To the editors,
We read the recent published letter by Setor K. Kunutsor and colleagues in journal of infection with great interest, which performed a single arm meta-analysis of 17 studies (n = 5815) to address issues on the association between cardiovascular complications and COVID-19, the incidence of cardiovascular complications, and whether patients with previous cardiovascular morbidities are more likely to have cardiovascular complications.1 Until now, the potential mechanisms for cardiovascular complications are still yet to be elucidated. The state-of-art evidence suggests that most common cardiovascular complications of COVID-19 are heart failure, myocardial injury and cardiac arrhythmias, with pooled incidence of 17.6%, 16.3% and 9.3%, respectively 1. The incidence of myocardial injury may be associated with pre-existing hypertension but not cardiovascular disease 1. However, not all cardiovascular complications have been well described in previous studies and the risk factors associated with cardiovascular complications among COVID-19 patients remain unclear. Therefore, in this retrospective cohort study, we aimed to characterize the COVID-19 patients who developed cardiac arrhythmia during hospitalization and explore the performance of risk factors in predicting cardiac arrhythmia.
A total of 234 laboratory-confirmed COVID-19 patients were retrospectively included from three designated hospitals of China. We collected the demography, comorbidities (pre-existing hypertension, cardiovascular disease, diabetes, chronic liver disease and chronic lung disease) and laboratory characteristics from medical records. Arrhythmia was defined as rapid ventricular tachycardia lasting >30 s, inducing hemodynamic instability and/or ventricular fibrillation.2 We defined the severity of COVID-19 according to the newest COVID-19 guidelines released by the National Health Commission of China3 and the guidelines of American Thoracic Society for community-acquired pneumonia.4 The comparison of baseline characteristics between COVID-19 patients with and without cardiac arrhythmia using T test or Mann-Whitney U or Chi-squared or Fisher's exact test. The optimal cutoff of risk factors for discriminating patients with or without arrhythmia was determined using receiver operating characteristic (ROC) curve and by maximizing the Youden index. Logistic regression analysis was used to identify risk factors for arrhythmia. This study was approved by an ethics committee of our institution, with a waiver of informed consent.
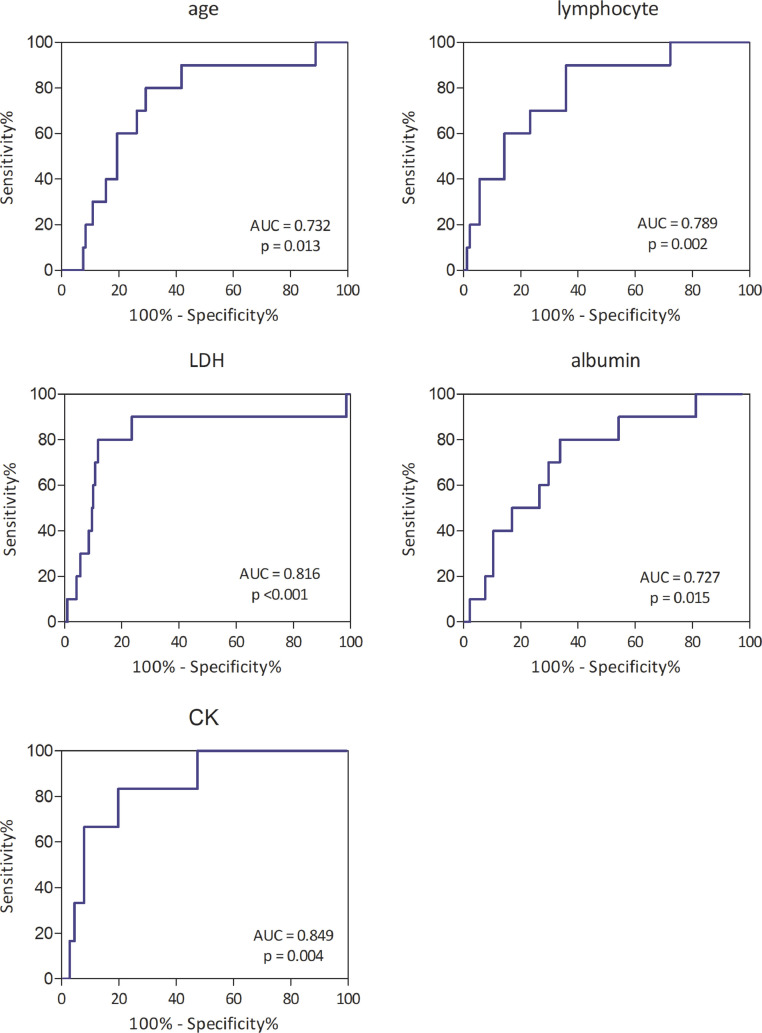
