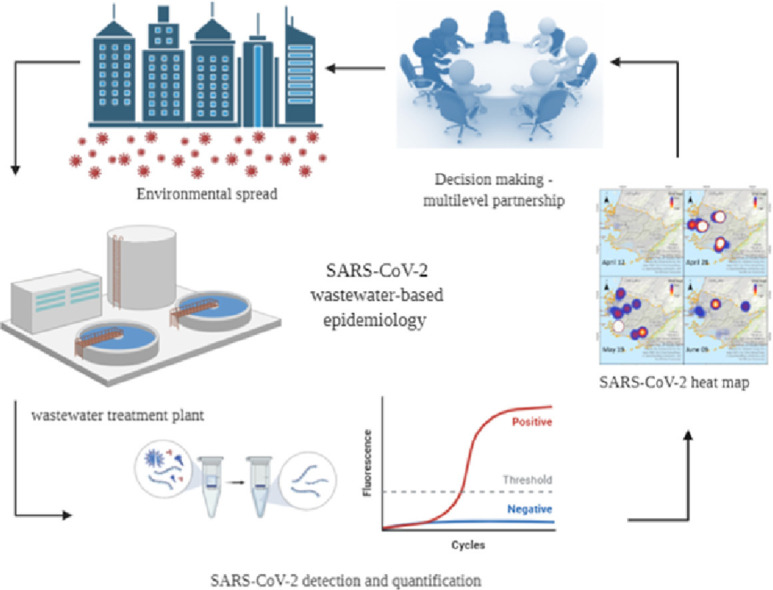
Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) wastewater-based epidemiology (WBE) has been applied as a complementary approach for spatial tracking of coronavirus disease 2019 cases (COVID-19) as well as early warning of the occurrence of infected populations. The present study presents the result of the monitoring of sanitary sewerage in the municipality of Niterói, a metropolitan region of Rio de Janeiro (Brazil) and its use as a complementary indicator in the surveillance of COVID-19 cases, thus assisting actions of public health from local authorities. Twelve composite raw sewage samples were weekly collected from two wastewater treatment plants (WWTPs) and alternately from 17 sewer pipes (SP) from surrounding neighbourhoods and slums throughout 20 weeks (April 15th to August 25th, 2020). Two hundred twenty-three samples were concentrated using the ultracentrifugation-based method and SARS-CoV-2 RNA detected and quantified by RT-qPCR using primers and probe targeting the N2 genome. SARS-CoV-2 RNA was detected in 84.3% (188/223) of samples with a positive rate ranging from 42% (5/12) in the first week of monitoring to 100% during the peak of epidemic with viral concentration ranging from 3.1 to 7.1 log10 genome copies /100 mL throughout the studied period. Positive rates were higher in WWTPs when compared to SP, being useful tool for monitoring trends in the evolution of the COVID-19 curve, while SP data were more effective when health public interventions were needed. Whole-genome sequencing using Illumina MiSeq System confirmed the lineage of three genomes as B.1.1.33 (clade G) containing the nucleotide substitutions observed in strains that circulate in the Rio de Janeiro during the period of this study. In addition, geoprocessing tool was used to build heat maps based on SARS-CoV-2 data from sewage samples, which were weekly updated and available online to the general population as an indicator of the ongoing epidemic situation in Niterói city, raising public awareness.
Keywords: COVID-19, Public health policies, SARS-CoV-2, Wastewater-based epidemiology, Whole-genome sequencing
Graphical abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) brought an unprecedented impact worldwide. After the first cases reported in November 2019 in Wuhan, Hubei province, China (Zhu et al., 2020), SARS-CoV-2, a new beta-coronavirus, rapidly spread to other regions in China and then to many other countries worldwide. On 31st January 2020, the WHO declared the outbreak of COVID-19 to be of Public Health Emergency of International Concern posing a high risk especially to countries with vulnerable health systems (WHO, 2020). According to WHO reports, on December 18th, the number of reported cases and deaths reached 73,275,943 and 1650,348, respectively (WHO, https://covid19.who.int/).
In Brazil, the first COVID-19 case was confirmed in São Paulo on February 26th, and a week later, Rio de Janeiro confirmed its first case. As major Brazilian metropolis, both cities are important in the entry flow of transmissible diseases into the country. As of December 14th, 2020, over 180,000 COVID-19 confirmed deaths have been officially reported by the Brazilian Ministry of Health (MS, https://covid.saude.gov.br/), and Brazil is the third country with the largest number of confirmed cases to date (WHO, https://covid19.who.int/).
The main route of SARS-CoV-2 transmission is through respiratory droplets and aerosols generated by coughing, sneezing and speaking, and although less likely, contaminated fomites could be implicated in the transmission of SARS-CoV-2 (Chia et al., 2020; Liu et al., 2020; Goldman, 2020). SARS-CoV-2 infection can affect not only the respiratory system, causing fever, cough, rhinorrhoea, dyspnoea or severe pneumonia, but may also cause other clinical symptoms like lethargy, muscle ache, headache, neurologic manifestation or gastrointestinal symptoms such as diarrhoea (Foladori et al., 2020). Diarrhoea has been reported in a significant number of cases (incidences varying from 3.8% to 80% of cases), with positive viral RNA in stools ranging from 15% to 83% of patients (Foladori et al., 2020). SARS-CoV-2 RNA titers in stool may be highly variable as well (from 103 to 108 RNA copies/g of stool) depending on the day of sampling post onset of symptoms (Foladori et al., 2020; Kitajima et al., 2020).
Detection of SARS-CoV-2 in faeces and early shedding demonstrated by virus detection in faeces (He et al., 2020) resulted in use of an environmental approach called wastewater-based epidemiology (WBE) as an additional tool to support COVID-19 prevention and control actions in several regions (Farkas et al., 2020; Hart and Halden, 2020; Mao et al., 2020; Thompson et al., 2020). According to Polo et al. (2020), WBE consists on the assumption that any stable substance that is excreted by humans and in wastewater can be used to back-calculate the original concentration excreted by the serviced population. The same concept can be used for analysis of pathogens circulation in sanitary sewers in a given population, when excreted in the faeces/urine of infected people (Mao et al., 2020; Polo et al., 2020). Such an approach is a useful tool especially where resources for clinical diagnosis are limited and when reporting systems are unavailable or inefficient (Kitajima et al., 2020; Hart and Halden, 2020; Thompson et al., 2020).
