over between treatment assignments (17 assigned to the continuation group and seven assigned to the discontinuation group). Among participants who crossed over between treatment assignments, the median time to crossing over after enrolment was 5 days (IQR 3–7) in the continuation group and 5 days (3–6) in the discontinuation group. In the continuation group, discontinuation of ACEIs or ARBs occurred in 11 participants due to hypotension, two participants due to hyperkalaemia, and four participants at the clinical discretion of the care provider (eg, due to poor oral intake with concern for volume depletion). In the discontinuation group, re-initiation of ACEIs or ARBs occurred in six participants due to hypertension and one participant due to acutely worsening heart failure. In sensitivity analyses in which participants were censored at the time of crossover, no significant differences in study endpoints were observed between treatment groups (appendix 2 p 10).
Discussion
Continuation of ACEI or ARB therapy among patients admitted to hospital with COVID-19 had no overall effect on severity of COVID-19 disease course. We observed no significant difference in the primary hierarchical endpoint between participants who continued their ACEI or ARB therapy compared with those who discontinued therapy. Secondary and subgroup analyses were otherwise consistent with our primary endpoint, showing no difference in length of COVID-19 hospitalisation, need for intensive care, invasive mechanical ventilation, or death between treatment groups. Multiorgan dysfunction, as depicted by the AUC of the modified SOFA score, was also similar across treatment groups. Furthermore, we observed no difference in blood pressure control, serum potassium, or serum creatinine over the course of follow-up among patients whose ACEI or ARB therapy was continued versus discontinued.
We selected the global hierarchical rank score for our primary endpoint because it incorporates biomarkers and important clinical events into an integrated metric in which participants are ranked by the severity of their disease course.21, 22 Our hierarchical global rank score incorporates information about each of the highest-priority events in COVID-19, but allows these events to be prioritised. For instance, the principal outcome of interest is death, but even if there is no difference in rate of death across treatment groups, we would still be interested in a shorter duration of invasive respiratory support. Accordingly, individuals in the study were directly contrasted with one another and ranked according to their time to death, duration of invasive mechanical ventilation, duration of renal replacement therapy or vasopressors, multiorgan failure, and duration of hospitalisation. Given the urgency of understanding factors that influence COVID-19-related outcomes while infection rates continue to increase in many countries, the global rank score benefits from considerably higher statistical power compared with other commonly used approaches (such as 28-day ventilator-free days, time-to-death, and the WHO COVID-19 ordinal endpoint; appendix 2 p 14), allowing for greater efficiency in trial implementation.23, 24, 25, 26 It also provides insights into a combination of factors that are important during a global pandemic, including patient-centred hospitalisation outcomes and health-resource use.23, 24, 25, 26
In designing the trial, we considered that there was clinical equipoise regarding continuation versus discontinuation of ACEI or ARB therapy in patients with COVID-19. The reason for this assessment was the existence of insufficient information on the effects of renin–angiotensin system inhibition on ACE2 expression, and on the effect of ACE2 expression on COVID-19-related outcomes. Experimental animal models have shown that ACEIs and ARBs increase ACE2 expression in several organs,7, 8, 9, 10 which could theoretically amplify the capacity of SARS-CoV-2 to enter cells in the lungs and other organs, thus increasing the risk of acute respiratory distress syndrome, multiorgan dysfunction, and death.5 However, several studies have shown no difference in ACE2 expression and activity caused by ACEI or ARB therapy in either animals11 or humans.27, 28 Furthermore, experimental animal models of SARS-CoV-1 have shown that increased ACE2 expression might protect against acute lung injury.29 These studies suggest that ACEIs and ARBs enhance host defence and mitigate the hyperinflammatory response to SARS-CoV-2, resulting in a reduction in short-term target organ injury in addition to the known long-term benefits of these medications in the heart and kidneys.29 Accordingly, several ongoing randomised trials (eg, NCT04335786, NCT04311177, NCT04328012) are evaluating the effect of de novo introduction of ACEI or ARB therapy versus placebo for the treatment of COVID-19.
