Abstract
Coumarins are naturally occurring molecules with a versatile range of activities. Their structural and physicochemical characteristics make them a privileged scaffold in medicinal chemistry and chemical biology. Many research articles and reviews compile information on this important family of compounds. In this overview, the most recent research papers and reviews from 2020 are organized and analyzed, and a discussion on these data is included. Multiple electronic databases were scanned, including SciFinder, Mendeley, and PubMed, the latter being the main source of information. Particular attention was paid to the potential of coumarins as an important scaffold in drug design, as well as fluorescent probes for decaging of prodrugs, metal detection, and diagnostic purposes. Herein we do an analysis of the trending topics related to coumarin and its derivatives in the broad field of drug discovery.
Keywords: coumarins, biological applications, drug discovery, fluorescent probes
1. Introduction
Coumarins are molecules that belong to a very special family. Their conjugated double ring system makes them interesting molecules for different fields of research. Coumarins can be found in industry as cosmetics and perfume ingredients, as food additives, and especially in the pharmaceutical industry in the synthesis of a large number of synthetic pharmaceutical products [1]. This last application is the main focus of our overview.
Coumarin (Figure 1) is found in nature in a wide variety of plants, particularly in high concentration in the tonka bean (Dipteryx odorata). It can also be found in sweet woodruff (Galium odoratum), vanilla grass (Anthoxanthum odoratum), and sweet grass (Hierochloe odorata), among others. This explains the great interest in the extraction and characterization techniques of natural coumarins, and in the synthesis of their derivatives. In addition, the simplicity of its chemical backbone is very attractive, as well as the reactivity of the benzene and pyrone rings. Conjugated double bonds are responsible for an electronic environment that plays a very important role in this family of compounds.
Figure 1.
Basic classification of coumarins: chemical structures of the three main classes.
This review is based on the most relevant literature that comprises new data from recent research articles and overviews on the development of new therapeutic solutions and fluorescent probes based on the coumarin scaffold. The research articles and reviews organized to prepare this manuscript have been compiled from various electronic databases, including SciFinder, Mendeley, and PubMed. The latter was the main source of information, due to its specificity in the biomedical field.
2. Discussion
Searching for the word “coumarin” in Mendeley, PubMed, and SciFinder in early November 2020, and filtering by year “2020”, more than a thousand references appeared. Another search was conducted in late December to include as much information as possible in this manuscript. A diversity of journals from different fields publishes research articles and reviews related to the biological interest of both plant extracts containing coumarins and/or synthetic molecules based on this scaffold. Hybrid molecules containing different pharmacophores [2,3] like piperazines or pyrazolines [4] are at the top of the list. For simplicity, in the current review the information is organized taking into account the potential pharmacological/biological applications of coumarin derivatives.
2.1. Anticancer Activity
The activity of coumarins as anticancer agents is at the top of reviews published in 2020 [5,6,7,8], as well as research papers. Potent inhibitors (Figure 2, general structure I) of aldo–keto reductase (AKR) presenting an iminocoumarin scaffold, with activities between 25 and 56 nM, have been described for the treatment of prostatic cancer [9]. The design of sulfamide 3-benzylcoumarin hybrids bearing an oxadiazole ring at position 7 (Figure 2, general structure II) has allowed the preparation of new multitarget mitogen-activated protein kinase (MEK) inhibitors and nitric oxide (NO) donors, both with antiproliferative properties [10]. In other cases, the anticancer profile has been directed to other targets. Such is the case of new inhibitors of cyclin-dependent kinases, specifically CDK9, designing hybrids that incorporate an aminopyrimidine fragment to coumarin, both pharmacophores of known activity on these therapeutic targets [11]. We highlight here compound III (Figure 2), with high activity and selectivity for these receptors in comparison with other kinases.
Figure 2.
Structures of coumarin derivatives as anticancer agents.
Other important targets for cancer treatment, especially lymphomas, are histone deacetylases (HDACs). A series of coumarins (Figure 2, general structure IV) exhibiting a hydroxamate structure similar to HDACi vorinostat (SAHA) has been published [12]. The compounds show inhibitory activity in the nanomolar range, being higher in the case of propyl or methoxypropyl derivatives.
In addition, it is worth highlighting the design of hybrids in which one part of the molecule provides fluorescent properties, and another provides therapeutic action (theranostic). Such is the case of the fusion of a 7-aminocoumarin fluorescent ring with a chalcone fragment (Figure 2, compound V). This molecule is an inhibitor of thioredoxin reductases (TrxRs), presenting high antitumor activity (IC50 = 3.6 μM), and is also used as a diagnostic agent [13]. Coumarin scaffold fluorescence is being explored extensively in biomedicine, as described at the end of this review.
The preparation of photo-triggered drug delivery systems (PTDDSs, Figure 2, general structure VI) has also been described, in which the chlorambucil pharmacophore is incorporated into more complex carbazole–coumarins (electron donor and electron acceptor fragments, respectively), carriers of a mitochondrial triphenylphosphonium ligand. This system allows, by irradiation, the controlled release of the chemotherapeutic agent [14].
Finally, coumarins are widely used as ligands in the formation of metal complexes, as described in a very recent review [15] focusing on their application as anticancer agents. Such is the case of complexes with platinum, palladium, gold, copper, or ruthenium, many of which are also used as described below, in the design of antimicrobial agents.
Within the group of compounds with anticancer activity, coumarins exhibiting an antiglioma profile may be highlighted. Simple coumarins such as osthole, umbelliferone, esculin, and 4-hydroxycoumarin, combined with sorafenib (a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive acute myeloid leukemia (AML), and radioactive iodine-resistant advanced thyroid carcinoma) were studied [16]. The same group also studied a combination of the same simple coumarins with temozolomide (used in the treatment of brain tumors such as glioblastoma multiforme or anaplastic astrocytoma) [17].
