Abstract
Background
Psychiatric morbidities have been associated with a risk of severe infections through compromised immunity, health behaviours, or both. However, data are scarce on the association between multiple types of pre-pandemic psychiatric disorders and COVID-19. We aimed to assess the association between pre-pandemic psychiatric disorders and the subsequent risk of COVID-19 using UK Biobank.
Methods
For this cohort analysis, we included participants from UK Biobank who were registered in England and excluded individuals who died before Jan 31, 2020, (the start of the COVID-19 outbreak in the UK) or had withdrawn from UK Biobank. Participants diagnosed with a psychiatric disorder before Jan 31 were included in the group of individuals with pre-pandemic psychiatric disorders, whereas participants without a diagnosis before the outbreak were included in the group of individuals without pre-pandemic psychiatric disorders. We used the Public Health England dataset, UK Biobank hospital data, and death registers to collect data on COVID-19 cases. To examine the relationship between pre-pandemic psychiatric disorders and susceptibility to COVID-19, we used logistic regression models to estimate odds ratios (ORs), controlling for multiple confounders and somatic comorbidities. Key outcomes were all COVID-19, COVID-19 specifically diagnosed in inpatient care, and COVID-19-related deaths. ORs were also estimated separately for each psychiatric disorder and on the basis of the number of pre-pandemic psychiatric disorders. As a positive disease control, we repeated analyses for hospitalisation for other infections.
Findings
We included 421 014 UK Biobank participants in our study and assessed their COVID-19 status between Jan 31 and July 26, 2020. 50 809 participants were diagnosed with psychiatric disorders before the outbreak, while 370 205 participants had no psychiatric disorders. The mean age at outbreak was 67·80 years (SD 8·12). We observed an elevated risk of COVID-19 among individuals with pre-pandemic psychiatric disorders compared with that of individuals without such conditions. The fully adjusted ORs were 1·44 (95% CI 1·28–1·62) for All COVID-19 cases, 1·55 (1·34–1·78) for Inpatient COVID-19 cases, and 2·03 (1·59–2·59) for COVID-19-related deaths. We observed excess risk, defined as risk that increased with the number of pre-pandemic psychiatric disorders, across all diagnostic categories of pre-pandemic psychiatric disorders. We also observed an association between psychiatric disorders and elevated risk of hospitalisation due to other infections (OR 1·74, 95% CI 1·58–1·93).
Interpretation
Our findings suggest that pre-existing psychiatric disorders are associated with an increased risk of COVID-19. These findings underscore the need for surveillance of and care for populations with pre-existing psychiatric disorders during the COVID-19 pandemic.
Funding
National Natural Science Foundation of China.
Introduction
The 2020 COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is posing an unprecedented crisis worldwide.1 Given its rapid transmission and the substantial proportion of severe cases needing intensive medical care, this new and potent infectious disease has put major pressure on the global health-care system and the general public. According to WHO, SARS-CoV-2 has spread to over 200 countries, infected over 36 million people, and led to more than one million deaths as of Oct 8, 2020,2 and the numbers keep increasing.
Psychiatric morbidities, such as depression, anxiety, and stress-related disorders, have been associated with an elevated risk of various somatic diseases, including autoimmune diseases,3 respiratory diseases,4 and severe infections,5 possibly due to altered immune responses.6 Therefore, it is plausible that a pre-existing psychiatric disorder, diagnosed before the pandemic, might also alter individual susceptibility to COVID-19. Emerging evidence has shown a strong genetic association between having at least one psychiatric diagnosis and the occurrence of infections, which lends additional support to this notion.7 Suboptimal behaviours (eg, smoking)8 and socioeconomic status (eg, deprivation)9 among individuals with psychiatric disorders might also contribute to an increased vulnerability to COVID-19. Indeed, several COVID-19 studies, most of which used self-reported data or moderate sample sizes, have shown that unhealthy lifestyles,10 psychosocial factors (including self-reported psychological distress),11 and certain psychiatric disorders (ie, depression12, substance abuse and schizophrenia13) might increase the risk of COVID-19.
Research in context.
Evidence before this study
Psychiatric morbidities have been associated with a risk of severe infections through compromised immunity, health behaviours, or both. Hence, it is plausible that past psychiatric problems can alter a person's susceptibility to COVID-19. We searched PubMed up to Aug 10, 2020, with no language restrictions, using the search terms: ((“COVID-19” or “SARS-nCoV-2” or “novel coronavirus”) and (“psychiatric disorder” or “mental illness” or “depression” or “anxiety” or “stress disorder” or “substance misuse” or “psychotic disorders”) and (“cohort” or “longitudinal”)). The search yielded 77 records, including five eligible prospective cohort studies, two of which observed an elevated risk of hospitalisation for COVID-19 among individuals with self-reported psychological problems, such as psychological distress. Similarly, the other three studies reported associations between selected psychiatric disorders (depression and schizophrenia) and the risk of COVID-19. However, we did not find any comprehensive assessment of the role of multiple types of clinically confirmed pre-pandemic psychiatric disorders on susceptibility to COVID-19, and little knowledge has been obtained about the possible underlying mechanism linking pre-pandemic psychiatric disorders to COVID-19.
Added value of this study
To the best of our knowledge, this is the first study, which used the large community-based dataset in the UK Biobank, to provide evidence of an increased risk of COVID-19, especially severe and fatal COVID-19, among individuals with clinically confirmed pre-pandemic diagnoses of psychiatric disorders, independent of many confounders. A similar association was observed between pre-pandemic psychiatric disorders and hospitalisation due to other infections during the COVID-19 outbreak, suggesting a shared pathway between psychiatric disorders and different infections, which might involve altered immune responses.
Implications of all the available evidence
These findings underscore the need for surveillance of and care for populations with pre-existing psychiatric disorders during the COVID-19 pandemic.
Yet, to our knowledge, no comprehensive assessment of the role of multiple types of clinically confirmed pre-pandemic psychiatric disorders on COVID-19 susceptibility has been done to date using longitudinal data.
Taking advantage of the rich information on phenotypes relevant to psychiatric disorders, sociodemographic, socioeconomic, and lifestyle factors and the continuously updated data on COVID-19 infection in the UK Biobank, we aimed to determine the association between pre-pandemic psychiatric disorders and the subsequent risk of COVID-19.
Methods
Study design
The UK Biobank is a prospective cohort study of 502 507 middle-aged (40–69 years) participants from England, Scotland, and Wales who were recruited between 2006 and 2010. Information about socio-demographic characteristics, lifestyle, and health-related factors was collected at recruitment. Health-related outcomes were obtained through periodically linked data from multiple national datasets, with the participants' consent.14 Inpatient hospital data were mapped across England, Scotland, and Wales based on Hospital Episode Statistics (HES) in England, the Scottish Morbidity Record, and the Patient Episode Database for Wales. Data on mortality are updated from the National Health Service (NHS) Digital for participants in England and Wales and from the NHS Central Register for participants in Scotland.15 After the global outbreak of COVID-19, the UK Biobank has also been linked to Public Health England (PHE), where results of COVID-19 tests done by RT-PCR (RdRp gene assay) from oral swabs were documented.16 For this cohort analysis, we only included UK Biobank participants who were registered in England because no data on COVID-19 tests were available for participants in Scotland or Wales.