The results of this trial contribute novel information to our knowledge of the effects of continuing versus discontinuing ACEI and ARB therapy in patients with COVID-19. Our findings, derived from a prospective, multicentre, randomised, controlled design, are consistent with previously published observational studies and unpublished trial evidence, which have generally shown no difference in the risk of SARS-CoV-2 infection and COVID-19 severity among patients who are treated with ACEIs or ARBs compared with those who are not.13, 14, 15, 16, 30 For example, in a case-control study of 6272 patients with COVID-19 compared with 30 759 controls, Mancia and colleagues14 observed no association of ACEI or ARB use with a positive SARS-CoV-2 test (ACEI adjusted odds ratio [OR] 0·96 [95% CI 0·87–1·07]; ARB adjusted OR 0·95 [0·86–1·05]) or COVID-19 disease severity (ACEI adjusted OR 0·91 [0·69–1·21]; ARB adjusted OR 0·83 [0·63–1·10]). Similarly, in a retrospective, propensity score-matched, cohort study of 12 594 patients tested for SARS-CoV-2, Reynolds and colleagues15 found no association of ACEI or ARB use with likelihood of SARS-CoV-2 test positivity or COVID-19 severity. The authors of many of the existing observational studies took important steps to minimise the effects of bias and confounding; however, these studies were unable to draw causal inferences about the relationship between ACEI or ARB use and COVID-19-related outcomes, and could not assess inpatient exposure to ACEI or ARB therapy following the onset of SARS-CoV-2 infection.3
Similar to the current trial, unpublished results from the BRACE CORONA trial, a pragmatic, registry-based trial of 659 participants admitted to hospital for COVID-19 in Brazil, show no difference in days alive and out of the hospital at 30 days (mean ratio 0·95 [95% CI 0·90–1·01]) or risk of death (nine deaths in each treatment group; HR 0·97 [95% CI 0·38–2·52]) among participants randomly assigned to continue versus discontinue ACEI or ARB therapy.30 The BRACE CORONA trial was characterised by a single-country design and a notably younger (mean age 55 years) and less comorbid (33% with diabetes; 5% with cardiovascular diseases) patient population than the current study, with fewer total deaths despite the larger sample size (3% mortality in the BRACE CORONA trial vs 14% mortality in our study).30 Thus, the participants in the BRACE CORONA trial might not be fully generalisable to patients typically admitted to hospital with COVID-19 and hypertension globally.1, 2, 3, 5, 13, 14, 15, 16 Additionally, 11% of participants enrolled in the BRACE CORONA trial were excluded after randomisation due to protocol deviations, absence of informed consent, or Good Clinical Practice violation.30, 31
Strengths of our study include that, to our knowledge, it is the only registered, multicentre, international trial aiming to evaluate continuation versus discontinuation of ACEI or ARB use in patients with COVID-19. Participants were recruited across low-resource and high-resource settings and had clinical characteristics similar to those reported in most studies of hospitalised patients with COVID-19.1, 2, 13, 14, 15, 16 Thus, the results are likely to be widely generalisable to patients on ACEIs or ARBs for the management of hypertension. Of note, due to concerns surrounding the safety of discontinuing ACEIs or ARBs in patients with heart failure with reduced ejection fraction, these individuals were excluded from the trial. The trial was pragmatic, applying clinician implementation of the treatment strategy during routine inpatient care, further supporting the generalisability of the findings. Our novel hierarchical endpoint provides sufficient statistical power to evaluate the study question with a smaller sample size than is required for typical binary endpoints and offers greater insight into the breadth of COVID-19-related adverse outcomes (ie, time to death; duration of mechanical ventilation, renal replacement therapy, vasopressor therapy, and hospitalisation; and severity of multiorgan dysfunction using information available in low-resource settings) than other commonly used endpoints.23, 24, 25, 26
