Abstract
Objectives
Obesity is a modifiable risk factor for coronavirus disease 2019 (COVID-19)–related mortality. We estimated excess mortality in obesity, both ‘direct’, through infection, and ‘indirect’, through changes in health care, and also due to potential increasing obesity during lockdown.
Study design
The study design of this study is a retrospective cohort study and causal inference methods.
Methods
In population-based electronic health records for 1,958,638 individuals in England, we estimated 1-year mortality risk (‘direct’ and ‘indirect’ effects) for obese individuals, incorporating (i) pre-COVID-19 risk by age, sex and comorbidities, (ii) population infection rate and (iii) relative impact on mortality (relative risk [RR]: 1.2, 1.5, 2.0 and 3.0). Using causal inference models, we estimated impact of change in body mass index (BMI) and physical activity during 3-month lockdown on 1-year incidence for high-risk conditions (cardiovascular diseases, diabetes, chronic obstructive pulmonary disease and chronic kidney disease), accounting for confounders.
Results
For severely obese individuals (3.5% at baseline), at 10% population infection rate, we estimated direct impact of 240 and 479 excess deaths in England at RR 1.5 and 2.0, respectively, and indirect effect of 383–767 excess deaths, assuming 40% and 80% will be affected at RR = 1.2. Owing to BMI change during the lockdown, we estimated that 97,755 (5.4%: normal weight to overweight, 5.0%: overweight to obese and 1.3%: obese to severely obese) to 434,104 individuals (15%: normal weight to overweight, 15%: overweight to obese and 6%: obese to severely obese) would be at higher risk for COVID-19 over one year.
Conclusions
Prevention of obesity and promotion of physical activity are at least as important as physical isolation of severely obese individuals during the pandemic.
Keywords: Obesity, Coronavirus, Physical activity, Cardiovascular, Diabetes
Introduction
By 13 June 2020, there were 429,878 deaths and 7,800,566 people infected worldwide in the coronavirus disease 2019 (COVID-19) pandemic.1 More than one-third of the world's population (more than 3 billion people) is estimated to be under some form of ‘lockdown’, describing policy-driven, population-level physical isolation to avoid spread of infection.2
It is clear that the pandemic presents a profound insult directly through infection but also indirectly through behaviour change at the individual level (e.g. due to changes to employment and income, access to education and social isolation)3 and at the health system level.4 Moreover, as countries plan strategies to exit or scale down lockdown, policies to tackle COVID-19 may have unintended consequences on health care and outcomes for non-COVID-19 diseases, which we reported for cancer and cardiovascular diseases (CVDs).5 , 6
On 16 March 2020, the UK government specified ‘high-risk’ (now ‘vulnerable’) subgroups in case of infection,7 moving to the pandemic's ‘delay’ phase with stringent ‘physical distancing’ measures. On 22 March 2020, it announced 12 weeks of ‘shielding’ for 1.5 million ‘extremely vulnerable’ people in England with underlying conditions (including severe COPD) but without data underpinning the list of ‘extremely vulnerable’ or ‘high-risk’ conditions.8 On 23 March, the UK lockdown was announced, which is being eased since 11 May.
Among high-risk individuals, severe obesity (body mass index [BMI] ≥40) is a risk factor modifiable in relevant timescales.9 Obesity is common among patients with COVID-19, associated with increasing severity of the disease.10 , 11 Thus, physical isolation seems reasonable, but impact of age, sex and underlying conditions needs to be better characterised to better tailor policies if further lockdown periods are necessary. Regardless of COVID-19, obesity is associated with increased risk of several chronic conditions, including CVD, diabetes, chronic obstructive pulmonary disease (COPD) and chronic kidney disease (CKD).12 Prolonged periods of physical isolation could reduce physical activity and increase obesity,13 leading to more people being at high risk during the pandemic.
Using population-based electronic health records (EHRs) in England, we estimated (i) background mortality in severe obesity by underlying risk factors; (ii) direct and indirect excess deaths in individuals with severe obesity; and (iii) impact of BMI gain and physical activity on the incidence of the most common high-risk diseases for COVID-19 mortality as defined by National Health Service (NHS) guidance.
Methods
Data sources
We used EHR from primary care (Clinical Practice Research Datalink, CPRD-GOLD), hospitals (Hospital Episodes Statistics, HES), and death registry (Office of National Statistics, ONS) with prospective recording and follow-up; linked by CPRD and NHS Digital using unique healthcare identifiers.14 More than 99% of England's population is general practice (GP) registered. CPRD is representative by sociodemography and ethnicity.15 Approval was given by the Independent Scientific Advisory Committee (18_010R) of the UK Medicines and Healthcare products Regulatory Agency in accordance with the Declaration of Helsinki.