Since the beginning of the COVID-19 pandemic this environmental approach for tracking SARS-CoV-2 has been used in several countries such as Australia, Netherlands, France, Spain, Italy, Japan, China, India, and the USA (Ahmed et al., 2020a; Farkas et al., 2020; Haramoto et al., 2020; Hart and Halden, 2020; Kumar et al., 2020; La Rosa et al., 2020a; Mao et al., 2020; Medema et al., 2020; Randazzo et al., 2020; Wurtzer et al., 2020). Based on this concept and in addition to rapid action to face the epidemic in Niterói, this municipality established a partnership with Oswaldo Cruz Foundation – Fiocruz (under the Brazilian Ministry of Health) aiming at promoting an environmental surveillance project based on detection of SARS-CoV-2 in sewers. The intention was to provide data on the spread of these viruses in the municipality alone and to assist actions carried out by the local Health Secretariat. Niterói reported the first COVID-19 case on March 12th, a few weeks after the first confirmed case in the country. The success in detecting SARS-CoV-2 in sewerage samples in this pilot project (Prado et al., 2020) led us to expand sampling throughout the city thus adding environmental surveillance data to support decision making of local public health policies.
In this study, we present data from SARS-CoV-2 detected in the sewerage system over a 20-week monitoring period, in samples obtained from wastewater treatment plants (WWTPs) and sewer pipes (SP) in locations with demographic density and of variable income levels (neighbourhoods and slums) throughout COVID-19 epidemic in Niterói. The SARS-CoV-2 RNA was characterized by complete genome sequencing using the Illumina MiSeq platform, which represented an important report of SARS-CoV-2 sewage sequencing data in Brazil to evaluate its local circulation compared to strains distributed globally. Local public health actions to control the epidemic are also described, as well as an attempt to formulate an environmental health indicator based on data from the WBE.
2. Methods
2.1. Study area, period of study and sampling sites
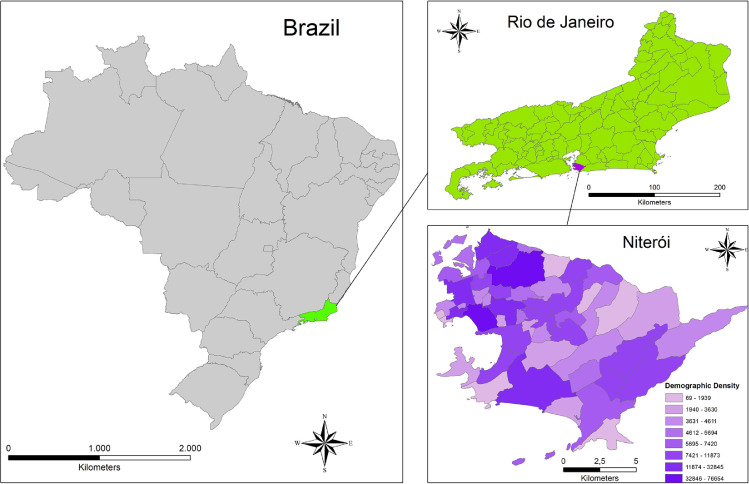
The present study was carried out in Niterói municipality, state of Rio de Janeiro, Southeast region of Brazil. The city is connected to Rio de Janeiro capital (second largest Brazilian city) by a 13.3 km-bridge thus integrating the Metropolitan Region of Rio de Janeiro (Fig. 1 A). Niterói houses an estimated population of 513,584 inhabitants, occupying an area of 133,757 km², with the highest Municipal Human Development Index of Rio de Janeiro state. The city is divided into five administrative regions named: Praias da Baía (Guanabara Bay beaches - Central), Oceânica (Oceanic), Leste (East), Norte (North) and Pendotiba, with demographic density ranging from 69 to 76,654 inhabitants/Km2 (Fig. 1B). Based on data from 2014, the city figures in the 12th national position of basic sanitation coverage, with 100% of treated water supply. Regarding sewerage system, the rate of sewage collection and treatment is of 94.5% (SNIS, 2020).
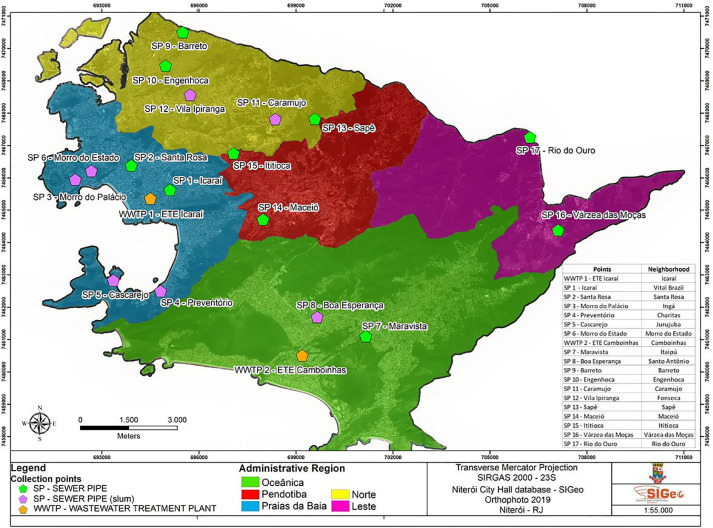
Fig. 1.
(A). Location of Niterói on maps of Brazil and Rio de Janeiro State, indicating range of demographic density. (1B). Sampling points distributed in Niterói municipality–location map.
The collection strategy for monitoring followed criteria prioritized by the Municipal Health Secretariat, such as densely populated areas, more exposed areas in relation to the SARS-CoV-2 transmission and areas with social vulnerability covered by the Family Medical Program (FMP). FMP is part of the Brazilian Universal Public Health System that provides health services by a “general practitioner” or “family doctor” or “primary care physician” in low-income areas.
Between April and August 2020, 12 raw sewage samples were weekly processed for SARS-CoV-2 investigation. In total, 19 sites were monitored being, two WWTP, 10 SPs from neighbourhood and seven SPs from slums (Fig. 1B). Five fixed collection points were monitored weekly, being two WWTP and three SPs located at the most populous region of the city (Praias da Baía). The other seven samples were obtained from 14 SPs, where sampling occurred fortnightly, in order to monitor all regions of the municipality.
Each raw sewage sample was collected as a 10h-composite sample (1 L) from each sampling point (Fig. 1B). Samples were collected in sterile polypropylene bottles and transported at 4 °C until processing at the Laboratory of Comparative and Environmental Virology, Oswaldo Cruz Foundation.