2.2. Antimicrobial Activity
There is also an abundant bibliography related to the interest of coumarins as antimicrobials. Most of the projects are still inspired by the classic antibiotic novobiocin. There are several works in which antibacterial activity is found due to the presence of an azole ring introduced in different positions of the coumarin system. Articles have been published recently on the antibacterial activity of azole–coumarins, as well as 3/4/7 substituted arylcoumarins (Figure 3, general structures VII and VIII), especially active on Gram-positive and negative bacteria according to substitution patterns [15,18,19,20]. In other cases, thiazolidinedione–coumarin hybrids have been described (Figure 3, general structure IX) that show activity on methicillin-resistant Staphylococcus aureus (MRSA) [21].
Figure 3.
Structures of coumarin derivatives as antimicrobial agents.
Interestingly, coumarin metal complexes also show antibacterial activity. Such is the case of 3-arylcoumarins that present general structures X (Figure 3), coordinated with Re(I), active against MRSA in nanomolar concentrations [22]; or the complexes of general structure XI, a coordination of coumarin–quinoline hybrids with Cu(I), with activity against Flavobacterium psychrophilum, a Gram-negative bacterium that causes significant septicemia in fish, causing devastating economic problems in aquaculture [23].
In addition to the antibacterial activity, in a recent and comprehensive review on coumarins, activity against protozoa of the genus Leishmania was described [24]. The most promising compounds are prenylated, glycosylated, furan/pyranocoumarins, or simple hydroxy- or methoxy-substituted coumarins, along with the natural coumarin mammea A/BB (Figure 3, structure XII). Derivatives of this natural product have been prepared and substitutions at positions 6 and 8, as well as the phenyl ring at position 4, turned out to be mandatory for the studied activity. This structure–activity relationship (SAR) study led to the synthesis of the simplest and most lipophilic analogue XIII (Figure 3), the most promising member of the group as an antileishmanial agent. Similar structures, some also derived of the Mammea genus, have been evaluated against Mycobacterium tuberculosis, an activity that also shows simpler synthetic analogues derived from 4-hydroxycoumarin (Figure 3, general structure XIV) [25].
Finally, it is worth mentioning two articles reported this year on the design and preparation of coumarin derivatives with potential antiviral activity. This is the case of the dual hybrid inhibitors inspired by the antiviral activity of calanolide, known as reverse transcriptase (RT) inhibitor. With this in mind, dual inhibitors of HIV-1 RT and protease (PR) have been designed, in which the coumarin fragment responsible for RT activity is linked to the fragment of the antiretroviral darunavir, active against PR of the HIV, through different amide, carbamate, or amine linkers (Figure 3, general structure XV) [26]. The second case described the introduction of a piperidine ring through a linker in position 7 of the coumarin scaffold, originating compounds with outstanding activity against certain filoviruses such as Marburg virus (MARV) or Ebolavirus (EBOV). From the SAR studied, it is interesting to highlight the role of substitution in para position with a trifluoromethoxy group that originated compound XVI (Figure 3) with IC50 = 0.5 μM and 1.2 μM against EBOV and MARV, respectively [27].
2.3. Antioxidant and Anti-Inflammatory Activities
Although we have found very few publications related to these activities, in some cases the antioxidant activity of coumarin derivatives of both natural [28] and synthetic [29] origin has been reported. This is the case of NOs inhibitors, an activity described for coumarins that bind through different linkers to phenolic fragments capable of acting as radical scavengers (Figure 4, general structure XVII), hybrids that can therefore be used in the treatment of immunomodulatory diseases.
Figure 4.
Structures of coumarin derivatives as antioxidant and anti-inflammatory agents.
Regarding the anti-inflammatory activity, it is worth mentioning a review on the coumarins of natural origin (simple coumarins, prenylcoumarins, furocoumarins, coumestans, and benzocoumarins) with a detailed anti-inflammatory activity due to the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2 factor) that protects cells against stress oxidative [30]. In other cases, the anti-inflammatory activity found for coumarin esters (Figure 4, general structure XVIII) as inhibitors of the Kallikrein-related peptidase 9 (KLK9) involved in inflammatory processes of the skin is reported [31]. Finally, the replacement of the carboxylic group by a sulfone or sulfoxide group (Figure 4, general structure XIX) gives rise to new inhibitors of cyclooxygenase-2 (COX-2) with activities comparable, in many cases, to indomethacin [32].
2.4. Adenosine Ligands
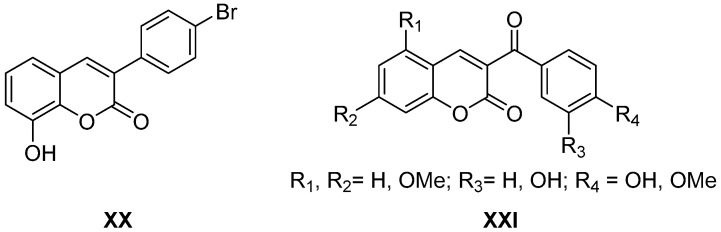
The affinity of the coumarin system for adenosine receptors has also been published recently. The 3-arylcoumarins (Figure 5, compound XX) have been described as antagonists of hA3 receptors, showing a high affinity (in the low nanomolar range) and selectivity for this subtype [33], while the 3-aroylcoumarins (Figure 5, general structure XXI) have been described as dual hA1/hA3 antagonists in the low micromolar range [34]. These works are aligned with the already known potential of these derivatives as modulators of the different adenosine receptors, published in the last decade.
