Abstract
Background
To date, influenza epidemics have been considered suitable for use as a model for the COVID-19 epidemic, given that they are respiratory diseases with similar modes of transmission. However, data directly comparing the two diseases are scarce.
Methods
We did a nationwide retrospective cohort study using the French national administrative database (PMSI), which includes discharge summaries for all hospital admissions in France. All patients hospitalised for COVID-19 from March 1 to April 30, 2020, and all patients hospitalised for influenza between Dec 1, 2018, and Feb 28, 2019, were included. The diagnosis of COVID-19 (International Classification of Diseases [10th edition] codes U07.10, U07.11, U07.12, U07.14, or U07.15) or influenza (J09, J10, or J11) comprised primary, related, or associated diagnosis. Comparisons of risk factors, clinical characteristics, and outcomes between patients hospitalised for COVID-19 and influenza were done, with data also stratified by age group.
Findings
89 530 patients with COVID-19 and 45 819 patients with influenza were hospitalised in France during the respective study periods. The median age of patients was 68 years (IQR 52–82) for COVID-19 and 71 years (34–84) for influenza. Patients with COVID-19 were more frequently obese or overweight, and more frequently had diabetes, hypertension, and dyslipidaemia than patients with influenza, whereas those with influenza more frequently had heart failure, chronic respiratory disease, cirrhosis, and deficiency anaemia. Patients admitted to hospital with COVID-19 more frequently developed acute respiratory failure, pulmonary embolism, septic shock, or haemorrhagic stroke than patients with influenza, but less frequently developed myocardial infarction or atrial fibrillation. In-hospital mortality was higher in patients with COVID-19 than in patients with influenza (15 104 [16·9%] of 89 530 vs 2640 [5·8%] of 45 819), with a relative risk of death of 2·9 (95% CI 2·8–3·0) and an age-standardised mortality ratio of 2·82. Of the patients hospitalised, the proportion of paediatric patients (<18 years) was smaller for COVID-19 than for influenza (1227 [1·4%] vs 8942 [19·5%]), but a larger proportion of patients younger than 5 years needed intensive care support for COVID-19 than for influenza (14 [2·3%] of 613 vs 65 [0·9%] of 6973). In adolescents (11–17 years), the in-hospital mortality was ten-times higher for COVID-19 than for influenza (five [1·1% of 458 vs one [0·1%] of 804), and patients with COVID-19 were more frequently obese or overweight.
Interpretation
The presentation of patients with COVID-19 and seasonal influenza requiring hospitalisation differs considerably. Severe acute respiratory syndrome coronavirus 2 is likely to have a higher potential for respiratory pathogenicity, leading to more respiratory complications and to higher mortality. In children, although the rate of hospitalisation for COVID-19 appears to be lower than for influenza, in-hospital mortality is higher; however, low patient numbers limit this finding. These findings highlight the importance of appropriate preventive measures for COVID-19, as well as the need for a specific vaccine and treatment.
Funding
French National Research Agency.
Introduction
In December, 2019, a new disease now known as COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged as a major world threat. Considering its ability to spread and the relatively low case-fatality rates initially reported in China, SARS-CoV-2 was thought to be more similar to influenza than SARS-CoV-1.1 The preventive measures for this new infection were based on a comparison with the influenza virus because the viruses have similar modes of transmission and cause respiratory disease. Patients with COVID-19 were also anticipated to benefit from the clinical knowledge and care management of influenza.
A global pandemic was declared on March 11, 2020. By July 10, 2020, more than 12 million cases of COVID-19 and 554 000 COVID-19-related deaths had been identified worldwide. In France, it had already caused more than 30 000 deaths, nearly 20 000 of which were in hospital.2 At this point, COVID-19 had developed into a pandemic associated with substantial morbidity and mortality, putting great strain on hospitals and the health system in general.
Research in context.
Evidence before this study
We searched PubMed with the terms “COVID-19” and “influenza” for articles in any language published up to July 16, 2020, that compared clinical characteristics, intensive care unit (ICU) admission, morbidity, and mortality of patients hospitalised for COVID-19 and for seasonal influenza. After excluding co-infection studies and studies of influenza pandemics, we identified five studies. An international study compared comorbidity and previous medications in adult patients (≥18 years) hospitalised either for COVID-19 or for seasonal influenza over the 2014–19 period. Another study focused on the risk of ischaemic stroke, including ICU admission and in-hospital mortality, of adult patients visiting emergency departments or hospitalised for COVID-19 in 2020 in two New York hospitals compared with adult patients with seasonal influenza from 2016 to 2018. The three other studies identified by our search had smaller populations. One small study was done in a French hospital and compared adult patients, and another focused on children younger than 1 year, comparing those hospitalised for influenza in the past few years to published features of COVID-19 in low-income and middle-income countries. Finally, another small study compared the clinical characteristics and treatments of children under the age of 5 years hospitalised in Wuhan for COVID-19 or influenza. Therefore, little information is available on the comparative burden of COVID-19 and seasonal influenza, particularly in children, who are considered to be at low risk of severe disease.
Added value of this study
This study provides novel information about hospitalised patients with COVID-19. Our analysis uses a large national database including more than 89 000 patients with COVID-19 and more than 45 000 patients with seasonal influenza, including patients of all ages. We identified excess mortality associated with COVID-19 relative to seasonal influenza, with an age-standardised mortality ratio of 2·8. We evaluated differences between the characteristics of the two epidemics by age group, and more specifically in children aged 0–5 years (around 600 patients with COVID-19 and 7000 with influenza) and 6–17 years (around 600 patients with COVID-19 and 2000 with influenza).