Study population and EHR phenotypes
Eligible individuals were 18–69 years old and registered with a GP between 1 January 1998 and 30 June 2016 with BMI and weight data and at least one year of follow-up. Demographic (age, gender, index of multiple deprivation quintiles and geographic region) and baseline characteristics were recorded at study entry (1 year after latest GP registration).16 We excluded individuals aged ≥70 years to focus on high-risk individuals due to obesity alone.
Weight (kg), height (m) and BMI (kg/m2) are recorded during GP registration, health checks and at clinical discretion.17 For those with >1 BMI measurement, we selected an eligible BMI record at random during the study period. We excluded BMI records (Fig. S1 in the supplementary material) in pregnancy if 2 same day observations differed by > 0.5 kg/m2, if an individual's highest BMI was more than double their lowest BMI record and where absolute difference between recorded and calculated BMI on the same date was >1 kg/m2. We defined underweight (BMI<18.5 kg/m2), normal weight (BMI = 18.5–24.9 kg/m2), overweight (BMI = 25–29.9 kg/m2), obesity (BMI≥30 kg/m2) and severe obesity (BMI≥40 kg/m2) (Web supplement ).
Estimating 1-year mortality
We estimated pre-COVID-19 1-year mortality risk with and without severe obesity using Kaplan-Meier analyses stratified by comorbidities (0, 1, 2 and 3+), scaling from CPRD (N = 1,958,184) to the whole English population, aged 18–69 years (n = 36,621,520 in 2018).18
Estimating 1-year direct and indirect excess deaths in people with severe obesity
Excess deaths were considered as direct (due to or with infection) or indirect (due to changes in health services). Direct excess deaths were estimated by applying relative risks [RRs] of 1.2, 1.5, 2 and 3, based on a published model and consistent with hazard ratios for CVD and COVID-19 deaths,4 , 19 in the absence of cohort studies of clinical cohorts of patients with obesity investigating all-cause mortality in those with and without infection. We modelled 10% infection rate (population prevalence of previous infection) based on recent seroprevalence estimates.20 , 21 Although infection rate will change depending on pandemic phase, we assumed infection rate over 1 year in line with the first wave. For the calculation of the excess number of deaths, we first calculated the cumulative risk of mortality over a one-year mortality risk from the ONS18 (in 2018, there were 106,656 deaths in 36,621,520 individuals aged 18–69 years, i.e., 1-year mortality risk = 0.2912%) and we used a bias correction factor to account for the fact that we expected higher mortality in people with at least one BMI measurement in CPRD. We applied a bias correction factor because we did not want to assume that the mortality rate in individuals who measured their BMI in the practice (between 1998 and 2016) was the same as that of (healthier) individuals who did not.
For direct and indirect excess deaths, we modelled 40% and 80% ‘population affected’ rates, where 10% were infected and affected directly and 30% and 70%, respectively, were uninfected but affected indirectly at corresponding RRs. Based on the ONS estimates of risk of excess non-COVID-19 deaths (indirect effect: RR 1.29) and likely long-term effects on mortality, we estimated direct and indirect excess deaths together by applying RR of 1.2 to 40%–80% of the population. Thus, we provide low (infection rate 10%; no indirect effect at RR 1.5, 2.0 and 3.0), medium (infection rate 10%; 30% indirectly affected at RR 1.2) and high (infection rate 10%; 70% indirectly affected at RR 1.2) estimates, projected to the whole English population.
Estimating direct and indirect effect of severe obesity on mortality
In Table S1, we estimated associations between BMI≥40 kg/m2 and 1-year mortality using Cox regression models, adjusting for (1) age and sex; (2) model 1 + 16 combinations of baseline CVD, diabetes, COPD and CKD; and (3) model 2 + baseline hypertension, gout, rheumatoid arthritis and diuretic use. We estimated the severe obesity effect on mortality that is mediated through combinations of chronic diseases (indirect effect) by subtracting the model 1 estimate [(hazard ratio-1)%] from that of models 2 and 3 (i.e. the difference method22).
