Abstract
Carbapenemase-producing Enterobacterales (CPE) are significant contributors to the global public health threat of antimicrobial resistance. OXA-48-like enzymes and their variants are unique carbapenemases with low or null hydrolytic activity toward carbapenems but no intrinsic activity against expanded-spectrum cephalosporins. CPEs have been classified by the WHO as high-priority pathogens given their association with morbidity and mortality and the scarce number of effective antibiotic treatments. In Spain, the frequency of OXA-48 CPE outbreaks is higher than in other European countries, representing the major resistance mechanism of CPEs. Horizontal transfer of plasmids and poor effective antibiotic treatment are additional threats to the correct prevention and control of these hospital outbreaks. One of the most important risk factors is antibiotic pressure, specifically carbapenem overuse. We explored the use of these antibiotics in Spain and analyzed the frequency, characteristics and prevention of CPE outbreaks. Future antibiotic stewardship programs along with specific preventive measures in hospitalized patients must be reinforced and updated in Spain.
Keywords: OXA-48, carbapenem, carbapenemase, Spain, outbreak, multiresistant, enterobacteria, antibiotic stewardship
1. Introduction
Enterobacteriaceae were identified as a public health threat since the discovery of their ability to acquire molecular resistance through extended-spectrum β-lactamases (ESBLs) [1]. In 2016, large-scale genomic sequencing data led to reclassification of various species, originally included in the family Enterobacteriaceae, in an order called Enterobacterales [2].
In order to counteract the menace of antibiotic resistant Enterobacterales, carbapenems were developed and introduced into the therapeutic arsenal during the decade of 1990 [3]. Since then, these drugs have been widely used as first-line empirical antibiotic treatment [4]. Nevertheless, this strategy resulted in an even greater problem since the lack in carbapenem stewardship has led to the development of carbapenem-resistant Enterobacterales (CRE) [5,6]. Specifically, the first carbapenemase (NmcA) producer was identified in 1993 in a clinical isolate of Enterobacter cloacae [7]. Since then, numerous CRE have been reported [8].
CRE present three main mechanisms of carbapenem resistance: enzyme production, efflux pumps and porin mutations [9]. Of these, enzyme production is the most frequent resistance mechanism and OXA-48-like enzymes represent one of the most common CRE enzymes worldwide [10]. The family takes its name from the first identified enzyme, OXA-48, and includes several sequence variants transmissible via plasmids.
The aim of this review is to analyze the frequency, characteristics and prevention of OXA-48 CRE outbreaks in Spain.
2. OXA-48-Like Enzymes: Mechanism of Resistance
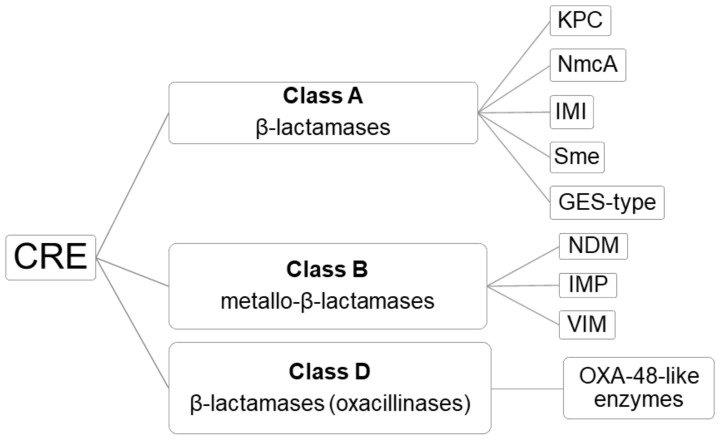
According to the Ambler classification, three classes of carbapenemases can be distinguished in CRE: A, B and D (Figure 1) [11]. According to the Bush–Jacoby functional system [12], carbapenemases in class A include β -lactamases, which are inhibited by clavulanic or boronic acid; class B include metallo- β -lactamases capable of hydrolyzing all ß-lactam antibiotics except aztreonam and inhibited by EDTA and dipicolinic acid; and class D include β-lactamases (oxacillinases) including all OXA-48-like enzymes (e.g., OXA-48, OXA-72 and OXA-244), capable of hydrolyzing carbapenems but not (or weakly hydrolyzing) cephalosporins, and not inhibited by classical inhibitors [13,14,15]. However, there are several OXA-48-like variants, such as OXA-163, that completely lose their ability to hydrolyze carbapenems and display an ESBL phenotype.
Figure 1.
Classification of carbapenemases produced by carbapenem-resistant Enterobacterales (CRE), based on the Ambler classification.
The kinetics of OXA-48-like enzymes shows high-level hydrolytic activity against penicillins and low hydrolytic activity toward imipenem and meropenem compared to ertapenem, which is the best substrate for these enzymes [16,17]. Several variants of OXA-48 have been reported in clinical samples since its first identification (Table 1) [15,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Nevertheless, 96 different OXA-48-like enzymes have been reported to date according to the Beta-Lactamase DataBase [39], 35 of which have a definite assignment name by the National Center for Biotechnology Information (NCBI), as shown in the Supplementary Materials (Table S1) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Table 1.
OXA-48-like enzymes reported in human clinical samples.
| Enzyme | First Clinical Identification | Reference |
|---|---|---|
| OXA-48 | Turkey, 2001 | [18] |
| OXA-54 | France, 2003 | [19] |
| OXA-162 | Turkey, 2012 | [20] |
| OXA-163 | Argentina, 2008 | [21] |
| OXA-181 | India, 2010 | [22] |
| OXA-199 | China, 2012 | [23] |
| OXA-204 | Tunisia/France, 2012 | [24] |
| OXA-232 | India/France, 2011 | [25] |
| OXA-244 | Spain, 2012 | [26] |
| OXA-245 | Spain, 2012 | [26] |
| OXA-247 | Argentina, 2010 | [27] |
| OXA-252 | Canada, 2014 | [28] |
| OXA-370 | Brazil, 2013 | [29] |
| OXA-405 | France, 2014 | [30] |
| OXA-416 | Italy, 2013 | [31] |
| OXA-436 | Denmark, 2015 | [32] |
| OXA-438 | Argentina, 2020 | [33] |
| OXA-484 | United Kingdom, 2015 | [34] |
| OXA-505 | USA, 2018 | [35] |
| OXA-519 | Belgium, 2015 | [36] |
| OXA-535 | France, 2018 | [37] |
| OXA-566 | New Zealand, 2017 | [38] |
Updated from the information provided by Mairi et al. [15] and the Beta-Lactamase Database [38] available at: http://www.bldb.eu/BLDB.php?prot=D#OXA.
It has been suggested that the progenitor gene of OXA-48 was encoded in Shewanella spp., a waterborne bacterium. To date, more than fifteen OXA-48-like variants have been identified in clinical samples, but classical OXA-48 remains as the most frequent form globally. The emergence of this enzyme is mediated by the rapid spread of a broad host-range conjugative plasmid sheltering the blaOXA-48 gene, located within a composite transposon (Tn1999), which flanks the carbapenemase gene and helps mobilize an intervening DNA segment [40,41]. Additionally, five variants of Tn1999 have been identified to date (Tn1999.1, Tn1999.2, Tn1999.3, Tn1999.4 and Tn1999.5) [41,42,43]. Given the wide transmission and spread of this plasmid, the range of species in which OXA-48 has been identified as part of hospital outbreaks is increasingly higher (Table 2) [44,45,46,47,48,49,50,51,52,53,54,55,56]. This fact led to suggest that transmission of hospital outbreaks might be explained not only by the nosocomial spread of bacteria but also by the horizontal transfer of plasmids between species in certain hospital areas [57]. Apart from human clinical samples, a wide range of OXA-48-like enzymes have been detected in animals or the water environment [39].
Table 2.
OXA-48-like enzymes detected in different Enterobacterales species in clinical samples.
| First Clinical Identification | References |
|---|---|
| Klebsiella pneumoniae | [44,45,46,47,48] |
| Klebsiella oxytoca | [44,45,48,49] |
| Kluyvera spp. | [48,50] |
| Escherichia coli | [44,45,48,51] |
| Proteus mirabilis | [44,45,48,52] |
| Serratia marcescens | [53] |
| Enterobacter cloacae | [44,45,48,54] |
| Enterobacter aerogenes | [44,45,48] |
| Enterobacter sakasakii | [15] |
| Citrobacter freundii | [44,45,48,55] |
| Citrobacter koseri | [44,45,48] |
| Citrobacter braakii | [15] |
| Salmonella enterica | [44,45,48,56] |
| Morganella morganii | [44,45,48] |
| Providencia rettgeri | [15] |
| Raoultella planticola | [15] |
Updated from the information provided by Suay-García et al. [10].