Figure 5.
Structures of coumarin derivatives as adenosine ligands.
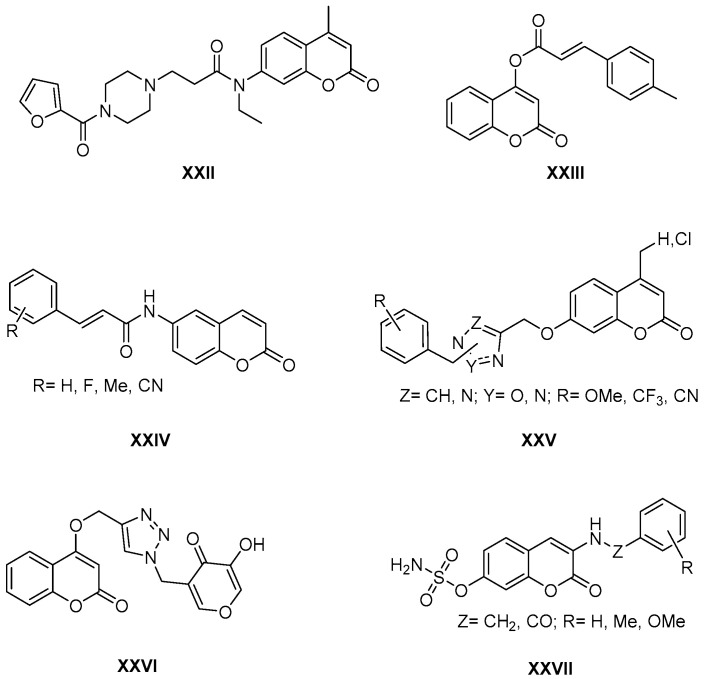
2.5. Enzymatic Inhibitory Activity: α-Glucosidase, Carbonic Anhydrase, Tyrosinase, Sulfatase, and Xanthine Oxidase
The activity of coumarin derivatives on α-glucosidase was also reviewed in 2020 [35], in a study in which the influence of the substitution pattern was evaluated, and an important SAR was established. In addition to α-glucosidase, aldehyde dehydrogenase 1A1 (ALDH1A1) is another target for the treatment of diabetes and obesity, and 3-amidocoumarins have been described as inhibitors of this enzyme, with compound XXII (Figure 6) being a very promising derivative (IC50 = 3.87 mM) [36]. This activity has also recently been found for hybrids of coumarin and cinnamic acid, with compound XXIII (Figure 6) being described as a very promising derivative (IC50 = 12.98 mM) [37].
Figure 6.
Structures of coumarin derivatives as enzymatic inhibitors.
Closely related to these structures, works have been published on coumarin derivatives with inhibitory activity on carbonic anhydrase IX and XII. These are coumarins that incorporate arylacrylamide substituents at position 3 (Figure 6, general structure XXIV) that showed inhibitory activity in the nanomolar range [38]. In other cases, anhydrase inhibitory activity was reported for hybrids connected by a methyleneoxy linker at position 7, oxadiazole heterocycles [39] that the same authors extend to the triazole ring (Figure 6, general structure XXV) [40].
These last structures are closely related to others that present tyrosinase inhibitory activity in the sub-micromolar range. This is the case of coumarins (umbelliferone and other phenolic analogues) that incorporate a kojic acid fragment through a triazole linker at position 4 (Figure 6, compound XXVI), both fragments with demonstrated tyrosinase inhibitory activity [41].
Likewise, the introduction of a sulfamate group in the coumarin scaffold originates a hybrid prototype (Figure 6, general structure XXVII) that presents a high inhibitory activity of the steroid sulfatase (best compound of the series with IC50 = 0.13 μM), which is of interest in the treatment of hormone-dependent breast cancers [42].
During 2020, 3-phenylcoumarins were also studied as xanthine oxidase inhibitors [43]. Methoxy and nitro substituents were introduced into the framework. The best compound in the series proved to be 3-(4-methoxyphenyl)-6-nitrocoumarin, with an IC50 = 8.4 μM, being also non-cytotoxic in B16F10 cells.
2.6. Anti-Neurodegenerative Diseases Activity: MAO and AChE/BChE Inhibitors
The role played by coumarin derivatives as agents that exhibit biological activities associated with neurodegenerative diseases, such as Alzheimer’s disease, is very important. Throughout this year, a large number of manuscripts related to this field have been found. Due to the multidirectional nature of these diseases, there are also many works on hybrid coumarins directed at different pharmacological targets, such as monoamine oxidase B (MAO-B) or acetylcholinesterase (AChE), amyloid aggregation, or oxidative stress, among others. Hybrids of general structure XXVIII (Figure 7) have been described, in which the rasagiline fragment with MAO-B inhibitory activity and neuroprotection properties is incorporated into the coumarin scaffold also with demonstrated MAO-B inhibitory activity, antioxidant, and neuroprotective properties [44]. The incorporation at position 3 of a pyridazine ring (Figure 7, general structure XXIX) is another case of hybrid structures as selective MAO-B inhibitors [45]. The incorporation of isoxazole-type heterocycles in carboxamide–coumarins (Figure 7, general structure XXX) allowed obtaining derivatives with significant inhibitory activities of AChE/BuChE and beta-secretase 1 (BACE1) [46]. In other cases, taking into account the importance of metals in the pathogenesis of Alzheimer’s disease, a pyridinone fragment (Figure 7, general structure XXXI) with iron-chelating properties was incorporated [47].
