Abstract
The aims of this research were to observe yield extract, phytochemical content, the antidiabetic and anticancer activity of Chinese water chestnut. This research method used an experimental laboratory, and data analysis was carried out descriptively. The stages carried of this research are sample extraction, extract yield, phytochemical analysis (flavonoid and phenolic content), antidiabetic, and anticancer activity. The results showed that the yield of extract using ethanol on Chinese water chestnut was higher than that extracts using n-hexane and ethyl acetate solvents. Phenolic and flavonoids compounds are polar so that the results of extracts with ethanol solvents show higher values than that extracts using n-hexane and ethyl acetate solvents. The result of antidiabetic activity has the highest value at a concentration of 2000 ppm using ethanol value of 65.74%. The results of the anticancer activity were expressed by (IC50), the IC50 value of the n-hexane extract was 1.231.74 ppm, ethyl acetate 941.04 ppm, and ethanol 2.261.90 ppm.
Key words: Anticancer, antidiabetic, Chinese water chestnut, phytochemical
INTRODUCTION
Swamp waters have various types of plants, including Chinese water chestnut (Eleocharis dulcis). Chinese water chestnut is wild plants that can live in swamp waters. Chinese water chestnut has been used as a traditional medicine to treat hypertension, constipation, and chronic nephritis.[1] Whereas Chinese water chestnut has been carried out qualitatively by phytochemical tests and Chinese water chestnut extract can function as a natural antibacterial.[2] The economic potential of Chinese water chestnut plants in the health sector is not well known and studied. This potential is related to the utilization of components contained in it. With a variety of substances contained in Chinese water chestnut, it is hoped this plant can function to prevent various diseases such as diabetes mellitus (DM).
DM is a group of metabolic disorders characterized by hyperglycemia and metabolic disorders of carbohydrates, fats, and proteins. Type 1 diabetes occurs because the insulin-producing cells of the pancreas (beta cells) are damaged. Type 2 diabetes, the pancreas makes insulin, but it either does not produce enough, or the insulin does not work properly. Insulin resistance is manifested by increased hepatic glucose production, decreased skeletal glucose muscle uptake, and increased lipolysis and free fatty acid production.[3]
Cancer is a disease caused by abnormal tissue growth due to the loss of cell control mechanisms and is a leading cause of death in developing countries.[4] There are many medicinal plants that are known to be beneficial and can be used as antidiabetic and anticancer. Chinese water chestnut has phenolic, tannin, and flavonoid bioactive compounds that are thought to play a role in inhibiting the enzyme α-glucosidase, but scientifically it is not yet known the inhibitory power of Chinese water chestnut as an antidiabetic agent. Therefore, it is necessary to further study the antidiabetic activity test by stratified extraction using n-hexane, ethyl acetate, and ethanol in Chinese water chestnut. The purpose of multilevel extraction is to attract bioactive compounds that have not been interested in the extraction process from the solvent stage using n-hexane, ethyl acetate, and ethanol. N-hexane solvents will attract nonpolar compounds, ethyl acetate solvents will attract semi-polar compounds, and ethanol solvents will attract polar bioactive compounds.[5]
MATERIALS AND METHODS
Materials
The tools used in this study are vacuum rotary evaporator (B-one model RE 1000 VN, Germany), incubator (Memmert, Germany), oven (Salvis Lab, Swiss), and spectrophotometer (Pharmacia Novaspec II, The Netherlands).
The main raw material used in this study was Chinese water chestnut. The chemicals used for analysis are ethanol, n-hexane (Merck), ethyl acetate (Merck), Na2CO3 (Merck), p-Nitrophenyl-α-D-glucopyrranoside (Merck), potassium acetate, aquades, Folin-Ciocalteau (Merck), methanol (Merck), FeC13 (Merck), AlCl3 (Merck), α-glucosidase (Sigma-Aldrich), and akarbose (Sigma-Aldrich).