Limitations of the study include the small sample size, which limits the ability to draw conclusions from the secondary outcomes. Nonetheless, relative to the sample size, we observed a large number of deaths (n=21) and participants requiring intensive care unit admission or invasive mechanical ventilation (n=30), determined by masked outcome adjudication. These findings are consistent with other trials of COVID-19 interventions among patients admitted to hospital, and facilitate adequate statistical power to evaluate our prespecified primary endpoint despite the small sample size.25 Additionally, our randomisation scheme achieved successful balance of baseline covariates across the assigned groups except with respect to previous use of ACEI (as opposed to ARB) therapy, which was higher in the discontinuation group (49% vs 33% in the continuation group). Nonetheless, our protocol had prespecified analyses planning to adjust for ACEI versus ARB use, which showed no difference in the overall findings. Furthermore, we observed no effect modification by ACEI versus ARB use. Experimental data in animals suggest that ACEIs and ARBs have differential effects on ACE2 expression and activity,7 although this might not be consistent across organs.11 Although it is possible that differences in their effect on ACE2 could cause ACEIs and ARBs to impose distinctive effects on COVID-19-related outcomes, our analyses suggest no meaningful differences in effect across these two classes. Additional limitations to consider are inherent limitations of the pragmatic prospective, randomised, open-label, blinded endpoint design. We did not control participant ACEI or ARB dosing or other medication exposures during the trial, although we observed that ACEI and ARB dosing as well as off-label and adjuvant COVID-19 therapies administered during follow-up were similar across the two groups. Also, although outcome adjudicators were masked to the randomly assigned groups when determining clinical endpoints, providers caring for the patients were aware of the group the patient was assigned to. It is possible that the open-label nature of the study might have introduced information bias or influenced provider behaviour. Nonetheless, this pragmatic approach enhances the generalisability of the findings to routine patient care.
In conclusion, among patients admitted to hospital with COVID-19, continuation and discontinuation of renin–angiotensin system inhibitors have similar effects on acute hospitalisation outcomes. Consistent with current international society recommendations, providers should continue to prescribe these medications in patients admitted to hospital with COVID-19 unless there is a distinct medical contraindication to ongoing therapy. Ongoing trials will determine whether de novo use of these medications is effective for the treatment of COVID-19.
Data sharing
De-identified data collected during the conduct of the trial, including anonymised individual participant data, and study documents will be made available for collaborative analyses upon request from the time of publication, after the execution of appropriate data sharing agreements. Proposals should be directed to the REPLACE COVID trial data coordinating centre (JBC: jco@pennmedicine.upenn.edu). Data will be provided electronically after review and approval of research proposals by the trial data coordinating centre and enrolment site investigators, as allowed by existing local regulations and data sharing agreements.
Acknowledgments
Acknowledgments
We thank the members of the Data Safety Monitoring Board for their oversight of the trial: John Younger; Raymond R Townsend; Gustavo A Heresi; Todd A Miano; and Jesse Y Hsu. We also thank the following individuals for their invaluable contributions to the trial: Jaime Heier; Jessica Koshinski; Caroline Margo; Bianca Pourmussa; Candy Greczylo; Michael Harhay; Zeba Hashmath; Alisha Jamil; Ashwin Murthy; Lauren Boccagno; Ghazal Quinn; Ira Blau; Jordan Shaffer; Esther Pak; Sarah Ahmad; Sobeyda Lizzette Cruz; Rosalina Quiroga; John A Stroster; Saul A Garcia; Sara Salce; Bijin Thajudeen; Sanjay Polisetty; Lani Lucente Demchak; Vivek Bhalla; Andrea Berrido; Kavita Shah; Cynthia Redd; Matthew Sinclair; Barbara Lang; Adedapo Iluyomade; Maria Delgado-Lelievre; Kevin Gregg; Monica Grilli; Cecilia Solchaga; and those individuals who contributed to the REPLACE COVID Trial Social Fundraising Campaign. This trial was sponsored by the investigators from the various enrolment centres; the REPLACE COVID Trial Social Fundraising Campaign supported a portion of enrolment at the University of Pennsylvania (Philadelphia, PA, USA); FastGrants supported enrolment at the University of Michigan (Ann Arbor, MI, USA). Several US investigators have National Institutes of Health funding for research effort outside of this work. JBC has received grants NIH-NHLBI K23-HL133843 and R01-HL153646; TCH has received grant NIH-NHLBI T32-HL007891; JAC has received grants NIH-NHLBI R01-HL 121510-01A1, R61-HL-146390, 1R01-HL104106, P01-HL094307, R03-HL146874-01, R56-HL136730, and NIH-NIA R01-AG058969; CRV has received grant NIH-NIDDK T32-DK07785; and JBB has received a FastGrant from Emergent Ventures; none of these funding sources had a role in the conduct of the study.