Implications of all the available evidence
In-hospital case mortality was found to be higher in younger patients with COVID-19 compared with younger patients with influenza, even though children were at a lower risk of hospitalisation for COVID-19 than for influenza (as observed in our study and previously). However, the findings relating to deaths in children with COVID-19 are based on small numbers and should be treated with caution. More generally, the severe forms of COVID-19 and seasonal influenza requiring hospitalisation differ considerably. This underlines the need for additional studies to better characterise and understand the role and relative importance of risk factors associated with COVID-19 mortality, such as obesity, particularly in adolescents. This study also reinforces the importance of preventive and curative measures against both diseases. These data are particularly relevant as the epidemic continues to grow around the world and several countries prepare for potential overlapping of the seasonal influenza and COVID-19 epidemics.
The case-fatality rate in COVID-19 appears to be higher than in seasonal influenza, even though both diseases mainly affect older adults (>65 years) with frailty. The higher case-fatality of COVID-19 could be due to differences in underlying comorbidities of patients, the pathogenicity of the virus, population immunity, and host responses to infection. For example, vaccines and approved treatments are available for influenza but not for COVID-19. Moreover, the considerable strain that was put on hospitals within a short period of time led to limitations in available care for the patients who were most frail.
Few studies have directly compared the respective burdens of the COVID-19 and influenza epidemics.3, 4, 5 A comprehensive assessment of the outcomes and mortality of the two diseases and risk factors for hospitalisation and mortality could be used to identify specific at-risk populations, to strengthen and focus specific preventive measures in these populations, and to help define the future needs of health-care facilities. The aim of the present study was to compare, using French nationwide data, the population hospitalised for COVID-19 and the population hospitalised for influenza during the 2018–19 season, and to assess the differences in terms of risk factors, clinical characteristics, and outcomes.
Methods
Study design and participants
We did a retrospective cohort study using the national Programme de médicalisation des systèmes d'information (PMSI) database, which is designed to include discharge summaries for all inpatients admitted to public and private hospitals in France. Inspired by the American diagnosis-related group (DRG) model,6 the gathering of national administrative health data was established in France in 1991 and extended to all French health-care facilities in 1997. This coding system was initially designed to analyse hospital activity and to contribute to the development of strategic health-care plans. Since 2008, each hospital's budget depends on the medical activity described in a specific computer program that compiles discharge abstracts for all admissions. The information in these abstracts is anonymous and covers both medical and administrative data. Diagnoses identified during the hospital stay are coded according to the 10th edition of the International Classification of Diseases (ICD-10), and procedures done during hospitalisation are coded according to the French Common Classification of Medical Procedures. Each facility produces its own standardised anonymous dataset, and these are compiled to create a national dataset.
In France, stage 1 of the COVID-19 epidemic was declared on Feb 23, 2020; stage 2 was declared on Feb 29; and stage 3 was declared on March 14. The peak of the epidemic in France occurred during the second week of April, 2020. Although during stages 1 and 2 all patients with COVID-19 were hospitalised regardless of clinical presentation, only those in a serious clinical condition were admitted to hospital during stage 3. A PMSI database was developed for COVID-19, and hospitals were asked to perform accelerated data transmission for patients with COVID-19 from March, 2020, onwards at the request of the government, according to the decree dated April 21, 2020.
For the COVID-19 cohort in this study, all patients hospitalised for COVID-19 from March 1 to April 30, 2020, were included, regardless of their age. Patients were followed up until the end of their hospital stay, even if that date was after April. Hospital stays for COVID-19 were identified by primary diagnoses, related diagnoses, or associated diagnoses, with ICD-10 codes U07.10, U07.11, U07.12, U07.14, or U07.15.7 For the influenza cohort, all patients in the PMSI database hospitalised during the 2018–19 influenza outbreak period (admitted from Dec 1, 2018, to Feb 28, 2019), and identified by ICD-10 codes J09, J10, or J11 (as primary, related, or associated diagnoses) were included, regardless of their age.
This study was approved by the Comité Ethique et Scientifique pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé (CESREES) and L'Institut national des données de santé (INDS, registration number 1611357) and authorised by the Commission nationale de l'informatique et des sibertés (CNIL, registration number DR-2020–250).
Variables
The following variables were extracted for each inpatient stay: age, sex, transfer to an intensive care unit (ICU), and hospital death. We also included all diagnoses (ICD-10 codes for primary, related, and associated diagnoses) recorded in the discharge abstracts for the included hospital stays (COVID-19 and influenza) to analyse comorbidities (hypertension, diabetes, dementia, HIV, heart failure, chronic respiratory and kidney diseases, cirrhosis, peripheral vascular disease, overweight (body-mass index ≥25 kg/m2), dyslipidaemia, deficiency anaemia, pulmonary bacterial infection, immunocompromised status) and complications (acute respiratory and kidney diseases, stroke, myocardial infarction, atrial fibrillation, venous thrombosis including pulmonary embolism). Deficiency anaemia included iron anaemia and other causes of deficiency anaemia. Immunocompromised patients were those with agranulocytosis, immunodeficiency, medullar aplasia and cancer treated by chemotherapy. We also identified ventilation procedures. The Charlson comorbidity index8 and the Elixhauser comorbidity score9 were retrieved. We also recorded the social deprivation index (considering the socioeconomic environment of the patient).10 In theory, this index ranges from –6·4 for the least deprived areas to 21·2 for the most deprived areas. Thus, the higher the score, the more disadvantaged the municipality. We divided this index into four quartiles, with the lowest quartile representing the least deprived group.
Nine age categories were assessed: younger than 18 years, 18–30 years, 31–40 years, 41–50 years, 51–60 years, 61–70 years, 71–80 years, 81–90 years, and older than 90 years. For patients younger than 18 years, four subcategories were created: younger than 1 year, 1–5 years, 6–10 years, and 11–17 years. All identified medical conditions (both baseline comorbidities and complications) were accounted for in relation to the patient. If a patient had a condition at least once during one of the stays for COVID-19 or for influenza, then the patient was considered to have had the condition.