Transition to the increased BMI group and incidence of high-risk conditions
We investigated the following three transitions to the higher BMI group: (i) normal weight (BMI: 18.5–24.9kg/m2(x6)) to overweight (BMI: 25.0–29.9kg/m2), (ii) overweight (BMI: 25.0–29.9kg/m2) to obese (BMI: ≥30kg/m2) and (iii) obese (BMI: 30.0–39.9kg/m2) to severely obese (BMI: ≥40kg/m2). To calculate 1-year incidence of CVD, COPD, diabetes and CKD, with all and none of the population increasing BMI group, we applied the parametric g-formula, separately in normal weight, overweight and non-severely obese individuals depending on whether they transitioned to a higher BMI group (Fig. S2). Using logistic regression, adjusted for age, sex, mental health conditions (recorded before first BMI measurement and during three months of BMI change), hypertension (recorded before first BMI measurement and during three months of BMI change), use of diuretics (recorded before first BMI measurement and during three months of BMI change) and cancer (recorded before first BMI measurement), we investigated associations between transition to the higher BMI group and 1-year incidence and mortality for the combined outcome. We used coefficients of the model to predict risk of the outcome, if transition was set to zero (i.e. no transition to the higher BMI group) and one (i.e. transition to the higher BMI group) for all individuals, with standardisation (the mean of all predicted values) and non-parametric bootstrapping from 500 samples to calculate confidence intervals. To estimate incidence of the combined outcome, we assumed different 3-month transition scenarios: normal weight to overweight (5.4%–15%), overweight to obese (5.0%–15%) and obese to severely obese (1.3%–6%) (Table 2). The 1st scenario of Table 1 , that is, 5.4%, 5.0% and 1.4%, was the observed one from our data set (pre-COVID transitions). We used 3 months in line with lockdown periods across different countries. In the same logistic regression models, we added physical activity in four categories (low, gentle, moderate and vigorous) to estimate its incremental association with 1-year incidence of CVD, diabetes, COPD and CKD. All analyses were performed using Stata 16 MP and R (version 3.4.3).
Table 2.
Individuals developing high-risk conditions for COVID-19a in 1 year in England, by BMI group and overall, under different scenarios of transition to higher BMI groups over 3 months.
| BMI group | Scenario 1 Normal weight: 5.4% Overweight: 5.0% Obese: 1.4%b |
Scenario 2 Normal weight: 8% Overweight: 8% Obese: 2% |
Scenario 3 Normal weight: 10% Overweight: 10% Obese: 3% |
Scenario 4 Normal weight: 12% Overweight: 12% Obese: 4% |
Scenario 5 Normal weight: 15% Overweight: 15% Obese: 6% |
|---|---|---|---|---|---|
| Normal weight | 325 | 479 | 598 | 718 | 898 |
| Overweight | 4256 | 6865 | 8581 | 10,298 | 12,872 |
| Obese | 93,174 | 140,111 | 210,167 | 280,222 | 420,334 |
| Total | 97,755 | 147,455 | 219,346 | 291,238 | 434,104 |
BMI, body mass index; COVID-19, coronavirus disease 2019; CI, confidence interval.
Cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease or severe obesity (BMI≥40 kg/m2).
Observed in 2 million individuals in England.
Table 1.
Transition to the higher BMI group over 3 months and 1-year incidence of high-risk conditions for COVID-19b.
| BMI group | Transition to the higher BMI groupa within 3 months vs no transition (odds ratio) (95% CI) | 1-year risk for high-risk conditions for COVID-19 ⴕ (%, 95% CI) |
|
|---|---|---|---|
| No individuals transition to the higher BMI group | All individuals transition to the higher BMI group | ||
| Normal weight N = 85,159 |
1.07 (0.78–1.48) | 0.64 (0.58–0.69) | 0.68 (0.48–0.90) |
| Overweight N = 81,066 |
1.44 (1.16–1.80) | 1.65 (1.56–1.75) | 2.35 (1.88–2.80) |
| Obese N = 87,534 |
1.50 (1.06–2.13) | 2.39 (2.28–2.50) | 3.52 (2.39–4.66) |
BMI, body mass index; COVID-19, coronavirus disease 2019; CI, confidence interval.
Transition to the higher BMI group: normal weight (BMI: 18.5–24.9 kg/m2) to overweight (BMI: 25.0–29.9 kg/m2), overweight (BMI: 25.0–29.9 kg/m2) to obese (BMI: ≥30 kg/m2) and obese (BMI: 30.0–39.9 kg/m2) to severely obese (BMI: ≥40 kg/m2).
Incidence of cardiovascular disease, chronic obstructive pulmonary disease, diabetes and chronic kidney disease in 1 year.
Results
Prevalence of underlying conditions
Figs. S1 and S2 illustrate the numbers of individuals used in our analyses. The proportion of individuals without comorbidities with BMI<40 kg/m2 was 84% and with BMI≥40 kg/m2 was 70% (Fig. S3). The prevalence of CVD, diabetes, COPD and CKD and all their combinations were higher among people with severe obesity (Fig. S3).