Water chestnut extraction
Chinese water chestnut plants that have been prepared and then carried out an extraction process. Active ingredient extraction is done by referring to the modified of Santoso et al.[6] The extraction method used in this study is a multilevel extraction method.
The stages of extraction as stratified are as follows: Swamp plant powder was weighed as much as 250 g and put into an Erlenmeyer, then the solvent is added until the final volume reaches 1000 mL with a ratio of 1:4 (w/v), extracted by stratified maceration using n-hexane solvent (nonpolar), ethyl acetate (semipolar), and ethanol (polar), respectively, for 2 × 24 h, filtering was carried out using Whatman paper. Extracts obtained from the three types of solvents were concentrated with a Rotary evaporator.
Phenolic content
Analysis of phenolic content carried out according to the method of Andarwulan and Shetty[7] is as follows: A total of 50 mg samples plus 2.5 mL of 95% ethanol in a test tube, then centrifuged 3500 rpm for 10 min. A volume of 1 mL of the supernatant was put into a test tube containing 1 ml of ethanol and 5 mL aquadest, then add 0.5 mL of Folin-Ciocalteu reagent, let stand 5 min. 1 mL Na2CO3 5% was divortexed and left in a dark room for 60 min. The sample was again homogenized to measure its absorbance at a wavelength of 725 nm.
Flavonoids content
Analysis of flavonoids owas carried out according to the method of Chang et al.,[8] which is as follows: 200 mg of sample extracts were dissolved in 1 ml of ethanol, then diluted with 3 replications. Total flavonoids from ethanol extract were calculated based on the colorimetric method. Every 0.2 ml of the sample solution was added 3.7 ml of 95% ethanol, 0.1 ml of 10% AlCl3, 0.1 ml of 1 M potassium acetate and added to 5 ml of distilled water, then mixed with homogeneous and allowed to stand for 30 min. Then, the absorbance was measured at a wavelength of 437 nm. Quercetin is used as a calibration curve with a concentration of 100–400 μg/ml.
Antidiabetic activity (inhibiting α-glucosidase)
The antidiabetic activity test of Chinese water chestnut was carried out according to Sancheti,[9] as follows: Phosphate buffer (pH 7) 0.1 M for 50 μL 0.5 mM 4-nitrophenyl α-D-glucopyranoside, homogeneous. Then, 10 ml of test samples were added with a concentration of 100 ppm, 500 ppm, 1000 ppm, 1500 ppm, and 2000 ppm. Then, the reaction was added 25 μL α-glucosidase, the solution was homogenized and incubated at 37°C for 30 min. Then the reaction was added with 100 μL of sodium carbonate 0.2 M. Then, the reaction was calculated by using the uv-vis 410 nm spectrophotometry.
Anticancer test with the MTT assay method
Anticancer test with the MTT assay method. MTT was applied to assess cell viability as described in a previous study.[10] Cells were grown at a concentration of 5000 cells in 100 μl of media. Stocks of extract concentrations are 125, 250, 500, 1000, and 2000 ppm. The sample was added after the cells reached 50% confluent (24 h). The MTT test was carried out on the 3rd day, by adding 10 μl of MTT (5 mg/ml) per well, then incubated for 4 h at 37°C. Formazan crystals dissolved in 0.1 N HCl in isopropanol. Optical density readings were performed using a microplate reader at a wavelength of 565 nm.
Statistical analysis
The IC50 value obtained in this research was obtained from the linear regression analysis in the Microsoft Excel Program.
RESULTS AND DISCUSSION
Phytochemical compounds
Phytochemical testing of Chinese water chestnut was carried out after the sample was macerated using multilevel maceration method. Phytochemical compounds tested were phenolic, and flavonoid content. Quantitative phytochemical from Chinese water chestnut extract can be seen in Table 1.
Table 1.