Although only participants registered in England were included, we used all available linked data to retrieve information for individuals with records in multiple datasets. We excluded individuals who died before Jan 31, 2020 (ie, when the first COVID-19 case was diagnosed in the UK) or had withdrawn from the UK Biobank. Individuals with a clinical diagnosis of a psychiatric disorder before Jan 31 (the start of the COVID-19 outbreak in the UK) were included in the group of individuals with pre-pandemic psychiatric disorders, whereas participants without a diagnosis before the outbreak were included in the group without pre-pandemic psychiatric disorders.
All the UK Biobank participants gave written informed consent before data collection. The UK Biobank has full ethical approval from the NHS National Research Ethics Service (16/NW/0274), and this study was approved by the biomedical research ethics committee of West China Hospital (2020.661).
Pre-pandemic psychiatric disorders
We retrieved information about the diagnoses of psychiatric disorders from the UK Biobank inpatient hospital data, available since 1981.15 A pre-pandemic psychiatric disorder was defined as any hospital admission before Jan 31, 2020, with a diagnosis of a psychiatric disorder, including depression (according to the International Classification of Diseases [ICD] 10th edition [ICD-10] codes F32–F33; ICD 9th edition [ICD-9] codes 296.1, 300.4, and 311), anxiety (ICD-10 F40–F41; ICD-9 300.0 and 300.2), stress-related disorder (ICD-10 F43; ICD-9 308 and 309), substance misuse (ICD-10 F10–F19; ICD-9 291 and 303–305), and psychotic disorders (ICD-10 F20–F29; ICD-9 295, 297, and 298). The administrative data for psychiatric diagnoses, from HES in England and the equivalent datasets in Scotland and Wales, have been validated against detailed clinical evaluations, showing a good validity for some psychiatric diagnoses, especially psychotic categories (positive predictive value 73–80%) and depression (about 75%),17, 18 but less robust validity for anxiety and substance use (<60%).17
To capture individuals with less severe psychiatric disorders, we also analysed a subgroup of the study population with available primary care data, which were obtained from multiple data suppliers, including the Phoenix Partnership and Egton Medical Information Systems.15 For this analysis, we additionally defined a pre-pandemic psychiatric disorder as any primary care visit with a diagnosis of the previously mentioned psychiatric disorders.
Ascertainment of COVID-19
The ascertainment of COVID-19 mainly relied on the PHE dataset, which contained information about the specimen date, origin (inpatient or not), and results (positive or negative) of all COVID-19 tests done in England between March 16 and July 26, 2020.16 Additionally, we used UK Biobank inpatient hospital data and death registers—any diagnosis or cause of death recorded as U07.1 or U07.2 (ICD-10)—to ensure the inclusion of all COVID-19 cases. Cases identified by either of these approaches were classified as All COVID-19.
Notably, the testing strategy varied over time in the UK. The initial tests were largely restricted to inpatients with symptoms, and a positive result was, therefore, a reasonable proxy for severe COVID-19. As testing capacity increased (after April 27, 2020), more community testing was introduced and all patients admitted to a hospital for an overnight stay, including asymptomatic ones, were tested. Accordingly, besides All COVID-19 cases, we did analyses specifically for Inpatient COVID-19 cases, defined as a hospital admission with a diagnosis of COVID-19 according to the UK Biobank inpatient hospital data, or a positive test from PHE, provided that the origin of the test was marked as “inpatient”.
We defined COVID-19-related death as a death with COVID-19 (ICD-10 U07.1 and U07.2) as a cause of death according to data from the death registers (updated up to June 28, 2020).
Hospitalisation for other infections
To test our hypothesis that individuals with pre-pandemic psychiatric disorders are more susceptible to all severe infections5 including COVID-19 through, for instance compromised immunity, we used other infections that required hospital care during the study period as a positive disease control. Individuals hospitalised for other infections were defined as free of COVID-19 (either tested negative or were not tested [assumed negative]) and admitted to a hospital with a main diagnosis of other infections (ICD codes in the appendix, p 1) between Jan 31 and May 31, 2020, on the basis of UK Biobank inpatient hospital data.
Covariates
Data on sociodemographic characteristics (birth year, sex, and race or ethnicity), socioeconomic factors (Townsend deprivation index, educational attainment, and annual household income), and lifestyle factors (smoking status) were collected at baseline through questionnaires. Body- mass index (BMI) was calculated from height and weight measurements taken during the initial visit to an assessment centre. The postal codes of study participants were used to generate the Townsend deprivation index, which is widely used as a measure of area-level socioeconomic deprivation, with higher scores representing greater deprivation.19 Additionally, history of somatic diseases that could affect COVID-19 susceptibility or course (ie, chronic cardiac disease, diabetes, chronic pulmonary disease, chronic kidney disease, and asthma; ICD codes listed in the appendix, p 1) were selected as covariates according to previous literature20 and were also extracted from the UK Biobank inpatient hospital data.
Statistical analysis
We examined the association between pre-pandemic psychiatric disorders and the risk of COVID-19 using odds ratios (ORs) with 95% CIs, derived from logistic regression models. We first did analyses for All COVID-19 cases, and then Inpatient COVID-19 cases and COVID-19-related deaths. Models were partly (models 1–3) or fully (model 4) adjusted for birth year, sex (men or women), race or ethnicity (White, Asian, Black, others, or unknown), the Townsend deprivation index (as a continuous variable), educational attainment (college degree, A-level, O-level, Certificate of Secondary Education [CSE] or equivalent, National Vocation Qualifications [NVQ] or equivalent, other professional qualifications, or unknown), annual household income (<£18 000, £18 000–30 999, £31 000–51 999, £52 000–100 000, >£100 000, or prefer not to answer), BMI (<18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2, ≥30·0 kg/m2, or unknown), smoking status (never, ever, or unknown), and a history of chronic cardiac disease (yes or no), diabetes (yes or no), chronic pulmonary disease (yes or no), chronic kidney disease (yes or no), and asthma (yes or no).
Subgroup analyses calculated ORs by age at the time of the outbreak (ie, Jan 31, 2020, by tertiles ≤64, 65–72, or ≥73 years), sex (men or women), race or ethnicity (White, Black, or others), the Townsend deprivation score (by tertiles low [≤–3·15], middle [–3·16 to ≤–0·63], or high [≥–0·64]), educational attainment (college degree, A-level, O-level, CSE or equivalent, NVQ or equivalent, or other professional qualifications), annual household income (<£18 000, £18 000–51 999, or ≥£52 000), BMI (<18·5 kg/m2, 18·5–24·9 kg/m2, 25·0–29·9 kg/m2, or ≥30·0 kg/m2), smoking status (never or ever), and the number of somatic comorbidities (0, 1, 2, or ≥3). Furthermore, we explored whether the effect of pre-pandemic psychiatric disorders on susceptibility to COVID-19 varied during different stages of the outbreak, by analysing the associations in different time periods (until March 31, 2020, between April 1 and May 31, and from June 1 onward). Additionally, we separately investigated the associations according to time since the first diagnosis of a psychiatric disorder (<1, 1–2, 3–4, 5–9, or ≥10 years) and the number of pre-pandemic psychiatric disorders (1, 2, or ≥3). The differences in ORs were assessed by introducing an interaction term to the logistic models or by Wald test.