Statistical analysis
Qualitative variables are provided as frequencies (percentages), and quantitative variables are provided as the mean (SD) or median (IQR). The different variables studied in the cohort of patients hospitalised for COVID-19 were compared with variables of the influenza population, when applicable, using the χ2 test or the Fisher's exact test (for qualitative variables) and Student's t-test or Mann-Whitney U test (for quantitative variables), to evaluate whether some populations were more represented among COVID-19 cases. These comparisons were made for all patients and then by age group.
To account for a possible age difference between the two populations, we also stratified the results by age. Furthermore, we estimated an age-standardised mortality ratio by calculating a mortality ratio between the observed number of hospital deaths in the COVID-19 population and the number of deaths that would be expected with the same influenza age-specific case-fatality rates. We also estimated the relative risk of death between patients with COVID-19 and patients with influenza patients, with the associated 95% CI. Considering the potential heterogeneity of patients admitted to hospital in the three stages of the epidemic, we did a sensitivity analysis restricted to stage 3 of the epidemic (March 14, 2020, onwards).
The statistical significance threshold was set at p<0·05. All analyses were done using SAS version 9.4.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author and JC had full access to all of the data in the study and had final responsibility for the decision to submit for publication. All authors had access to all the data reported in the study.
Results
This retrospective, nationwide cohort study included 89 530 patients who were hospitalised for COVID-19 between March 1 and April 30, 2020, and 45 819 patients who were hospitalised for seasonal influenza between Dec 1, 2018, and Feb 28, 2019. Almost twice as many patients were hospitalised for COVID-19 than for seasonal influenza, over a shorter time period (2 months for COVID-19 and 3 months for seasonal influenza). The baseline characteristics of patients are presented in table 1 . Patients hospitalised for COVID-19 were more often male than were those hospitalised for influenza. The age distribution also differed, with a median age of 68 years (IQR 52–82) for COVID-19 and 71 (34–84) for influenza. The proportions of patients at the extreme ends of the age categories were significantly greater for influenza (8942 [19·5%] <18 years and 15 366 [33·5%] >80 years) than for COVID-19 (1227 [1·4%] <18 years and 24 242 [27·0%] >80 years).
Table 1.
Main baseline characteristics of patients hospitalised in France for COVID-19 or seasonal influenza
| COVID-19 (n=89 530) | 2018–19 seasonal influenza (n=45 819) | p value | |||
|---|---|---|---|---|---|
| Sex | |||||
| Female | 42 035 (47·0%) | 23 701 (51·7%) | <0·0001 | ||
| Male | 47 495 (53·0%) | 22 118 (48·3%) | .. | ||
| Age, years | |||||
| Mean (SD) | 65 (20) | 59 (32) | <0·0001 | ||
| Median (IQR) | 68 (52 to 82) | 71 (34 to 84) | .. | ||
| Range | 0 to 106 | 0 to 108 | .. | ||
| Age category, years | |||||
| <18 | 1227 (1·4%) | 8942 (19·5%) | <0·0001 | ||
| 18–30 | 4384 (4·9%) | 1836 (4·0%) | .. | ||
| 31–40 | 6065 (6·8%) | 1568 (3·4%) | .. | ||
| 41–50 | 8892 (9·9%) | 1626 (3·5%) | .. | ||
| 51–60 | 13 110 (14·6%) | 3164 (6·9%) | .. | ||
| 61–70 | 15 345 (17·1%) | 5624 (12·3%) | .. | ||
| 71–80 | 16 265 (18·2%) | 7693 (16·8%) | .. | ||
| 81–90 | 17 841 (19·9%) | 11 276 (24·6%) | .. | ||
| >90 | 6401 (7·1%) | 4090 (8·9%) | .. | ||
| Social deprivation score | |||||
| Mean (SD) | −0·27 (1·78) | −0·17 (1·56) | <0·0001 | ||
| Median (IQR) | −0·15 (−1·23 to 0·92) | −0·01 (−0·96 to 0·87) | .. | ||
| Social deprivation quartile* | |||||
| First (least deprived) | 22 922 (26·9%) | 9195 (21·2%) | <0·0001 | ||
| Second | 20 741 (24·3%) | 11 223 (25·9%) | .. | ||
| Third | 19 926 (23·4%) | 12 608 (29·1%) | .. | ||
| Fourth (most deprived) | 21 593 (25·3%) | 10 256 (23·7%) | .. | ||
| Comorbidity scores | |||||
| Charlson comorbidity index | |||||
| Mean (SD) | 1·19 (1·85) | 1·23 (1·78) | <0·0001 | ||
| Median (IQR) | 0 (0 to 2) | 1 (0 to 2) | .. | ||
| 0 | 45 237 (50·5%) | 21 715 (47·4%) | <0·0001 | ||
| 1 | 19 730 (22·0%) | 10 539 (23·0%) | .. | ||
| 2 | 10 654 (11·9%) | 6132 (13·4%) | .. | ||
| ≥3 | 13 909 (15·5%) | 7433 (16·2%) | .. | ||
| Elixhauser comorbidity score | |||||
| Mean (SD) | 2·01 (1·96) | 2·01 (2·03) | 0·59 | ||
| Median (IQR) | 1 (0 to 2) | 2 (0 to 3) | .. | ||
| 0 | 25 701 (28·7%) | 14 080 (30·7%) | <0·0001 | ||
| 1 | 17 497 (19·5%) | 8691 (19·0%) | .. | ||
| 2 | 15 458 (17·3%) | 7280 (15·9%) | .. | ||
| ≥3 | 30 874 (34·5%) | 15 768 (34·4%) | .. | ||
| Comorbidities | |||||
| Hypertension | 29 622 (33·1%) | 12 921 (28·2%) | <0·0001 | ||
| Dementia | 6742 (7·5%) | 3345 (7·3%) | 0·12 | ||
| HIV | 432 (0·5%) | 205 (0·5%) | 0·37 | ||
| Heart failure | 7134 (8·0%) | 6266 (13·7%) | <0·0001 | ||
| Chronic respiratory disease | 1433 (1·6%) | 1830 (4·0%) | <0·0001 | ||
| Chronic kidney disease | 7458 (8·3%) | 3821 (8·3%) | 0·95 | ||
| Cirrhosis | 718 (0·8%) | 489 (1·1%) | <0·0001 | ||
| Diabetes | 17 050 (19·0%) | 7352 (16·0%) | <0·0001 | ||
| Peripheral vascular disease | 2870 (3·2%) | 1614 (3·5%) | 0·0021 | ||
| Dyslipidaemia | 4489 (5·0%) | 2072 (4·5%) | <0·0001 | ||
| Deficiency anaemia | 3501 (3·9%) | 1946 (4·2%) | 0·0029 | ||
| Pulmonary bacterial infection | 6286 (7·0%) | 3142 (6·9%) | 0·26 | ||
| Immunocompromised | 3395 (3·8%) | 2033 (4·4%) | <0·0001 | ||
| Overweight, BMI ≥25 kg/m2 | 10 116 (11·3%) | 2811 (6·1%) | <0·0001 | ||
| Obese, BMI ≥30 kg/m2 | 8611 (9·6%) | 2491 (5·4%) | <0·0001 | ||
Data are n (%) unless otherwise indicated. Data are for patients who were hospitalised for COVID-19 between March 1 and April 30, 2020, and patients who were hospitalised for seasonal influenza between Dec 1, 2018, and Feb 28, 2019. BMI=body-mass index.
