Abstract
Calcium hydroxide induces chronic inflammation and pulp tissue necrosis due to its high pH value. Ellagic acid is an anti-inflammatory and antioxidant flavonoid. Therefore, the effect of combining calcium hydroxide and ellagic acid must be researched to reduce cell damage due to the application of calcium hydroxide. The objective of the study was to determine the cytotoxicity and proliferation of fibroblasts after combining calcium hydroxide and ellagic acid with ratios of 99:1, 98:2, 97:3, 96:4, and 95:5. Calcium hydroxide and ellagic acid with different ratios were mixed with water and stirred. Rat gingival fibroblasts were prepared and incubated in two 96-well microplates. The control group and treatment groups (16 samples) were placed in the microplate and incubated for 1 and 3 days. An MTT assay test was performed, and the absorbance was observed using the ELISA reader with a wavelength of 540 nm. Following that, the cell viability was calculated. The results were tabulated and analyzed using a one-way ANOVA. For all treatment groups, the fibroblast cells showed a viability of higher than 50%. There was a significant increase (P < 0.05) in the fibroblast cell proliferation after combining calcium hydroxide and ellagic acid with ratios of 99:1 and 97:3. The combination of calcium hydroxide and ellagic acid is nontoxic. The treatment groups with ratios of 99:1 and 97:3 showed increased fibroblast cell proliferation.
Key words: Calcium hydroxide, cytotoxicity, ellagic acid, MTT assay, proliferation of fibroblast cells
INTRODUCTION
Calcium hydroxide is a substance that is often used in endodontic therapy because it protects the pulp against thermal stimulation, stimulates the formation of reparative dentin, and has antibacterial properties.[1] The mechanism of the action of calcium hydroxide in tissues encourages the deposition of mineralized tissue, which is an important aspect because calcium hydroxide has a biological compatibility.[2] However, calcium hydroxide has many disadvantages such as having a weak bond to dentin, material reabsorption, high solubility,[3] and has a high pH of approximately 12.5–12.8.[4] Due to calcium hydroxide's pH, it causes the necrosis of pulp tissue, chronic inflammation, and decreased viability of fibroblasts.[3,5]
One of the natural ingredients that have been investigated for reducing inflammation and cell damage due to the use of calcium hydroxide is ellagic acid. Ellagic acid has a broad spectrum of biological activities including antibacterial, antioxidant, and anti-inflammatory properties.[6] Ellagic acid is a natural phenol antioxidant that is found in various fruits and vegetables such as extracts of pomegranate, strawberry, and blackberry. Among the natural ingredients used in traditional Chinese medicine, ellagic acid has been used to cure pain of dental caries. Ellagic acid has been shown to be a powerful antioxidant and protects against cell death because it has anti-inflammatory properties,[7] able to induce fibroblast proliferation and accelerate the healing process.[6] Previous research shows that ellagic acid used in rat wounds can accelerate the wound healing process.[8]
One of the requirements for materials used in dentistry is that it is biocompatible, which means that material must meet the requirements for use in tissues that is not harmful to the pulp and soft tissue, does not contain substances that can cause a systemic response when diffused or absorbed into the circulation system, and has no carcinogenic potential. The most frequently used method for toxicity testing is the direct counting method using the trypan blue and MTT assay method. MTT assay has long been used to analyze the cytotoxicity of dental material.[9]
This study combines calcium hydroxide with ellagic acid with ratios of 99:1, 98:2, 97:3, 96:4, and 95:5 to analyze the effect of the combination in decreasing inflammation induced by calcium hydroxide by looking at the cytotoxicity of the combination and proliferation fibroblast cells. The aim of this study is to determine the cytotoxicity and proliferation of fibroblasts after combining calcium hydroxide and ellagic acid.
MATERIALS AND METHODS
Ethically approved
This research is experimental laboratory research. It has passed ethical clearance with the serial numbers 619/HRECC.FODM/X/2019 and 649/HRECC.FODM/X/2019. The sample is a combination of calcium hydroxide and ellagic acid with five different ratios (99:1, 98:2, 97:3, 96:4, and 95:5). The size of each sample is 16 samples.