Klebsiella pneumoniae was the first OXA-48 CRE described, and remains the most common global bacterium related to healthcare-associated infections [46,47], followed by Enterobacter spp.
An important aspect that may cause delay in the detection and treatment errors is the attenuated carbapenem hydrolyzing ability of OXA-48 CRE. Given that these bacteria present lower carbapenem hydrolyzing capacity than Ambler type A or B CRE, they sometimes do not exceed the level points for the detection of phenotypic resistance. Therefore, without genotypic diagnostic tools (e.g., polymerase chain reaction (PCR)), OXA-48 CRE may go unnoticed and appear susceptible to carbapenems. However, patients do not show improvement after treatment with carbapenems. This could be explained by the sum of low-level resistance mechanisms and selection of especially resistant subpopulations favored by antibiotic pressure [58].
Detection of OXA-48 CRE
OXA-48 CRE is of major concern due to the difficulties in their detection and their association with treatment failure. These bacteria might be detected through screening tests, phenotypic detection assays and molecular-based detection methods [48]. The main phenotypic assays include inhibitor-based synergy tests, such as the double-disk synergy test (DDST) or combined disk test (CDT); the Carba NP test; colorimetric tests like the β-CARBA test (Bio-Rad, Marne la Coquette, France); the carbapenem inactivation method (CIM) in water containing a meropenem disk; indirect carbapenemase tests in paper test devices; the lateral flow immunoassays like the OXA-48 K-SeT assay (Coris Bioconcept, Gembloux, Belgium), which have been upgraded to detect multiple enzymes (e.g., KPC, NDM or VIM); electrochemical assays like the BYG Carba test; spectrophotometry and mass spectrometry like matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based methods.
The genotypic molecular-based detection methods include PCR, in-house multiplex PCR assays for detecting different carbapenemase genes, commercial assays like microarray-based assays, fully automated systems based on PCR or microarray techniques and whole-genome sequencing based on next generation sequencing technology.
A comprehensive description of all the different detection methods for OXA-48 CRE can be consulted in the recent review by Pitout et al. [48].
3. Worldwide Spread
In the last years, surveillance studies have pointed that OXA-48-like cabarpenemases are the most common carbapenemases in several areas of the world [10,15,16,17]. Moreover, they are increasingly being introduced into non-endemic regions where they cause nosocomial outbreaks [48].
A search in PubMed using the terms “OXA-48” and “outbreak” reveals an increasing number of reports in the last years, from 3 in 2010 to 76 in 2020.
The first identification of this enzyme was reported in Klebsiella pneumoniae isolated from a urinary tract infection sample in 2001 in Turkey [18]. Since then, a number of nosocomial outbreaks of these bacteria have been reported in this country [59,60]. A rapid spread led to first-identification reports in colonization or infection samples in many areas of the world, especially the Mediterranean region [61].
In Europe, the first case of OXA-48 identification was reported in Belgium in 2008 [62]. In Africa, reports of carbapenem-hydrolyzing OXA-48 β-lactamase in K. pneumoniae were communicated in Tunisia in 2010 [63]. In Asia, reports of OXA-48 presence were published in 2012 in Kuwait [64]. In North America, it was first described in the United States in 2013 [65], whereas in South America, the first identification was reported in Brazil in 2014 [29]. In total, more than 50 countries have reported outbreaks of OXA-48-producing bacteria to date [48,66]. In Spain, reports of OXA-48-producing bacteria in outbreaks were published in 2013 [26,27,67]. Since then, several outbreaks have been identified and OXA-48-producing bacteria have become one of the major causal agents of hospital outbreaks in our country [10,42,50].
4. OXA-48 CRE Outbreaks in Spain
4.1. Frequency
According to the report from Spain on the prevalence of healthcare-related and community infections and the use of antibiotics (EPINE-EPPS study) [68], the prevalence of CRE colonization or infections in Spanish hospital settings is continuously increasing. This study analyzed the prevalence of healthcare-associated infections in one month of the year throughout several hospitals across all the national territory. The prevalence of CRE in Spanish hospitals in 2019 is shown in Table 3. Data from 2020 are not available because the study was stopped due to the COVID-19 pandemic, which especially affected healthcare professionals [69] and hospitalized patients [70] in Spain.
Table 3.
Prevalence of CRE in hospital settings in 2019 in Spain.
| CRE Species | Nosocomial Infections | Community Infections | ||||
|---|---|---|---|---|---|---|
| IM | CR-IM | %CR | IM | CR-IM | %CR | |
| Escherichia coli | 607 | 10 | 2.1 | 1341 | 12 | 1.1 |
| Klebsiella pneumoniae | 316 | 30 | 11.2 | 305 | 30 | 11.5 |
| Klebsiella oxytoca | 60 | 2 | 4.1 | 79 | 3 | 5.0 |
| Klebsiella spp., other | 12 | 1 | 10.0 | 15 | 0 | 0.0 |
| Enterobacter aerogenes | 45 | 3 | 8.3 | 23 | 0 | 0.0 |
| Enterobacter cloacae | 126 | 9 | 8.3 | 92 | 2 | 2.3 |
| Enterobacter spp., other | 17 | 1 | 7.7 | 15 | 1 | 12.5 |
| Citrobacter spp. | 58 | 4 | 10.8 | 49 | 0 | 0.0 |
| Proteus spp. | 127 | 3 | 3.4 | 218 | 4 | 2.4 |
| Serratia marcescens | 66 | 5 | 9.6 | 58 | 1 | 2.1 |
| Serratia spp., other | 3 | 0 | 0.0 | 6 | 0 | 0.0 |
| Morganella spp. | 53 | 6 | 12.8 | 58 | 0 | 0.0 |
| Other Enterobacterales | 3 | 0 | 0.0 | 8 | 0 | 0.0 |
IM, number of isolated microorganisms; MR-IM, number of carbapenem-resistant microorganisms; %CR, percentage of carbapenem resistance.
It can be seen that the prevalence of CRE is high in Spanish hospitals, especially regarding nosocomial infections. The bacteria with a higher percentage of carbapenem resistance were Klebsiella spp., Citrobacter spp. and Enterobacter spp. Overall, OXA-48 represents the main enzymatic resistance mechanism, especially regarding Klebsiella spp. and Enterobacter spp. strains. Recent studies pointed Acinetobacter baumannii as a producer of different OXA carbapenemase encoding genes such as OXA-24-like genes [71]. However, as Pseudomonas spp. and Acinetobacter spp. are not Enterobacterales, we did not include them in the present review.
According to a multicenter study performed in 83 Spanish hospitals, OXA-48 was the most frequent carbapenemase produced by CRE (71.5%), followed by VIM-1 (25.3%) [72].
In the study by Hernández-García et al. [73], the incidence of colonization by CRE in a Spanish hospital in Madrid was 2% (161/8209), and more than 50% of them acquired colonization after admission. The main colonizing pathogen was K. pneumoniae (54%), followed by Escherichia coli (19%). The main resistance mechanism was the expression of OXA-48 (64%) and VIM-1 enzymes (27%), identified through PCR.
A recent study, also performed in Madrid [74], showed that the intestinal loads of OXA-48 colonized patients were high, suggesting an increasing replacement of the host microbiota by OXA-48-producing K. pneumoniae. The main factors associated with these loads were the previous antibiotic treatment and development of infection instead of colonization. Another study by Mateos et al. [75] also reported high frequency of Enterobacter spp. producing carbapenemase in hospitalized patients, with superimposition of OXA-48 over VIM producers in the last years.
In a previous study by our research group, we identified elderly patients living in residential care homes as a particularly vulnerable population for colonization by OXA-48 CRE [76]. Specifically, 34.5% of these patients showed positive rectal colonization by multiresistant bacteria, 27.5% of them by OXA-48-producing K. pneumoniae and 2.5% by OXA-48-producing E. coli.
According to the EUSCAPE study [77], a multinational study performed across different European countries, the rate of infections by carbapenemase-producing K. pneumoniae or E. coli in Spain was 4.01 per 10,000 admissions, one of the highest rates of all the 36 countries analyzed and far over the average rate (1.3/10,000). The ratio between K. pneumoniae and E. coli carbapenemase producers was approximately 7:1. Among them, OXA-48-like enzymes represented the major resistance mechanism (25.8% in Europe and 69.8% in Spain, the highest after Turkey, 79.0%, and Romania, 73.5%).
Therefore, Spanish hospitals present an alarming increase in OXA-48-producing CRE outbreaks compared to the rest of the European countries.