2.2. Virus concentration, recovery rates and replicates
Before concentration, sewage samples were pasteurized at 60 °C for 90 minutes for virus’ inactivation, as described by Wu et al. (2020). Viruses were concentrated using the ultracentrifugation-based method (Pina et al., 1998). Briefly, 42 mL of sewage samples were centrifuged at 100,000 × g for 1 h at 4 °C. After supernatant discharge, pellet was re-suspended in 4 mL of 0.25 N glycine buffer (pH 9.5) and incubated at 4 °C for 30 min, mixed by vortex each 5 min. The solution was then neutralized by adding 4 mL of 2 × phosphate-buffered saline (PBS, pH 7.2), and clarified by centrifugation at 12,000 × g for 20 min. Finally, supernatant samples (~ 8 mL) were centrifuged at 100,000 × g for 1 h at 4 °C and viral particles were re-suspended in 400 µL of 1 × PBS (pH 7.2) and processed immediately for nucleic acid extraction or stored at −80 °C until use.
Ultracentrifugation recovery rates were evaluated by spiking experiments using the enveloped bovine respiratory syncytial virus (BRSV/InforceTM 3, Zoetis, US) and a non-enveloped RNA bacteriophage (PP7, ATCC 15692-B2). RT-qPCR TaqMan System was used for genome copy (GC) quantification according to described protocols, respectively (Boxus et al., 2005; Rajal et al., 2007).
Viral recovery rates were calculated according to the following eq. (E):
E (%) = [number of GC/mL) of PP7/BRSV after concentration/ number of GC/mL of spiked PP7/BRSV] x 100.
Technical and biological replicates were performed by testing eight 42-ml aliquots from the same sample, and by testing paralleled samples collected within 10-min intervals, respectively.
2.3. RNA extraction and RT-qPCR for virus detection
For viral RNA extraction (SARS-CoV-2, BRSV and PP7), 140 µL of the suspended viral concentrates were extracted using the QIAamp® Viral RNA Mini kit (QIAGEN, CA, USA) and a QIAcube® automated system (QIAGEN). SARS-CoV-2 RNA was detected and quantified by RT-qPCR using primers and probe targeting the N2 genome, according to CDC protocols (Lu et al., 2020). Reactions were performed with 5 µL of the extracted RNA in a final volume of 20 µL, using the SuperScriptTM III PlatinumTM One-Step qRT-PCR Kit (Invitrogen) and 1.5 µL of N2 primers and probe mixture (2019-nCoV RUO Kit, Integrated DNA Technologies, Coralville, IA, USA) using Applied Biosystems ViiA 7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). PP7 and BRSV RT-qPCR reactions were performed using same conditions as described above with primers and probes concentrations according to previously published protocols described by Rajal et al. (2007) and Boxus et al. (2005), respectively. Quality controls included the use of different rooms (master mix preparation, nucleic acid extraction, qPCR setting up, and product analysis) to prevent cross-contamination, as well as positive control included in the kit (2019-nCoV RUO Kit, Integrated DNA Technologies) and negative controls (DNA/RNAse free water).
SARS-CoV-2 titers were estimated using a standard curve (y = –3.533x + 41.627) prepared by five 10-fold serial dilutions (105–101 GC per reaction) of a double-stranded DNA fragment (gBlock Gene Fragment, Integrated DNA Technologies) containing the SARS-CoV-2 amplification region sequence. All RNA samples obtained from sewage were tested in duplicate as well as undiluted and 10-fold diluted in order to observe the presence of inhibitors. All samples that crossed the threshold line showing a characteristic sigmoid curve with a cycle threshold (Ct) value <40 (Wang et al., 2020) for at least two of the four wells tested were regarded as positive. SARS-CoV-2 viral concentration was expressed as GC per 100 mL (GC/100 mL), considering the volumes of the sample, the concentrate (eluate), the nucleic acid extracts and the RT-qPCR reaction. Recovery rates were not employed to estimate the concentration of SARS-CoV-2 in the samples.
2.4. Whole genome sequencing
Aiming at recovering SARS-CoV-2 genomes from sewage samples, 11 µL of the viral RNA from positive samples (cycle threshold - Ct ranging 24.5 to 38.8) was submitted to a previously developed protocol (Resende et al., 2020a). A RT was conducted using the SuperScript® IV First-Strand Synthesis System (Invitrogen) followed by two multiplex PCR containing primers to cover the whole SARS-CoV-2 genome. After the amplification, DNA amplicons were purified using AMPure XP PCR Purification magnetic beads (Beckman Coulter) and quantified by Qubit™ dsDNA HS Assay Kit (Thermo Fisher Scientific). The library was constructed using the Nextera XT DNA Library Preparation Kit (Illumina) and submitted to Illumina MiSeq System using MiSeq Reagent Kit v3 (600-cycle, Illumina) to recover at least 1000 depth coverage of these genomes.
2.5. Pipelines to obtain the consensus file and lineage classification
The bioinformatic pipeline to obtain the assembled reads, to observe the viral variants and to recover the consensus was performed using the CLC Genomics Workbench platform (Qiagen). The consensus file was generated and submitted to EpiCoV GISAID platform after a manual curation (Global initiative on sharing avian flu data) under the accession numbers: EPI_ISL_541397 to EPI_ISL_541400. For lineage classification, the consensus file was submitted to Pangolin software (Phylogenetic Assignment of Named Global Outbreak LINeages) (https://github.com/cov-lineages/pangolin) (Rambaut et al., 2020).
2.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism v.8.4.1 (GraphPad Software, San Diego, CA, USA). Data of viral concentrations were grouped by type of sampling points: WWTPs, neighbourhoods and slums. Mann–Whitney U test was used for comparison of median viral concentration values obtained for WWTPs, neighbourhoods and slums. For analyses, a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Evaluation of ultracentrifugation-based method for viral recovery
The ultracentrifugation-based method recoveries were assessed by spiking 42 mL of raw sewage samples with BRSV (n = 23) and PP7 (n = 6) concentrations previously quantified by RT-qPCR (Supplementary Material Table S1). Both viruses were detected in 100% of spiked samples. For BRSV, recovery rates varying from 11.4% to 40.2%, with mean of 27.4% ± 8.64 were found (standard deviation, sd); for PP7, recovery rates varied from 6.7% to 24.9%, with mean of 18.5% ± 7.46. Technical and biological replicates to assess the performance of the used method were also carried out. Eight replicates were collected from two different samples and SARS-CoV-2 was detected and quantified from these replicates. SARS-CoV-2 was detected in 100% of replicates from both samples tested. For sample #1, viral concentration varied from 4.8 to 5.1 log10 GC/100 mL, among the eight replicates tested (Ct values varied from 32.7 to 34). For sample #2, viral concentration varied from 5.2 to 5.6 log10 GC/100 mL (Ct values varied from 31.1 to 32.4). For biological replicates, we analysed three composite samples collected within 10-min intervals between the collections. We found minor variations among three biological replicates. For sample #1, Ct values of SARS-CoV-2 were 30.4, 30.5 and 30.7; and for sample #2, Ct values were 33.2, 33.4 and 33.5. Detailed information of recovery efficiency and technical and biological replicates is shown in Table S1 and Fig. S1 in the Supplementary Material.