Figure 7.
Structures of coumarin derivatives as anti-neurodegenerative diseases agents.
Other multitarget structures are the benzotriazole–coumarin (Figure 7, general structure XXXII) and carbazole–coumarin (Figure 7, general structure XXXIII) hybrids [48,49]. In both cases, the molecules show antioxidant activity, as well as AChE and β-amyloid aggregation inhibitory properties. Finally, it is worth mentioning another type of hybrid, this time a furocoumarin that incorporates two fragments of resveratrol (Figure 7, general structure XXXIV) [50]. Compounds containing this scaffold present AChE and BACE1 inhibitory properties related to the furocoumarin fragment, and antioxidant (radical scavenging) and COX-2 inhibition, related to the resveratrol [50].
The 7-amidocoumarins (Figure 7, general structure XXXV) have recently been published for their potential against monoamine oxidase A (hMAO-A), hMAO-B, hBACE1, hAChE, and butyrylcholinesterase (hBuChE) [51]. The research project is based on a screening of compounds with potential activity against Alzheimer’s and Parkinson’s diseases, since these multifactorial pathologies share some of their pharmacological targets. Five derivatives of the studied series were described as potent and selective hMAO-B inhibitors in the nanomolar range; six turned out to be hMAO-A inhibitors in the low micromolar range; one showed inhibitory activity of hBACE1, and another one hAChE inhibitory activity, both in the micromolar range. In addition to the enzymatic inhibition, all of the studied molecules proved to be non-cytotoxic to neurons in the motor cortex. As a main conclusion, results suggest that by modulating the substitution pattern at position 7 of the scaffold, selective or multitarget molecules can be achieved.
The 3-arylcoumarins are a family of compounds with proven activity on different targets related to neurodegenerative diseases, especially Alzheimer’s and Parkinson’s diseases, the two most prevalent. In the last decade, several manuscripts described very promising activities of this scaffold, both as selective and multitarget compounds. SAR studies were performed, and important conclusions were drawn based on the substitution patterns in the main scaffold. Due to the large number of molecules based on this scaffold currently synthetized and studied as hMAO inhibitors, in 2020 a theoretical work was published comparing different QSAR models and docking calculations, in order to predict the hMAO-B activity of the 3-arylcoumarins [52]. Based on the predictions, a small series of compounds was synthetized and evaluated against both hMAO-A and hMAO-B, and the most promising models were validated. Selective activities were found in the low nanomolar range against this isoenzyme for 6 and 8 methyl-substituted 3-arylcoumarins, also presenting methoxy groups or bromine atoms in different positions of the 3-phenyl ring (Figure 7, general structure XXXVI). These advancements may represent robust tools in the design of potent and selective derivatives.
Analogues of 3-phenylcoumarins were also published during 2020. The discovery and optimization of 3-thiophenylcoumarins (Figure 7, general structure XXXVII) as novel and promising agents against Parkinson’s disease have been described [53]. This study explores, for the first time, the potential of these structures as in vitro and in vivo agents against this disease. The inhibitory activities of hMAO-A and hMAO-B, antioxidant profile, neurotoxicity in neurons of the motor cortex, and neuroprotection against hydrogen peroxide production were studied. The in vivo effect on locomotor activity was also evaluated by an open field test (OFT) for the most potent, selective and reversible hMAO-B inhibitor of the series: 3-(4′-bromothiophen-2′-yl)-7-hydroxycoumarin (IC50 = 140 nM). In reserpinized mice pre-treated with levodopa and benserazide, this molecule exhibited a slightly better in vivo profile than selegiline, currently a therapeutic option for Parkinson’s disease. The results suggested that the 7-position substitution of the coumarin scaffold is interesting for enzyme inhibition. Furthermore, the presence of a catechol at positions 7 and 8 exponentially increases the antioxidant potential and the neuroprotective properties.
The neuroprotective effects of xanthotoxin and umbelliferone on streptozotocin (STZ)-induced cognitive dysfunction in rats were evaluated [54]. Alzheimer’s disease was induced in these animals and both compounds were administrated, proving to prevent cognitive deficits in the Morris water maze and object recognition tests. In addition, both compounds reduced the activity of hippocampal AChE and the level of malondialdehyde, increasing the glutathione content. These coumarins also modulated neuronal cell death by reducing the level of proinflammatory cytokines, inhibiting the overexpression of inflammatory markers (nuclear factor κB and cyclooxygenase II), and upregulating the expression of NF-κB inhibitor (IκB-α). An attenuation of cognitive dysfunction by these compounds was observed. This effect can at least be attributed to the inhibition of AChE and the reduction of oxidative stress, neuroinflammation, and neuronal loss, opening a new door for these classic coumarins.
2.7. Anticoagulant Activity
The classic anticoagulant effect of specific coumarin derivatives, based on acenocoumarol and warfarin, also remains one of the classic applications for this family. During 2020, a review on this topic was published [55].
2.8. Fluorescent Probes
In addition to the interest of coumarins as a versatile scaffold in drug design, the important role that this scaffold plays as fluorescent probes to detect metals, enzymes, and biomaterials, among others, should be highlighted [56,57,58,59]. These fluorescent probes have a great imaging potential for the diagnosis of several pathologies.