4.2. Surgical Site Infections
The hospital outbreaks caused by OXA-48 CRE do not only occur in hospitalization rooms, but also in surgical patients. A recent study performed by Mora-Guzmán et al. [78] on 65 OXA-48 infected patients showed a significantly increased risk of intra-abdominal OXA-48 CRE infection after surgery following a broad-spectrum antibiotic consumption. The authors advocated a more targeted antibiotic approach to reduce this risk.
The Spanish national program called “Zero Surgical Infection” [79] was designed to reduce the risk of surgical site infections through the implementation of preventive measures such as:
Adequate antibiotic prophylaxis before surgery.
Skin antisepsis with 2% alcoholic chlorhexidine.
Correct hair removal when necessary, without causing injuries or irritation.
Adequate control of temperature and glycemia during the procedure.
Rapid identification of infection signs after the surgery.
Although few studies have specifically addressed the risk of CRE infection after surgical procedures [78,80], antibiotic pressure and long hospital stay have been reported to be the most important risk factors.
4.3. Colonization in the Intensive Care Unit (ICU)
There is a high prevalence of CRE isolates from clinical samples in ICU admitted patients in Spain. In a recent multicenter study performed in eight hospitals, 20% of all carbapenemase-producing K. pneumoniae infections were found in ICU patients [81].
A recent prospective cohort study reported that K. pneumoniae was the predominant CRE in a Spanish ICU (73.4%), with OXA-48 being the major resistant mechanism [82].
These alarming findings led the Spanish government to implement a program called “Zero-Resistance Program” aimed at rapid identification of these patients in order to perform the adequate isolation and prevention measures [83]. The program consists of performing a systematic rectal swab test in all ICU patients at admission and every 48–72 h and applying special preventive measures in CRE colonized patients.
Risk factors for OXA-48 CRE colonization in a Spanish ICU were recently analyzed by Maseda et al. [84]. In their study, OXA-48 was the main carbapenemase found (76.1%) and carriers of CRE showed increased rates of previous chronic diseases, previous digestive or biliary endoscopy, previous hospitalization, ICU admission, intra-abdominal surgery and higher (i.e., worst) clinical prognostic scores. They also presented higher rates of antibiotic intake according to multivariate analyses, especially third or fourth generation cephalosporins (OR = 27.96, 95%CI = 6.88, 113.58, p < 0.001) and β-lactam/β-lactamase inhibitors (OR = 11.71, 95%CI = 4.51, 30.43, p < 0.001) [85]. This study may provide relevant clues for developing prevention measures to reduce CRE carriers in Spanish ICUs.
In fact, effective preventive measures in the ICU have been reported. For example, successful control of two simultaneous CREs including OXA-48-producing Enterobacterales was described in a hospital in Madrid [85]. These measures included isolation of affected patients in individual confined areas with dedicated personnel to take care of them, 2% chlorhexidine soap for patient daily hygiene, contact precautions including correct hand hygiene (detailed in [86], according to each type of microorganism), in-depth cleaning of the ICU with chlorine solution and vaporized hydrogen peroxide and strict surveillance of environmental samples [85]. Therefore, environmental hygiene might play a crucial role in controlling OXA-48 CRE outbreaks in Spanish ICUs.
4.4. Plasmid Transfer
The epidemiology of OXA-48 CRE outbreaks is very complex because, in addition to the transmission of bacteria, horizontal transfer of plasmids is possible.
Some high-risk K. pneumoniae clones, like ST307 and ST147, acquired plasmids with various carbapenemases including OXA-48 in several European countries during the late 2000s [87]. Since then, plasmid transfer has become an important factor to explain the rapid spread of this CREs and even the transmission during hospital outbreaks. Therefore, colonization by ESBL-producing K. pneumoniae predisposes to future colonization by OXA-48-producing K. pneumoniae [84]. This mechanism of transmission must be considered when adopting preventive measures to control hospital outbreaks. During the year 2019, an outbreak in the Netherlands involving 148 OXA-48 CRE colonized patients suggested a horizontal transmission mechanism of this resistance gene by one or more plasmids [88]. Specifically, blaOXA-48 plasmids were identified in transposon Tn1999.2 and located on a ca 62 kb IncL/M conjugative plasmid in 14 different species. Accordingly, a ca 62 kb plasmid was responsible for the OXA-48 outbreak [89]. These outbreaks provide strong evidence of both within-host interspecies and between-host dissemination of plasmid-based OXA-48 during a nosocomial outbreak. In Iran, K. pneumoniae blaOXA-48 plasmids were successfully transferred to an E. coli K12-recipient strain, which highlights the importance of horizontal gene transfer in the dissemination of these genes [89]. However, most reported plasmids are conjugative but absent in multiple countries or species, suggesting limited interspecies and interboundary transmission of a common plasmid [90]. In this regard, factors of incompatibility of similar plasmids might be considered, as firstly described in 1973 [91].
Clearly, the transfer of resistance plasmids calls for improved infection control measures to prevent further spread of multiresistant pathogens in future hospital outbreaks in Spain.
4.5. Risk Factors for Acquiring OXA-48 CREs
There are limited data regarding the main preventable risk factors for carrying or acquiring OXA-48 CREs. The main reservoir is the gastrointestinal tract [15], thus rectal swab tests represent an adequate sample for their identification [48,92]. As discussed above, admission to ICU is a risk factor for acquiring these strains, probably due to several reasons, such as long-term ventilator use [93], antibiotic pressure or presence of CREs or plasmids in the ICU area. A recent case-control study performed in Korea [94] showed that pneumonia/chronic pulmonary disease, previous fluoroquinolone use and previous use of nasogastric tube were the significant risk factors for CRE infection or colonization in ICU-admitted patients, although no OXA-48-like CRE were identified in their study.
Use of antacids [95] and traveling to endemic countries [96] are also possible risk factors [15]. Finally, vulnerable conditions such as current chemotherapy use or dialysis in the previous 12 months and history of an overnight stay in a healthcare setting or epidemiological linkage to a known carrier also increase the risk for carriage of CRE [97]. However, the main factor associated with new colonization and transmission of CREs is, undoubtedly, antibiotic treatment [95], especially inappropriate carbapenem and aminoglycoside stewardship [93].
In Spain, overuse of carbapenems in hospital settings has been widely reported [98,99].
An overall increase in the use of carbapenems in acute care hospitals has been reported (88.4% higher from 2008 to 2015). They are mainly used empirically (76.2%) for the treatment of urinary tract and intra-abdominal infections in the context of suspicion of polymicrobial or mixed infection (27.4%) and severity (25.4%) [97]. The national Program for Optimizing the Use of Antibiotics (PROA) [100] aims to adequately control the use of antibiotics in our country. For instance, to avoid overuse of carbapenems, other antibiotics have been proposed as an alternative for the treatment of ESBL-producing Enterobacterales [99,101]. The massive consumption of antibiotics, especially in the ICU, calls for a deep reflection and implementation of antibiotic stewardship programs, as highlighted by Timsit et al. [102]. The worrying increase in carbapenem use found in other Spanish hospitals led us to investigate the rate of consumption of each antibiotic in our context. Accordingly, to add further information to the recent studies that have been previously mentioned, we analyzed the original data regarding antibiotic stewardship in hospitalized patients in Spain, available at the EPINE-EPPS Study [68] (Table 4). We included the most used antibiotics in Spain. However, prevalence of other commonly used antibiotics like erythromycin (0.6%), cefepime (0.6%), teicoplanin (0.6%), rifampicin (0.4%) or colistin (0.3%), among others, should be also taken into account.
Table 4.
Prevalence of the most frequent antibiotics used in hospitalized patients in 2019 in Spain.
| Antibiotic Group | % |
| Penicillins including β-lactamase inhibitors | 27.5 |
| Fluoroquinolones | 15.4 |
| Third generation cephalosporins | 12.4 |
| Carbapenems | 7.0 |
| Macrolides | 3.1 |
| First generation cephalosporins | 2.3 |
| Antibiotic | % |
| Amoxicillin and clavulanic acid | 14.4 |
| Ceftriaxone | 9.8 |
| Piperacillin and tazobactam | 8.5 |
| Levofloxacin | 7.8 |
| Cefazolin | 7.4 |
| Meropenem | 5.7 |
| Ciprofloxacin | 4.7 |
| Linezolid | 2.8 |
| Cotrimoxazole | 2.1 |
| Vancomycin | 2.0 |
| Metronidazole | 2.0 |
| Clindamycin | 1.9 |
| Cefuroxime | 1.8 |
| Azithromycin | 1.7 |
| Gentamycin | 1.7 |
| Ampicillin | 1.4 |
| Cefotaxime | 1.2 |
| Ertapenem | 1.2 |
| Daptomycin | 1.1 |
| Ceftazidime | 1.0 |
| Imipenem and cilastatin | 1.0 |
| Cloxacillin | 0.9 |
| Amikacin | 0.9 |
| Amoxicillin | 0.8 |
| Fosfomycin | 0.6 |
The table was designed based on the information provided by the report from Spain on the prevalence of healthcare-related and community infections and the use of antibiotics (EPINE-EPPS Study) [68].