3.2. SARS-CoV-2 in sewerage systems from Niterói, Rio de Janeiro
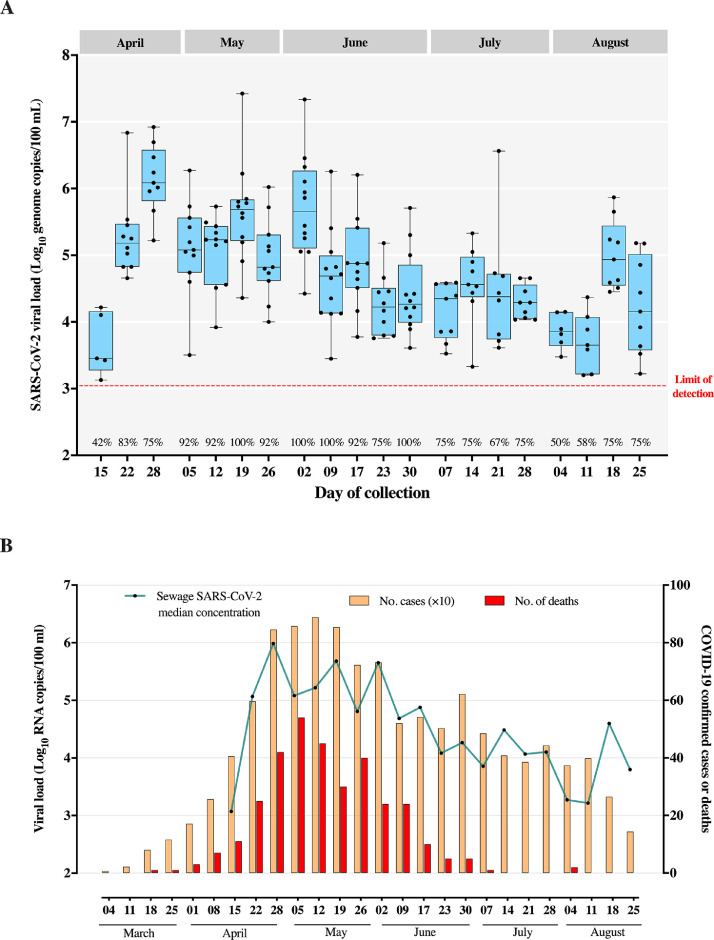
A total of 223 composite raw sewage samples were collected weekly between April 15th and August 25th, and SARS-CoV-2 was detected in 84.3% (188/223) of samples. SARS-CoV-2 positive rate varied from 42% (5/12) in the first week of monitoring, in 100% (12/12) of samples, observed for the first time in 19th May (Fig. 2 A). We observed high positive rates (>90% of positivity among weekly tested samples) between 5th May and 30th June. From the beginning of July onwards, SARS-CoV-2 positive decreased to ≤75% (Fig. 2A).
Fig 2.
(A). Box Plot of viral concentrations obtained per collection point in each sampling date in Niterói municipality (April–August, 2020). (B) COVID-19 weekly confirmed cases and deaths versus median viral concentrations of SARS-CoV-2 present in wastewaters from Niterói.
Fig. 2A also shows the variation of SARS-CoV-2 RNA titers detected throughout the 20-week study. The lowest RNA concentrations were observed in the first week of the study (April 15), when SARS-CoV-2 RNA median value was of 3.4 log10 GC/100 mL. RNA titers peaked in the third week of monitoring (28th April) with median of 6.1 log10 GC/100 mL, being the only median recorded above 6 log10. From May to early-June, median values remained high, varying between 4.8 and 5.7 log10 GC/100 mL, and from mid-June to beginning of August, a decreasing trend in SARS-CoV-2 RNA concentration in sewage samples was observed (median values from 3.6 to 4.6 log10 GC/100 mL). In the last two weeks of August, SARS-CoV-2 RNA titers in sewage increased again in some areas with median values of 4.9 and 4.2 log10 GC/100 mL (Fig. 2A).
We analysed SARS-CoV-2 RNA titers (by median values) with confirmed COVID-19 cases and deaths registered in Niterói by the Municipal Health Secretariat (Fig. 2B). To calculate median values, we included all the samples collected in 12 weeks , using a value equal to the LOD of N2 assay (3.1 log10) divided by square root of 2 (Croghan and Egeghy, 2003), when a sample was non-detected (bellow the LOD). Overall, high titers of SARS-CoV-2 RNA in sewage samples coincided with the period when the city registered the highest numbers of COVID-19 cases and related deaths (end of April to mid-June).