Quantitative phytochemical from Chinese water chestnut extract
| Phytochemical compounds | Solvent |
||
|---|---|---|---|
| n-Hexane | Ethyl acetate | Ethanol | |
| Phenolic (ppm) | 30.00 | 74.00 | 86.00 |
| Flavonoids (mg GAE/g) | 16.63 | 27.88 | 48.56 |
In this study, it can be seen from the results of multilevel maceration of the solvent used to see the content of bioactive components found in Chinese water chestnut plants that have the highest bioactive component, namely extraction using ethanol as a solvent. Based on these data, it is suspected that ethanol dissolves more bioactive compounds in Chinese water chestnut extract. The high quantitative value of extract with ethanol solvents explains that the characteristics of the phenolic and flavonoid bioactive compounds are more precisely extracted using ethanol solvents.
Phenolic and flavonoids compounds are polar so that the results of extracts with ethanol solvents show higher values than extracts using n-hexane and ethyl acetate solvents because polar solvents can attract polar compounds. Flavonoids are polar compounds because they have hydroxyl groups, or sugar so that flavonoids are quite soluble in polar solvents such as ethanol.[11]
The extraction process that uses solvents with different polarity will extract different compounds. The solubility of the bioactive component in the material or sample will determine the composition of the extract obtained. Siedel[5] states that the selection of solvents and the appropriate extraction method will affect the yield of secondary metabolite compounds that can be extracted. The selection of extraction solvents generally uses the principle of like dissolves like, where nonpolar compounds will dissolve in nonpolar solvents while polar compounds will dissolve in polar solvents.
The difference in the amount and composition of secondary metabolite compounds depends on the type of extraction, extraction time, temperature, natural condition of the solvent, solvent concentration, and polarity.[12] The selection of the appropriate solvent in the extraction process and the conditions at the time of the extract preparation can also affect the compounds contained in the extract from Chinese water chestnut.
Antidiabetic activity (inhibition of α-glucosidase enzyme)
The results of inhibition of the α-glucosidase enzyme in n-hexane, ethyl acetate, and ethanol solvent can be seen in Table 2.
Table 2.
The inhibition of α-glucosidase enzyme of Chinese water chestnut extract
| Sample concentration (ppm) | Inhibition (%) |
||
|---|---|---|---|
| n-hexane | Ethyl acetate | Ethanol | |
| 100 | - | - | 8.14 |
| 500 | - | 3.64 | 20.81 |
| 1000 | - | 5.48 | 37.09 |
| 1500 | - | 12.98 | 55.05 |
| 2000 | - | 21.99 | 65.74 |
-: Not identified
Inhibition of α-glucosidase enzyme of the third stage of Chinese water chestnut extract using ethanol solvent has a higher inhibitory value than using ethyl acetate and n-hexane solvents. Chinese water chestnut (Eleocharis dulcis) can be seen in Figure 1. This can be seen from quantitative phytochemical testing, which has a higher value compared to using ethyl acetate and n-hexane solvents.
Figure 1.