The proportion of COVID-19 patients who developed cardiac arrhythmia was 4.3% (10/234) in this study. The median age of 234 patients was 58 years (interquartile range, 42–67 years) and the median age of patients with cardiac arrhythmia was 69 years (interquartile range, 63.8–77.5 years). Of the 10 patients with arrhythmia, only 3 had pre-existing hypertension and 2 had pre-existing coronary heart disease. Eight patients were critically ill, one was severe and one was moderate. The optimal cutoff value of age was 64.5 years, with an area under the ROC curve (AUC) of 0.732 (95% CI: 0.584–0.880), sensitivity of 80%, and specificity of 68.3%. The optimal cutoff value of lymphocyte was 0.915 × 109/L, with an AUC of 0.789 (95% CI: 0.655–0.923), sensitivity of 90.0%, and specificity of 61.9%. The optimal cutoff value of albumin was 35.0 g/L, with an AUC of 0.727 (95% CI: 0.578–0.875), sensitivity of 80%, and specificity of 66.1%. The optimal cutoff value of lactic dehydrogenase (LDH) was 401.5 U/L, with an AUC of 0.816 (95% CI: 0.644–0.988), sensitivity of 80%, and specificity of 88.3%. The optimal cutoff value of creatine kinase (CK) was 101.5 U/L, with an AUC of 0.849 (95% CI: 0.720–0.978), sensitivity of 83.3%, and specificity of 80.1%. Thus, admission CK had the highest performance in predicting cardiac arrhythmia among patients with COVID-19, followed by LDH. The results showed that age >64.5 years (OR 8.62, 95% CI: 1.79–41.63), lymphocyte <0.915 × 109/L (OR 12.13, 95% CI: 1.51–97.35), albumin <35.0 g/L (OR 7.15, 95% CI: 1.48–34.49), LDH >401.5 U/L (OR 30.1, 95% CI: 6.05–149.69, p <0.001) and CK >101.5 U/L (OR 20.0, 95% CI: 2.26–176.7) were risk factors of arrhythmia. (Fig. 1 )
Fig. 1.
Receiver operating characteristic curve of risk factors of cardiac arrhythmia in patients with COVID-19.
This study might suggest that cardiac arrhythmia was associated with COVID-19 because most of patients had no previous cardiovascular morbidities. In addition to pre-existing cardiovascular comorbidities lead to worse outcomes in COVID-19 patients, cardiovascular complications have also been shown to be associated with increased risk of severe/critical COVID-19 5, 6, 7. The occurrence of cardiac arrhythmia was related to older age, higher levels of LDH and CK, and lower levels of lymphocyte and albumin. However, larger cohort study is warranted to be conducted to validate these findings. Monitoring of biomarkers of cardiac arrhythmia for COVID-19 could help in the identification of patients with potential cardiac manifestations, in order to enable early and more aggressive intervention. (Table 1 )
Table 1.
Comparison of baseline characteristics between COVID-19 patients with or without complication of arrhythmia.