As shown in the table, carbapenems are the fourth most frequently used group of antibiotics in Spanish hospitals (7%), with meropenem being the most common (5.7%), followed by ertapenem (1.2%) and the combination of imipenem and cilastatin (1%).
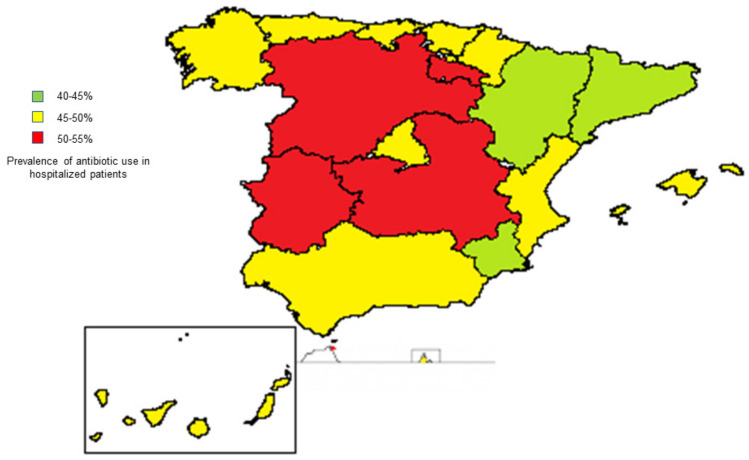
The overall use of antibiotics in hospitalized patients in Spain is approximately 50% [68]. However, there are some regional differences (Figure 2), ranging from 40.5% in Catalonia to 57.1% in Ceuta.
Figure 2.
Prevalence of antibiotic use in hospitalized patients in the different autonomous communities in Spain in 2019. Ceuta (red) and Melilla (yellow) are located in North Africa. The percentages represent the prevalence of antibiotic use in hospitalized patients registered in the report from Spain on the prevalence of healthcare-related and community infections and the use of antibiotics (EPINE-EPPS study) in the year 2019 [68].
These data reinforce the need for developing stewardship interventions in Spain in order to homogenize antibiotic use in hospitalized patients and prevent carbapenem overuse to reduce CRE outbreaks.
To achieve this, all the healthcare professionals should be involved in these interventions. At the community level, apart from primary care professionals, pharmacists have been suggested to play an important role in antibiotic use programs [103]. At the hospital level, professionals from all the services, not only ICU, infectious diseases or surgical settings should be aware and conscious of antibiotic stewardship programs and interhospital or interservices sessions should be continuously reinforced and updated. In this regard, infectious diseases, microbiology and preventive medicine and public health services should join forces to design these strategies for ensuring the adequate use of antibiotics and controlling multiresistant outbreaks in Spanish hospitals.
5. Treatment
Optimizing therapy for CRE infections represents a fundamental and increasing research field, continuously updating and adapting to the epidemiological contexts.
The OXA-48 enzyme hydrolyzes carbapenems but shows very weak activity against extended-spectrum cephalosporins such as cefepime and ceftazidime [15], although isolates are frequently multidrug resistant as they combine multiple resistance mechanisms [104].
CRE have historically been susceptible to polymyxins, tigecycline or aminoglycosides (especially gentamicin). Accordingly, these treatments have been widely used as antibiotics of choice for CRE infections. However, varying rates of resistance to all of them have been reported, thus different antibiotics are continuously being tested. Treatment of OXA-48 CRE with ceftazidime-avibactam could be effective according to a study conducted in Italy [105], which is consistent with the resistance profile of an OXA-48 CRE outbreak in Russia [106]. A study performed in Australia also reported effectiveness of other avibactam combinations (imipenem-avibactam or aztreonam-avibactam), suggesting that avibactam may be the most potent β-lactamase inhibitor [107]. Although the authors stated that combined therapy is more effective, successful outcomes were observed in 70% of the patients treated with ceftazidime-avibactam in monotherapy. Carbapenem in combination with amikacin or colistin may be useful in certain cases, but recent reports of resistance are concerning [106].
In Spain, treatment for CRE with ceftazidime-avibactam has proven to be promising, and other novel combinations including meropenem-vaborbactam, imipenem-relebactam, plazomicin, cefiderocol, eravacycline and aztreonam-avibactam [108]. However, meropenem-vaborbactam [109] and imipenem-relebactam [110] are not effective against OXA-48 producers, and limited data are available on eravacycline against CRE [111]. Therefore, the β-lactamase inhibitor with more contrasted efficacy on OXA-48 producers is avibactam. Regarding cephalosporins, despite the paucity of clinical data and the fact that OXA-48 CRE are associated with ESLBs (which implies resistance to cephalosporins), a recent Spanish systematic review suggested that ceftazidime without avibactam could be a therapeutic option [101]. Plazomicin is another therapeutic alternative, which has been reported to be effective in some CREs [112], although it is not commercialized in Spain. Cefiderocol showed encouraging results in terms of efficacy and safety in a clinical trial [113], covering a very wide spectrum of bacteria. Given its siderophore mechanism of action, based on the use of ferric iron transporter systems [114], cefiderocol has been referred to as a “Trojan horse” antibiotic. This drug is available in Spain and has been advocated as a promising future therapeutic option. Finally, ertapenem and meropenem have shown in vitro synergistic activity against CRE [115], although they are not currently recommended for the treatment of OXA-48 producers. Other new drugs with activity against some CPE isolates are at different stages of development [101] and will probably be incorporated into the commercialized therapeutic arsenal in Spain in the next years.
It is important to note that several OXA-48-like variants, such as OXA-163, completely lose their ability to hydrolyze carbapenems and display an ESBL phenotype. Accordingly, they hydrolyze ceftazidime and confer high levels of resistance to this drug when expressed in Enterobacterales.
Overall, the therapeutic approach to OXA-48 CRE infections must be individualized according to susceptibility, type and severity of infection, and to the characteristics of the patient. Limited data are currently available on the best strategy for OXA-48 CRE, thus future studies might be crucial for optimizing the therapeutic approaches.
6. Conclusions
The frequency of CRE colonization or infection is increasing in hospital outbreaks around the world. In Spain, this frequency is higher than the European average. OXA-48 is the most common enzymatic resistance mechanism of CRE in Spain [116]. Colonization in the ICU and infections of the surgical site are especially complicated and, therefore, specific preventive measures should be implemented in these settings. Plasmid transfer between different species has been described, and the presence of possible host bacteria in patients should be considered in order to implement appropriate isolation and preventive measures when an OXA-48 outbreak is identified. One of the most preventable risk factors for hospital outbreaks caused by OXA-48 bacteria is a lack of adequate antibiotic stewardship. In Spain, an overall high frequency of carbapenem overuse (especially ertapenem) has been described in 2019, although regional differences in the percentage of antibiotic use in hospitalization were also reported. Accordingly, specific programs focused on optimizing antibiotic stewardship are required. Finally, preventive control measures need to be reinforced and continuously updated and adapted to identify and control OXA-48 CRE in Spanish hospitals with the aim to stop this growing global health threat.
Acknowledgments
We acknowledge the health workers of the Service of Preventive Medicine and Public Health, San Cecilio University Hospital, for their kind support and ideas to improve this work, the Chair of Teaching and Research in Family Medicine SEMERGEN-UGR for supporting this work and Ángela Rivera-Izquierdo for improving the use of English of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/10/1/89/s1, Table S1: OXA-48-like enzymes reported to date.