Chinese water chestnut (Eleocharis dulcis)
The results of Anisah et al.,[13] analysis of chemical components with gas chromatography-mass spectrometry showed that the most active extracts of ethanol extract on Jabon leaves contained phenolic compounds (quinic acid, catechol) and fatty acid derivatives (hexadecanoic acid) which had potential antidiabetic activity. Quinic acid phenolic compounds have antidiabetic activity in experimental animals[14] and can inhibit the α-glucosidase enzyme in vitro.[15] Hexadecanoic acid can reduce blood sugar levels.[16]
In the study, Hernawan et al.[17] tannins of gallotanin and ellagitanin types can increase the activity of glucose transport into adipose cells in vitro. Gallotanin can increase glucose uptake while inhibiting adipogenesis. Ellagitanin derivatives, on the other hand, have properties similar to the hormone insulin. According to Nawwar et al.,[18] soursop leaves containing quavetin type flavonoids can prevent an increase in blood glucose values so that it can stimulate β-cells to produce more insulin. The results of Kumar[19] flavonoid type anthocyanin, isoflavones, and flavonols are able to inhibit the α-glucosidase enzyme. OPLS analysis results indicate that flavonoid compounds of sinensitin type can also inhibit the α-glucosidase enzyme. Sinensitin with the chemical structure, there are five most methoxy groups of cat whiskers. Besides OH and C = O groups are thought to have a tendency to increase the inhibition of α-glucosidase by flavonoids.[20]
Anticancer activity
The anticancer analysis was carried out with the aim to determine the cytotoxic effects of n-hexane, ethyl acetate, and ethanol extracts of Chinese water chestnut against HeLa cells (cervical cancer) with IC50 parameters. The in vitro cytotoxicity test is a model commonly used as a basis for studying the effects of anticancer molecules of a medicinal plant.[21]
Cells were grown at a concentration of 5000 cells in 100 μl of media. Stocks of collagen peptide concentrations are 125, 250, 500, 1000, and 2000 ppm. The sample was added after the cells reached 50% confluent (24 h). The MTT test was carried out on the 3rd day, by adding 10 μl of MTT (5 mg/ml) per well, then incubated for 4 h at 37°C. Formazan crystals dissolved in 0.1 N HCl in isopropanol. The IC50 value is a concentration value that results in the inhibition of cancer cell proliferation by 50% and shows the potential for the oxidation of a compound to cells.[22] IC50 value is determined from the equation formed from the linear regression curve between probit percent inhibition and log concentration. The linear equation obtained is used to find the IC50 value. The results of anticancer activity tests stated by (IC50) can be seen in Figure 2.
Figure 2.
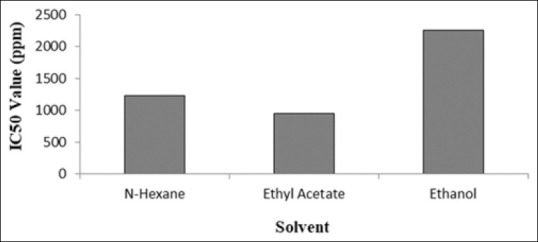
IC50 value of water chestnut extract
From Figure 2, it can be seen that the IC50 value of extract with n-hexane was 1,231.74 ppm, ethyl acetate was 941.04 ppm, and ethanol was 2,261.90 ppm. Other plants that have habitat in swamp waters and function as anticancer against HeLa cancer cells by using MTT Assay from Sala (Cynometra ramiflora Linn) which was tested on the bark and leaves using ethanol solvent obtained IC50 values on the bark were 0, 90 and 6.29 ppm and IC50 values obtained were 1.92; 6.37 and 0.41 ppm, respectively.[23]
CONCLUSION
Phenolic and flavonoid bioactive compounds from Chinese water chestnut are more precisely extracted using ethanol as a solvent. Chinese water chestnut extract has a very little inhibitory effect against the enzyme α-glucosidase. The results of anticancer activity tests stated by (IC50), the IC50 value of extract with n-hexane was 1,231.74 ppm, ethyl acetate was 941.04 ppm, and ethanol was 2,261.90 ppm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by competitive grant 2018 from Universitas Sriwijaya and Ministry of Research, Technology and Higher Education, Republic of Indonesia.