Baseline severe obesity and COVID-19–related excess 1-year mortality
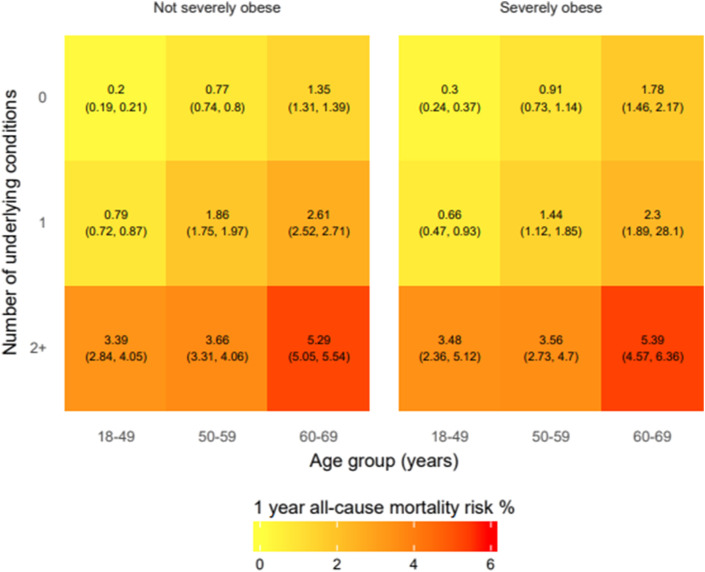
Baseline 1-year mortality risk was 0.83% overall, so we used a bias correction factor of 0.83/0.2912 = 2.85 in our analysis for the excess death calculations (i.e., 0.2912 is the mortality from the ONS in 2018 for individuals aged 18–69 years). Mortality at baseline was the highest in severely obese individuals aged 60–69 years with ≥2 underlying conditions (Fig. 1 ). The total effect of BMI≥40 kg/m2 on 1-year mortality is 1.53 (1.42–1.66), after age and sex adjustment (Table S1). The proportion of obesity mediated through all combinations of CVD, diabetes, COPD and CKD is 51%. With addition of gout, rheumatoid arthritis, psychiatric diseases and diuretic use, this proportion is 73%.
Fig. 1.
One-year mortality in 1,958,184 individuals in England, according to severe obesity, age and the number of underlying conditions.
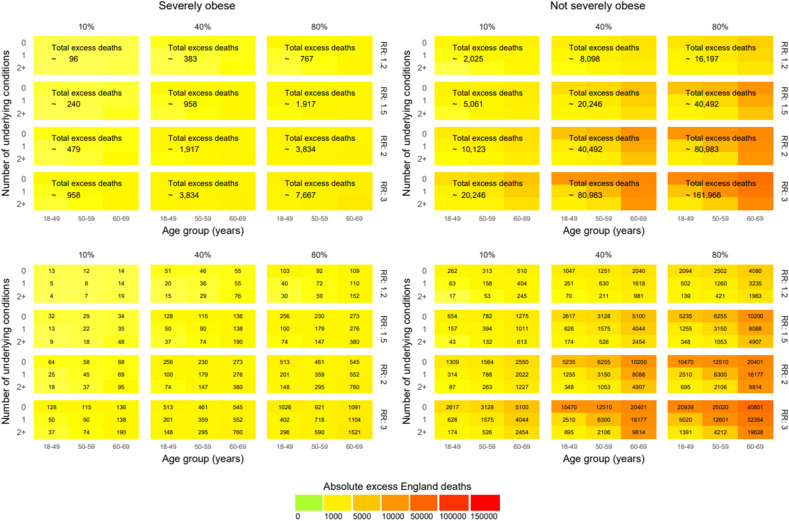
At 10% population prevalence of previous infection, there would 240, 479 and 958 excess deaths at RR 1.5, 2.0 and 3.0, respectively, in individuals with severe obesity. When 40% and 80% of the population is affected by the pandemic (directly and indirectly), at RR 1.2, the total excess deaths are estimated to be 383 and 767, respectively, for severe obesity (Fig. 2 ).
Fig. 2.
Excess deaths at 1 year in England in different pandemic scenarios by infection rate and relative risks (RRs) in people aged 18–69 years.