In addition to considering all pre-pandemic psychiatric disorders as one group, we did separate analyses for five subtypes of psychiatric disorders: depression, anxiety, stress-related disorder, substance misuse, and psychotic disorder. Furthermore, restricting analyses to participants with available data on both inpatient stay and primary care, we assessed the differences in ORs between pre-pandemic psychiatric disorders on the basis of different sources of diagnosis (ie, both inpatient and primary care, only inpatient care, or only primary care), and COVID-19-related outcomes.
We explored the association of pre-pandemic psychiatric disorders with susceptibility to other severe infections, excluding COVID-19, by re-running all analyses for the positive disease control outcome—hospitalisation for other infections. We then repeated all the main analyses using the primary diagnoses of psychiatric disorders only in the UK Biobank inpatient hospital data, to test the robustness of the results for this definition of pre-pandemic psychiatric disorders. Moreover, because the testing strategy in the UK changed considerably on April 27, 2020, we did another sensitivity analysis that only included data from the study period before this date. Lastly, to test the effect of surveillance bias (ie, the increased probability of detecting outcome in individuals with psychiatric disorders due to increased medical surveillance, compared with those without psychiatric disorders), we did a bias analysis. By assuming 5–50% under-ascertainment of COVID-19 outcomes in individuals without psychiatric disorders but no such under-ascertainment in those with psychiatric disorders (ie, extreme scenario), we plotted the changes of ORs, on the basis of average estimates derived from 100 datasets with some proportion of misclassified cases randomly selected from individuals without psychiatric disorders. All the analyses were done with R software, version 3.6. A 2-sided p<0·05 was considered statistically significant.
Role of the funding source
The sponsor of the study had no role in study design, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
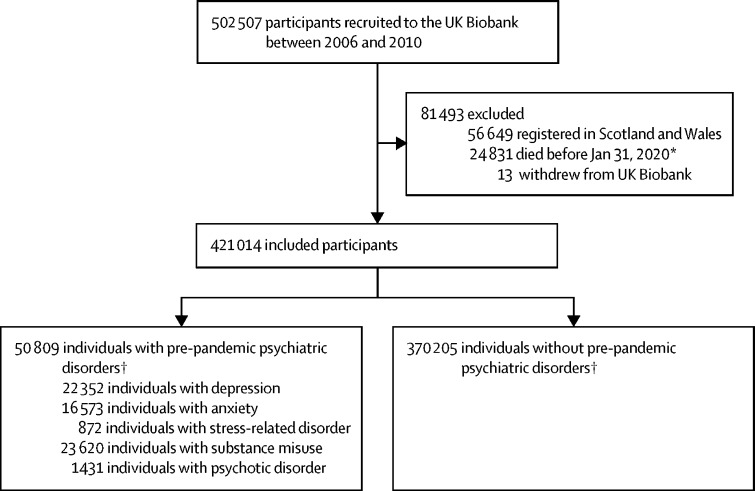
Of the 445 858 UK Biobank participants from England, 24 844 (5·6%) died before Jan 31, 2020, or had withdrawn from UK Biobank, resulting in 421 014 participants included in the analysis (figure 1 ). The mean age at the time of the COVID-19 outbreak was 67·80 years (SD 8·12) and 188 862 (44·9%) of study participants were men (table 1 ). We identified 50 809 (12·1%) individuals with a pre-pandemic psychiatric disorder, and 370 205 (87·9%) individuals without psychiatric disorders. While we observed no differences in age, sex, or race or ethnicity, we found higher proportions of individuals with pre-pandemic psychiatric disorders who were smokers, had higher BMI, and lower socioeconomic status compared with individuals without pre-pandemic psychiatric disorders (table 1). We also found higher proportions of individuals with pre-pandemic psychiatric disorders who had a history of severe somatic comorbidities, such as chronic cardiac disease and diabetes, compared with those without psychiatric disorders.
Figure 1.
Study design
*The first COVID-19 case was diagnosed on Jan 31, 2020, in the UK. †Defined as psychiatric disorders identified from the UK Biobank inpatient hospital data before Jan 31, 2020.
Table 1.
Characteristics of the study cohort
| Individuals with pre-pandemic psychiatric disorders (n=50 809) | Individuals without pre-pandemic psychiatric disorders (n=370 205) | Total (n=421 014) | |||
|---|---|---|---|---|---|
| Age at the outbreak*, years | 67·80 (8·12) | 67·70 (8·12) | 67·80 (8·12) | ||
| Age group, years | |||||
| ≤64 | 17 760 (35·0%) | 129 548 (35·0%) | 147 308 (35·0%) | ||
| 65–72 | 15 278 (30·1%) | 112 637 (30·4%) | 127 915 (30·4%) | ||
| ≥73 | 17 771 (35·0%) | 128 020 (34·6%) | 145 791 (34·6%) | ||
| Sex | |||||
| Men | 22 016 (43·3%) | 166 846 (45·1%) | 188 862 (44·9%) | ||
| Women | 28 793 (56·7%) | 203 359 (54·9%) | 232 152 (55·1%) | ||
| Race or ethnicity | |||||
| White | 47 670 (93·8%) | 346 013 (93·5%) | 393 683 (93·5%) | ||
| Asian | 985 (1·9%) | 9536 (2·6%) | 10 521 (2·5%) | ||
| Black | 839 (1·7%) | 6797 (1·8%) | 7636 (1·8%) | ||
| Others | 899 (1·8%) | 5887 (1·6%) | 6786 (1·6%) | ||
| Unknown | 416 (0·8%) | 1972 (0·5%) | 2388 (0·6%) | ||
| Townsend deprivation index | −0·38 (3·4) | −1·46 (3·0) | −1·33 (3·1) | ||
| Educational attainment | |||||
| College degree | 11 528 (22·7%) | 123 672 (33·4%) | 135 200 (32·1%) | ||
| A-level | 5052 (9·9%) | 41 703 (11·3%) | 46 755 (11·1%) | ||
| O-level | 10 767 (21·2%) | 79 083 (21·4%) | 89 850 (21·3%) | ||
| Certificate of Secondary Education or equivalent | 3587 (7·1%) | 20 732 (5·6%) | 24 319 (5·8%) | ||
| National Vocation Qualifications or equivalent | 3839 (7·6%) | 23 446 (6·3%) | 27 285 (6·5%) | ||
| Other professional qualifications | 2559 (5·0%) | 18 843 (5·1%) | 21 402 (5·1%) | ||
| Unknown | 13 477 (26·5%) | 62 726 (16·9%) | 76 203 (18·1%) | ||