REFERENCES
- 1.Zhan G, Pan L, Tu K, Jiao S. Antitumor, antioxidant, and nitrite scavenging effects of Chinese water chestnut (Eleocharis dulcis) peel flavonoids. J Food Sci. 2016;81:H2578–86. doi: 10.1111/1750-3841.13434. [DOI] [PubMed] [Google Scholar]
- 2.Baehaki A, Herpandi, Putra AA. Antibacterial activity of exttact from swamp plant, Eleocharis dulcis. Oriental J Chem. 2018;34:573–5. [Google Scholar]
- 3.Well GB, Dipiro TJ, Schwinghammer LT, Dipiro VC. Pharmacotheraphy Hamdbook Ninth Edition. New York: MCGraw-Hill Education; 2015. [Google Scholar]
- 4.Hill RP, Tannock IF. The Basic Science of Oncology. New York: Mc-Graw-Hill Inc; 1992. Cancer as a Cellular Disease. [Google Scholar]
- 5.Siedel P. The use of the stable radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. J Sci Technol. 2008;26:211–9. [Google Scholar]
- 6.Santoso J, Anwariyah S, Rumiantin RO, Putri AP, Ukhty N, Yoshie-Stark Y. Phenol content, antioxidant activity and fibers profile of four tropical seagrasses from Indonesia. J Coastal Develop. 2012;15:189–96. [Google Scholar]
- 7.Andarwulan N, Shetty K. Phenolic content in differentiated tissue cultures of untransformed and Agrobacterium-transformed roots of anise (Pimpinella anisum L.) J Agric Food Chem. 1999;47:1776–80. doi: 10.1021/jf981214r. [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82. [Google Scholar]
- 9.Sancheti S. Chaenomeles sinensis: A potent α-and-β-glucosidase inhibitor. Am J Pharm Toxic. 2009;4:8–11. [Google Scholar]
- 10.Kadan S, Rayan M, Rayan A. Anticancer activity of anise (Pimpinella anisum L.) seed extract. Open Nutraceuticals J. 2013;6:1–5. [Google Scholar]
- 11.Markham KR. Techniques of flavonoid identification. New York: Academic Press; 1982. [Google Scholar]
- 12.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Int Pharm Sci. 2011;1:1–9. [Google Scholar]
- 13.Anisah LN, Syafril W, Sari RK, Pari G. Antidiabetic activity of Jabon (Anthocephalus cadamba) ethanol extracts. J Ilmu Teknol Kayu Tropis. 2015;13:111–24. [Google Scholar]
- 14.Ong KW, Hsu A, Song L, Huang D, Tan BK. Polyphenols rich Vernonia amygdalina shows antidiabetic effects in streptozotocin induced diabetic Chinese water chestnut. J Ethnopharmacol. 2011;133:598–607. doi: 10.1016/j.jep.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Iwai K, Kim MY, Onodera A, Matsue H. Alpha-glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J Agric Food Chem. 2006;54:4588–92. doi: 10.1021/jf0606353. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan V, Dhas AS. Effect of active fraction isolated from the leaf extract of Dregea volubilis (Linn) Benth on plasma glucose concentration and lipid profile in streptozotocin induced diabetic Chinese water chestnut. Springer Plus. 2013;2:1–6. doi: 10.1186/2193-1801-2-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernawan UE, Sutarno S, Setyawan AD. Hypoglycaemic and hypolipidaemic activities of water extract of Lagerstroemia speciosa (L.) Pers. leaves in diabetic rat. Biofarmasi. 2004;2:15–23. [Google Scholar]
- 18.Nawwar M, Ayoub N, Hussein S, Hashim A, El-Sharawy R, Wende K, et al. A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Arch Pharm Res. 2012;35:761–7. doi: 10.1007/s12272-012-0501-4. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Narwal S, Kumar V, Prakash O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn Rev. 2011;5:19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hossain MA, Rahman SM. Isolation and characterisation of flavonoids from the leaves of me dicinal plant Orthosiphon stamineus. Arab J Chem. 2015;8:218–21. [Google Scholar]
- 21.Wozniak MA, Keely PJ. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cell. Biol Proced Online. 2005;7:144–61. doi: 10.1251/bpo112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son HL, Anh NP. Phytochemical composition, in vitro antioxidant and anticancer activities of quercetin from methanol extract of Asparagus cochinchinensis (LOUR.) Merr. tuber. Med Plant Res. 2013;7:3360–6. [Google Scholar]
- 23.Haryoto H, Muhtadi M, Indrayudha P, Azizah T, Suhendi A. Cytotoxic activity of sala (Cynometra ramiflora Linn) ethanol extract on HeLa, T47D, and WiDR Cells. J Penelitian Saintek. 2013;18:21–8. [Google Scholar]