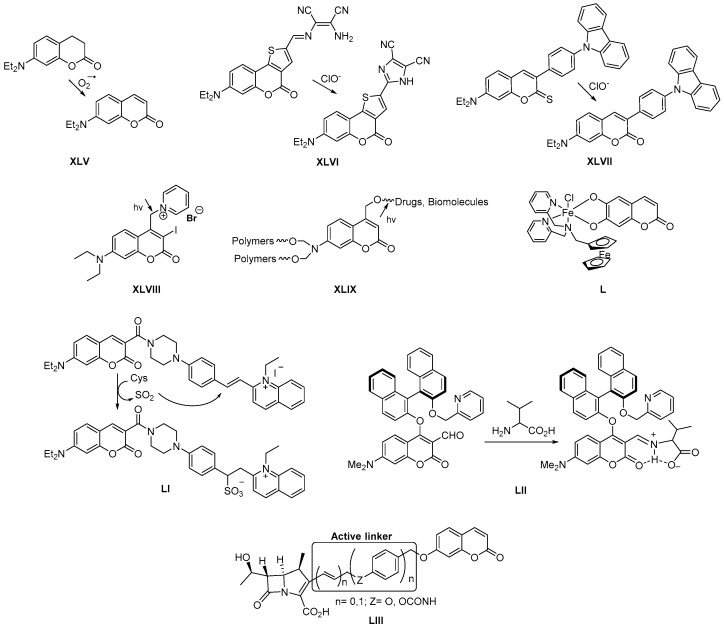
Coumarins are being used in the selective detection of metals such as copper (Figure 8, general structure XXXVIII) [60] or its determination in drinking water (Figure 8, compound XXXIX) [61,62]. A recent review focuses on the detection of iron in water and its applications [63]. Other works study the fluorescence determination of the presence of silver in aqueous medium (Figure 8, compound XL) [64]. In the case of mercury, there are also published works in which the selective determination in water is studied (Figure 8, general structure XLI) [65]. In some cases, this determination is selective, but in this case, the innovative methodology can be applied over a wide pH range (Figure 8, compound XLII) [66].
Figure 8.
Structures of coumarin derivatives as fluorescent probes.
In some cases, these metal complexes serve as probes for the detection of biothiols, as in the case of copper complexes with benzothiazoles (Figure 8, compound XLIII) used in the determination of cysteines [67], or coumarin–quinoline complexes used in the detection of glutathione (Figure 8, compound XLIV) [68]. In other cases, aromatization to form the coumarin ring is used as a fluorescence test to detect the superoxide anion (Figure 8, compound XLV) [69].
In addition to the determination of metals, in many cases coumarin derivatives are used as fluorescence probes for the detection of hypochlorite, as in the case of coumarin–thiophene complexes (Figure 8, compound XLVI) [70] or of 2-thiocoumarins in which the presence of ClO– allows the formation of a fluorescent coumarin (Figure 8, compound XLVII) [71].
Coumarins can also be used as photocleavable linkers in the controlled release of drugs or biomaterials. This is the case of the in vivo photolysis of the microtubule inhibitor 4-pyridinomethylcoumarin (Figure 8, compound XLVIII) [72]. 7-Hydroxymethyl substituted aminocoumarins are used as iron complexes (Figure 8, general structures XLIX and L), and can be used to photochemotherapeutically target the mitochondria in the treatment of cancer [60,73].
Other reviews report on the use of 7-hydroxycoumarin and its derivatives in determining the activity of cytochromes P450 (CYP) enzymes [74] as well as 7-aminocoumarin derivatives in determining amino acids from serine or cysteine proteases [58]. In other cases, they are used to detect the metabolism of mitochondrial cysteines, the oxidation of which is a measure of cellular oxidative stress (Figure 8, compound LI) [75]. The use of the chiral coumarin-BINOL hybrid allows the enantioselective detection of amino acids (Figure 8, compound LII) [76]. Finally, coumarins can be used for easy detection of bacterial carbapenamases in which the coumarin fluorophore binds to the carbapenemic structure via a reactive linker (Figure 8, general structure LIII) [77,78].
3. Perspectives
Coumarins are privileged structures for biological applications. Their conjugated double ring system allows different spots for chemical modifications, and a large number of derivatives can be obtained. Therefore, structure/activity studies appear to be the hottest emerging topic. During the year 2020, more than a thousand research articles and reviews related to coumarins could be found. This highlights the great potential that these molecules can have in different fields of research. For simplicity, our overview focused on the potential of coumarins in medicinal chemistry. The most relevant studies were included. The range of applications described in this document, and some others outside the scope of this general description (i.e., optoelectronic applications [79], polymers [80], etc.), reflect the versatility of this scaffold.
In our opinion, the potential of coumarins as fluorescent probes appears to be the most promising field of research for the next few years, since several coumarin derivatives have shown great potential in prodrug degradation (drug release) and diagnostics.
Due to the length of this general overview, synthetic strategies for obtaining new coumarins have not been discussed in detail. To find information on the most recent synthetic pathways, we recommend the manuscripts by Molnar and co-authors [81] and Kovač and co-authors [82], both from 2020. To find an overview of the wide range of biological activities of coumarins, a 2020 review by Pinto and co-authors [1] is strongly recommended. Finally, to find information on the most recent analytical methods (fundamentals, instrumentation, purification and quantification applications, optimization of experimental conditions, emerging ecological methods, etc.) we recommend the review by Xue-song and co-authors [83].
4. Conclusions
Coumarins belong to a privileged family for biomedical proposes. Their simplicity, chemical properties, and the efficiency of the synthetic routes to obtain a wide range of substitution patterns make these compounds highly attractive and versatile for medicinal and biological chemists. To date, and during 2020, more than a thousand research articles and reviews containing information on coumarins appear in the PubMed, SciFinder, and Mendeley databases. The number of research groups working on this scaffold, and the impact of the results, highlight the potential of these molecules. Special attention has been paid to the potential of coumarins in drug design, as well as to fluorescent probes. This last application seems to be the most promising field of research for the next few years, since several coumarin derivatives have shown great potential in the decaging of prodrugs (drug release) and for diagnostic purposes.