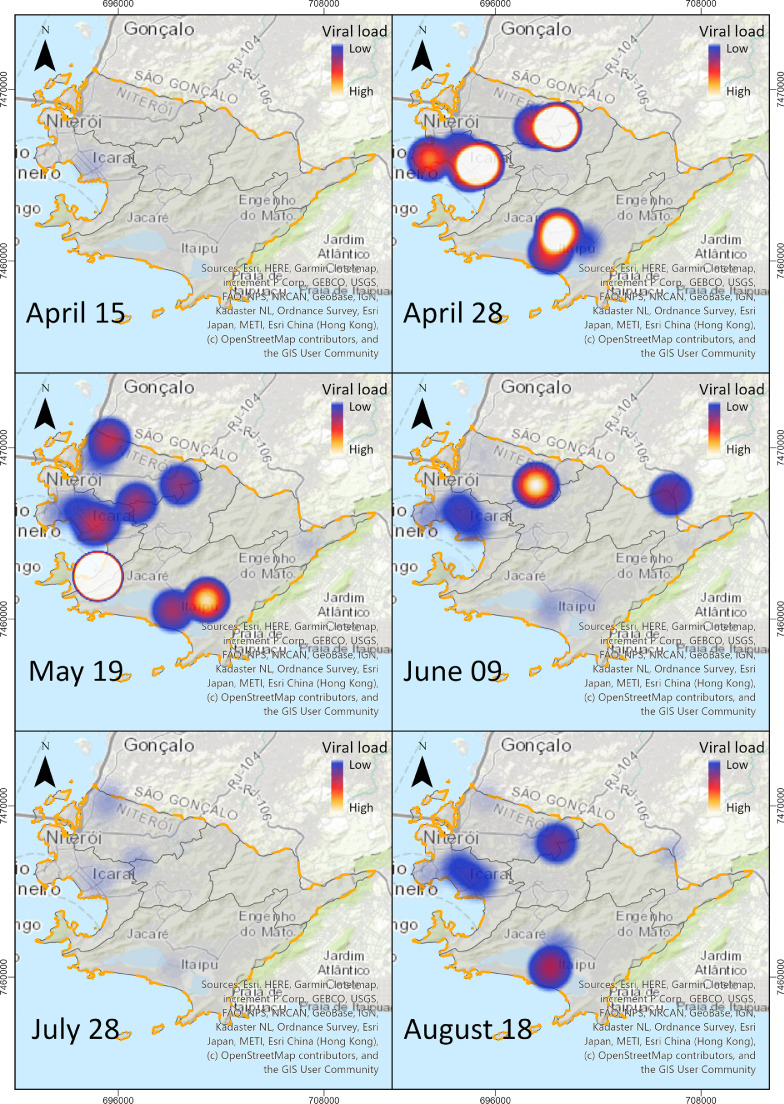
Fig. 3 shows the expansion of the affected areas in Niterói by detection of SARS-CoV-2 RNA from sewerage system along the studied period. These heat maps were designed based on SARS-CoV-2 sewage titers, and used as an environmental indicator of viral spread through different areas of the city (Fig. 3). The maps were built using ArcGIS Pro with the colour's scales varying from lowest viral RNA titer value (blue colour – moderate risk areas) to maximum value (white colour – higher risk areas). Colours gradations between the minimum and maximum viral concentrations were calculated according to the program's algorithm (https://pro.arcgis.com/en/pro-app/help/mapping/layer-properties/color-schemes.htm) (Fig. 3).
Fig. 3.
Heat maps showing SARS-CoV-2 spread (log10 RNA titers/100 mL) in different regions (hotspots areas) of Niterói municipality over the epidemic period.
At the beginning of monitoring, SARS-CoV-2 was detected in four sampling points at Praias da Baía (Guanabara Bay beaches) region, where the first cases were registered in the municipality (15th April). SARS-CoV-2 spread to other regions and reached 100% of detection on 19th May, during the peak of the epidemic. In the subsequent weeks, a reduction of positive rates and viral concentrations were observed between June and July. On 18th August, viruses were detected again in the sewers from five regions of the municipality, with an increase in viral concentration (Fig. 3).
Geodetic reference: SIRGAS2000; Projection System: UTM; Spindle: 23S; Scale: 1: 220,000.
3.3. Findings according to collection points
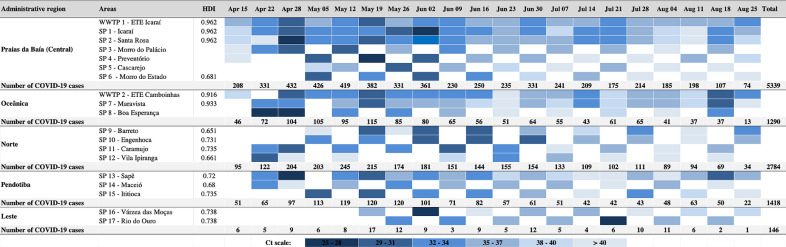
Fig. 4 illustrates SARS-CoV-2 RNA monitoring during the course of the epidemic through WWTP and decentralized sewage collection networks (SP), including areas with different socio-economic conditions (neighbourhoods, slums) considered of interest by the Municipal Health Secretary. We analysed RNA titers by 3-Ct intervals (from 25 to 40 that correspond to ~1 log10 GC decrease). It is possible to observe that low Ct values (high SARS-CoV-2 RNA titers) for all five administrative regions of Niterói were concentrated between April and mid-June, thus coinciding with the high numbers of COVID-19 confirmed cases in each region (Fig. 4).
Fig. 4.
SARS-CoV-2 spread in different areas of Niterói according to wastewater treatment plants (WWTPs) and sewer points (SP) from neighbourhoods and slums. HDI: Human Development Index; Squares in white: without sample collection; Number of COVID-19 cases were provided by Health Secretariat.
Collection points were also grouped in WWTPs, neighbourhoods and slums for descriptive statistics (Table 1 ).
Table 1.
Frequency of detection and median viral concentrations obtained for WWTPs, neighbourhoods and slums during period of monitoring (April to August 2020).
| Location (n) | Total samples analysed* | Positive samples | Frequency (%) | Range(min –max log10 GC/100 mL) | Median (log10 GC/100 mL) |
|---|---|---|---|---|---|
| WWTPs (n = 2) | 39 | 39 | 100.0 | 3.78 – 6.14 | 4.65 |
| Neighbourhoods (n = 10) | 120 | 104 | 86.6 | 3.43 – 6.54 | 4.48 |
| Slums (n = 7) | 64 | 45 | 70.3 | 3.07 – 7.12 | 4.31 |
| Total | 223 | 188 | 84.3 | 3.07 – 7.12 | 4.6 |
Results demonstrated that positive rates were higher in WWTPs compared to sewer pipes (neighbourhoods and slums) (Table 1). Median viral concentrations were significantly higher in WWTPs compared to slums (p ≤ 0.036), but no significant difference was found between WWTPs and neighbourhoods (p > 0.05). In the same way, no significant difference was observed between the median viral concentrations obtained between neighbourhoods and slums.
3.4. Genomic surveillance strains detected in the sewage
Twenty-seven SARS-CoV-2 positive samples were submitted to the protocol for genome amplification. However, only four of them presented enough viral material to assemble the library. Lineage of three genomes B.1.1.33 (clade G) containing the nucleotide substitutions described were recovered (Table 2 ). Details of the four partial genomes recovered are described in the Supplementary Material–Table S2.
Table 2.