Author Contributions
Conceptualization, M.R.-I.; methodology, M.R.-I., A.J.L.-R.-B., C.R.-I., J.L.-G., N.F.F.-M., P.R.-G., L.M.M.-d., V.M.-R., E.M.-R. and E.J.-M.; investigation, M.R.-I., A.J.L.-R.-B., C.R.-I., J.L.-G., N.F.F.-M., P.R.-G., L.M.M.-d., V.M.-R., E.M.-R. and E.J.-M.; writing—original draft preparation, M.R.-I.; writing—review and editing, M.R.-I., A.J.L.-R.-B., C.R.-I., J.L.-G., N.F.F.-M., P.R.-G., L.M.M.-d., V.M.-R., E.M.-R. and E.J.-M.; supervision, E.J.-M.; funding acquisition, E.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chair of Teaching and Research in Family Medicine SEMERGEN-UGR.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pana Z.D., Zaoutis T. Treatment of extended-spectrum ß-lactamase-producing Enterobacteriaceae (ESBLs) infections: What have we learned until now? F1000Res. 2018;7:1347. doi: 10.12688/f1000research.14822.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeolu M., Alnajar S., Naushad S.S., Gupta R. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016;66:5575–5599. doi: 10.1099/ijsem.0.001485. [DOI] [PubMed] [Google Scholar]
- 3.Hawkey P.M. The growing burden of antimicrobial resistance. J. Antimicrob. Chemother. 2008;62:i1–i9. doi: 10.1093/jac/dkn241. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo R.G., Johnson J.K., Bork J.T., Heil E.L. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin. Pharmacother. 2016;17:953–967. doi: 10.1517/14656566.2016.1154538. [DOI] [PubMed] [Google Scholar]
- 5.Sheu C.C., Lin S.Y., Chang Y.T., Lee C.Y., Chen Y.H., Hsueh P.R. Management of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae: Current evidence and future prospects. Expert Rev. Anti. Infect. Ther. 2018;16:205–218. doi: 10.1080/14787210.2018.1436966. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE)—November 2015 Update CRE Toolkit. [(accessed on 1 December 2020)]; Available online: https://www.cdc.gov/hai/organisms/cre/Cre-toolkit/index.html.
- 7.Nordmann P., Mariotte S., Naas T., Labia R., Nicolas M.H. Biochemical properties of a carbapenem-hydrolyzing betalactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob. Agents Chemother. 1993;37:939–946. doi: 10.1128/AAC.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naas T., Nordmann P. Analysis of a carbapenem hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc. Natl. Acad. Sci. USA. 1994;91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haidar G., Clancy C.J., Chen L., Samanta P., Shields R.K., Kreiswirth B.N., Nguyen M.H. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2017;61:e00642-17. doi: 10.1128/AAC.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suay-García B., Pérez-Gracia M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics. 2019;8:122. doi: 10.3390/antibiotics8030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambler R.P. The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980;289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 12.Bush K., Jacoby G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010;54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordmann P., Naas T., Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordmann P., Dortet L., Poirel L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012;18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Mairi A., Pantel A., Sotto A., Lavigne J.P., Touati A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:587–604. doi: 10.1007/s10096-017-3112-7. [DOI] [PubMed] [Google Scholar]
- 16.Evans B.A., Amyes S.G.B. OXA β-lactamases. Clin. Microbiol. Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirel L., Potron A., Nordmann P. OXA-48-like carbapenemases: The phantom menace. J. Antimicrob. Chemother. 2012;67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 18.Poirel L., Héritier C., Tolün V., Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004;48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel L., Héritier C., Nordmann P. Chromosome-encoded ambler class D beta-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob. Agents Chemother. 2004;48:348–351. doi: 10.1128/AAC.48.1.348-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasap M., Torol S., Kolayli F., Dundar D., Vahaboglu H. OXA-162, a novel variant of OXA-48 displays extended hydrolytic activity towards imipenem, meropenem and doripenem. J. Enzyme Inhib. Med. Chem. 2013;28:990–996. doi: 10.3109/14756366.2012.702343. [DOI] [PubMed] [Google Scholar]
- 21.Poirel L., Castanheira M., Carrër A., Rodriguez C.P., Jones R.N., Smayevsky J., Nordmann P. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 2011;55:2546–2551. doi: 10.1128/AAC.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potron A., Nordmann P., Lafeuille E., Al Maskari Z., Al Rashdi F., Poirel L. Characterization of OXA-181, a carbapenemhydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011;55:4896–4899. doi: 10.1128/AAC.00481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zong Z. Discovery of bla(OXA-199), a chromosome-based bla(OXA-48)-like variant, in Shewanella xiamenensis. PLoS ONE. 2012;7:e48280. doi: 10.1371/journal.pone.0048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potron A., Nordmann P., Poirel L. Characterization of OXA204, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013;57:633–636. doi: 10.1128/AAC.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potron A., Rondinaud E., Poirel L., Belmonte O., Boyer S., Camiade S., Nordmann P. Genetic and biochemical characterisation of OXA232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int. J. Antimicrob. Agents. 2013;41:325–329. doi: 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Oteo J., Hernández J.M., Espasa M., Fleites A., Sáez D., Bautista V., Pérez-Vázquez M., Fernández-García M.D., Delgado-Iribarren A., García-Picazo L., et al. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J. Antimicrob. Chemother. 2013;68:317–321. doi: 10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 27.Gomez S., Pasteran F., Faccone D., Bettiol M., Veliz O., De Belder D., Rapaport M., Gatti B., Petroni A., Corso A. Intrapatient emergence of OXA-247: A novel carbapenemase found in a patient previously infected with OXA-163-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2013;19:E233–E235. doi: 10.1111/1469-0691.12142. [DOI] [PubMed] [Google Scholar]
- 28.Mataseje L.F., Abdesselam K., Vachon J., Mitchel R., Bryce E., Roscoe D., Boyd D.A., Embree J., Katz K., Kibsey P., et al. Results from the Canadian Nosocomial Infection Surveillance Program on Carbapenemase-Producing Enterobacteriaceae, 2010 to 2014. Antimicrob. Agents Chemother. 2016;60:6787–6794. doi: 10.1128/AAC.01359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampaio J.L.M., Ribeiro V.B., Campos J.C., Rozales F.P., Magagnin C.M., Falci D.R., da Silva R.C.F., Falarosa M.G., Luz D.I., Vieira F.J., et al. Detection of OXA-370, an OXA-48- related class D β-lactamase, in Enterobacter hormaechei from Brazil. Antimicrob. Agents Chemother. 2014;58:3566–3567. doi: 10.1128/AAC.02510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dortet L., Oueslati S., Jeannot K., Tandé D., Naas T., Nordmann P. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum β-lactamase without significant carbapenemase activity. Antimicrob. Agents Chemother. 2015;59:3823–3828. doi: 10.1128/AAC.05058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelli A., Di Palo D.M., Galano A., Becciani S., Montagnani C., Pecile P., Galli L., Rossolini G.M. Intestinal carriage of Shewanella xiamenensis simulating carriage of OXA-48-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2015;82:1–3. doi: 10.1016/j.diagmicrobio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsen O., Hansen F., Aasnaes B., Hasman H., Lund B.A., Leiros H.S., Lilje B., Janice J., Jakobsen L., Littauer P., et al. Dissemination and characteristics of a novel plasmid-encoded carbapenem-hydrolyzing class D beta-lactamase, OXA-436, found in isolates from four patients at six different hospitals in Denmark. Antimicrob. Agents Chemother. 2018;62:e01260-17. doi: 10.1128/AAC.01260-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Belder D., Ghiglione B., Pasteran F., de Mendieta J.M., Corso A., Curto L., Di Bella A., Gutkind G., Gomez S.A., Power P. Comparative Kinetic Analysis of OXA-438 with Related OXA-48-Type Carbapenem-Hydrolyzing Class D β-Lactamases. ACS Infect. Dis. 2020;6:3026–3033. doi: 10.1021/acsinfecdis.0c00537. [DOI] [PubMed] [Google Scholar]
- 34.Findlay J., Hopkins K.L., Loy R., Doumith M., Meunier D., Hill R., Pike R., Mustafa N., Livermore D.M., Woodford N. OXA-48-like carbapenemases in the UK: An analysis of isolates and cases from 2007 to 2014. J. Antimicrob. Chemother. 2017;72:1340–1349. doi: 10.1093/jac/dkx012. [DOI] [PubMed] [Google Scholar]
- 35.Lutgring J.D., Zhu W., de Man T.J.B., Avillan J.J., Anderson K.F., Lonsway D.R., Rowe L.A., Batra D., Rasheed J.K., Limbago B.M. Phenotypic and Genotypic Characterization of Enterobacteriaceae Producing Oxacillinase-48-Like Carbapenemases, United States. Emerg. Infect. Dis. 2018;24:700–709. doi: 10.3201/eid2404.171377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dabos L., Bogaerts P., Bonnin R.A., Zavala A., Sacre P., Iorga B.I., Huang D.T., Glupczynski Y., Naas T. Genetic and biochemical characterization of OXA-519, a novel OXA-48-like beta-lactamase. Antimicrob. Agents Chemother. 2018;62:e00469-18. doi: 10.1128/AAC.00469-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dabos L., Jousset A.B., Bonnin R.A., Fortineau N., Zavala A., Retailleau P., Iorga B.I., Naas T. Genetic and Biochemical Characterization of OXA-535, a Distantly Related OXA-48-Like β-Lactamase. Antimicrob. Agents Chemother. 2018;62:e01198-18. doi: 10.1128/AAC.01198-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard J.C., Anderson T., Creighton J., Freeman J.T. Geographical and temporal clustering of OXA-48-producing Escherichia coli ST410 causing community-onset urinary tract infection in Christchurch, New Zealand. J. Antimicrob. Chemother. 2018;73:2900–2901. doi: 10.1093/jac/dky269. [DOI] [PubMed] [Google Scholar]