Contributors
Study conception and design was done by JBC, TCH, and JAC. Data acquisition was done by all authors. The statistical analyses were done by JBC, TCH, and JC. Interpretation of the analysis was done by JBC and JAC. JC and JBC accessed and verified the data. All authors contributed important intellectual content during manuscript drafting and revision.
Declaration of interests
In the past 2 years, JAC has received consulting honoraria from Sanifit, Bristol Myers Squibb, Edwards Lifesciences, Bayer, and Johnson & Johnson; research grants from the National Institutes of Health, Microsoft, Fukuda-Denshi, and Bristol Myers Squibb; compensation from the American Heart Association and the American College of Cardiology for editorial roles; and visiting speaker honoraria from Washington University and University of Utah, all outside of the submitted work. JS has received speaker honoraria from and is on the advisory boards for AstraZeneca, ViforPharma, and NovoNordisk, and has received speaker honoraria from AMGEN, all outside of the submitted work. JBB has received research grants from FastGrants for this study, as well as from the National Institutes of Health, outside of the submitted work. TIC has received funding paid by Janssen Pharmaceuticals to Stanford University; has served as a consultant for Bayer, Janssen Pharmaceuticals, Novo Nordisk, Fresenius Medical Care, Tricida, Gilead, and AstraZeneca; and has received grant support from Satellite Healthcare, the American Heart Association, and the National Institutes of Health, all outside of the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between COVID-19 mortality and the renin-angiotensin system? A call for epidemiologic investigations. Clin Infect Dis. 2020;71:870–874. doi: 10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. doi: 10.1016/j.cell.2020.02.052. 80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoufaly A, Poglitsch M, Aberle JH, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 8.Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. [DOI] [PubMed] [Google Scholar]
- 9.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 10.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 11.Wysocki J, Lores E, Ye M, Soler MJ, Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: implications for COVID-19. J Am Soc Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the national health and nutrition examination survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 13.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JB, Hanff TC, Corrales-Medina V, et al. Randomized elimination and prolongation of ACE inhibitors and ARBs in coronavirus 2019 (REPLACE COVID) trial protocol. J Clin Hypertens. 2020;22:1780–1788. doi: 10.1111/jch.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grissom CK, Brown SM, Kuttler KG, et al. A modified sequential organ failure assessment score for critical care triage. Disaster Med Public Health Prep. 2010;4:277–284. doi: 10.1001/dmp.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julious SA. Sample sizes for clinical trials with normal data. Stat Med. 2004;23:1921–1986. doi: 10.1002/sim.1783. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 21.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circ Heart Fail. 2010;3:643–646. doi: 10.1161/CIRCHEARTFAILURE.109.926030. [DOI] [PubMed] [Google Scholar]
- 22.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- 24.Harhay MN, Hill AS, Wang W, et al. Measures of global health status on dialysis signal early rehospitalization risk after kidney transplantation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao B, Wang Y, Wen D, et al. A Trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson RL, Vock DM, Babiker A, et al. Comparison of an ordinal endpoint to time-to-event, longitudinal, and binary endpoints for use in evaluating treatments for severe influenza requiring hospitalization. Contemp Clin Trials Commun. 2019;15 doi: 10.1016/j.conctc.2019.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 28.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes R. BRACE CORONA: continuing vs suspending ACE inhibitors and ARBs in COVID-19. Oral presentation. European Society of Cardiology Congress 2020; online; Sept 1, 2020.
- 31.Lopes RD, Macedo AVS, de Barros E Silva PGM, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—the BRACE CORONA trial. Am Heart J. 2020;226:49–59. doi: 10.1016/j.ahj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data collected during the conduct of the trial, including anonymised individual participant data, and study documents will be made available for collaborative analyses upon request from the time of publication, after the execution of appropriate data sharing agreements. Proposals should be directed to the REPLACE COVID trial data coordinating centre (JBC: jco@pennmedicine.upenn.edu). Data will be provided electronically after review and approval of research proposals by the trial data coordinating centre and enrolment site investigators, as allowed by existing local regulations and data sharing agreements.