Pangolin lineage classification and nucleotide substitution compared to the reference strain observed in sewage samples from Niterói, Rio de Janeiro State.
| Strain / collection points – date (EpiCoV GISAID accession number) | Genome coverage | % stretches of NNNs of overall genome | Nucleotide |
||||
|---|---|---|---|---|---|---|---|
| Position | Reference | Sample | Coverage depth | Frequency | |||
| hCoV-19/env/Brazil/RJ-4712/2020 lineage B.1.1.33 - Clade G/SP8 Boa Esperança - 22 April (EPI_ISL_541397) | 94,6% | 4,6% | 241 | C | T | 101 | 100 |
| 3037 | C | T | 126 | 100 | |||
| 3240 | A | G | 97 | 100 | |||
| 11,528 | G | A | 26 | 100 | |||
| 14,408 | C | T | 1951 | 99,74 | |||
| 22,583 | G | A | 31 | 100 | |||
| 23,403 | A | G | 872 | 99,43 | |||
| 27,299 | T | C | 2160 | 99,91 | |||
| 29,148 | T | C | 15 | 93,33 | |||
| hCoV-19/env/Brazil/RJ-4736/2020 lineage B.1.1.33 - Clade G/SP15 Ititioca - 05 May (EPI_ISL_541398) | 93,7% | 5,5% | 241 | C | T | 79 | 100 |
| 3037 | C | T | 159 | 99,37 | |||
| 14,408 | C | T | 603 | 100 | |||
| 23,403 | A | G | 730 | 99,18 | |||
| 27,299 | T | C | 699 | 99,86 | |||
| 28,881 | GGG | AAC | 21 | 100 | |||
| 29,148 | T | C | 15 | 100 | |||
| hCoV-19/env/Brazil/RJ-4830/2020 lineage B.1.1.33 - Clade G/SP2 Icaraí - 02 June (EPI_ISL_541399) | 98,3% | 1,2% | 241 | C | T | 733 | 99,73 |
| 3037 | C | T | 260 | 100 | |||
| 3689 | G | A | 273 | 98,17 | |||
| 8016 | C | T | 533 | 98,31 | |||
| 11,083 | G | T | 503 | 85,69 | |||
| 14,408 | C | T | 2480 | 99,76 | |||
| 22,028 | G | C | 11 | 100 | |||
| 23,403 | A | G | 428 | 100 | |||
| 27,299 | T | C | 1027 | 100 | |||
| 28,087 | C | T | 1825 | 99,78 | |||
| 28,881 | GGG | AAC | 652 | 99,69 | |||
| 29,148 | T | C | 694 | 99,57 | |||
| 29,648 | G | T | 363 | 100 | |||
| hCoV-19/env/Brazil/RJ-4841/2020* / SP16 Várzea das Moças - 02 June (EPI_ISL_541400) | 34,5% | 63,9% | 14,408 | C | T | 14 | 100 |
4. Discussion
4.1. Environmental surveillance of SARS-CoV-2
In the present study, we successfully used an environmental approach to track SARS-CoV-2 presence, concentration and genetic diversity from wastewater samples obtained in different areas of Niterói municipality, Brazil. Following biosafety measures previously recommended wastewater samples were thermal inactivated prior to viral concentration, once there is no evidence that this procedure causes a considerable loss of viral RNA in these samples (Wu et al., 2020; La Rosa et al., 2020b)
The mean recovery rate of BRSV, used as a surrogate of SARS-CoV-2, obtained in our study was 27.4%, similar to values ranging from ≤6% to 33.5%, previously obtained for enveloped viruses using the ultracentrifugation-based method (Ye et al., 2016; Ahmed et al., 2020b; Rusiñol et al., 2020). For PP7, the mean recovery rate found was of 18.5%, similar to the one found for mengovirus (11 ± 2.1%), a non-enveloped picornavirus (Randazzo et al., 2020). Other concentration methods resulted in lower recovery rates (range: 6.6% to 11%) using surrogates for human coronavirus (Randazzo et al., 2020; Gonzales et al., 2020), although variable rates were observed by Ahmed et al. (2020b) comparing different types of viral concentration methods.
The confirmation of positive RT-qPCR signals by sequencing analysis or the use or more than one target region is highly recommended until assay specificities have been validated for environmental samples (Kitajima et al., 2020; Michael-Kordatou et al., 2020). Despite no consensus on the best methodology to detect SARS-CoV-2, we used N2 primes and probe based on studies that described this target region as the most suitable to detect these viruses (Nalla et al., 2020; Gonzalez et al., 2020).
Over the period of five months, we analysed 223 raw sewage samples weekly collected from different sampling collection points (WWTP and SP), and SARS-CoV-2 was detected in 84.3% of them presenting Ct values ranging from 24.8 and 39.8 and detection frequencies between 42% and 100%. During the surveillance, viral concentrations ranged from 3.1 to 7.1 log10 GC/100 mL. Concentrations found in our study were higher than those found in Australia, USA, India, Israel and Turkey (Ahmed et al., 2020a; Gonzalez et al., 2020; Kocamemi et al., 2020; Kumar et al., 2020). However, it is worth mentioning that most of these studies analysed a limited number of samples. In Australia and Turkey, nine samples were analysed, and two and seven were positive for SARS-CoV-2, respectively, presenting Ct values higher than 34 (Ahmed et al., 2020a; Kocamemi et al., 2020). Our results are more aligned with SARS-CoV-2 concentrations found in Spain and France (Randazzo et al., 2020; Wurtzer et al., 2020). As stated by Gonzalez et al. (2020), we believe that several factors affect different findings of viral concentration in sewage samples, especially the ongoing circumstances of COVID-19 pandemic in the studied region.
From 188 SARS-CoV-2-positive samples, we selected 27 samples based on lower Ct values to perform whole-genome sequencing. From these, we successfully obtained the almost complete genome of four samples, all presenting Ct values < 30. Three out of four genomic sequences were identified as lineage B.1.1.33 (clade G). This lineage was the most prevalent SARS-CoV-2 lineage in Rio de Janeiro state in the early phase of the pandemic (late February to late April) (Resende et al., 2020b), probably established before the detection of the first reported SARS-CoV-2 case in the country. Comparing SARS-CoV-2 genomes obtained from our samples with other genomes available in EpiCoV GISAID, we observed that the strains recovered from sewage samples in Niterói held a close identity with SARS-CoV-2 strains circulating locally in Rio de Janeiro capital.