Fibroblast cell culture
The fibroblast cells that have been used are rat gingival fibroblasts. The four rats that were used were male, over 9 months old and weighed on average 250–300 g. Fibroblast cells were obtained from rat maxillary tissue. Rat gingival fibroblast cells that were cultured until they were homogeneous were inserted into two 96-well microplates with a density of 2 × 105 cells/ml for 50 μL and incubated for 24 h.
Calcium hydroxide and ellagic acid preparation
Calcium hydroxide and ellagic acid were weighed and mixed with sterile water and stirred according to Table 1.
Table 1.
The calcium hydroxide and ellagic acid combinations
| Group | CH powder (g) | EA powder (g) | Sterile water (ml) | Total (g) |
|---|---|---|---|---|
| Pure CH (control) | 0.2 | - | 0.2 | 0.2 |
| Combination A | 0.198 | 0.002 | 0.2 | 0.2 |
| Combination B | 0.196 | 0.004 | 0.2 | 0.2 |
| Combination C | 0.194 | 0.006 | 0.2 | 0.2 |
| Combination D | 0.192 | 0.008 | 0.2 | 0.2 |
| Combination E | 0.190 | 0.010 | 0.2 | 0.2 |
Combination A: CH and EA ratio 99:1, Combination B: CH and EA ratio 98:2, Combination C: CH and EA ratio 97:3, Combination D: CH and EA ratio 96:4, Combination E: CH and EA ratio 95:5. CH: Calcium hydroxide, EA: Ellagic acid
Toxicity test
The combination of calcium hydroxide and ellagic acid was put into a 96-well microplate containing 50 μl Eagle's minimum essential medium (MEM) culture media and 50 μl rat gingival fibroblasts. The division of groups on the microplate consisted of five treatment groups, one media control group, and one cell control group.
The microplate was incubated in a 37°C incubator for 24 and 72 h. The cell growth media were then removed and washed with 200 μl phosphate-buffered saline and repeated twice. 40 μl of Eagle's MEM media were added to the well. MTT was added to every well that contained 10 μl of culture media, then re-incubated for 4 h at 37°C. For each well, 50 μl of dimethyl sulfoxide was added.
At 24 and 72 h after incubation, the microplate was shaken using a plate shaker for 5 min until the formazan crystals had dissolved. Fibroblast living cells were colored with formazan purplish blue, as the dead cells do not turn the purplish-blue color. The formazan absorbance was read using an ELISA reader with a wavelength of 540 nm. The more concentrated the color, the higher the absorbance value, and the higher the number of living cells. The percentage of living fibroblasts cells was calculated using the following formula:[10]
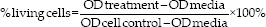
% living cells: percentage of the number of fibroblasts living after testing
Optical density (OD) treatment: the OD values in each sample after testing
OD media: the OD values in the control media
OD cell control: the OD values in the cell control group.
Statistical analysis
The data obtained were tabulated, and the one-way ANOVA test was performed using the SPSS Statistics for Macintosh Version 23.0 (IBM, New York, USA) and post hoc Tukey's honestly significant difference test to find out the significant differences between the groups with a significance of P < 0.05.
RESULTS
Toxicity test
Twenty-four hours obtained the highest percentage of living cells with combination E (calcium hydroxide and ellagic acid with a ratio of 95:5). Seventy-two hours after treatment, the highest percentage of living cells was combination A (calcium hydroxide and ellagic acid with a ratio of 99:1) [Table 2].
Table 2.