- 39.Naas T., Oueslati S., Bonnin R.A., Dabos M.L., Zavala A., Dortet L., Retailleau P., Iorga B.I. Beta-Lactamase DataBase (BLDB)—Structure and Function. J. Enzyme Inhib. Med. Chem. 2017;32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubert D., Naas T., Héritier C., Poirel L., Nordmann P. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 2006;188:6506–6514. doi: 10.1128/JB.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beyrouthy R., Robin F., Dabboussi F., Mallat H., Hamzé M., Bonnet R. Carbapenemase and virulence factors of Enterobacteriaceae in North Lebanon between 2008 and 2012: Evolution via endemic spread of OXA-48. J. Antimicrob. Chemother. 2014;69:2699–2705. doi: 10.1093/jac/dku181. [DOI] [PubMed] [Google Scholar]
- 42.Argente M., Miró E., Martí C., Vilamala A., Alonso-Tarrés C., Ballester F., Calderón A., Gallés C., Gasós A., Mirelis B., et al. Molecular characterization of OXA-48 carbapenemase-producing Klebsiella pneumoniae strains after a carbapenem resistance increase in Catalonia. Enferm. Infecc. Microbiol. Clin. 2019;37:82–88. doi: 10.1016/j.eimc.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Skalova A., Chudejova K., Rotova V., Medvecky M., Studentova V., Chudackova E., Lavicka P., Bergerova T., Jakubu V., Zemlickova H., et al. Molecular Characterization of OXA-48-Like-Producing Enterobacteriaceae in the Czech Republic and Evidence for Horizontal Transfer of pOXA-48-Like Plasmids. Antimicrob. Agents Chemother. 2017;61:e01889-16. doi: 10.1128/AAC.01889-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okoche D., Asiimwe B.B., Katabazi F.A., Kato L., Najjuka C.F. Prevalence and Characterization of Carbapenem-Resistant Enterobacteriaceae Isolated from Mulago National Referral Hospital, Uganda. PLoS ONE. 2015;10:e0135745. doi: 10.1371/journal.pone.0135745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boutal H., Vogel A., Bernabeu S., Devilliers K., Creton E., Cotellon G., Plaisance M., Oueslati S., Dortet L., Jousset A., et al. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMPand VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018;73:909–915. doi: 10.1093/jac/dkx521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Jonge B.L., Karlowsky J.A., Kazmierczak K.M., Biedenbach D.J., Sahm D.F., Nichols W.W. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM Global Surveillance Study (2012 to 2014) Antimicrob. Agents Chemother. 2016;60:3163–3169. doi: 10.1128/AAC.03042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlowsky J.A., Lob S.H., Kazmierczak K.M., Badal R.E., Young K., Motyl M.R., Sahm D.F. In vitro activity of imipenem against carbapenemasepositive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J. Clin. Microbiol. 2017;55:1638–1649. doi: 10.1128/JCM.02316-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitout J., Peirano G., Kock M.M., Strydom K.A., Matsumura Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin. Microbiol. Rev. 2019;33:e00102-19. doi: 10.1128/CMR.00102-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aquino-Andrade A., Merida-Vieyra J., de la Garza E.A., Arzate-Barbosa P., De Colsa Ranero A. Carbapenemase-producing Enterobacteriaceae in Mexico: Report of seven non-clonal cases in a pediatric hospital. BMC Microbiol. 2018;18:38. doi: 10.1186/s12866-018-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez-Garcia M., Leon-Sampedro R., Perez-Viso B., Morosini M.I., Lopez-Fresnena N., Diaz-Agero C., Coque T.M., Ruiz-Garbajosa P., Canton R. First report of an OXA-48- and CTX-M-213-producing Kluyvera species clone recovered from patients admitted in a university hospital in Madrid, Spain. Antimicrob. Agents Chemother. 2018;62:e01238-18. doi: 10.1128/AAC.01238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauthier L., Dortet L., Cotellon G., Creton E., Cuzon G., Ponties V., Bonnin R.A., Naas T. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob. Agents Chemother. 2018;62:e00266-18. doi: 10.1128/AAC.00266-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L., Al Laham N., Chavda K.D., Mediavilla J.R., Jacobs M.R., Bonomo R.A., Kreiswirth B.N. First report of an OXA-48-producing multidrugresistant Proteus mirabilis strain from Gaza, Palestine. Antimicrob. Agents Chemother. 2015;59:4305–4307. doi: 10.1128/AAC.00565-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regev-Yochay G., Smollan G., Tal I., Zade N.P., Haviv Y., Nudelman V., Gal-Mor O., Jaber H., Zimlichman E., Keller N., et al. Sink traps as the source of transmission of OXA-48-producing Serratia marcescens in an intensive care unit. Infect. Control Hosp. Epidemiol. 2018;39:1307–1315. doi: 10.1017/ice.2018.235. [DOI] [PubMed] [Google Scholar]
- 54.Peirano G., Matsumura Y., Adams M.D., Bradford P., Motyl M., Chen L., Kreiswirth B.N., Pitout J. Genomic epidemiology of global carbapenemase-producing Enterobacter spp., 2008–2014. Emerg. Infect. Dis. 2018;24:1010–1019. doi: 10.3201/eid2406.171648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedenic B., Slade M., Starcevic L.Ž., Sardelic S., Vranic-Ladavac M., Bencic A., Atalic V.Z., Bogdan M., Bubonja-Šonje M., Tomic-Paradžik M., et al. Epidemic spread of OXA-48 beta-lactamase in Croatia. J. Med. Microbiol. 2018;6:1031–1041. doi: 10.1099/jmm.0.000777. [DOI] [PubMed] [Google Scholar]
- 56.Seiffert S.N., Perreten V., Johannes S., Droz S., Bodmer T., Endimiani A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob. Agents Chemother. 2014;58:2446–2449. doi: 10.1128/AAC.02417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woerther P.-L., Jardak T., Ben Hassine I., Forget S., Chachaty E., Arlet G., Decré D. A long-term study of the diversity of OXA-48-like carbapenemase-producing bacterial strains in infected patients and carriers. Microb. Drug Resist. 2018;24:181–189. doi: 10.1089/mdr.2017.0060. [DOI] [PubMed] [Google Scholar]
- 58.Kidd J.M., Livermore D.M., Nicolau D.P. The difficulties of identifying and treating Enterobacterales with OXA-48-like carbapenemases. Clin. Microbiol. Infect. 2020;26:401–403. doi: 10.1016/j.cmi.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Carrër A., Poirel L., Eraksoy H., Cagatay A.A., Badur S., Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 2008;52:2950–2954. doi: 10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azap O., Otlu B., Yeşilkaya A., Yakupoğulları Y. Detection of OXA-48-like Carbapenemase-Producing Klebsiella pneumoniae in a Tertiary Care Center in Turkey: Molecular Characterization and Epidemiology. Balk. Med. J. 2013;30:259–260. doi: 10.5152/balkanmedj.2013.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Djahmi N., Dunyach-Remy C., Pantel A., Dekhil M., Sotto A., Lavigne J.-P. Epidemiology of carbapenemase-producing Enterobacteriaceae and Acinetobacter baumannii in Mediterranean Countries. Biomed. Res. 2014;2014:305784. doi: 10.1155/2014/305784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuzon G., Naas T., Bogaerts P., Glupczynski Y., Huang T.D., Nordmann P. Plasmid-encoded carbapenem-hydrolyzing beta-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob. Agents Chemother. 2008;52:3463–3464. doi: 10.1128/AAC.00543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cuzon G., Naas T., Lesenne A., Benhamou M., Nordmann P. Plasmid-mediated carbapenem-hydrolysing OXA-48 betalactamase in Klebsiella pneumoniae from Tunisia. Int. J. Antimicrob. Agents. 2010;36:91–93. doi: 10.1016/j.ijantimicag.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Poirel L., Carbonnelle E., Bernabeu S., Gutmann L., Rotimi V., Nordmann P. Importation of OXA-48-producing Klebsiella pneumoniae from Kuwait. J. Antimicrob. Chemother. 2012;67:2051–2052. doi: 10.1093/jac/dks167. [DOI] [PubMed] [Google Scholar]
- 65.Lascols C., Peirano G., Hackel M., Laupland K.B., Pitout J.D. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: First report of OXA-48-like enzymes in North America. Antimicrob. Agents Chemother. 2013;57:130–136. doi: 10.1128/AAC.01686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taggar G., Attiq Rheman M., Boerlin P., Diarra M.S. Molecular Epidemiology of Carbapenemases in Enterobacteriales from Humans, Animals, Food and the Environment. Antibiotics. 2020;9:693. doi: 10.3390/antibiotics9100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oteo J., Saez D., Bautista V., Fernández-Romero S., Molina J.M.H., Pérez-Vázquez M., Aracil B., Campos J., Spanish Collaborating Group for the Antibiotic Resistance Surveillance Program Carbapenemase producing Enterobacteriaceae in Spain in 2012. Antimicrob. Agents Chemother. 2013;57:6344–6347. doi: 10.1128/AAC.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estudio EPINE-EPSS n°30, 2019: Estudio de Prevalencia de las Infecciones Nosocomiales en España [EPINE-EPSS Study, n°30, 2019: Study of Prevalence of Nosocomial Infections in Spain] [(accessed on 1 December 2020)];2019 Available online: https://epine.es/api/documento-publico/2019%20EPINE%20Informe%20Espa%C3%B1a%2027112019.pdf/reports-esp.