| Annual household income, £ | |||||
| <18 000 | 15 256 (30·0%) | 62 957 (17·0%) | 78 213 (18·6%) | ||
| 18 000–30 999 | 11 123 (21·9%) | 79 032 (21·4%) | 90 155 (21·4%) | ||
| 31 000–51 999 | 9018 (17·8%) | 84 704 (22·9%) | 93 722 (22·3%) | ||
| 52 000–100 000 | 5303 (10·4%) | 68 606 (18·5%) | 73 909 (17·6%) | ||
| >100 000 | 995 (2·0%) | 19 030 (5·1%) | 20 025 (4·7%) | ||
| Prefer not to answer | 9114 (17·9%) | 55 876 (15·1%) | 64 990 (15·4%) | ||
| Body-mass index, kg/m2 | |||||
| <18·5 | 338 (0·7%) | 1757 (0·5%) | 2095 (0·5%) | ||
| 18·5–24·9 | 14 046 (27·6%) | 124 194 (33·6%) | 138 240 (32·8%) | ||
| 25·0–29·9 | 20 274 (39·9%) | 157 785 (42·6%) | 178 059 (42·3%) | ||
| ≥30·0 | 15 636 (30·8%) | 84 536 (22·8%) | 100 172 (23·8%) | ||
| Unknown | 515 (1·0%) | 1933 (0·5%) | 2448 (0·6%) | ||
| Smoking status | |||||
| Never | 17 911 (35·3%) | 214 971 (58·1%) | 232 882 (55·3%) | ||
| Ever | 32 478 (63·9%) | 153 182 (41·4%) | 185 660 (44·1%) | ||
| Unknown | 420 (0·8%) | 2052 (0·6%) | 2472 (0·6%) | ||
| Chronic cardiac disease | |||||
| Yes | 10 999 (21·7%) | 28 915 (7·8%) | 39 914 (9·5%) | ||
| No | 39 810 (78·4%) | 341 290 (92·2%) | 381 100 (90·5%) | ||
| Diabetes | |||||
| Yes | 7557 (14·9%) | 23 398 (6·3%) | 30 955 (7·4%) | ||
| No | 43 252 (85·1%) | 346 807 (93·7%) | 390 059 (92·7%) | ||
| Chronic pulmonary disease | |||||
| Yes | 7261 (14·3%) | 12 141 (3·3%) | 19 402 (4·6%) | ||
| No | 43 548 (85·7%) | 358 064 (96·7%) | 401 612 (95·4%) | ||
| Chronic kidney disease | |||||
| Yes | 3575 (7·0%) | 9893 (2·7%) | 13 468 (3·2%) | ||
| No | 47 234 (93·0%) | 360 312 (97·3%) | 407 546 (96·8%) | ||
| Asthma | |||||
| Yes | 8820 (17·4%) | 29 180 (7·9%) | 38 000 (9·0%) | ||
| No | 41 989 (82·6%) | 341 025 (92·1%) | 383 014 (91·0%) | ||
| Number of somatic comorbidities | |||||
| 0 | 26 304 (51·8%) | 291 683 (78·8%) | 317 987 (75·5%) | ||
| 1 | 14 923 (29·4%) | 59 146 (16·0%) | 74 069 (17·6%) | ||
| 2 | 6391 (12·6%) | 14 758 (4·0%) | 21 149 (5·0%) | ||
| ≥3 | 3191 (6·3%) | 4618 (1·3%) | 7809 (1·9%) | ||
| COVID-19 status and severity | |||||
| All COVID-19 | |||||
| Yes | 442 (0·9%) | 1509 (0·4%) | 1951 (0·5%) | ||
| No | 50 367 (99·1%) | 368 696 (99·6%) | 419 063 (99·5%) | ||
| Inpatient COVID-19 | |||||
| Yes | 338 (0·7%) | 1012 (0·3%) | 1350 (0·3%) | ||
| No | 50 471 (99·3%) | 369 193 (99·7%) | 419 664 (99·7%) | ||
| COVID-19-related death | |||||
| Yes | 120 (0·2%) | 256 (0·1%) | 376 (0·1%) | ||
| No | 50 689 (99·8%) | 369 949 (99·9%) | 420 638 (99·9%) | ||
| Hospitalisation for other infections† | |||||
| Yes | 700 (1·4%) | 1852 (0·5%) | 2552 (0·6%) | ||
| No | 50 109 (98·6%) | 368 353 (99·5%) | 418 462 (99·4%) | ||
Data are n (%) or mean (SD).
The first COVID-19 case in the UK was diagnosed on Jan 31, 2020.
Hospitalisation for other infections was defined as a hospital admission with a main diagnosis of any other infections than COVID-19 between Jan 31 and May 31, 2020, according to the UK Biobank inpatient hospital data; we excluded all individuals with confirmed COVID-19.
As of July 26, 2020, 13 502 (3·2%) of 421 014 study participants were tested for COVID-19. We identified 1951 participants with a COVID-19 positive test or diagnosis or COVID-19 as cause of death, 1350 (69·2%) of whom were Inpatient COVID-19 cases. 1650 participants were identified as having COVID-19 because of tests, corresponding to a positive rate of 12·2% (1650 of 13 502) among all tested participants. There were 376 COVID-19-related deaths up to June 28 (table 1).
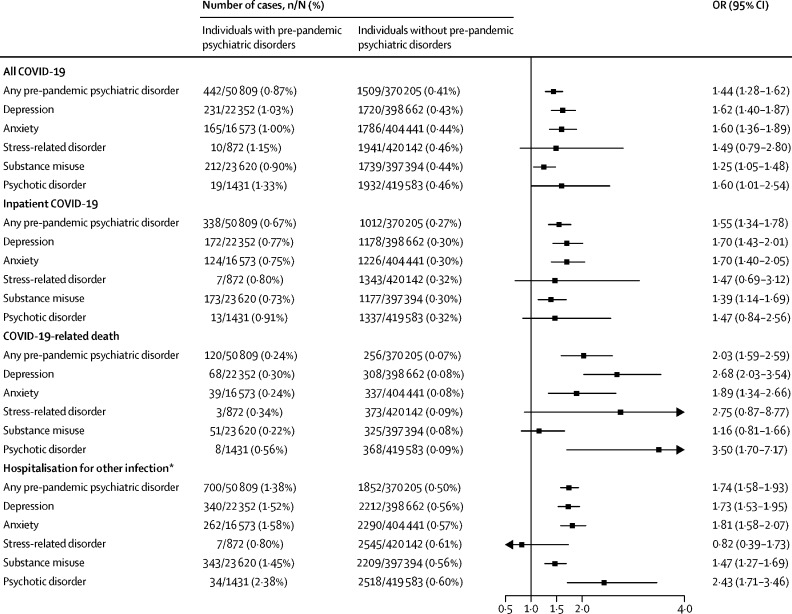
We observed an elevated risk of COVID-19 among individuals with pre-pandemic psychiatric disorders compared with individuals without such conditions (figure 2 ). Of 50 809 individuals with pre-pandemic psychiatric disorders, 442 (0·9%) were identified as All COVID-19 cases, of whom 338 (0·7%) were Inpatient COVID-19 cases, and 120 (0·2%) had COVID-19-related death. By contrast, of 370 205 individuals without pre-pandemic psychiatric disorders, 1509 (0·4%) were identified as All COVID-19 cases, 1012 (0·3%) were Inpatient COVID-19 cases, and 256 (0·1%) had COVID-19-related death. These proportions in the group of people with psychiatric disorders corresponded to a birth year-adjusted and sex-adjusted OR of 2·16 (95% CI 1·94–2·40) for All COVID-19 cases (model 1, appendix p 2), which decreased to 1·85 (1·65–2·07) when adjusting for more confounders (models 2 and 3) and then 1·44 (1·28–1·62) when adding somatic comorbidities into the models (model 4). The fully adjusted OR (model 4) was 1·55 (95% CI 1·34–1·78) for Inpatient COVID-19 and 2·03 (95% CI 1·59–2·59) for COVID-19-related death (figure 2). The observed ORs were similar between all studied subtypes of pre-pandemic psychiatric disorders, with the highest point estimates observed for depression, psychotic disorder, and anxiety (figure 2). Similar patterns of results were observed for hospitalisation for other infections (figure 2). The fully adjusted OR of hospitalisation for other infections was 1·74 (95% CI 1·58–1·93) for any pre-pandemic psychiatric disorder.
