Dear Editor,
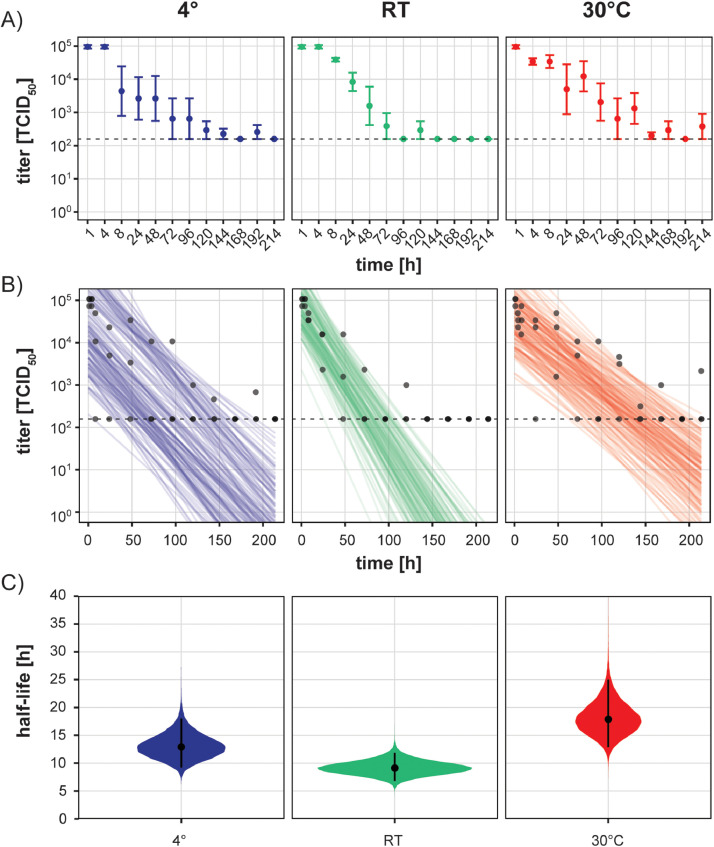
The ongoing severe respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic puts a large strain on public healthcare systems worldwide. Given that effective treatments and vaccines are not yet available, it is of utmost importance to elucidate potential routes of SARS-CoV-2 transmission to device effective transmission-based precautions. Ye et al. recently described SARS-CoV-2 contaminated surfaces in COVID-19 patient care areas in a hospital environment.1 Moreover, infectious virus has been shown to persist on surfaces for several hours to days at room temperature (RT).2 Despite the current consideration of respiratory droplets as the main route of SARS-CoV-2 transmission,3 contaminated surfaces could indicate the possibility of surface contact transmission. Importantly, temperature variation has been shown to influence the surface stability of SARS-CoV4 and moreover, recently differences on temperature-dependent SARS-CoV-2 stability in solution were reported.5 This raises the question whether seasonal changes which are accompanied by temperature fluctuations might actively influence virus stability. We examined the stability of SARS-CoV-2 on inanimate surfaces at 4°C, RT and 30°C in order to understand seasonal temperature variation of possible surface transmission. Surface stability over time was assessed with a carrier test on metal discs for 4h, 8h, 24h and subsequently every 24h up to 9 days at a humidity of 30-40%. Virus suspensions were mixed with 0.3 % bovine serum albumin (BSA) as interfering substance and dried on metal discs for 1h at RT. Initial virus (SARS-CoV-2/München-1.1/2020/929) concentration was 1.58 × 107 50% tissue culture infectious dose per milliliter [TCID50/mL] and declined to 9.63 × 104 TCID50/mL after 1h drying. At each individual time point after drying, the inoculated area was incubated for 1 min with sterile water and subsequently mixed with cold Dulbecco's modified minimal essential medium. The resulting suspension was serially diluted, and the TCID50/mL values were determined by crystal violet staining. Half-lives and decay rates of viable virus were calculated using a previously published Bayesian regression model2 (https://github.com/dylanhmorris/sars-cov-2-stability). Since the results of the model are strongly depended on the values assumed for the initial inoculum, we used the titers of the dried virus at t=1h as initial values.2 In contrast to the high stability of SARS-CoV-2 in solution5 the infectivity of the virus was strongly reduced upon drying. After 1h of drying on a metal disc, the measured viral titers were reduced up to 100-fold. However, after the initial loss of infectivity, the recovered virus titers remained stable over the next 4h to 8h with only minimal decline at 30°C and a larger variability at 4°C (Fig. 1 A). Beyond 8h we observed a stable, slow decline of viral titers at all temperatures over several days (Fig. 1A). We were able to recover detectable amounts of infectious virus even after 180h on metal surface. At all temperatures tested, we observed an exponential decay rate, which prompted us to use a previously developed algorithm to model possible decay rates and estimates for viral half-lives under the tested circumstances (Fig. 1B). Due to a higher variance in actually measured titers at 4°C and 30°C, the modelled regression lines follow a rather broad spectrum also mirrored in the greater confidence intervals for predicted half-lives (Fig. 1C). We estimated the half-lives of the three different ambient temperatures (Fig. 1C). At room temperature the median half-life is predicted to be 9.1h and thereby slightly higher than previous reports.2 These differences most originate from different initial titers of the inoculum and different experimental setups .The decay rate at 4°C was slower with an estimated