Interestingly, Brazilian SARS-CoV-2 strains were clustered into three major clades (1, 2 and 3), and currently, clade 2 also characterized as lineage B.1.1.33 (B.1.1.BR) is described by two nucleotide substitutions in ORF6 (T27299C) and nucleoprotein (T29148C) (Resende et al., 2020b). Lineage B.1.1.33 is considered the most spatially widespread lineage in Rio de Janeiro state (Resende et al., 2020b). In this study, three complete genome of the strains recovered from sewage samples showed the same nucleotide mutations. The most recent common ancestor of the Brazilian lineage B.1.1.33 was dated from 22nd February, indicating that the community-driven transmission was already stablished in Brazil by early March (Resende et al., 2020b).
4.2. Wastewater-based epidemiology to support public health policies
During the pandemic of COVID-19, several studies have demonstrated how an environmental approach was useful to monitor SARS-CoV-2 spread in a given geographic area (Gonzalez et al., 2020; Medema et al., 2020; Randazzo et al., 2020). However, there are few examples reporting how WBE data were used by local authorities to assist public health interventions at controlling the epidemic or how sewage monitoring programs could be incorporated into public health policies.
This intersectional project, initiated by the City Hall of Niterói, involved several municipal government departments (Environment, Health, Planning and Transport), as well as the Water and Sanitation Company (Águas de Niterói) and Fiocruz as a research institute. Throughout this study, the interaction of these groups took place through weekly meetings with the participation of different representatives of these institutions to discuss the data obtained and plan health interventions.
In the first week of sewage monitoring, higher COVID-19 number of cases was found in Praias da Baía (Guanabara Bay beaches – Central), one of the regions with the highest HDI and population density in the municipality. At this moment, we also tested raw sewage from two hospitals in this area, however, SARS-CoV-2 RNA was not detected (data not shown). The use of diapers in patients and/or hospital cleaning procedures may explain the absence of detectable viruses in hospital sewage. After the first week, the other regions (eastern and northern) reported an increase in the number of cases reaching lower-income populations and several poorer communities in the municipality. The expansion of the disease to more vulnerable communities was accompanied by virus research in sewers, which explains the increase in SPs analyzed and in relation to the pilot project (Prado et al., 2020).
The idea of investigating SP in addition to WWTP was supported by the need for new indicators to guide allocation of resources and investments to control the spread of the disease, promoting interventions in the most affected areas. One example of this action plan took place in a specific area located at the Oceânica region, Boa Esperança community. The community houses 610 families (2083 people) registered in the FMP. SARS-CoV-2 was firstly detected in sewage (Ct value of ~26) on April 22, when no COVID-19 cases had been reported in this community. Based on sewage findings, the first intervention was to launch an active surveillance to search for individuals showing COVID-19 related symptoms, identifying infected individuals by testing and to implement measures to control the spread of the disease. Based on this scenario, supplementary assistance services were conducted, such as guidance of appropriate support treatment, as well as tracking contacts and advising of family members on disease prevention. In addition, payment for hotel accommodation was also offered to families without physical spaces in their homes to promote social isolation.
The ongoing environmental surveillance in Niterói demonstrated an increase of SARS-CoV-2 concentration in sewage followed by progressive decrease that reflected the epidemic control measures implemented by Niterói City Hall. However, from August towards, the progressive lifting of social distancing and re-opening of commerce and services have promoted an increase of SARS-CoV-2 concentration, observed in the last monitoring campaign carried out on 18th August 2020, when the city presented average values of social isolation below 50%. However, the increase of SARS-CoV-2 in sewage did not follow a proportionally great number of reported COVID-19 cases or related deaths by the Niterói health system. In this case, we have two hypotheses. It is likely that a large part of the new wave of infection is occurring in young people who usually have mild symptoms and who attend bars, gyms and collective sports activities, dispensing medical or hospital care in most cases. The second hypothesis is related to underreporting or delay in updating the number of daily/weekly cases reported by the health system.
Our findings highlight the use of WBE approach as an early warning system for the emergence of new COVID-19 cases enabling faster prevention and public health actions by the State, as emphasized recently (Farkas et al., 2020; Medema et al., 2020; Polo et al., 2020; Randazzo et al., 2020; Thompson et al., 2020). The success of the intervention carried out in the Boa Esperança community, resulted in the inclusion of SARS-CoV-2 sewage data as a complementary indicator to other traditionally health indicators used, such as growth rates of new cases and hospitalizations, mortality rate, number of available hospital beds and bed occupancy rate. Moreover, SARS-CoV-2 environmental data as well as heat maps showing a time line were published and weekly updated in the official control panel of the Niterói City Hall webpage (https://experience.arcgis.com/experience/305269f3cdd24839b263c5ab346e1aa7sed), as an additional information tool encouraging disease prevention actions.
Although national or municipal responses to control COVID-19 are different according to institutional arrangements that compounds the public policies of each government (Capano et al., 2020), the decision to include the investigaton of SARS-CoV-2 in sewage to support strategies for controlling the epidemic in Niterói was pionner in the country. This action provides evidences to identify regions with unreported cases of the disease, mainly in areas of social vulnerability and to define regionalized coping strategies in priority areas to focus on the distribution of tests and other resources. The development of geoinformation management system (SiGeo), including "smart cities" tools citizen service (mobile app), was implemented to share scientific information among the general public. Scientific evidence-based public actions supported by multilevel partnership between Public Health research institution, Sanitation Company and Government Secretariats were fundamental to the success of this project.
5. Conclusion
In this study, we demonstrated comprehensive aspects of WBE and how its results have been converted into more effective public health policies for the population. Environmental monitoring during the course of the epidemic through decentralized sewage collection networks (SP), including areas of uneven socio-economic conditions (neighbourhoods and slums) considered of interest by Municipal Health Secretariat allowed quick and targeted actions carried out in areas of greatest risk and social vulnerability.
WWTPs does not offer the same accuracy for identifying outbreaks and rapid intervention for controlling of the disease, but their higher detection rates (100% during monitoring program), are more effective for monitoring trends, that is, for assessing whether SARS-CoV-2 concentrations are increasing or decreasing in a broader population, indicating a risk for the occurrence of the disease in communities. Moreover, whole-genome sequencing demonstrated that SARS-CoV-2 lineage circulating in Niteroi municipality is closest related to the Brazilian strains, more specifically, with strains circulating in Rio de Janeiro. Community transmission should have occurred before the first cases were notified in the city.
The successful experience of WBE and public health interventions adopted by Niterói municipality could be replicated at the national level as well as in other countries interested in using SARS-CoV-2 environmental surveillance data as part of the COVID-19 combat strategies.