Figure 2.
Risk of COVID-19 and other infections among individuals with any or specific pre-pandemic psychiatric disorders compared with that of individuals without such disorders
ORs (95% CI) were derived from logistic regression models, adjusted for birth year, sex, race or ethnicity, Townsend deprivation index, educational attainment, annual household income, body-mass index, smoking status, and history of chronic cardiac disease, diabetes, chronic pulmonary disease, chronic kidney disease, and asthma. OR=odds ratio. *Hospitalisation for other infections was defined as a hospital admission with a main diagnosis of any infection other than COVID-19 between Jan 31 and May 31, 2020, according to the UK Biobank inpatient hospital data; we excluded all individuals with confirmed COVID-19.
The elevated risk of COVID-19 in individuals with pre-pandemic psychiatric disorders did not differ by sex, race or ethnicity, Townsend deprivation index, educational attainment, annual household income, BMI, smoking status, number of somatic comorbidities, or the stage of the COVID-19 outbreak (table 2 ). However, the ORs were somewhat higher among individuals older than 64 years for All COVID-19 cases (pinteraction <0·0001), Inpatient COVID-19 cases (pinteraction <0·0001), and COVID-19-related death (pinteraction =0·011; table 2).
Table 2.
Risk of COVID-19 among individuals with pre-pandemic psychiatric disorders by different characteristics, compared with that of individuals without such conditions
|
All COVID-19 |
Inpatient COVID-19 |
COVID-19-related deaths |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individuals with pre-pandemic psychiatric disorders | Individuals without pre-pandemic psychiatric disorders | OR (95% CI) | pinteraction | Individuals with pre-pandemic psychiatric disorders | Individuals without pre-pandemic psychiatric disorders | OR (95% CI) | pinteraction | Individuals with pre-pandemic psychiatric disorders | Individuals without pre-pandemic psychiatric disorders | OR (95% CI) | pinteraction | ||
| By age, years | <0·0001 | <0·0001 | 0·011 | ||||||||||
| ≤64 | 96/17 760 (0·5%) | 603/129 548 (0·5%) | 0·93 (0·73–1·17) | 68/17 760 (0·4%) | 338/129 548 (0·3%) | 1·10 (0·82–1·47) | 13/17 760 (0·1%) | 19/129 548 (0·01%) | 2·27 (0·96–5·33) | ||||
| 65–72 | 125/15 278 (0·8%) | 292/112 637 (0·3%) | 1·83 (1·44–2·34) | 104/15 278 (0·7%) | 208/112 637 (0·2%) | 1·96 (1·49–2·59) | 34/15 278 (0·2%) | 41/112 637 (0·04%) | 3·07 (1·80–5·25) | ||||
| ≥73 | 221/17 771 (1·2%) | 614/128 020 (0·5%) | 1·74 (1·46–2·06) | 166/17 771 (0·9%) | 466/128 020 (0·4%) | 1·64 (1·35–2·00) | 73/17 771 (0·4%) | 196/128 020 (0·2%) | 1·69 (1·25–2·28) | ||||
| By sex | 0·25 | 0·73 | 0·52 | ||||||||||
| Men | 244/22 016 (1·1%) | 787/166 846 (0·5%) | 1·58 (1·34–1·86) | 193/22 016 (0·9%) | 566/166 846 (0·3%) | 1·61 (1·34–1·94) | 74/22 016 (0·3%) | 167/166 846 (0·1%) | 1·99 (1·46–2·72) | ||||
| Women | 198/28 793 (0·7%) | 722/203 359 (0·4%) | 1·30 (1·09–1·55) | 145/28 793 (0·5%) | 446/203 359 (0·2%) | 1·47 (1·19–1·82) | 46/28 793 (0·2%) | 89/203 359 (0·04%) | 2·14 (1·43–3·20) | ||||
| By race or ethnicity | 0·53 | 0·93 | 0·90 | ||||||||||
| White | 408/47 670 (0·9%) | 1288/346 013 (0·4%) | 1·54 (1·36–1·74) | 307/47 670 (0·6%) | 856/346 013 (0·3%) | 1·64 (1·41–1·90) | 108/47 670 (0·2%) | 225/346 013 (0·1%) | 2·06 (1·59–2·68) | ||||
| Black | 17/839 (2·0%) | 81/6797 (1·2%) | 1·27 (0·70–2·29) | 16/839 (1·9%) | 57/6797 (0·8%) | 1·69 (0·90–3·17) | 6/839 (0·7%) | 17/6797 (0·3%) | 1·92 (0·67–5·49) | ||||
| Others | 12/1884 (0·6%) | 129/15 423 (0·8%) | 0·48 (0·25–0·91) | 11/1884 (0·6%) | 92/15 423 (0·6%) | 0·59 (0·30–1·17) | 5/1884 (0·3%) | 10/15 423 (0·1%) | 2·91 (0·85–9·92) | ||||
| By Townsend deprivation score | 0·079 | 0·18 | 0·46 | ||||||||||
| Low | 84/12 793 (0·7%) | 394/127 548 (0·3%) | 1·60 (1·24–2·06) | 64/12 793 (0·5%) | 250/127 548 (0·2%) | 1·88 (1·39–2·53) | 24/12 793 (0·2%) | 63/127 548 (0·1%) | 2·23 (1·33–3·75) | ||||
| Middle | 121/14 884 (0·8%) | 467/125 417 (0·4%) | 1·54 (1·23–1·92) | 84/14 884 (0·6%) | 316/125 417 (0·3%) | 1·51 (1·16–1·98) | 28/14 884 (0·2%) | 79/125 417 (0·1%) | 1·89 (1·17–3·07) | ||||
| High | 237/23 132 (1·0%) | 648/117 240 (0·6%) | 1·34 (1·13–1·59) | 190/23 132 (0·8%) | 446/117 240 (0·4%) | 1·46 (1·20–1·77) | 68/23 132 (0·3%) | 114/117 240 (0·1%) | 2·00 (1·43–2·81) | ||||
| By educational attainment | 0·30 | 0·21 | 0·39 | ||||||||||
| College degree | 73/11 528 (0·6%) | 394/123 672 (0·3%) | 1·39 (1·06–1·83) | 54/11 528 (0·5%) | 266/123 672 (0·2%) | 1·53 (1·11–2·12) | 16/11 528 (0·1%) | 57/123 672 (0·1%) | 1·43 (0·77–2·66) | ||||
| A-level | 34/5052 (0·7%) | 128/41 703 (0·3%) | 1·68 (1·10–2·57) | 28/5052 (0·6%) | 78/41 703 (0·2%) | 2·31 (1·42–3·74) | 6/5052 (0·1%) | 17/41 703 (0·04%) | 2·05 (0·73–5·75) | ||||