Social deprivation index was missing for 4348 patients in the COVID-19 group and 2537 patients in the influenza group.
The distribution of social deprivation scores differed between the two groups, but there was no distinct trend because the two extremes (least deprived and most deprived) were both more frequently observed in the COVID-19 group than in the influenza group (table 1). In patients older than 40 years, including the oldest age groups, the deprivation score was significantly lower in the COVID-19 group than the influenza group (−0·31 [SD 1·77] vs –0·20 [1·59], p<0·0001). Conversely, in patients aged 18–40 years, social deprivation score was significantly higher in patients with COVID-19 (0·01 [1·84] vs –0·21 [1·62], p<0·0001).
The Charlson comorbidity index was higher in the influenza group than in the COVID-19 group, but there was no significant difference between groups in Elixhauser comorbidity score (table 1). More specifically, patients with COVID-19 were more often obese or overweight, and more often had diabetes, hypertension, and dyslipidaemia, whereas patients with influenza more often had heart failure, peripheral vascular disease, chronic respiratory disease, cirrhosis, and deficiency anaemia. There was no significant difference between groups in the proportion of patients with HIV. However, fewer patients were immunocompromised in the COVID-19 group than in the influenza group. A similar proportion of patients was diagnosed with pulmonary bacterial infection in the two groups. The results are presented by age group in the appendix (pp 1–3). Patients with COVID-19 younger than 80 years were more often obese or overweight, whereas those older than 50 years more frequently had hypertension compared with patients with influenza. By contrast, patients with COVID-19 less frequently had heart failure or chronic respiratory disease in all age groups except those younger than 18 years. Indeed, in children, patients with COVID-19 more often had hypertension, chronic respiratory disease, heart failure, and pulmonary bacterial infection, and were more often obese or overweight.
Regarding the clinical evolution after admission (table 2 ), hospitalised patients with COVID-19 were more likely to develop acute respiratory failure, pulmonary embolism, or septic shock, but less likely to develop myocardial infarction or atrial fibrillation. Haemorrhagic strokes were more common among patients with COVID-19 compared with patients with influenza, but there were no significant differences in other types of stroke.
Table 2.
Main outcomes of patients hospitalised in France for COVID-19 or seasonal influenza
| COVID-19 (n=89 530) | 2018–19 seasonal influenza (n=45 819) | p value | |
|---|---|---|---|
| Acute respiratory failure | 24 317 (27·2%) | 7977 (17·4%) | <0·0001 |
| Pulmonary embolism | 3086 (3·4%) | 412 (0·9%) | <0·0001 |
| Venous thrombosis (including pulmonary embolism) | 4367 (4·9%) | 766 (1·7%) | <0·0001 |
| Septic shock | 2551 (2·8%) | 918 (2·0%) | <0·0001 |
| Myocardial infarction | 558 (0·6%) | 506 (1·1%) | <0·0001 |
| Atrial fibrillation | 11 129 (12·4%) | 7222 (15·8%) | <0·0001 |
| Stroke | 1068 (1·2%) | 569 (1·2%) | 0·44 |
| Haemorrhagic stroke | 253 (0·3%) | 93 (0·2%) | 0·0061 |
| Ischaemic stroke | 714 (0·8%) | 405 (0·9%) | 0·097 |
| Transient ischaemic attack | 161 (0·2%) | 92 (0·2%) | 0·40 |
| Acute kidney failure | 5761 (6·4%) | 2227 (4·9%) | <0·0001 |
| Invasive mechanical ventilation | 8684 (9·7%) | 1833 (4·0%) | <0·0001 |
| Admission to ICU | 14 585 (16·3%) | 4926 (10·8%) | <0·0001 |
| Mean (SD) stay in ICU, days | 15 (14) | 8 (9) | <0·0001 |
| Median (IQR) stay in ICU, days | 10 (4–21) | 5 (2–10) | <0·0001 |
| Mechanical ventilation among ICU patients | 10 430/14 585 (71·5%) | 3004/4926 (61·0%) | <0·0001 |
| In-hospital death | 15 104 (16·9%) | 2640 (5·8%) | <0·0001 |
| Expected in-hospital death with influenza age-specific mortality | 5355 (6·0%) | .. | .. |
| Standardised mortality ratio | 2·82 | .. | .. |
| In-hospital death among patients in ICU | 3949/14 585 (27·1%) | 885/4926 (18·0%) | <0·0001 |
| Mean (SD) stay in ICU among non-deceased patients, days | 15 (15) | 8 (9) | <0·0001 |
| Median (IQR) stay in ICU among non-deceased patients, days | 10 (3–21) | 5 (2–9) | <0·0001 |
| In-hospital death among patients in ICU with mechanical ventilation | 3312/10 430 (31·8%) | 780/3004 (26·0%) | <0·0001 |
| In-hospital death among non-ventilated patients in ICU | 477/2773 (17·2%) | 81/1496 (5·4%) | <0·0001 |
Data are n (%) or n/N (%) unless otherwise indicated. ICU=intensive care unit. Data are for patients who were hospitalised for COVID-19 between March 1 and April 30, 2020, and for patients who were hospitalised for seasonal influenza between Dec 1, 2018, and Feb 28, 2019.