- 69.Rivera-Izquierdo M., Valero-Ubierna M., Martínez-Diz S., Fernández-García M.Á., Martín-Romero D.T., Maldonado-Rodríguez F., Sánchez-Pérez M.R., Martín-delosReyes L.M., Martínez-Ruiz V., Lardelli-Claret P., et al. Clinical Factors, Preventive Behaviours and Temporal Outcomes Associated with COVID-19 Infection in Health Professionals at a Spanish Hospital. Int. J. Environ. Res. Public Health. 2020;17:4305. doi: 10.3390/ijerph17124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rivera-Izquierdo M., Valero-Ubierna M.D., R-delAmo J.L., Fernández-García M.Á., Martínez-Diz S., Tahery-Mahmoud A., Rodríguez-Camacho M., Gámiz-Molina A.B., Barba-Gyengo N., Gámez-Baeza P., et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLoS ONE. 2020;15:e0235107. doi: 10.1371/journal.pone.0235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El-Kazzaz W., Metwally L., Yahia R., Al-Harbi N., El-Taher A., Hetta H.F. Antibiogram, Prevalence of OXA Carbapenemase Encoding Genes, and RAPD-Genotyping of Multidrug-Resistant Acinetobacter baumannii Incriminated in Hidden Community-Acquired Infections. Antibiotics. 2020;9:603. doi: 10.3390/antibiotics9090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oteo J., Ortega A., Bartolomé R., Bou G., Conejo C., Fernández-Martínez M., González-López J.J., Martínez-García L., Martínez-Martínez L., Merino M., et al. GEIH-GEMARA (SEIMC) and REIPI. Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob. Agents Chemother. 2015;59:3406–3412. doi: 10.1128/AAC.00086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernández-García M., Pérez-Viso B., Turrientes M.C., Díaz-Agero C., López-Fresneña N., Bonten M., Malhotra-Kumar S., Ruiz-Garbajosa P., Cantón R. Characterization of carbapenemase-producing Enterobacteriaceae from colonized patients in a university hospital in Madrid, Spain, during the R-GNOSIS project depicts increased clonal diversity over time with maintenance of high-risk clones. J. Antimicrob. Chemother. 2018;73:3039–3043. doi: 10.1093/jac/dky284. [DOI] [PubMed] [Google Scholar]
- 74.Lázaro-Perona F., Rodríguez-Tejedor M., Ruiz-Carrascoso G., Díaz-Pollán B., Loeches B., Ramos-Ramos J.C., Mingorance J. Intestinal loads of OXA-48-producing Klebsiella pneumoniae in colonized patients determined from surveillance rectal swabs. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.09.054. in press. [DOI] [PubMed] [Google Scholar]
- 75.Mateos M., Hernández-García M., Del Campo R., Martínez-García L., Gijón D., Morosini M.I., Ruiz-Garbajosa P., Cantón R. Emergence and Persistence over Time of Carbapenemase-Producing Enterobacter Isolates in a Spanish University Hospital in Madrid, Spain (2005–2018) Microb. Drug Resist. 2020 doi: 10.1089/mdr.2020.0265. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Rivera-Izquierdo M., Benavente-Fernández A., López-Gómez J., Láinez-Ramos-Bossini A.J., Rodríguez-Camacho M., Valero-Ubierna M., Martín-delosReyes L.M., Jiménez-Mejías E., Moreno-Roldán E., Lardelli-Claret P., et al. Prevalence of Multi-Resistant Microorganisms and Antibiotic Stewardship among Hospitalized Patients Living in Residential Care Homes in Spain: A Cross-Sectional Study. Antibiotics. 2020;9:324. doi: 10.3390/antibiotics9060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grundmann H., Glasner C., Albiger B., Aanensen D.M., Tomlinson C.T., Andrasević A.T., Cantón R., Carmeli Y., Friedrich A.W., Giske C.G., et al. European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017;17:153–163. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 78.Mora-Guzmán I., Rubio-Perez I., Domingo-Garcia D., Martín-Pérez E. Infecciones asociadas a enterobacterias productoras de carbapenemasas OXA-48 en pacientes quirúrgicos: Consumo de antibióticos y evolución de sensibilidades [Infections by OXA-48 carbapenemase-producing Enterobacteriaceae in surgical patients: Antibiotic consumption and susceptibility patterns] Rev. Esp. Quimioter. 2020;33:448–452. doi: 10.37201/req/081.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ministerio de Sanidad Proyecto Infección Quirúrgica Zero [Zero Surgical Infection] [(accessed on 10 December 2020)]; Available online: https://infeccionquirurgicazero.es/es/
- 80.Bartsch S.M., McKinnell J.A., Mueller L.E., Miller L.G., Gohil S.K., Huang S.S., Lee B.Y. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin. Microbiol. Infect. 2017;23:48.e9–48.e16. doi: 10.1016/j.cmi.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.García-Fernández S., García-Castillo M., Bou G., Calvo J., Cercenado E., Delgado M., Pitart C., Mulet X., Tormo N., López Mendoza D., et al. Activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and Enterobacterales isolates recovered from intensive care unit patients in Spain: The SUPERIOR multicentre study. Int. J. Antimicrob. Agents. 2019;53:682–688. doi: 10.1016/j.ijantimicag.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 82.López-González L., Viñuela-Prieto J.M., Rodriguez-Avial I., Manzano R., Candel F.J. Description of carbapenemase-producing Enterobacteriaceae isolates in a Spanish tertiary hospital. Epidemiological analysis and clinical impact. Rev. Esp. Quimioter. 2019;32:254–262. [PMC free article] [PubMed] [Google Scholar]
- 83.Ministerio de Sanidad Proyecto Resistencia Zero [Zero Resistance Project] [(accessed on 10 December 2020)]; Available online: https://www.seguridaddelpaciente.es/es/practicas-seguras/seguridad-pacientes-criticos/proyectoresistencia-zero/
- 84.Maseda E., Salgado P., Anillo V., Ruiz-Carrascoso G., Gómez-Gil R., Martín-Funke C., Gimenez M.J., Granizo J.J., Aguilar L., Gilsanz F. Risk factors for colonization by carbapenemase-producing enterobacteria at admission to a Surgical ICU: A retrospective study. Enferm. Infect. Microbiol. Clin. 2017;35:333–337. doi: 10.1016/j.eimc.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 85.Robustillo-Rodela A., Pérez-Blanco V., Ruiz M.A.E., Carrascoso G.R., Iglesias J.C.F., Martín D.A. Successful control of 2 simultaneous outbreaks of OXA-48 carbapenemase-producing Enterobacteriaceae and multidrug-resistant Acinetobacter baumannii in an intensive care unit. Am. J. Infect. Control. 2017;45:1356–1362. doi: 10.1016/j.ajic.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention Infection Control Isolation Precautions. [(accessed on 10 December 2020)]; Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.