The fibroblast cells after treatment with the combination of calcium hydroxide and ellagic acid
| Groups | Absorbance |
Percentage of living cells (%) |
||
|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | |
| Control cell | 0.278±0.023 | 0.280±0.011 | 100 | 100 |
| Combination A | 0.234±0.014 | 0.282±0.042 | 77.3 | 101 |
| Combination B | 0.273±0.017 | 0.234±0.021 | 97.5 | 76.6 |
| Combination C | 0.218±0.045 | 0.264±0.014 | 69.5 | 91.9 |
| Combination D | 0.279±0.012 | 0.270±0.012 | 100.6 | 94.9 |
| Combination E | 0.318±0.043 | 0.273±0.015 | 120.9 | 96.5 |
Combination A: CH and EA ratio 99:1, Combination B: CH and EA ratio 98:2, Combination C: CH and EA ratio 97:3, Combination D: CH and EA ratio 96:4, Combination E: CH and EA ratio 95:5. CH: Calcium hydroxide, EA: Ellagic acid
Within 24 h after treatment, the percentage of living cells in combinations D and E (calcium hydroxide and ellagic acid with ratios of 96:4 and 95:5) increased, whereas the other groups decreased. Within 72 h after treatment, only combination A (calcium hydroxide and ellagic acid with a ratio of 99:1) showed an increase in the percentage of living cells [Table 2].
Twenty-four hours after exposure, combination A (calcium hydroxide and ellagic acid with a ratio of 99:1), C (calcium hydroxide and ellagic acid with a ratio of 97:3), and E (calcium hydroxide and ellagic acid with a ratio of 95:5) showed a lower percentage of living cells compared to the control cell (P = 0.001, P = 0.000, and P = 0.003, respectively). Combination E (calcium hydroxide and ellagic acid with a ratio of 95:5) showed a higher percentage of living cells compared to combination D (calcium hydroxide and ellagic acid with a ratio of 96:4) (P = 0.005), combination C (calcium hydroxide and ellagic acid with a ratio of 97:3) (P = 0.000), combination B (calcium hydroxide and ellagic acid with a ratio of 98:2) (P = 0.001), and combination A (calcium hydroxide and ellagic acid with a ratio of 99:1) (P = 0.000) [Figure 1].
Figure 1.
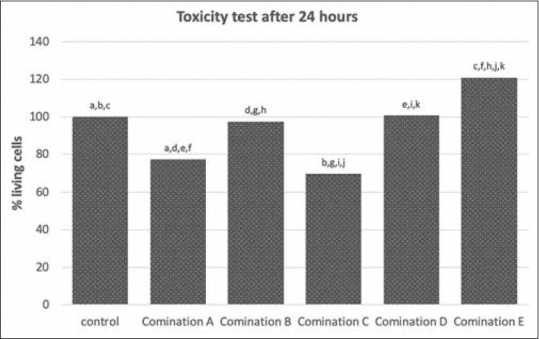
Toxicity test on calcium hydroxide and ellagic acid combination after 24 h exposure. The same character on top each bar indicated a significant different with P < 0.05
At 72 h after exposure, combination B (calcium hydroxide and ellagic acid with a ratio of 98:2) showed a lower percentage of living cells compared to the control (P = 0.000). Combination A (calcium hydroxide and ellagic acid with a ratio of 99:1), combination C (calcium hydroxide and ellagic acid with a ratio of 97:3), combination D (calcium hydroxide and ellagic acid with a ratio of 96:4), and combination E (calcium hydroxide and ellagic acid with a ratio of 95:5) were higher than combination B (calcium hydroxide and ellagic acid with a ratio of 98:2) (P = 0.006, P = 0.000, and P = 0.000, respectively) [Figure 2].
Figure 2.
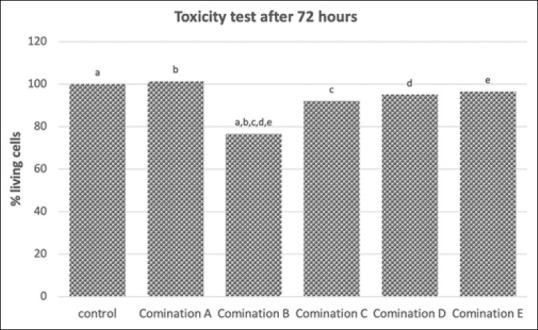
Toxicity test on calcium hydroxide and ellagic acid combination after 72 h exposure. The same character on top each bar indicated a significant different with P < 0.05
DISCUSSION
Ellagic acid is a natural flavonoid in fruits and plant extract. It has therapeutic effects such as anti-inflammation, antibacterial properties, and antioxidant activity.[6] Calcium hydroxide has a high pH, which leads to pulp tissue necrosis and chronic inflammation.[5] Therefore, researchers are trying to investigate the effect of ellagic acid when it is combined with calcium hydroxide to see whether this combination can reduce inflammation and speed up the wound healing process.