| Characteristics | without arrhythmia (n = 224) | with arrhythmia (n = 10) | p-value |
|---|---|---|---|
| Age (years) | 57.0 (42.0–67.0) | 69.0 (63.8–77.5) | 0.013 |
| Sex, n (%) | |||
| Male | 121 (54.0) | 8 (80.0) | 0.192 |
| Female | 103 (46.0) | 2 (20.0) | |
| Comorbidities | |||
| Hypertension, n(%) | 50 (22.3) | 3 (30.0) | 0.698 |
| Coronary heart disease, n(%) | 17 (7.6) | 2 (20.0) | 0.190 |
| Diabetes, n(%) | 27 (12.1) | 1 (10.0) | 1.000 |
| Chronic liver diseases, n(%) | 7 (3.1) | 1 (10.0) | 0.299 |
| Chronic lung diseases, n(%) | 18 (8.0) | 2 (20.0) | 0.206 |
| Others, n(%) | 47 (21.0) | 4 (40.0) | 0.231 |
| Disease severity, n(%) | |||
| Mild | 29 (12.9) | 0 | 0.617 |
| Moderate | 106 (47.3) | 1 (10.0) | 0.023 |
| Severe | 53 (23.7) | 1 (10.0) | 0.461 |
| Critical | 36 (16.1) | 8 (80.0) | <0.001 |
| Laboratory findings | |||
| WBC (× 109/L) | 5.3 (4.1–6.6) | 6.3 (3.6–8.8) | 0.590 |
| Neutrophil (× 109/L) | 3.4 (2.6–4.8) | 5.2 (2.5–8.2) | 0.266 |
| Lymphocyte (× 109/L) | 1.1 (0.7–1.6) | 0.6 (0.4–0.9) | 0.002 |
| LDH (U/L) | 195.0 (152.0–279.5) | 425.5 (378.5–564.5) | 0.001 |
| Hemoglobin (g/L) | 129.0 (119.0–143.0) | 135.5 (125.3–144.5) | 0.413 |
| Platelet (g/L) | 203.0 (149.0–252.0) | 146.5 (89.5–201.8) | 0.054 |
| Albumin (g/L) | 37.6 (32.4–41.0) | 31.7 (29.1–35.7) | 0.014 |
| AST (U/L) | 23.0 (16.0–36.0) | 37.2 (25.5–60.0) | 0.035 |
| ALT (U/L) | 22.0 (15.0–37.0) | 21.5 (9.8–33.8) | 0.417 |
| DBIL (μmol/L) | 3.5 (2.5–4.8) | 4.6 (3.5–5.7) | 0.062 |
| IBIL (μmol/L) | 7.4 (5.2–10.3) | 5.6 (4.5–7.4) | 0.256 |
| TBIL (μmol/L) | 11.0 (8.2–15.1) | 5.5 (4.6–8.8) | 0.990 |
| APTT (s) | 34.2 (31.7–37.0) | 10.6 (8.1–15.0) | 0.936 |
| PT (s) | 13.3 (12.5–14.3) | 34.7 (31.2–37.7) | 0.519 |
| D-dimer (μg/ml) | 0.2 (0.1–0.5) | 0.8 (0.2–7.5) | 0.033 |
| Creatinine (μmol/L) | 70.0 (58.0–82.0) | 75.5 (70.0–94.8) | 0.081 |
| CK (U/L) | 55.0 (37.0–84.0) | 196.0 (92.0–331.5) | 0.004 |
| CK-MB (U/L) | 9.0 (6.0–14.0) | 14.0 (8.0–36.0) | 0.090 |
| hs-CRP (mg/L) | 9.4 (1.1–35.7) | 31.4 (25.6–35.9) | 0.134 |
| Procalcitonin (ng/ml) | 0.08 (0.06–0.17) | 0.2 (0.1–0.4) | 0.033 |
| FBG (mmol/L) | 5.5 (4.8–6.9) | 9.5 (6.8–12.0) | <0.001 |
| NTproBNP (pg/mL) | 127.0 (41.6–420.0) | 422.1 (105.5–1218.2) | 0.079 |
Note: Data were number (percentage) or median (interquartile range). Abbreviations: WBC, white blood cells; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBIL, Total Bilirubin; DBIL, Direct Bilirubin; IBIL, indirect bilirubin; APTT, activated partial thromboplastin time; PT, prothrombin time; CK, creatine kinase; CK-MB, Creatine kinase isoenzyme; hs-CRP, high-sensitivity C-reactive protein; FBG, fasting blood glucose; NTproBNP, N-terminal portion of proBNP.
Declaration of Competing Interest
The authors declare no competing interests.
References
- 1.Kunutsor Setor K., Laukkanen Jari A. Cardiovascular complications in COVID-19: a systematic review and meta-analysis. J Infection. 2020 doi: 10.1016/j.jinf.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T., Fan Y., Chen M. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection (trial version 7) (in Chinese) National Health Commission of the People's Republic of China; March 04, 2020. [DOI] [Google Scholar]
- 4.Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the american thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunutsor S.K., Laukkanen J.A. Markers of liver injury and clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. J Infection. 2020 doi: 10.1016/j.jinf.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahajan K., Chand Negi P., Ganju N. Cardiac biomarker-based risk stratification algorithm in patients with severe COVID-19. Diabetes Metab Syndr. 2020;14:929–931. doi: 10.1016/j.dsx.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]