Author Contributions
Conceptualization, M.J.M. and L.S.; methodology, A.C., M.J.M., E.U., and L.S.; formal analysis, A.C., M.J.M., E.U., and L.S.; investigation, A.C., M.J.M., E.U., and L.S.; resources, M.J.M., E.U., and L.S.; writing—original draft preparation and editing, M.J.M. and L.S.; writing—review and editing, M.J.M., L.S., and E.U.; visualization, M.J.M., E.U., and L.S.; supervision, L.S.; project administration, M.J.M., E.U., and L.S.; funding acquisition, M.J.M., E.U., and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Xunta de Galicia (Galician Plan of Research, Innovation and Growth 2011–2015, Plan I2C, ED481B 2014/027-0, ED481B 2014/086–0 and ED481B 2018/007) and Fundação para a Ciência e Tecnologia (FCT, CEECIND/02423/2018 and UIDB/00081/2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented is original and not inappropriately selected, manipulated, enhanced, or fabricated.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Annunziata F., Pinna C., Dallavalle S., Tamborini L., Pinto A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020;21:4618. doi: 10.3390/ijms21134618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fotopoulos I., Hadjipavlou-Litina D. Hybrids of coumarin derivatives as potent and multifunctional bioactive agents: A review. Med. Chem. 2020;16:272–306. doi: 10.2174/1573406415666190416121448. [DOI] [PubMed] [Google Scholar]
- 3.Feng D., Zhang A., Yang Y., Yang P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020;353:e1900380. doi: 10.1002/ardp.201900380. [DOI] [PubMed] [Google Scholar]
- 4.Matiadis D., Sagnou M. Pyrazoline hybrids as promising anticancer agents: An up-to-date overview. Int. J. Mol. Sci. 2020;21:5507. doi: 10.3390/ijms21155507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song X.F., Fan J., Liu L., Liu X.F., Gao F. Coumarin derivatives with anticancer activities: An update. Arch. Pharm. 2020;353:e2000025. doi: 10.1002/ardp.202000025. [DOI] [PubMed] [Google Scholar]
- 6.Akkol E.K., Genç Y., Karpuz B., Sobarzo-Sánchez E., Capasso R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers. 2020;12:1959. doi: 10.3390/cancers12071959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Warhi T., Sabt A., Elkaeed E.B., Eldehna W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020;103:104163. doi: 10.1016/j.bioorg.2020.104163. [DOI] [PubMed] [Google Scholar]
- 8.Goud N.S., Kumar P., Bharath R.W. Recent developments of target based coumarin derivatives as potential anticancer agents. Mini-Rev. Med. Chem. 2020;20:1754–17668. doi: 10.2174/1389557520666200510000718. [DOI] [PubMed] [Google Scholar]
- 9.Endo S., Oguri H., Segawa J., Kawai M., Hu D., Xia S., Okada T., Irie K., Fujii S., Gouda H., et al. Development of novel AKR1C3 inhibitors as new potential treatment for castration-resistant prostate cancer. Med. Chem. 2020;63:10396–10411. doi: 10.1021/acs.jmedchem.0c00939. [DOI] [PubMed] [Google Scholar]
- 10.Wang C., Xi D., Wang H., Niu Y., Liang L., Xu F., Peng Y., Xu P. Hybrids of MEK inhibitor and NO donor as multitarget antitumor drugs. Eur. J. Med. Chem. 2020;196:112271. doi: 10.1016/j.ejmech.2020.112271. [DOI] [PubMed] [Google Scholar]
- 11.Xu J., Li H., Wang X., Huang J., Li S., Liu C., Dong R., Zhu G., Duan C., Jiang F., et al. Discovery of coumarin derivatives as potent and selective cyclin-dependent kinase 9 (CDK9) inhibitors with high antitumour activity. Eur. J. Med. Chem. 2020;200:112424. doi: 10.1016/j.ejmech.2020.112424. [DOI] [PubMed] [Google Scholar]
- 12.Zhao N., Yang F., Han L., Qu Y., Ge D., Zhang H. Development of coumarin-based hydroxamates as histone deacetylase inhibitors with antitumor activities. Molecules. 2020;25:717. doi: 10.3390/molecules25030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang U., Zhang W., Dong J., Gao J. Design, synthesis, and bioactivity evaluation of coumarin-chalcone hybrids as potential anticancer agents. Bioorg. Chem. 2020;95:103530. doi: 10.1016/j.bioorg.2019.103530. [DOI] [PubMed] [Google Scholar]
- 14.Wang B.Y., Lin Y.C., Lai Y.T., Ou J.Y., Chang W.W., Chu C. Targeted photoresponsive carbazole–coumarin and drug conjugates for efficient combination therapy in leukemia cancer cells. Bioorg. Chem. 2020;100:103904. doi: 10.1016/j.bioorg.2020.103904. [DOI] [PubMed] [Google Scholar]
- 15.Qin H.L., Zhang Z.W., Ravindar L., Rakesh K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020;207:112832. doi: 10.1016/j.ejmech.2020.112832. [DOI] [PubMed] [Google Scholar]