This experiment was conducted to investigate the cytotoxicity and proliferative effects of combining calcium hydroxide and ellagic acid, which was given in five different ratios to fibroblast cells after 24 h and 72 h. The parameter used in this study is the inhibitory concentration 50% (IC50). IC50 represents the concentration that is required to inhibit 50% of the proliferation.[11] The percentage of living rat gingival fibroblast indicates the level of toxicity for the sample groups. If the percentage of living fibroblasts increases, it indicates high cell viability and fibroblast cell proliferation. If the material used is not toxic, the dehydrogenase enzyme will be active, and formazan crystals will form.[12]
According to the experiment's results after 24 h, the percentage of living fibroblast cells was more than 50% for all combinations. The combination of calcium hydroxide and ellagic acid with a ratio of 95:5 showed a significant increase in cell viability compared to the control group. This might be due to the different ratios of ellagic acid that was used in every treatment group. Therefore, it can be concluded that the combination of calcium hydroxide and ellagic acid is not toxic when it is applied to fibroblast cells. At 72 h after exposure, there was a significant decrease in fibroblast cell viability after combining calcium hydroxide and ellagic acid with a ratio of 98:2. On the other hand, a comparison of fibroblast cell viability at 24 h and 72 h showed that there was a significant fibroblast cell proliferation after combining calcium hydroxide and ellagic acid with a ratio of 99:1 and 97:3.
Calcium hydroxide has a high pH, which can lead to mitochondria dysfunction and increase the cell respiration rate. This will increase the radical superoxide. Under these conditions, superoxide diffuses from cytosol to the mitochondrial membrane and causes the de-energization of mitochondria or cell apoptosis.[10] Reactive oxygen species (ROS) appears after tissue damage has occurred.[13] As ellagic acid is an antioxidant, it can reduce the free radicals that are produced due to calcium hydroxide and prevent oxidative stress damage to fibroblasts.[14] Ellagic acid can reduce the intracellular ROS level on the gingival fibroblast undergoing oxidative stress due to ultraviolet-B radiation.[15]
Moreover, combining calcium hydroxide and ellagic acid can increase cell viability and fibroblast cell proliferation because ellagic acid is an anti-inflammatory agent. Calcium hydroxide activates nuclear factor kappa beta, which promotes inflammation.[10,16] Ellagic acid can inhibit COX; therefore, it is a strong anti-inflammatory agent. Ellagic acid inhibits COX-2, tumor necrosis factor-α (TNF-α), and interleukin (IL)-6.[17] Furthermore, ellagic acid can also reduce the NF-kB expression and pro-inflammatory cytokines production such as TNF-α and IL-1 β.[18,19] Therefore, ellagic acid can reduce inflammation induced by calcium hydroxide, which can increase fibroblast cell viability. When cell viability increases, it means that combining calcium hydroxide and ellagic acid is not toxic and promotes fibroblast cell proliferation.
CONCLUSION
In summary, fibroblast cells that are given a combination of calcium hydroxide and ellagic acid with ratios of 99:1 and 97:3 show high cell viability and cell proliferation. It can be concluded that combining calcium hydroxide and ellagic acid is not toxic. This research confirmed that the combination has nontoxic properties and able to stimulate the proliferation of dental pulp. This combination may also have ideal characteristics as pulp capping materials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Universitas Airlangga and Stem Cell Research and Development Center, Universitas Airlangga, Surabaya, Indonesia, for their support and for providing the research facilities.