Patients with COVID-19 were more likely to need intensive care, and the mean length of stay in the ICU for COVID-19 was twice as long (15 days [SD 14] for COVID-19 vs 8 days [9] for influenza; table 2). A quarter of patients with COVID-19 remained in the ICU for more than 3 weeks (table 2). Patients with COVID-19 were more likely to require invasive mechanical ventilation than patients with influenza. If admitted to the ICU, patients with COVID-19 were also more likely to need mechanical ventilation than patients with influenza.
In-hospital mortality was higher in patients hospitalised for COVID-19 than patients hospitalised for influenza, with a relative risk of death of 2·9 (95% CI 2·8–3·0). We found a standardised mortality ratio of 2·82. Therefore, the number of observed deaths was considerably higher than what would be expected if the COVID-19 population had the same probability of dying as the influenza population. Mortality was also higher in patients with COVID-19 who were admitted to the ICU, whether they were mechanically ventilated or not. After stratifying patients according to the main comorbidities, the in-hospital mortality for patients with COVID-19 was roughly three-times higher than that of patients with influenza, for all the main comorbidities except pulmonary bacterial coinfection, for which in-hospital mortality was two-times higher for patients with COVID-19 (appendix p 4).
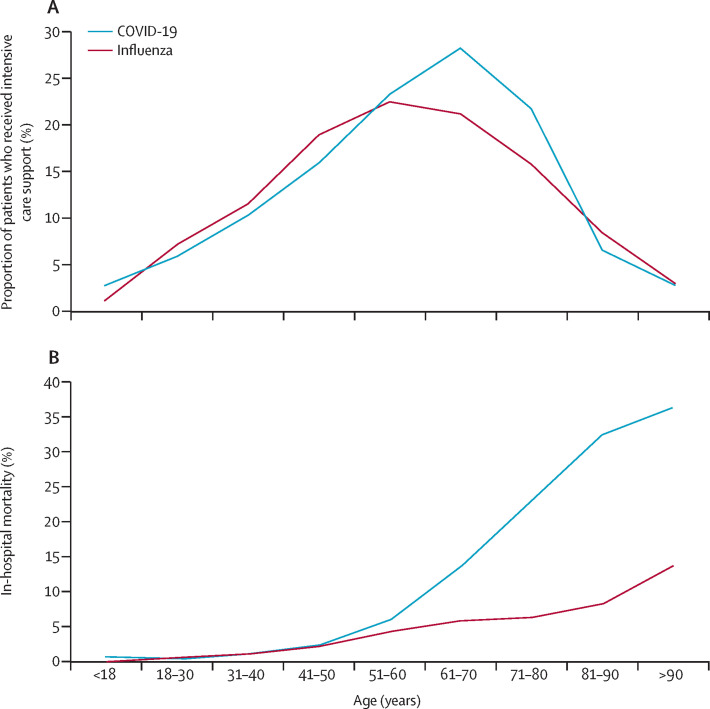
After stratification by age (figure 1 , appendix pp 5–6), the proportion of patients in the ICU was significantly higher in the COVID-19 group than in the influenza group for patients younger than 18 years and those aged 61 to 80 years. By contrast, the proportion of patients in the ICU was lower in the COVID-19 group than in the influenza group for patients older than 80 years. In patients older than 50 years and younger than 18 years, in-hospital mortality was higher in the COVID-19 group than the influenza group.
Figure 1.
Intensive care support and mortality of patients hospitalised in France for COVID-19 or seasonal influenza, by age at admission
Date are for patients who were hospitalised for COVID-19 between March 1 and April 30, 2020, and for patients who were hospitalised for seasonal influenza between Dec 1, 2018, and Feb 28, 2019.
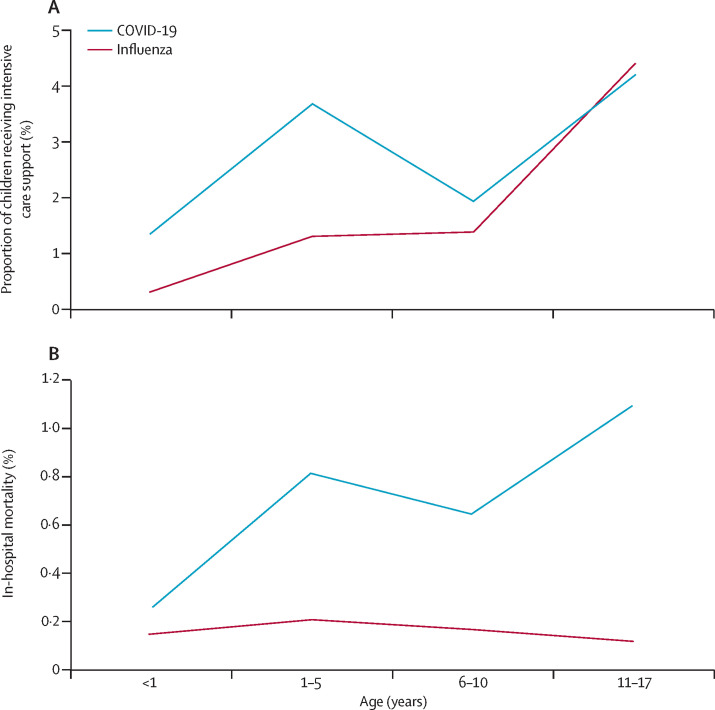
We identified 1227 children hospitalised for COVID-19 and 8942 children hospitalised for influenza (figure 2 , appendix pp 7–8). The need for intensive care in patients younger than 5 years was more common in cases of COVID-19 than influenza (14 [2·3%] of 613 for COVID-19 vs 65 [0·9%] of 6973 for influenza), but in-hospital mortality in this age group was did not significantly differ between the two diseases (three [0·5%] vs 13 [0·2%]). In patients aged 11–17 years, in-hospital mortality was ten-times higher for patients with COVID-19 than those with influenza (five [1·1%] of 458 vs one [0·1%] of 804). Patients aged 11–17 years who were hospitalised for COVID-19 were more likely to be obese (12 [2·6%] of 458 vs four [0·5%] of 804, p<0·0001) or overweight (16 [3·5%] vs nine [1·1%], p=0·0036) than those hospitalised for influenza. By contrast, they were less likely to have diabetes (seven [1·5%] vs 33 [4·1%], p=0·012) or chronic respiratory disease (three [0·7%] vs 17 [2·1%], p=0·046; appendix p 9).