- 16.Sumorek-Wiadro J., Zając A., Langner E., Skalicka-Woźniak K., Maciejczyk A., Rzeski W., Jakubowicz-Gil J. Antiglioma potential of coumarins combined with Sorafenib. Molecules. 2020;25:5192. doi: 10.3390/molecules25215192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumorek-Wiadro J., Zając A., Bądziul D., Langner E., Skalicka-Woźniak K., Maciejczyk A., Wertel I., Rzeski W., Jakubowicz-Gil J. Coumarins modulate the anti-glioma properties of temozolomide. Eur. J. Pharmacol. 2020;881:173207. doi: 10.1016/j.ejphar.2020.173207. [DOI] [PubMed] [Google Scholar]
- 18.Liu H., Xia D.G., Chu Z.W., Hu R., Cheng X., Lv X.H. Novel coumarin-thiazolyl ester derivatives as potential DNA gyrase Inhibitors: Design, synthesis, and antibacterial activity. Bioorg. Chem. 2020;100:103907. doi: 10.1016/j.bioorg.2020.103907. [DOI] [PubMed] [Google Scholar]
- 19.Sutar S.M., Savanur H.M., Malunavar S.S., Pawashe G.M., Aridoss G., Kim K.M., Lee J.Y., Kalkhambkar R.G. Synthesis and molecular modelling studies of coumarin and 1-aza-coumarin linked miconazole analogues and their antimicrobial properties. ChemistrySelect. 2020;5:1322–1330. doi: 10.1002/slct.201903572. [DOI] [Google Scholar]
- 20.Lnufaie R., Hansa R.K.C., Alsup N., Whitt J., Chambers S.A., Gilmore D., Alam M.A. Synthesis and antimicrobial studies of coumarin-substituted pyrazole derivatives as potent anti-Staphylococcus aureus agents. Molecules. 2020;25:2758. doi: 10.3390/molecules25122758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C.F., Zhang P.L., Sui Y.F., Lv J.S., Ansari M.F., Battini N., Li S., Zhou C.H., Geng R.X. Ethylenic conjugated coumarin thiazolidinediones as new efficient antimicrobial modulators against clinical methicillin-resistant. Bioorg. Chem. 2020;94:103434. doi: 10.1016/j.bioorg.2019.103434. [DOI] [PubMed] [Google Scholar]
- 22.Nasiri Sovari S., Vojnovic S., Skaro Bogojevic S., Crochet A., Pavic A., Nikodinovic-Runic J., Zobi F. Design, synthesis and in vivo evaluation of 3-arylcoumarin derivatives of rhenium (I) tricarbonyl complexes as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA) Eur. J. Med. Chem. 2020;204:112533. doi: 10.1016/j.ejmech.2020.112533. [DOI] [PubMed] [Google Scholar]
- 23.Aldabaldetrecu M., Parra M., Soto S., Arce P., Tello M., Guerrero J., Modak B. New Copper (I) complex with a coumarin as ligand with antibacterial activity against Flavobacterium psychrophilum. Molecules. 2020;25:3183. doi: 10.3390/molecules25143183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonçalves G.A., Spillere A.R., das Neves G.M., Kagami L.P., von Poser G.L., Canto R.F.S., Eifler-Lima V.L. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020;203:112514. doi: 10.1016/j.ejmech.2020.112514. [DOI] [PubMed] [Google Scholar]
- 25.Pires C.T.A., Scodro R.B.L., Cortez D.A.G., Brenzan M.A., Siquiera V.L.D., Caleffi-Ferracioli K.R., Vieira L.C.C., Monteiro J.L., Corrêa A.G., Cardoso R.F. Structure–activity relationship of natural and synthetic coumarin derivatives against Mycobacterium tuberculosis. Future Med. Chem. 2020;11:1533–1546. doi: 10.4155/fmc-2018-0281. [DOI] [PubMed] [Google Scholar]
- 26.Zhu M., Ma L., Wen J., Dong B., Wang Y., Wang Z., Zhou J., Zhang G., Wang J., Guo Y., et al. Rational design and structure-activity relationship of coumarin derivatives effective on HIV-1 protease and partially on HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2020;186:111900. doi: 10.1016/j.ejmech.2019.111900. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y., Cheng H., Khan S., Xiao G., Rong L., Bai C. Development of coumarin derivatives as potent anti-filovirus entry inhibitors targeting viral glycoprotein. Eur. J. Med. Chem. 2020;204:112595. doi: 10.1016/j.ejmech.2020.112595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minhas R., Bansal G., Bansal Y. Novel coupled molecules from active structural motifs of synthetic and natural origin as immunosuppressants. Med. Chem. 2020;16:544–554. doi: 10.2174/1573406415666190409111459. [DOI] [PubMed] [Google Scholar]
- 29.Salar U., Khan K.M., Jabeen A., Faheem A., Naqvi F., Ahmed S., Iqbal E., Ali F., Kanwal, Perveen S. ROS inhibitory activity and cytotoxicity evaluation of benzoyl, acetyl, alkyl ester, and sulfonate ester substituted coumarin derivative. Med. Chem. 2020;16:1099–1111. doi: 10.2174/1573406415666190826153001. [DOI] [PubMed] [Google Scholar]
- 30.Hassanein E.H.M., Sayed A.M., Hussein O.E., Mahmoud A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxidative Med. Cell. Longev. 2020;2020:1675957. doi: 10.1155/2020/1675957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanke S., Tindall C.A., Pippel J., Ulbricht D., Pirotte B., Reboud-Ravaux M., Heiker J.T., Sträter N. Structural studies on the inhibitory binding mode of aromatic coumarinic esters to human Kallikrein-related peptidase 7. J. Med. Chem. 2020;63:5723–57336. doi: 10.1021/acs.jmedchem.9b01806. [DOI] [PubMed] [Google Scholar]