REFERENCES
- 1.Modena KC, Casas-Apayco LC, Atta MT, Costa CA de S, Hebling J, Sipert CR, et al. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. Appl Oral Sci. 2009;17:544–54. doi: 10.1590/S1678-77572009000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dammaschke T. A new bioactive cement for direct. Int Dent Afr Ed. 2010;2:64–9. [Google Scholar]
- 3.Poggio C, Ceci M, Dagna A, Beltrami R, Colombo M, Chiesa M. In vitro cytotoxicity evaluation of different pulp capping materials: A comparative study. Arch Ind Hyg Toxicol. 2015;66:181–8. doi: 10.1515/aiht-2015-66-2589. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 5.Zare Jahromi M, Ranjbarian P, Shiravi S. Cytotoxicity evaluation of Iranian propolis and calcium hydroxide on dental pulp fibroblasts. J Dent Res Dent Clin Dent Prospects. 2014;8:130–3. doi: 10.5681/joddd.2014.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Obaidi MMJ, Al-Bayaty FH, Al Batran R, Hassandarvish P, Rouhollahi E. Protective effect of ellagic acid on healing alveolar bone after tooth extraction in rat-A histological and immunohistochemical study. Arch Oral Biol. 2014;59:987–99. doi: 10.1016/j.archoralbio.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Loo WT, Jin LJ, Cheung MN, Chow LW. Evaluation of ellagic acid on the activities of oral bacteria with the use of adenosine triphosphate (ATP) bioluminescence assay. Afr J Biotechnol. 2010;9:3938–43. [Google Scholar]
- 8.Yuniarti WM, Primarizky H, Lukiswanto BS. The activity of pomegranate extract standardized 40% ellagic acid during the healing process of incision wounds in albino rats (Rattus norvegicus) Vet World. 2018;11:321–6. doi: 10.14202/vetworld.2018.321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Küçükkaya S. Cytotoxic effect of endodontic irrigants in vitro. Med Sci Monit Basic Res. 2014;20:22–6. doi: 10.12659/MSMBR.890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paramitta VA, Heni TH, Susilowati S. The effect of calcium hydroxide on fibroblast cells viability. Indones J Dent Res. 2015;1:105–11. [Google Scholar]
- 11.Nevozhay D. Quintas LEM, editor. Cheburator software for automatically calculating drug inhibitory concentrations from in vitro screening assays. PLoS One. 2014;9:e106186. doi: 10.1371/journal.pone.0106186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariani MD, Yuliati A, Adiarto T. Toxicity testing of chitosan from tiger prawn shell waste on cell culture. Dent J (Majalah Kedokt Gigi) 2009;42:15–21. [Google Scholar]
- 13.Kandemir FM, Sagliyan A, Ozkaraca M, Gunay C, Han MC, Benzer F. Effects of oral administrations of pomegranate seed extract on surgical wound healing in rabbits. Rev Med Vet (Toulouse) 2013;164:400–8. [Google Scholar]
- 14.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44:984–93. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Baek B, Lee SH, Kim K, Lim HW, Lim CJ. Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J Physiol Pharmacol. 2016;20:269–77. doi: 10.4196/kjpp.2016.20.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardhana AS, Nirwana I, Budi HS, Surboyo MDC. Role of Hydroxyapatite and Ellagic Acid in the Osteogenesis. Eur J Dent [Internet] 2020;1:6. doi: 10.1055/s-0040-1714039. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-0040-1714039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usta C, Ozdemir S, Schiariti M, Puddu PE. The pharmacological use of ellagic acid-rich pomegranate fruit. Int J Food Sci Nutr. 2013;64:907–13. doi: 10.3109/09637486.2013.798268. [DOI] [PubMed] [Google Scholar]
- 18.Nirwana I, Agustantina TH, Soekartono RH. Nf-Kb expressions on rat dental pulp mechanically exposured after pomegranate fruit extract administration. J Int Dent Med Res. 2017;10:123–7. [Google Scholar]
- 19.Surboyo MDC, Arundina I, Rahayu RP, Mansur D, Bramantoro T. Potential of distilled liquid smoke derived from coconut (Cocos nucifera L) shell for traumatic ulcer healing in diabetic rats. Eur J Dent. 2019;13:271–9. doi: 10.1055/s-0039-1693527. [DOI] [PMC free article] [PubMed] [Google Scholar]