- 87.Peirano G., Chen L., Kreiswirth B.N., Pitout J. Emerging Antimicrobial-Resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antimicrob. Agents Chemother. 2020;64:e01148-20. doi: 10.1128/AAC.01148-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hidalgo L., de Been M., Rogers M., Schürch A.C., Scharringa J., van der Zee A., Bonten M., Fluit A.C. Sequence-based epidemiology of an OXA-48 plasmid during a hospital outbreak. Antimicrob. Agents Chemother. 2019;63:e01204-19. doi: 10.1128/AAC.01204-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solgi H., Nematzadeh S., Giske C.G., Badmasti F., Westerlund F., Lin Y.L., Goyal G., Nikbin V.S., Nemati A.H., Shahcheraghi F. Molecular Epidemiology of OXA-48 and NDM-1 Producing Enterobacterales Species at a University Hospital in Tehran, Iran, Between 2015 and 2016. Front. Microbiol. 2020;11:936. doi: 10.3389/fmicb.2020.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kopotsa K., Sekyere J.O., Mbelle N.M. Plasmid evolution in carbapenemase-producing Enterobacteriaceae: A review. Ann. N. Y. Acad. Sci. 2019;1457:61–91. doi: 10.1111/nyas.14223. [DOI] [PubMed] [Google Scholar]
- 91.Datta N., Hedges R.W. R factors of compatibility goup A. J. Gen. Microbiol. 1973;74:335–336. doi: 10.1099/00221287-74-2-335. [DOI] [PubMed] [Google Scholar]
- 92.Lee T.D., Adie K., McNabb A., Purych D., Mannan K., Azana R., Ng C., Tang P., Hoang L.M. Rapid Detection of KPC, NDM, and OXA-48-Like Carbapenemases by Real-Time PCR from Rectal Swab Surveillance Samples. J. Clin. Microbiol. 2015;53:2731–2733. doi: 10.1128/JCM.01237-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mittal G., Gaind R., Kumar D., Kaushik G., Gupta K.B., Verma P.K., Deb M. Risk factors for fecal carriage of carbapenemase producing Enterobacteriaceae among intensive care unit patients from a tertiary care center in India. BMC Microbiol. 2016;16:138. doi: 10.1186/s12866-016-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim Y.A., Lee S.J., Park Y.S., Lee Y.J., Yeon J.H., Seo Y.H., Lee K. Risk Factors for Carbapenemase-Producing Enterobacterales Infection or Colonization in a Korean Intensive Care Unit: A Case–Control Study. Antibiotics. 2020;9:680. doi: 10.3390/antibiotics9100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reuland E.A., Al Naiemi N., Kaiser A.M., Heck M., Kluytmans J.A., Savelkoul P.H., Elders P.J.M., Vandebroucke-Grauls C.M.J.E. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J. Antimicrob. Chemother. 2016;71:1076–1082. doi: 10.1093/jac/dkv441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruppé E., Armand-Lefèvre L., Estellat C., El-Mniai A., Boussadia Y., Consigny P.H. Acquisition of carbapenemaseproducing Enterobacteriaceae by healthy travellers to India, France, February 2012 to March 2013. Eurosurveillance. 2014;19:20768. doi: 10.2807/1560-7917.ES2014.19.14.20768. [DOI] [PubMed] [Google Scholar]
- 97.Magiorakos A.P., Burns K., Baño J.R., Borg M., Daikos G., Dumpis U., Lucet J.C., Moro M.L., Tacconelli E., Simonsen G.S., et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control. 2017;6:113. doi: 10.1186/s13756-017-0259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grau S., Fondevilla E., Echeverría-Esnal D., Alcorta A., Limon E., Gudiol F., VINCat Program Group Widespread increase of empirical carbapenem use in acute care hospitals in Catalonia, Spain. Enferm. Infecc. Microbiol. Clin. 2019;37:36–40. doi: 10.1016/j.eimc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Tamma P.D., Rodriguez-Bano J. The Use of Noncarbapenem β-Lactams for the Treatment of Extended-Spectrum β-Lactamase Infections. Clin. Infect. Dis. 2017;64:972–980. doi: 10.1093/cid/cix034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Plan Nacional de Resistencia a Antibióticos (PRAN). Programs of Optimization of Antibiotic Use (PROA) [(accessed on 10 December 2020)]; Available online: https://www.resistenciaantibioticos.es/es/programas-de-optimizacion-de-uso-de-los-antibioticos-proa.
- 101.Rodríguez-Baño J., Gutiérrez-Gutiérrez B., Machuca I., Pascual A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018;31:e00079-17. doi: 10.1128/CMR.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Timsit J.F., Bassetti M., Cremer O., Daikos G., de Waele J., Kallil A., Kipnis E., Kollef M., Laupland K., Paiva J.-A., et al. Rationalizing antimicrobial therapy in the ICU: A narrative review. Intensive Care Med. 2019;45:172–189. doi: 10.1007/s00134-019-05520-5. [DOI] [PubMed] [Google Scholar]
- 103.Napolitano F., Della Polla G., De Simone C., Lambiase C., Pelullo C.P., Angelillo I.F. The Knowledge, Attitudes, and Practices of Community Pharmacists in their Approach to Antibiotic Use: A Nationwide Survey in Italy. Antibiotics. 2019;8:177. doi: 10.3390/antibiotics8040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Evren E., Azap O.K., Colakoglu S., Arslan H. In vitro activity of fosfomycin in combination with imipenem, meropenem, colistin and tigecycline against OXA 48-positive Klebsiella pneumoniae strains. Diagn. Microbiol. Infect. Dis. 2013;76:335–338. doi: 10.1016/j.diagmicrobio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 105.Tumbarello M., Losito A.R., Giamarellou H. Optimizing therapy in carbapenem-resistant Enterobacteriaceae infections. Curr. Opin. Infect. Dis. 2018;31:566–577. doi: 10.1097/QCO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 106.Shaidullina E., Shelenkov A., Yanushevich Y., Mikhaylova Y., Shagin D., Alexandrova I., Ershova O., Akimkin V., Kozlov R., Edelstein M. Antimicrobial Resistance and Genomic Characterization of OXA-48- and CTX-M-15-Co-Producing Hypervirulent Klebsiella pneumoniae ST23 Recovered from Nosocomial Outbreak. Antibiotics. 2020;9:862. doi: 10.3390/antibiotics9120862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stewart A., Harris P., Henderson A., Paterson D. Treatment of Infections by OXA-48-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018;62:e01195-18. doi: 10.1128/AAC.01195-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martínez-Martínez L., González-López J.J. Carbapenemases in Enterobacteriaceae: Types and molecular epidemiology. Enferm. Infecc. Microbiol. Clin. 2014;32:4–9. doi: 10.1016/S0213-005X(14)70168-5. [DOI] [PubMed] [Google Scholar]
- 109.Gill C.M., Asempa T.E., Nicolau D.P. Efficacy of human-simulated exposures of meropenem/vaborbactam and meropenem against OXA-48 β-lactamase-producing Enterobacterales in the neutropenic murine thigh infection model. J. Antimicrob. Chemother. 2021;76:184–188. doi: 10.1093/jac/dkaa344. [DOI] [PubMed] [Google Scholar]
- 110.Karlowsky J.A., Lob S.H., Kazmierczak K.M., Hawser S.P., Magnet S., Young K., Motyl M.R., Sahm D.F. In vitro activity of imipenem/relebactam against Gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J. Antimicrob. Chemother. 2018;73:1872–1879. doi: 10.1093/jac/dky107. [DOI] [PubMed] [Google Scholar]
- 111.Clark J.A., Kulengowski B., Burgess D.S. In vitro activity of eravacycline compared with tigecycline against carbapenem-resistant Enterobacteriaceae. Int. J. Antimicrob. Agents. 2020;56:106178. doi: 10.1016/j.ijantimicag.2020.106178. [DOI] [PubMed] [Google Scholar]
- 112.Livermore D.M., Warner M., Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013;68:2286–2290. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 113.Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., Lodise T.P., Naas T., Niki Y., Paterson D.L., et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30796-9. online ahead of print. [DOI] [PubMed] [Google Scholar]
- 114.Ito A., Nishikawa T., Matsumoto S., Yoshizawa H., Sato T., Nakamura R., Tsuji M., Yamano Y. Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016;60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fredborg M., Sondergaard T.E., Wang M. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2017;88:355–360. doi: 10.1016/j.diagmicrobio.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 116.Sánchez-López J., Cantón R. Current status of ESKAPE microorganisms in Spain: Epidemiology and resistance phenotypes. Rev. Esp. Quimioter. 2019;32:27–31. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.