Funding
This study was supported by Instituto Oswaldo Cruz (PAEF/2), Ação 21C0, and Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) [grant number 202.796/2019, TMF – Jovem Cientista do Nosso Estado and 202.821/2018, MPM – Cientista do Nosso Estado programs], and The Brazilian National Council for Scientific and Technological Development (CNPq grant number 306655/2018-7). This research study is under the scope of the activities of the Oswaldo Cruz Foundation (Fiocruz) as a Collaborating Centre of PAHO/WHO of Public and Environmental Health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interest or conflict of interest related to this work.
Acknowledgement
The authors would like to thank the Municipality of Niterói for supporting this research and providing data, especially to the Health Secretariat; Secretariat for Planning, Budget and Management Modernization; Secretariat for the Environment, Water Resources and Sustainability. They also thank the company Águas de Niterói for their support in collecting samples and to Sérgio de Silva e Mouta Júnior for helping with sample processing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2021.116810.
Appendix. Supplementary materials
References
- Ahmed A., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;1 doi: 10.1016/j.scitotenv.2020.138764. 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bersch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxus M., Letellier C., Kerkhofs P. Real time RT-PCR for the detection and quantitation of bovine respiratory syncytial virus. J. Virol. Methods. 2005;125(2):125–130. doi: 10.1016/j.jviromet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Capano G., Howlett M., Jarvis D.S.L., Ramesh M., Goyald N. Mobilizing policy (in)capacity to fight COVID-19: understanding variations in state responses. Policy Soc. 2020 doi: 10.1080/14494035.2020.1787628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., Lim A.S., Sutjptol S., Lee P.H., Son T.T., Young B.E., Milton D.K., Gray G.C., Schuster S., Barkham T., Pratim P., Vasoo S., Chan M., Ang B.S.P., Tan B.H., Leo Y-S., Ng O., Wong M.S.Y. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croghan C.W., Egeghy P.P. US-EPA, Research Triangle Park, NC; 2003. Methods of Dealing with Values below the Limit of Detection Using SAS. [Google Scholar]
- Farkas K., Hillary L.S., Malhan S.K., McDonald J.E., Jones D.L. Wastewater and public health: the potential of wastewater surveillance for monitoring COVID-19. Environ. Sci. Health. 2020;17:14–20. doi: 10.1016/j.coesh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020;20(8):892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., Thompson H., Keeling D., Mitchell J., Gonzalez D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;13(186) doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;1 doi: 10.1016/j.scitotenv.2020.140405. 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O., Halden R.U. Computacional analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;15 doi: 10.1016/j.scitotenv.2020.138875. 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Review. Sci Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi, B.A., Kurt, H., Hacioglu, S., Yarali, C., Saatci, A.M., Pakdemirli, B., 2020. First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey. MedRxiv., doi: 10.1101/2020.05.03.20089417. [DOI]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;28(746) doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredino E. First detection of SARS-CoV-2 in untreated wastewater in Italy. Sci. Total Environ. 2020;20(736) doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods–a scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.-F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lu X., Wang L., Sakthivel S.K., Whitaker B., Murray J., Kamili S., Lynch B., Malapati L., Burke S.A., Harcourt J., Tamin A., Thornburg N.J., Villanueva J.M., Lindstrom S. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26(8):1654–1665. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K, Zhang K, Du W, Ali W, Feng X, Zhang H. The potential of wastewater based epidemiology as surveillance and early warning of infectious disease outbreaks. Curr. Opin. Environ. Sci. Health. 2020;17:1–7. doi: 10.1016/j.coesh.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla A.K., Casto A.M., Huang M.-L.W., Perchetti G.A., Sampoleo R., Shrestha L., Wei Y., Zhu H., Jerome K.R., Greninger A.L. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina S., Jofre J., Emerson S.U., Purcell R.H., Girones R. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl. Environ. Microbiol. 1998;64:4485–4488. doi: 10.1128/AEM.64.11.4485-4488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D., Quintela-Baluja M., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde J.L. Making waves: wastewater-based epidemiology for COVID-19–approaches and challenges for surveillance and prediction. Water Res. 2020;1 doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020;115 doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajal V.B., McSwain B.S., Thompson D.E., Leutenegger C.M., Kildare B.J., Wuertz S. Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Res. 2007;41:1411–1422. doi: 10.1016/j.watres.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O’Toole A., Hill V., McCrone J.T., Ruis C., du Plessis L., Pybus O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuervas-Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in alow prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende, P.C., Motta, F.C., Roy, S., Appolinario, L., Fabri, A., Xavier, J., Harris, K., Caetano, A.R.M.B., Orgeswalska, M., Miranda, M., Garcia, C., Willians, A.A.R., Breuer, J., Siqueira, M.M., 2020a. SARS-CoV-2 genomes recovered by long amplicon tiling multiplex approach using nanopore sequencing and applicable to other sequencing platforms. bioRxiv preprint doi: 10.1101/2020.04.30.069039. [DOI]
- Resende, P.C., Delatorre, E., Graf, T., Mir, D., Motta, F.C., Appolinario, L.R., Paixão, A.C.D., Ogrzewalska, M., Caetano, B., dos Santos, M.C., Ferreira, J.A., Junior, E.C.S., da Silva, S.P., Fernandes, S.B., Vianna, L.A., Souza, L.C., Ferro, J.F.G., Nardy, V.B., Croda, J., Oliveira, W.K., Abreu, A., Bello, G., Siqueira, M.M., 2020b. Genomic surveillance of SARS-CoV-2 reveals community transmission of a major lineage during the early pandemic phase in Brazil. bioRxiv preprint doi: 10.1101/2020.06.17.158006. [DOI]
- Rusiñol M., Martínez-Pucholl S., Forés E., Itarte M., Girones R., Bofill-Mas S. Concentration methods for the quantification of coronavirus and other potentially pandemic enveloped virus from wastewater. Environ. Sci. Health. 2020;17:21–28. doi: 10.1016/j.coesh.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNIS–Sistema Nacional de Informações em Saneamento. 2020. Série Histórica. Available: http://app4.mdr.gov.br/serieHistorica/. Last access: 04 Dec, 2020.
- Thompson J.R., Nancharaiahd Y.V., Gu X., Lee W.L., Rajal V.B., Hainesc M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Making waves: wastewater surveillance of SARS-CoV-2 for population based health management. Water Res. 2020;1(184) doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;12(18):1843–1844. doi: 10.1001/jama.2020.3786. 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. Statement on the second meeting of the International Health Regulations (2005) emergency committee regarding the outbreak of novel coronavirus (2019-nCoV) [WWW Document].
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Moulin L. [DOI]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Phil D., Tan W. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.