Figure 2.
Intensive care support and mortality of children younger than 18 years hospitalised in France for COVID-19 or seasonal influenza, by age at admission
Date are for patients who were hospitalised for COVID-19 between March 1 and April 30, 2020, and for patients who were hospitalised for seasonal influenza between Dec 1, 2018, and Feb 28, 2019.
Our sensitivity analysis, which was restricted to stage 3 of the COVID-19 epidemic (March 14, 2020, onwards), provided similar results (appendix pp 10–14).
Discussion
In this nationwide cohort study comparing the COVID-19 epidemic to seasonal influenza, almost twice as many patients were admitted to hospital for COVID-19 over a 2-month period than were admitted for seasonal influenza over a 3-month period. There is a small possibility that some patients hospitalised in 2020 were misclassified as COVID-19 when they actually had influenza. However, the risk of misclassification is low because the period studied in 2020 was at the end of the influenza epidemic in France. In addition, the results of the sensitivity analysis, which was limited to stage 3 of the epidemic, were similar to those of the main analysis. The observed difference in admissions is likely to be an underestimation, considering that the COVID-19 epidemic peaked in the first week of April, 2020, and universal lockdown measures were already in place in France by March 17, contributing to a reduction in the rate of hospitalisation in the following weeks.11 However, influenza vaccination coverage against seasonal influenza in France was 29·7% for those under 65 years and 51·0% for those over 65 years in 2018–19, according to the National Public Health Agency.12 Therefore, the influenza vaccine probably contributed to lower rates of hospitalisation for seasonal influenza and associated mortality.13 These factors suggest that the difference in numbers of COVID-19 and seasonal influenza cases could be higher in other settings or periods, or if the residual population immunity acquired from previous seasonal influenzas (which cannot be assessed) is lower than usual.
We found that the in-hospital mortality for COVID-19 was nearly three-times higher than for seasonal influenza, with an age-standardised mortality ratio of 2·82. In addition, patients with COVID-19 were twice as likely to receive invasive mechanical ventilation, and COVID-19 patients hospitalised in the ICU stayed nearly twice as long as those with influenza. Of note, the 2018–19 period had the highest case-fatality rate for seasonal influenza in France within the past 5 years (12 300 deaths, including 8100 directly attributable to influenza).13 Therefore, the excess mortality observed for COVID-19 was not the result of an influenza season that was less severe than usual.
Another potential explanation for the higher mortality of COVID-19 is that the sudden influx of patients over a short period of time created medical structural constraints, and care teams were led to prioritise patients based on clinical status and prognosis. This hypothesis is supported by the lower rate of transfer to ICU in patients older than 80 years with COVID-19, which strongly contrasts with the higher mortality in these same patients.
We did not identify strong associations between disease outcomes and social deprivation score. Considering France's national health insurance system does not require individuals to pay upfront for health care, the lower proportion of patients being admitted to the ICU with COVID-19 than influenza is likely to be related to poorer prognoses rather than socioeconomic considerations. Nevertheless, we cannot exclude the possibility that social deprivation is a risk factor in younger adults because deprivation score was significantly higher in patients with COVID-19 aged 18 to 40 years than in their counterparts hospitalised with influenza.
The higher in-hospital mortality observed in younger COVID-19 patients suggests that COVID-19 is intrinsically more severe than influenza. Although children seemed to have a lower risk of being hospitalised for COVID-19 (as shown here by the low rate of hospitalisation for COVID-19 compared with seasonal influenza in patients younger than 18 years), the in-hospital mortality of these children was more than four-times higher than it was for children with influenza. This contrasts with recent reports stating that the clinical manifestations of COVID-19 are very often mild in children.14, 15, 16 The clinical manifestations of COVID-19 appear to be milder than those of influenza in children younger than 5 years,17 and do not seem more severe than other coronavirus or influenza infections in children younger than 1 year.18 In our study, excess in-hospital mortality in our study seemed to be partially avoided by an increased use of intensive care support for children younger than 5 years. The higher rate of mortality associated with COVID-19 in patients aged 11–17 years might indicate that there is a need to be particularly vigilant in young people with overweight or obesity. The increase in mortality in children might be linked in part to paediatric inflammatory multisystem syndrome (also known as Kawasaki-like syndrome). Indeed, an unexpectedly high incidence of this syndrome has been observed, including in adolescents with COVID-19,19, 20 but it remains rare and probably does not explain the increased risk of mortality. In addition, although this syndrome can sometimes lead to death, no association with overweight has been reported to date.