| O-level | 58/10 767 (0·5%) | 293/79 083 (0·4%) | 1·16 (0·85–1·57) | 42/10 767 (0·4%) | 194/79 083 (0·3%) | 1·20 (0·84–1·73) | 19/10 767 (0·2%) | 42/79 083 (0·1%) | 2·46 (1·36–4·45) | ||||
| CSE or equivalent | 28/3587 (0·8%) | 103/20 732 (0·5%) | 1·23 (0·77–1·97) | 16/3587 (0·5%) | 63/20 732 (0·3%) | 0·95 (0·51–1·76) | 2/3587 (0·1%) | 9/20 732 (0·04%) | 0·69 (0·13–3·73) | ||||
| NVQ or equivalent | 39/3839 (1·0%) | 123/23 446 (0·5%) | 1·58 (1·05–2·36) | 32/3839 (0·8%) | 73/23 446 (0·3%) | 2·07 (1·29–3·30) | 6/3839 (0·2%) | 18/23 446 (0·1%) | 1·46 (0·51–4·20) | ||||
| Other professional qualifications | 32/2559 (1·3%) | 94/18 843 (0·5%) | 1·71 (1·09–2·68) | 22/2559 (0·9%) | 64/18 843 (0·3%) | 1·62 (0·94–2·79) | 15/2559 (0·6%) | 18/18 843 (0·1%) | 4·42 (2·09–9·36) | ||||
| By annual household income, £ | 0·30 | 0·25 | 0·58 | ||||||||||
| <18 000 | 183/15 256 (1·2%) | 347/62 957 (0·6%) | 1·64 (1·34–2·01) | 145/15 256 (1·0%) | 245/62 957 (0·4%) | 1·70 (1·35–2·16) | 56/15 256 (0·4%) | 86/62 957 (0·1%) | 2·08 (1·42–3·03) | ||||
| 18 000–51 999 | 128/20 141 (0·6%) | 634/163 736 (0·4%) | 1·22 (0·99–1·50) | 90/20 141 (0·5%) | 409/163 736 (0·3%) | 1·31 (1·02–1·69) | 29/20 141 (0·1%) | 90/163 736 (0·1%) | 1·90 (1·20–3·01) | ||||
| ≥52 000 | 31/6298 (0·5%) | 266/87 636 (0·3%) | 1·26 (0·85–1·88) | 20/6298 (0·3%) | 170/87 636 (0·2%) | 1·23 (0·75–2·01) | 9/6298 (0·1%) | 25/87 636 (0·03%) | 2·67 (1·12–6·33) | ||||
| By BMI level, kg/m2 | 0·68 | 0·41 | 0·46 | ||||||||||
| <18·5 | 1/338 (0·3%) | 7/1757 (0·4%) | 0·41 (0·03–6·18) | 1/338 (0·3%) | 5/1757 (0·3%) | 0·32 (0·01–7·51) | 0/338 (0%) | 3/1757 (0·2%) | .. | ||||
| 18·5–24·9 | 108/14 046 (0·8%) | 343/124 194 (0·3%) | 1·75 (1·36–2·26) | 81/14 046 (0·6%) | 206/124 194 (0·2%) | 2·04 (1·50–2·77) | 27/14 046 (0·2%) | 42/124 194 (0·03%) | 2·41 (1·34–4·34) | ||||
| 25·0–29·9 | 160/20 274 (0·8%) | 654/157 785 (0·4%) | 1·41 (1·16–1·71) | 120/20 274 (0·6%) | 441/157 785 (0·3%) | 1·49 (1·19–1·87) | 42/20 274 (0·2%) | 107/157 785 (0·1%) | 2·04 (1·37–3·04) | ||||
| ≥30·0 | 164/15 636 (1·1%) | 491/84 536 (0·6%) | 1·29 (1·06–1·57) | 128/15 636 (0·8%) | 351/84 536 (0·4%) | 1·34 (1·07–1·67) | 45/15 636 (0·3%) | 99/84 536 (0·1%) | 1·75 (1·18–2·58) | ||||
| By smoking status | 0·051 | 0·069 | 0·022 | ||||||||||
| Never | 152/17 911 (0·9%) | 776/214 971 (0·4%) | 1·60 (1·33–1·93) | 110/17 911 (0·6%) | 490/214 971 (0·2%) | 1·75 (1·41–2·19) | 40/17 911 (0·2%) | 100/214 971 (0·1%) | 2·91 (1·97–4·30) | ||||
| Ever | 281/32 478 (0·9%) | 719/153 182 (0·5%) | 1·26 (1·08–1·46) | 221/32 478 (0·7%) | 512/153 182 (0·3%) | 1·35 (1·14–1·61) | 78/32 478 (0·2%) | 152/153 182 (0·1%) | 1·64 (1·22–2·20) | ||||
| By the number of somatic comorbidities | 0·54 | 0·62 | 0·47 | ||||||||||
| 0 | 142/26 304 (0·5%) | 927/291 683 (0·3%) | 1·57 (1·30–1·89) | 96/26 304 (0·4%) | 581/291 683 (0·2%) | 1·70 (1·35–2·13) | 27/26 304 (0·1%) | 111/291 683 (0·04%) | 2·34 (1·50–3·66) | ||||
| 1 | 119/14 923 (0·8%) | 333/59 146 (0·6%) | 1·37 (1·09–1·72) | 94/14 923 (0·6%) | 231/59 146 (0·4%) | 1·56 (1·20–2·02) | 31/14 923 (0·2%) | 78/59 146 (0·1%) | 1·81 (1·16–2·83) | ||||
| 2 | 98/6391 (1·5%) | 155/14 758 (1·1%) | 1·61 (1·22–2·13) | 78/6391 (1·2%) | 122/14 758 (0·8%) | 1·55 (1·13–2·12) | 33/6391 (0·5%) | 38/14 758 (0·3%) | 2·23 (1·34–3·71) | ||||
| ≥3 | 83/3191 (2·6%) | 94/4618 (2·0%) | 1·38 (0·99–1·91) | 70/3191 (2·2%) | 78/4618 (1·7%) | 1·38 (0·96–1·98) | 29/3191 (0·9%) | 29/4618 (0·6%) | 1·73 (0·98–3·06) | ||||
| By time periods | 0·46 | 0·47 | 0·85 | ||||||||||
| Until March 31, 2020 | 111/50 809 (0·2%) | 336/370 205 (0·1%) | 1·57 (1·23–2·00) | 107/50 809 (0·2%) | 321/370 205 (0·1%) | 1·59 (1·24–2·04) | 39/50 809 (0·1%) | 88/370 205 (0·02%) | 1·92 (1·25–2·95) | ||||
| April 1 to May 31, 2020 | 301/50 698 (0·6%) | 1044/369 869 (0·3%) | 1·43 (1·24–1·65) | 214/50 702 (0·4%) | 662/369 884 (0·2%) | 1·49 (1·25–1·77) | 77/50 770 (0·2%) | 159/370 117 (0·04%) | 2·12 (1·56–2·88) | ||||
| June 1, 2020 onward | 30/50 397 (0·1%) | 129/368 825 (0·04%) | 1·14 (0·73–1·77) | 17/50 488 (0·03%) | 29/369 222 (0·01%) | 2·28 (1·17–4·47) | 4/50 693 (0·01%) | 9/369 958 (0·002%) | 1·51 (0·44–5·22) | ||||
Data are n/N (%), unless otherwise specified. ORs (95% CI) were derived from logistic regression models, adjusted for birth year, sex, race or ethnicity, Townsend deprivation index, educational attainment, annual household income, BMI, smoking status, and history of chronic cardiac disease, diabetes, chronic pulmonary disease, chronic kidney disease, and asthma. BMI=body-mass index. CSE=Certificate of Secondary Education. NVQ=National Vocation Qualifications. OR=odds ratio.