median half-life of 12.9h. Surprisingly, virus incubation at 30°C after drying showed the highest predicted half-life with 17.9h. Overall, our results demonstrate that SARS-CoV-2 infectivity is strongly reduced during the initial drying process; however, afterwards the virus remains infectious in a dried state for several days regardless of ambient temperature changes. Of note, one caveat of this study is the constant humidity. Previous studies have shown that CoV survival on inanimate surfaces was dependent on the humidity with high (80%) and low (20%) humidity increasing survival compared to medium (50%) humidity.6
Fig. 1.
Temperature-dependent Infectivity of SARS-CoV-2 on inanimate surfaces. A) Measured TCID50/mL values of recovered virus at indicated timepoints in hours at 4°C (blue panel), room temperature (green panel) and 30°C (red panel). Dots indicate mean of three independent experiments along with the standard error shown as bars. Dashed lines mark the lower limit of quantification. B) Regression plots indicating the predicted decay of virus over time. Dots (partially off-set along the x-axes to reduce overlap) show individual TCID50/mL values of single experiments. Fifty lines per replicate represent possible decay patterns for each experimental condition2. C) Violin plots of estimated half-life ranges of the virus at indicated temperatures. The dots indicate the posterior median estimates with 95% confidence intervals.
We found that the surface stability of SARS-CoV-2 does not display major differences at 4°C, RT and 30°C. Our results challenge the previously suspected temperature-dependent virus surface stability, especially with regard to seasonality of the SARS-CoV-2 transmission. Our data, as well as other models implicate that higher temperatures (up to 30°C) do not necessarily inactivate SARS-CoV-2.2 Nevertheless, other human and environmental factors such as viral load, humidity, and solar radiation which were not considered in our controlled laboratory settings might further influence SARS-CoV-2 surface stability and thus cause variations in seasonal SARS-CoV-2 surface transmission.
Contributor's Statement
A. Kratzel, S. Steiner: study design, data collection, data interpretation, writing; D. Todt: data analysis, data interpretation, figure, writing; P. V'kovski: study design, data collection; Y. Brueggemann: study design, writing; J. Steinmann: study design, writing; E. Steinmann: study design, writing; V. Thiel: study design, writing; S. Pfaender: study design, data interpretation, writing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
We are grateful to Marcel Müller and Christian Drosten for providing us with cells and virus stocks. We thank the members of the Institute of Virology and Immunology, Bern, Switzerland and the Department for Molecular and Medical Virology, Ruhr University Bochum, Bochum, Germany for helpful suggestions and discussions.
Footnotes
Financial support: This study was supported by the Federal Ministry of Education and Research, Germany (BMBF; grant RAPID, #01KI1723A), and by the National Centre of Competence in Research (NCCR) RNA & Disease funded by the Swiss National Science Foundation.
References
- 1.Ye G. Environmental Contamination of SARS-CoV-2 in Healthcare Premises. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Doremalen N. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anfinrud P., Stadnytskyi V., Bax C.E., Bax A. Visualizing Speech-Generated Oral Fluid Droplets with Laser Light Scattering. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan S.M. Stability of SARS Coronavirus in Human Specimens and Environment and Its Sensitivity to Heating and UV Irradiation. Biomed. Environ. Sci. 2003 [PubMed] [Google Scholar]
- 5.Chin A.W.H. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010 doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]