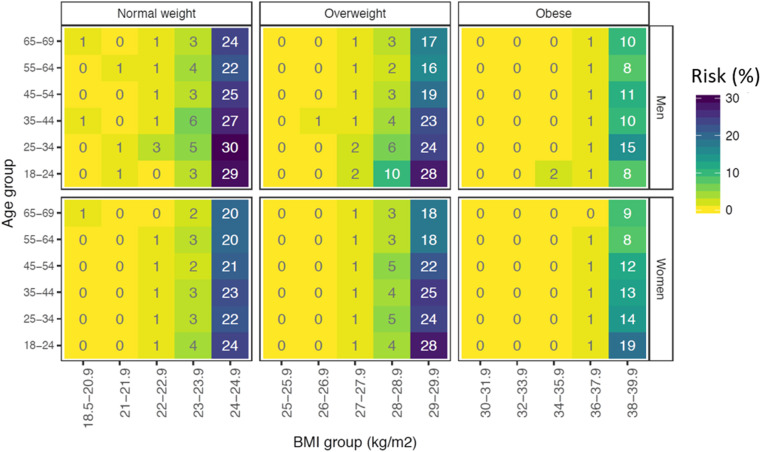
BMI transitions and 1-year incidence of high-risk conditions
The risk of transition to a BMI category over 3 months was higher in younger individuals (Fig. 3 ) and for those who were closer to the cut-offs of transition (Fig. 3). Transition to a higher BMI group was related to an increased risk for incidence of CVD, COPD, diabetes and CKD within 1 year: The odds ratios (ORs) were 1.07 (0.78–1.48), 1.44 (1.16–1.80) and 1.50 (1.06–2.13) for individuals with normal weight, overweight and obesity, respectively (Table 1). The risk of developing CVD, diabetes, COPD and CKD in 1 year would be 0.68% (0.48–0.90%), 2.35% (1.88–2.80%) and 3.52% (2.39–4.66%) if (due to lockdown) everybody transitioned from normal weight to overweight, from overweight to obesity and from obesity to severe obesity, respectively. Conversely, the risk of developing these diseases would be 0.64% (0.58–0.69%), 1.65% (1.56–1.75%) and 2.39% (2.28–2.50%) if normal weight, overweight and obese individuals, respectively, did not transition to higher BMI groups.
Fig. 3.
Risk of transition to a higher body mass index (BMI) category over 3 months in 85,159 normal weight, 81,066 overweight and 87,534 obese individuals in England, by age and sex.
Assuming that 3-month BMI transitions are in line with average 3-month transitions in CPRD (5.4% from normal weight to overweight, 5% from overweight to obese and 1.3% from obese to severely obese), there would be 97,755 individuals at higher risk for COVID-19 in one year. Assuming higher transition rates of 15% from normal weight to overweight, 15% from overweight to obese and 6% from obese to severely obese, we estimated 434,104 high-risk individuals (Table 2 ).
Physical activity and 1-year incidence of high-risk conditions
The incidence of high-risk conditions was reduced with moderate physical activity (Odds ratio (OR): 0.55, 0.30–0.99) but not with gentle (0.71, 0.38–1.31) and vigorous (0.74, 0.35–1.58) activity in the normal weight group. In overweight individuals, moderate (0.53, 0.38–0.74) and vigorous (0.50, 0.30–0.84) activity were associated with a reduced incidence of high-risk conditions but not gentle activity (0.76, 0.55–1.06). In obese individuals, gentle (0.78, 0.62–0.97), moderate (0.64, 0.51–0.81) and vigorous (0.49, 0.30–0.81) activity were associated with a reduced incidence of high-risk conditions (Table 3 ). Results remained similar, even after accounting for missing values for physical activity (data not shown).
Table 3.
Physical activity and 1-year incidence of high-risk conditions for COVID-19 in 85 308 individuals.a
| BMI group | Physical activity: odds ratio (95% CI) |
|||
|---|---|---|---|---|
| Low | Gentle | Moderate | Vigorous | |
| Normal weight N = 27,992 |
ref | 0.71 (0.38–1.31) | 0.55 (0.30–0.99) | 0.74 (0.35–1.58) |
| Overweight N = 28,436 |
ref | 0.76 (0.55–1.06) | 0.53 (0.38–0.74) | 0.50 (0.30–0.84) |
| Obese N = 28,880 |
ref | 0.78 (0.62–0.97) | 0.64 (0.51–0.81) | 0.49 (0.30–0.81) |
BMI, body mass index; COVID-19, coronavirus disease 2019; CI, confidence interval.
Incidence of cardiovascular disease, chronic obstructive pulmonary disease, diabetes and chronic kidney disease in 1 year.