We found no clear difference in ORs for time since the first diagnosis of a pre-pandemic psychiatric disorder. However, we observed an excess risk of COVID-19 with a greater number of pre-pandemic psychiatric disorders (ptrend <0·0001; table 3 ).
Table 3.
Risk of COVID-19 among individuals with pre-pandemic psychiatric disorders, by different psychiatric indicators, compared with that of individuals without such conditions
|
All COVID-19 |
Inpatient COVID-19 |
COVID-19-related deaths |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individuals with pre-pandemic psychiatric disorders | Individuals without pre-pandemic psychiatric disorders | OR (95% CI) | ptrend | Individuals with pre-pandemic psychiatric disorders | Individuals without pre-pandemic psychiatric disorders | OR (95% CI) | ptrend | Individuals with pre-pandemic psychiatric disorders | Individuals without pre-pandemic psychiatric disorders | OR (95% CI) | ptrend | ||
| Time since the first diagnosis of psychiatric disorders, years | 0·15 | 0·34 | 0·82 | ||||||||||
| <1 | 58/5207 (1·1%) | 1509/370 205 (0·4%) | 2·01 (1·54–2·63) | 49/5207 (0·9%) | 1012/370 205 (0·3%) | 2·42 (1·80–3·24) | 10/5207 (0·2%) | 256/370 205 (0·1%) | 1·72 (0·91–3·26) | ||||
| 1–2 | 83/9697 (0·9%) | 1509/370 205 (0·4%) | 1·54 (1·23–1·93) | 57/9697 (0·6%) | 1012/370 205 (0·3%) | 1·50 (1·14–1·97) | 25/9697 (0·3%) | 256/370 205 (0·1%) | 2·37 (1·55–3·61) | ||||
| 3–4 | 65/8777 (0·7%) | 1509/370 205 (0·4%) | 1·28 (0·99–1·66) | 40/8777 (0·5%) | 1012/370 205 (0·3%) | 1·11 (0·81–1·54) | 20/8777 (0·2%) | 256/370 205 (0·1%) | 2·01 (1·26–3·22) | ||||
| 5–9 | 152/18 646 (0·8%) | 1509/370 205 (0·4%) | 1·31 (1·10–1·57) | 123/18 646 (0·7%) | 1012/370 205 (0·3%) | 1·49 (1·21–1·82) | 41/18 646 (0·2%) | 256/370 205 (0·1%) | 1·81 (1·26–2·59) | ||||
| ≥10 | 84/8482 (1·0%) | 1509/370 205 (0·4%) | 1·40 (1·11–1·76) | 69/8482 (0·8%) | 1012/370 205 (0·3%) | 1·61 (1·24–2·08) | 24/8482 (0·3%) | 256/370 205 (0·1%) | 2·28 (1·47–3·54) | ||||
| Number of pre-pandemic psychiatric disorders | <0·0001 | <0·0001 | <0·0001 | ||||||||||
| 1 | 291/39 191 (0·7%) | 1509/370 205 (0·4%) | 1·29 (1·13–1·48) | 221/39 191 (0·6%) | 1012/370 205 (0·3%) | 1·38 (1·18–1·61) | 79/39 191 (0·2%) | 256/370 205 (0·1%) | 1·75 (1·33–2·30) | ||||
| 2 | 112/9418 (1·2%) | 1509/370 205 (0·4%) | 1·83 (1·49–2·24) | 87/9418 (0·9%) | 1012/370 205 (0·3%) | 2·01 (1·60–2·54) | 34/9418 (0·4%) | 256/370 205 (0·1%) | 3·17 (2·16–4·64) | ||||
| ≥3 | 39/2200 (1·8%) | 1509/370 205 (0·4%) | 2·33 (1·66–3·25) | 30/2200 (1·4%) | 1012/370 205 (0·3%) | 2·51 (1·71–3·68) | 7/2200 (0·3%) | 256/370 205 (0·1%) | 2·60 (1·19–5·68) | ||||
Data are n/N (%), unless otherwise specified. ORs (95% CI) were derived from logistic regression models, adjusted for birth year, sex, race or ethnicity, Townsend deprivation index, educational attainment, annual household income, body-mass index, smoking status, and history of chronic cardiac disease, diabetes, chronic pulmonary disease, chronic kidney disease, and asthma. OR=odds ratio.
178 661 (42·4%) of 421 014 participants had additional primary care data available. In the sub-analyses of these participants, we observed lower, but yet statistically significantly increased ORs for psychiatric disorders identified only in primary care (appendix p 3).
OR estimates were not modified by either defining pre-pandemic psychiatric disorders only on the basis of primary diagnosis in the UK Biobank inpatient hospital data, nor by restricting the analyses to the time period before April 27, 2020 (appendix pp 4–5). Furthermore, results of the surveillance bias analysis suggested that the observed associations stay valid when the outcome was underestimated up to 50% in individuals without pre-pandemic psychiatric disorders (appendix p 6).
Discussion
Using a large-scale community-based cohort in the UK, we found that individuals with clinically confirmed pre-pandemic psychiatric disorders were at elevated risk of COVID-19, particularly COVID-19-related hospitalisations and mortality. The associations between pre-pandemic psychiatric disorders and elevated risk of COVID-19 were independent of many potential confounders, such as socioeconomic status and smoking, but were somehow affected by history of somatic comorbidities. Additionally, the associations were stronger for individuals with multiple pre-pandemic psychiatric disorders, as well as for individuals diagnosed in inpatient care compared with primary care. Notably, we observed a similar increased risk of other infections requiring hospitalisation during the COVID-19 outbreak in individuals with psychiatric disorders, supporting the hypothesis that psychiatric disorders might alter the susceptibility to COVID-19 through multiple similar mechanisms, such as compromised immunity.
Although few comparable data exist in the setting of COVID-19, the association between psychiatric disorders and subsequent infections has been consistently shown in previous studies. Several experimental studies suggest a dose-dependent association between psychological stress and acute infectious respiratory illness.4, 21, 22 Similarly, studies using large population-based registers5, 23 have shown an association between psychiatric disorders, especially stress-related disorder, and subsequent severe infections in the general population. Recent studies have examined these associations in the context of the COVID-19 pandemic, indicating that a range of psychosocial factors,11 including self-reported symptoms of psychological distress, and certain psychiatric disorders (depression,12 substance use, and schizophrenia13) were associated with an increased risk of COVID-19. Nevertheless, the effect of multiple types of psychiatric disorders on COVID-19 was not addressed in those studies. Our findings of an association between clinically confirmed psychiatric disorders and an increased risk of COVID-19, especially severe and fatal COVID-19, underscore the need for surveillance of and care for populations with a history of psychiatric disorders during the COVID-19 outbreak.