Our findings suggest that severity could be linked to the comorbidities associated with each viral disease. The median age of patients hospitalised for COVID-19 in France was within the range reported in other large international studies.3, 21 The overall comorbidity scores tended to be lower in patients with COVID-19 than in patients with seasonal influenza, and the patients admitted with COVID-19 were more frequently male with fewer comorbidities, as previously observed.3 Whereas diabetes and overweight seem to be particular risk factors for COVID-19 hospitalisation,3, 21, 22, 23 major comorbidities such as heart failure, chronic respiratory disease, and cirrhosis, albeit frequently associated with COVID-19,3, 21, 22, 23 were more often observed in the seasonal influenza group. The higher short-term impact of these three conditions when compared with diabetes and obesity24 is another argument in favour of an intrinsic increased severity of COVID-19. Thus, although the differences in comorbidities might explain the difference in disease severity, the difference might also be due to an exaggerated immune response in COVID-19, or due to the exacerbation of comorbidities in influenza infection. Another point of interest is that people living with HIV in our study did not seem to be more affected by COVID-19 than seasonal influenza. This supports the hypothesis that virologically controlled (ie, antiretroviral-treated) HIV populations do not have a considerably higher risk of developing severe COVID-19 (as has been observed in countries with low antiretroviral rates25). Of note, people with HIV should also not be considered at a lower risk due to a potential preventive effect of antiretroviral therapy.26
Regarding the outcomes of hospitalised patients, respiratory complications were more common in COVID-19 cases than in seasonal influenza. Similar to other studies, we found that pulmonary bacterial co-infections were infrequent in COVID-19,21, 27 in contrast to what has been reported for influenza epidemics.28 However, in our study, the frequency of pulmonary bacterial co-infections was very similar for COVID-19 and influenza. It therefore seems that SARS-CoV-2 has a superior potential for respiratory pathogenicity, possibly by inducing upregulation of a small number of inflammatory mediators (including IL-6),29 and that respiratory complications are mainly responsible for the excess mortality observed in COVID-19. As previously reported, patients with COVID-19 have a higher risk of pulmonary embolism.30 A higher risk of haemorrhagic stroke (but not ischemic stroke, as has been more commonly observed31, 32) was also observed in our study, not only because of specific COVID-19-induced cerebral vasculitis, but also because of the high doses of anticoagulants used to prevent or reduce the risk of thromboembolism. Of note, we found that patients with COVID-19, who are often considered to have higher cardiac risks (including for acute coronary syndrome, myocarditis, arrhythmias, and cardiogenic shock),33 actually had lower risk of myocardial infarction and atrial fibrillation than patients with seasonal influenza. Patients with influenza were more likely to have chronic heart failure at baseline, but the question of whether the incidence and the impact of seasonal influenza-related myocarditis is underestimated requires further investigation.34
Although this study includes data collected on a national level from more than 100 000 hospitalised patients of all ages, we recognise that it has several limitations. First, the comparison between COVID-19 and seasonal influenza has some difficulties, such as possible testing biases and differences between viruses. More patients were likely to have been tested for COVID-19 in 2020 than for influenza during the 2018–19 season, but our study included only hospitalised patients. Tests systematically done in an ambulatory care setting for COVID-19 should have little influence on the number of patients hospitalised, particularly in ICUs. However, testing practices for influenza are likely to be highly variable across hospitals, whereas practices for COVID-19 may be more standardised (eg, all hospitalised patients require testing). The comparison between COVID-19 and seasonal influenza might also not be direct because the study periods are roughly 1 year apart. We cannot ascertain whether the 2018–19 seasonal influenza is representative of all seasonal influenzas, even though it was the most severe season in the past 5 years in France. Some patients with COVID-19 might also have had influenza at the same time, but this scenario is unlikely because influenza diagnostic procedures were accessible throughout the study period, and because the 2019–20 influenza epidemic was ending at the start of the COVID-19 epidemic. In addition, we found no differences between the sensitivity analyses restricted to stage 3 of the COVID-19 epidemic and the main analyses. In the stage 3 period (March 14, onwards), COVID-19 patients were unlikely to have influenza. The characteristics of patients who died from COVID-19 in places outside of the hospital setting, in a nursing home for example, could be different from those of the 15 104 individuals who died in our study. Another limitation is the potential for biases related to misclassification or under-detection, especially for comorbidities because they were only identified during the stays for COVID-19 or for influenza. Even so, this misclassification bias is likely to be non-differential for the majority of the comorbidities. We cannot always distinguish between acute and chronic conditions; for example, in heart failure the same code is used for chronic heart failure and for cardiac decompensation. Moreover, the PMSI inpatient database for COVID-19, with accelerated transmission of COVID-19-related patient data, was only recently developed on the request of the government (following the decree of April 21, 2020), and these data are still pending consolidation. This is, in particular, the consequence of strains on our health-care system, its mobilisation to care for sick individuals, and the willingness to act quickly. Information relating to do-not-resuscitate orders was not available in our database, nor was ethnicity data because its collection is not authorised in France. We were also lacking detailed data for some potentially relevant findings, such as the higher frequency of septic shock in COVID-19 patients or the underlying reasons for mortality in adolescents. We acknowledge that, despite the collection of nationwide data, the conclusions about ICU support and deaths in children are based on small numbers (eg, in children younger than 5 years, three deaths were associated with COVID-19 and 13 with influenza). Whether genetic characteristics could be associated with severity could not be assessed in these cases.35 Finally, for some variables, information is not completed in the discharge abstract when there is no direct impact on patient care during hospitalisation (eg, tobacco use). This is often the case for day-care hospitalisations but it did not apply to the hospitalisations considered in this study.