Discussion
In this large-scale, population-based EHR analysis regarding severe obesity, we investigated excess mortality due to COVID-19 and due to lockdown in England with four main findings. First, we found that severe obesity was generally not associated with high background mortality risk, other than with two or more underlying conditions. Second, relatively few excess deaths are likely to be directly or indirectly associated with severe obesity, compared with obesity overall. Third, lockdown could result in 97,755 to 434,104 individuals transitioning to ‘high-risk’ groups for COVID-19 infection, with significant excess deaths in future. Fourth, physical activity could be protective against adverse outcomes during lockdown.
Prevalence of obesity is increasing over the last decade in all countries, increasing the public health importance of both baseline and excess COVID-19–related risk.8 In obesity and severe obesity, the risk of diabetes and CVD is two and ten times higher than that for non-obese people, respectively,23 while weight gain is an independent risk factor for both diabetes and CVD.24, 25 We predict that, as at baseline, most of the excess mortality associated with COVID-19 in severely obese individuals will be in people with multimorbidity which should be the focus for prevention, early recognition and aggressive risk factor management. The management of chronic diseases is likely to be indirectly affected during the pandemic and so must be particularly prioritised in the context of obesity.
The largest UK primary care analysis to date showed that of 5683 COVID-19 deaths, 257 were in severely obese individuals (hazard ratio [HR]: 2.27, 1.99–2.58, compared with non-obese individuals), whereas 1631 were in non-severely obese people (HR: 1.56, 1.41–1.73).19 In the UK intensive care audit, 7.7% of patients were severely obese, whereas 31.3% have non-severe obesity.10 There are currently three postulated mechanisms for increased risk observed with obesity: (i) increased susceptibility to infection (e.g. modification of both innate and adaptive immune responses); (ii) increased risk associated with long-term chronic conditions (e.g. CVD, diabetes, COPD and CKD) and (iii) increased COVID-19–specific effects on obese individuals.26 Although we do not present COVID-19–specific data, our data suggest that obesity and its prevention should be viewed more holistically in terms of preventing onset of chronic diseases, rather than emphasising severe obesity. Moreover, these data also support the global picture where high BMI accounts for 4.0 million deaths, with nearly 40% occurring in non-obese individuals, and more than two-thirds due to CVD.8
The pandemic is having consequences far beyond infection on individuals, populations and health systems, including reductions in bariatric surgery.27 The rise of childhood obesity in Italy28 and the potential rise in diabetes in India29 during lockdown have been described, but to our knowledge, this is the first study to model BMI changes across all BMI categories and major chronic diseases together. We show that BMI increases during lockdown could significantly increase the population at high risk from COVID-19 and also lead to indirect future deaths through these incident diseases.
In the UK, as in other European countries, a study using tracking data from wearables and smartphones showed a dramatic increase in homestay duration and a sharp decrease in maximum distance from home and step count, starting from one week before lockdown.30 Physical activity is reduced in obese people during the pandemic,31 and lifestyle modification has been suggested to be an important adjunct to physical distancing and isolation measures.32 We add that exercise reduces incidence of high-risk conditions across the whole BMI range. Exercise should be encouraged during lockdown but also the impact of lockdown on physical activity should be taken into account as policies to ease lockdown are considered and implemented.
Strengths and limitations
Key strengths of this study include use of population-based EHRs, large sample sizes, utilisation of contemporary and clinically relevant measurements of weight and height, records of relevant comorbidities and causal inference methods (i.e. the g-formula). Normal weight, overweight and obese individuals have different underlying risk for the occurrence of these chronic conditions; hence, it is more appropriate to study them separately. Most observational studies cannot focus on this relationship in this resolution, mainly due to their sample size. Several limitations should be noted. Our observational study cannot exclude unmeasured confounding. Because we use EHRs, there may be differences between actual and recorded date of incidence of chronic diseases, which would result in bias due to reverse causation. However, this would actually result in underestimation of BMI gain and development of high-risk conditions, except when BMI loss is a result of the disease itself, which would cause potential increased mortality risk. Using EHRs, we made assumptions, for example, linear trend for BMI change between 2 body weight measurements recorded <6 months apart, and the last record for physical activity in the last 4 years. Another limitation of this article is that the individuals who were analysed for BMI transitioning in this study might not be a representative sample of the English population because they had 2 BMI measurements just a few months apart. It is likely that these people had preclinical diseases, even if they appeared healthy (i.e. there is difference between recorded and actual date of a chronic disease) The same problem also exists for physical activity that is measured infrequently.