Although the underlying mechanisms for the observed association remain unclear, the activation of the hypothalamic-pituitary-adrenal axis among individuals with psychiatric disorders has been widely reported.6, 24 This activation can lead to altered circulating glucocorticoids25 and, subsequently, suppressed cell-mediated and humoral immunity.26 As a result, the weakened immune system might increase a person's risk of being infected by SARS-CoV-2 when exposed. Furthermore, the overproduction of inflammatory cytokines induced by glucocorticoid receptor resistance can play an important role in the progression of severe infections.27 Indeed, increased cytokine levels have been reported to be correlated with disease deterioration and fatal COVID-19.28 Alternatively, individuals with psychiatric disorders are at increased risk of multiple serious somatic conditions, and our findings of attenuated ORs after adjusting for somatic comorbidities indicate that such comorbidities might contribute to the observed association with severe COVID-19 outcomes.
The major merit of our study is the use of longitudinal data for which information bias was minimised because the diagnosis and registration of psychiatric disorders before the outbreak and COVID-19-related outcomes were compiled prospectively and independently. The use of the positive control outcome (ie, hospitalisation for other infections) provides support for the existence of a pathway between psychiatric disorders and susceptibility to infections in general, shedding light on the potential underlying mechanism linking pre-pandemic psychiatric disorders to COVID-19 risk. The sample in UK Biobank enabled detailed analyses for all subgroups, and the availability of extensive phenotypic data allowed for the consideration of a wide range of confounding factors.
This study has several notable limitations. First, given that individuals with psychiatric disorders have established contact with the health-care system and thus might have more opportunities to report their respiratory symptoms and to be tested for COVID-19, surveillance bias might exist. Nevertheless, the obvious shortage of medical resources in the UK during the COVID-19 outbreak would have largely reduced the possibility of hospital admission for mild health conditions. Additionally, we observed even stronger associations for severe outcomes (ie, Inpatient COVID-19 and COVID-19-related death) throughout the outbreak period, and the bias analysis indicated that more than 50% underestimation of studied outcomes was needed to invalidate the observed associations, suggesting a limited influence of surveillance bias in the reported associations. Second, because many important variables, such as socioeconomic status and smoking, were only measured once at the baseline survey, misclassification due to no repeated measurements might exist. Additionally, due to the unclear temporal orders between covariates and the exposure, some mediators might have been included in the models, such as somatic comorbidities, leading to a potentially underestimated magnitude of the studied association. Third, the UK Biobank has some inherent limitations. For instance, it recruited only 5·5% of the invited population, and the participants were predominately white (94·6%).29 As such, the UK Biobank is not representative of the general population and, therefore, limited generalisation might be a concern. However, the reproducibility of the observed association for risk factors of various health endpoints—such as cardiovascular disease, cancer, and suicide—has been satisfactory.30 Finally, because this study mainly focused on patients who received a clinical diagnosis of psychiatric disorders through hospitalisation and primary care, and the validity of the diagnoses of some involved psychiatric disorders in register data was unsatisfiable, our findings might not be directly generalised to people with milder or subclinical psychiatric symptoms.
In conclusion, in the UK Biobank population, pre-pandemic psychiatric disorders were associated with a subsequent elevated risk of COVID-19, especially COVID-19-related hospitalisation and mortality. Although prospective research is required to confirm our findings, the similar risk observed for hospitalisation due to other infections suggests shared underlying mechanisms, including altered immune pathways. The increased COVID-19 susceptibility among individuals with pre-existing psychiatric disorders calls for improved clinical awareness and care among health professionals for this vulnerable group during the COVID-19 pandemic.
Data sharing
Data from UK Biobank are available to all researchers upon making an application. Part of this research was done using the UK Biobank Resource under Application 54803.
Acknowledgments
Acknowledgments
This work is supported by the National Natural Science Foundation of China (81971262 to HS), West China Hospital COVID-19 Epidemic Science and Technology Project (HX-2019-nCoV-014 to HS), Sichuan University Emergency Grant (2020scunCoVyingji10002 to HS), and EU Horizon 2020 Research and Innovation Action Grant (847776 to UAV and FF). We thank the team members involved in West China Biomedical Big Data Center for Disease Control and Prevention for their support.
Contributors
UAV, HS, and FF were responsible for the study's concept and design. YH, YS, ZY, and YQ did the data and project management. HY, WC, YC, YZ, and JH did the data cleaning and analysis. HY, WC, YC, YZ, DL, FF, UAV, and HS interpreted the data. HY, WC, DL, FF, UAV, and HS drafted the manuscript. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus disease (COVID-19) situation reports. October, 2020. 2020. http://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 3.Song H, Fang F, Tomasson G. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319:2388–2400. doi: 10.1001/jama.2018.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen A, Zachariae R, Bovbjerg DH. Influence of psychological stress on upper respiratory infection—a meta-analysis of prospective studies. Psychosom Med. 2010;72:823–832. doi: 10.1097/PSY.0b013e3181f1d003. [DOI] [PubMed] [Google Scholar]
- 5.Song H, Fall K, Fang F. Stress related disorders and subsequent risk of life threatening infections: population based sibling controlled cohort study. BMJ. 2019;367 doi: 10.1136/bmj.l5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 7.Nudel R, Wang Y, Appadurai V. A large-scale genomic investigation of susceptibility to infection and its association with mental disorders in the Danish population. Transl Psychiatry. 2019;9:283. doi: 10.1038/s41398-019-0622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry. 2015;2:718–725. doi: 10.1016/S2215-0366(15)00152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 10.Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batty GD, Deary IJ, Luciano M, Altschul DM, Kivimäki M, Gale CR. Psychosocial factors and hospitalisations for COVID-19: prospective cohort study based on a community sample. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.06.021. published online June 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins JL, Masoli JAH, Delgado J. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa183. published online July 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji W, Huh K, Kang M. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35:e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bycroft C, Freeman C, Petkova D. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Biobank Data providers and dates of data availability. 2020. https://biobank.ndph.ox.ac.uk/showcase/exinfo.cgi?src=Data_providers_and_dates
- 16.Armstrong J, Rudkin JK, Allen N. Dynamic linkage of COVID-19 test results between Public Health England's Second Generation Surveillance System and UK Biobank. Microb Genom. 2020;6 doi: 10.1099/mgen.0.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis KAS, Sudlow CLM, Hotopf M. Can mental health diagnoses in administrative data be used for research? A systematic review of the accuracy of routinely collected diagnoses. BMC Psychiatry. 2016;16:263. doi: 10.1186/s12888-016-0963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis KAS, Bashford O, Jewell A. Using data linkage to electronic patient records to assess the validity of selected mental health diagnoses in English Hospital Episode Statistics (HES) PLoS One. 2018;13 doi: 10.1371/journal.pone.0195002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend P, Phillimore P, Beattie A. Croom Helm; London: 1988. Health and deprivation: inequality and the North. [Google Scholar]
- 20.Docherty AB, Harrison EM, Green CA. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S. Psychosocial vulnerabilities to upper respiratory infectious illness: implications for susceptibility to coronavirus disease 2019 (COVID-19) Perspect Psychol Sci. 2020 doi: 10.1177/1745691620942516. published online July 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang T, Farkas DK, Ahern TP, Lash TL, Sørensen HT, Gradus JL. Posttraumatic stress disorder and incident infections: a nationwide cohort study. Epidemiology. 2019;30:911–917. doi: 10.1097/EDE.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller J, Gomez R, Williams G. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. 2017;22:527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 26.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Janicki-Deverts D, Doyle WJ. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry A, Littlejohns TJ, Sudlow C. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from UK Biobank are available to all researchers upon making an application. Part of this research was done using the UK Biobank Resource under Application 54803.