In conclusion, significant differences exist between patients with COVID-19 and seasonal influenza requiring hospitalisation. SARS-CoV-2 appears to have a higher potential for respiratory pathogenicity, leading to more respiratory complications in patients with fewer comorbidities, and it is associated with a higher risk of mortality, particularly in adolescents, although any conclusions for this age group must be treated with caution considering the small number of deaths. These findings were confirmed even after we considered the constraints linked to the sudden influx of patients during the epidemic. In future, the possibility of overlapping influenza and COVID-19 epidemics will certainly also increase the complexity of patient management. At a time when no treatment has been shown to be effective for the COVID-19 clinical course, this study highlights the importance of all measures of physical prevention and the need for a specific vaccine for SARS-CoV-2 and treatment for COVID-19.
Data sharing
The PMSI database was made available by the French national agency for the management of hospitalisation data (Agence technique de l'information sur l'hospitalisation, ATIH). The use of these data by our department was approved by CNIL. We are not permitted to share these data. PMSI data are available from ATIH to researchers who meet the criteria for access (requests for access are evaluated by CNIL).
Acknowledgments
Acknowledgments
This project was funded by the French National Research Agency. We thank Suzanne Rankin for reviewing the English and Gwenaëlle Periard for help with the layout and coordinating the preparation of this Article.
Contributors
LP, PB, PT-B, and CQ were involved in the conception and design of the study. CQ was the coordinator of the study. JC, A-SM, and CQ were responsible for the data collection. LP, PT-B, and CQ wrote the first draft. JC was in charge of the analysis. JC and CQ accessed and verified the data. All authors were involved in the interpretation, critically reviewed the first draft, and approved the final version.
Declaration of interests
LP reports travel grants from Janssen-Cilag, Merck Sharp & Dohme, Pfizer, and Gilead. PB reports personal fees and other fees from Roche (board participation, consulting fees, travel fees), Boehringer Ingelheim (board participation, consulting fees, conference fees), and Novartis (board participation, consulting fees, conference fees), personal fees from TEVA, AstraZeneca, and Sanofi, and other fees from Chiesi (travel fees) and Stallergene (conference fees). All other authors declare no competing interests.
Supplementary Material
References
- 1.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santé publique France L'épidémie de COVID-19 en chiffres. July 10, 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-9-juillet-2020
- 3.Burn E, You SC, Sena A, et al. Deep phenotyping of 34,128 patients hospitalised with COVID-19 and a comparison with 81,596 influenza patients in America, Europe and Asia: an international network study. medRxiv. 2020 doi: 10.1101/2020.04.22.20074336. published online June 28. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zayet S, Kadiane-Oussou NJ, Lepiller Q, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche–Comte cluster. Microbes Infect. 2020;22:481–488. doi: 10.1016/j.micinf.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg M, Jougla E, Fassa M, Padieu R, Quantin C. The French public health information system. Journal of the International Association for Official Statistics. 2012;28:31–41. [Google Scholar]
- 7.L'Agence technique de l'information sur l'hospitalisation Mise à jour des consignes de codage des séjours COVID-19. Oct 23, 2020. https://www.atih.sante.fr/mise-jour-des-consignes-de-codage-des-sejours-covid-19
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Rey G, Jougla E, Fouillet A, Hémon D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health. 2009;9:33. doi: 10.1186/1471-2458-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux J, Massonnaud C, Crépey P. COVID-19: one-month impact of the French lockdown on the epidemic burden. medRxiv. 2020 doi: 10.1101/2020.04.22.20075705. published online April 27. (preprint) [DOI] [Google Scholar]
- 12.Santé publique France Données de couverture vaccinale grippe par groupe d'âge. Oct 12, 2020. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-grippe-par-groupe-d-age
- 13.Equipes de surveillance de la grippe Influenza activity in France, season 2018–2019. Bull Epidemiol Hebd. 2019;28:552–563. [Google Scholar]
- 14.Sinha IP, Harwood R, Semple MG, et al. COVID-19 infection in children. Lancet Respir Med. 2020;8:446–447. doi: 10.1016/S2213-2600(20)30152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Wang H, Wang F, et al. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years. Int J Infect Dis. 2020;98:80–83. doi: 10.1016/j.ijid.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanhems P, Endtz H, Dananché C, Komurian-Pradel F, Sanchez Picot V. Comparison of the clinical features of SARS-CoV-2, other coronavirus and influenza infections in infants less than 1-year-old. Pediatr Infect Dis J. 2020;39:e157–e158. doi: 10.1097/INF.0000000000002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santé publique France, Direction de la Recherche, des Études, de l'Évaluation et des Statistiques L'état de santé de la population en France: rapport 2017. 2017. https://drees.solidarites-sante.gouv.fr/IMG/pdf/esp2017.pdf
- 25.Alcorn K. HIV raises the risk of death from COVID-19 in South Africa's Western Cape. July 8, 2020. https://www.aidsmap.com/news/jul-2020/hiv-raises-risk-death-covid-19-south-africas-western-cape
- 26.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. published online May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bengoechea JA, Bamford CGG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudd PA, Crawford JC, Turner JS, et al. Targeted immunosuppression distinguishes COVID-19 from influenza in moderate and severe disease. medRxiv. 2020 doi: 10.1101/2020.05.28.20115667. published online May 30. (preprint) [DOI] [Google Scholar]
- 30.Fauvel C, Weizman O, Trimaille A, et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 34.Datta R, Helou E, Tucker M, John B, Martinello RA, Malinis M. Detection of influenza myocarditis using national healthcare safety network surveillance definitions accounting for fever in older adults. Infect Control Hosp Epidemiol. 2018;39:1145–1147. doi: 10.1017/ice.2018.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S-Y, Zhang Q, Casanova J-L, Su HC. Severe COVID-19 in the young and healthy: monogenic inborn errors of immunity? Nat Rev Immunol. 2020;20:455–456. doi: 10.1038/s41577-020-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The PMSI database was made available by the French national agency for the management of hospitalisation data (Agence technique de l'information sur l'hospitalisation, ATIH). The use of these data by our department was approved by CNIL. We are not permitted to share these data. PMSI data are available from ATIH to researchers who meet the criteria for access (requests for access are evaluated by CNIL).