Conclusions
Our analyses here suggest that despite high prevalence of obesity and underlying conditions, the actual background and excess COVID-19–related mortality due to severe obesity is unlikely to be high compared with other high-risk conditions and is likely to be mediated through other comorbidities. Burden of chronic diseases, even with a 3-month lockdown, may lead to a greater burden of excess deaths, highlighting avoidance of BMI gain and physical activity as public health priorities during the pandemic.
Author statement
Ethical approval
None declared.
Funding
M.K. is funded by the British Heart Foundation (grant: FS/18/5/33319). A.B. is supported by research funding from the National Institute for Health Research (NIHR), British Medical Association, Astra-Zeneca and UK Research and Innovation. A.G.L. is supported by funding from the National Institute for Health Research (NIHR) University College London Hospitals Biomedical Research Centre (BRC714/HI/RW/101440), NIHR Great Ormond Street Hospital Biomedical Research Centre (19RX02) and the Health Data Research UK Better Care Catalyst Award. H.H. is an NIHR Senior Investigator and is funded by the NIHR University College London Hospitals Biomedical Research Centre. H.H. and R.J.B.D. are supported by Health Data Research UK (grant No. LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and Wellcome Trust. H.H., A.B. and R.J.B.D. are supported by The BigData@Heart Consortium, funded by the Innovative Medicines Initiative-2 Joint Undertaking under grant agreement No. 116074. R.J.B.D. is supported by1 NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, London, U.K.,2 the National Institute for Health Research University College London Hospitals Biomedical Research Centre,3 the UK Research and Innovation London Medical Imaging & Artificial Intelligence Centre for Value Based Healthcare and4 the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King's College Hospital NHS Foundation Trust.
Competing interests
None declared.
Author contributions
M.K. and A.B. answered research questions of the study. M.K., A.B., S.D. and H.H. contributed in funding. M.K., A.B., L.P. and A.L. contributed in study design and analysis plan of the study. L.P. and S.D. prepared data, including electronic health record phenotyping in the CALIBER open portal. M.K., L.P. and A.L. contributed in statistical analysis of the study. A.B. and M.K. drafted initial and final versions of the manuscript. All authors critically reviewed the early and final versions of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2020.12.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Worldometer . 2020. Coronavirus worldwide graphs.https://www.worldometers.info/coronavirus/worldwide-graphs/ [Google Scholar]
- 2.Business insider. 29 April 2020. https://www.businessinsider.com/countries-on-lockdown-coronavirus-italy-2020-3?r=US&IR=T [Google Scholar]
- 3.Douglas M., Katikireddi S.V., Taulbut M., McKee M., McCartney G. Mitigating the wider health effects of covid-19 pandemic response. BMJ. 2020 Apr 27;369:m1557. doi: 10.1136/bmj.m1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L. Estimating excess 1-year mortality from COVID-19 according to underlying conditions and age in England: a rapid analysis using NHS health records in 3.8 million adults. Lancet. 2020. May 30;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai A.G., Pasea L., Banerjee A., Hall G., Denaxas S., Chang W.H. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020 Nov 17;10(11) doi: 10.1136/bmjopen-2020-043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee A., Chen S., Pasea L., Lai A., Katsoulis M., Denaxas S. Excess deaths in people with cardiovascular diseases during the COVID-19 pandemic. Eur J Prev Cardiol. 2020 doi: 10.1093/eurjpc/zwaa155. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health England . 16 March 2020. Guidance on social distancing for everyone in the UK and protecting older people and vulnerable adults.https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults [Google Scholar]
- 8.Uk Government. https://www.gov.uk/government/news/major-new-measures-to-protect-people-at-highest-risk-from-coronavirus (accessed 29 April 2020).
- 9.GBD 2015 Obesity Collaborators. Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017 Jul 6;377(1):13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020 Apr 22 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frühbeck G., Baker J.L., Busetto L., Dicker D., Goossens G.H., Halford J.C.G. European association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts. 2020 Apr 27:1–5. doi: 10.1159/000508082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyberg S.T., Batty G.D., Pentti J., Virtanen M., Alfredsson L., Fransson E.I. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. Lancet Public Health. 2018 Oct;3(10):e490–e497. doi: 10.1016/S2468-2667(18)30139-7. Epub 2018 Sep. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G., Henry B.M., Bovo C., Sanchis-Gomar F. Health risks and potential remedies during prolonged lockdowns for coronavirus disease 2019 (COVID-19) Diagnosis (Berl) 2020 Apr 7 doi: 10.1515/dx-2020-0041. pii:/j/dx.ahead-of-print/dx-2020-0041/dx-2020-0041.xml, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Denaxas S., Gonzalez-Izquierdo A., Direk K., Fitzpatrick N.K., Fatemifar G., Banerjee A. UK phenomics platform for developing and validating electronic health record phenotypes: CALIBER. J Am Med Inf Assoc. 2019;26(12):1545–1559. doi: 10.1093/jamia/ocz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koudstaal S., Pujades-Rodriguez M., Denaxas S., Gho J.M.I.H., Shah A.D., Yu N. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population-based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail. 2017 Sep;19(9):1119–1127. doi: 10.1002/ejhf.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah A.D., Langenberg C., Rapsomaniki E., Denaxas S., Pujades-Rodriguez M., Gale C.P. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson B.D., Aveyard P., Bankhead C.R., Hamilton W., Hobbs F.D.R., Lay-Flurrie S. Determinants and extent of weight recording in UK primary care: an analysis of 5 million adults' electronic health records from 2000 to 2017. BMC Med. 2019 Nov 29;17(1):222. doi: 10.1186/s12916-019-1446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Office for National Statistics . Jan 17, 2020. Deaths by single year of age tables, UK.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesdeathsbysingleyearofagetables [Google Scholar]
- 19.The OpenSAFELY Collaborative. Williamson E., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E. Medrxiv; 7 May 2020. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients.https://www.medrxiv.org/content/10.1101/2020.05.06.20092999v1.full.pdf [Google Scholar]
- 20.Office of National Statistics Coronavirus (COVID-19) infection survey. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/coronaviruscovid19infectionsurveydata
- 21.Valenti L., Bergna A., Pelusi S., Facciotti F., Lai A., Tarkowski M. May 31 2020. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 Milan outbreak.https://www.medrxiv.org/content/10.1101/2020.05.11.20098442v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWeele T.J. A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology. 2013 Mar;24(2):224–232. doi: 10.1097/EDE.0b013e318281a64e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kivimäki M., Kuosma E., Ferrie J.E., Luukkonen R., Nyberg S.T., Alfredsson L. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017 May 19;2(6):e277–e285. doi: 10.1016/S2468-2667(17)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzi L., Radaelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020 Apr 5 doi: 10.1007/s00592-020-01522-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsoulis M., DeStavola B., Diaz-Ordaz K., Gomes M., Lai A., Lagiou P. Weight change and the incidence of cardiovascular diseases in adults with normal weight, overweight and obesity without chronic diseases. emulating trials using electronic health records Medrxiv. 2020. May 27:2020. https://www.medrxiv.org/content/10.1101/2020.05.14.20102129v2 [Google Scholar]
- 26.Sattar N., McInnes I.B., McMurray J.J.V. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 Apr 22;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 27.Rubino F., Cohen R.V., Mingrone G., le Roux C.W., Mechanick J.I., Arterburn D.E. Bariatric and metabolic surgery during and after the COVID-19 pandemic: DSS recommendations for management of surgical candidates and postoperative patients and prioritisation of access to surgery. Lancet Diabetes Endocrinol. 2020 Jul;8(7):640–648. doi: 10.1016/S2213-8587(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrobelli A., Pecoraro L., Ferruzzi A., Heo M., Faith M., Zoller T. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity. 2020 Apr 30;(4):319–323. doi: 10.1002/oby.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosal S., Sinha B., Majumder M., Misra A. Estimation of effects of nationwide lockdown for containing coronavirus infection on worsening of glycosylated haemoglobin and increase in diabetes-related complications: a simulation model using multivariate regression analysis. Diabetes Metab Syndr. 2020 Apr 10;14(4):319–323. doi: 10.1016/j.dsx.2020.03.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun S., Folarin A.A., Ranjan Y., Rashid Z., Conde P., Stewart C., RADAR-CNS consortium . Arxiv; 2020. Using smartphones and wearable devices to monitor behavioural changes during COVID-19.https://arxiv.org/ftp/arxiv/papers/2004/2004.14331.pdf 17 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers N.T., Waterlow N., Brindle H.E., Enria L., Eggo R.M., Lees S. Medrxiv; 2020. Behavioural change towards reduced intensity physical activity is disproportionately prevalent among adults with serious health issues or self-perception of high risk during the UK COVID-19 lockdown.https://www.medrxiv.org/content/10.1101/2020.05.12.20098921v1 May 18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho F.H., Celis-Morales C.A., Gray S.R., Katikireddi S.V., Niedzwiedz C.L., Hastie C. Medrxiv; 2020. Modifiable and non-modifiable risk factors for COVID-19: results from UK Biobank.https://www.medrxiv.org/content/10.1101/2020.04.28.20083295v1.full.pdf May 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.