- 32.Wang T., Peng T., Wen X., Wang G., Liu S., Sun Y., Zhang S., Wang L. Design, Synthesis and evaluation of 3-substituted coumarin derivatives as anti-inflammatory agents. Chem. Pharm. Bull. 2020;68:443–446. doi: 10.1248/cpb.c19-01085. [DOI] [PubMed] [Google Scholar]
- 33.Matos M.J., Vilar S., Vazquez-Rodriguez S., Kachler S., Klotz K.N., Buccioni M., Delogu G., Santana L., Uriarte E., Borges F. Structure-based optimization of coumarin hA3 adenosine receptor antagonists. J. Med. Chem. 2020;63:2577–2587. doi: 10.1021/acs.jmedchem.9b01572. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Rodriguez S., Vilar S., Kachler S., Klotz K.N., Uriarte E., Borges F., Matos M.J. Adenosine receptor ligands: Coumarin-chalcone hybrids as modulating agents on the activity of hARs. Molecules. 2020;25:4306. doi: 10.3390/molecules25184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tafesse T.B., Bule M.H., Khoobi M., Faramarzi M.A., Abdollahi M., Amini M. Coumarin-based scaffold as α-glucosidase inhibitory activity: Implication for the development of potent antidiabetic agents. Mini-Rev. Med. Chem. 2020;20:134–151. doi: 10.2174/1389557519666190925162536. [DOI] [PubMed] [Google Scholar]
- 36.Liang D., Fa Y., Yang Z., Zhang Z., Liu M., Liu L., Jiang C. Discovery of coumarin-based selective aldehyde dehydrogenase 1A1 inhibitors with glucose metabolism improving activity. Eur. J. Med. Chem. 2020;187:111923. doi: 10.1016/j.ejmech.2019.111923. [DOI] [PubMed] [Google Scholar]
- 37.Xu X.T., Deng X.Y., Chen J., Liang Q.M., Zhang K., Li D.L., Wu P.P., Zheng X., Zhou R.P., Jiang Z.Y., et al. Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2020;189:112013. doi: 10.1016/j.ejmech.2019.112013. [DOI] [PubMed] [Google Scholar]
- 38.Swain B., Angeli A., Singh P., Supuran C.T., Arifuddin M. New coumarin/sulfocoumarin linked phenylacrylamides as selective transmembrane carbonic anhydrase inhibitors: Synthesis and in-vitro biological evaluation. Bioorg. Med. Chem. Lett. 2020;28:115586. doi: 10.1016/j.bmc.2020.115586. [DOI] [PubMed] [Google Scholar]
- 39.Thacker P.S., Angeli A., Argulwar O.S., Tiwari P.L., Arifuddin M., Supuran C.T. Design, synthesis and biological evaluation of coumarin linked 1,2,4-oxadiazoles as selective carbonic anhydrase IX and XII inhibitors. Bioorg. Chem. 2020;98:103739. doi: 10.1016/j.bioorg.2020.103739. [DOI] [PubMed] [Google Scholar]
- 40.Thacker P.S., Goud N.S., Argulwa O.S., Soman J., Angeli A., Alvala M., Arifuddin M., Supuran C.T. Synthesis and biological evaluation of some coumarin hybrids as selective carbonic anhydrase IX and XII inhibitors. Bioorg. Chem. 2020;104:104272. doi: 10.1016/j.bioorg.2020.104272. [DOI] [PubMed] [Google Scholar]
- 41.Ashooriha M., Khoshneviszadeh M., Khoshneviszadeh M., Rafiei A., Kardan M., Yazdian-Robati R., Emami S. Kojic acid–natural product conjugates as mushroom tyrosinase inhibitors. Eur. J. Med. Chem. 2020;201:112480. doi: 10.1016/j.ejmech.2020.112480. [DOI] [PubMed] [Google Scholar]
- 42.Hng Y., Lin M.H., Lin T.S., Liu I.C., Lin I.C., Lu Y.L., Chang C.N., Chiu P.F., Tsai K.C., Chen M.J., et al. Design and synthesis of 3-benzylaminocoumarin-7-O-sulfamate derivatives as steroid sulfatase inhibitors. Bioorg. Chem. 2020;96:103618. doi: 10.1016/j.bioorg.2020.103618. [DOI] [PubMed] [Google Scholar]
- 43.Era B., Delogu G.L., Pintus F., Fais A., Gatto G., Uriarte E., Borges F., Kumar A., Matos M.J. Looking for new xanthine oxidase inhibitors: 3-phenylcoumarins versus 2-phenylbenzofurans. Inter. J. Biolog. Macromol. 2020;162:774–780. doi: 10.1016/j.ijbiomac.2020.06.152. [DOI] [PubMed] [Google Scholar]
- 44.Matos M.J., Herrera Ibatá D.M., Uriarte E., Viña D. Coumarin-rasagiline hybrids as potent and selective hMAO-B inhibitors, antioxidants, and neuroprotective agents. ChemMedChem. 2020;15:532–538. doi: 10.1002/cmdc.202000018. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Enríquez F., Costas-Lago M.C., Besada P., Alonso-Pena M., Torres-Terán I., Viña D., Fontenla J.A., Sturlese M., Moro S., Quezada E., et al. Novel coumarin-pyridazine hybrids as selective MAO-B inhibitors for the Parkinson’s disease therapy. Bioorg. Chem. 2020;104:104203. doi: 10.1016/j.bioorg.2020.104203. [DOI] [PubMed] [Google Scholar]
- 46.Saeedi M., Rastegari A., Hariri R., Mirfazli S.S., Mahdavi M., Edraki N., Firuzi O., Akbarzadeh T. Design and synthesis of novel arylisoxazole-chromenone carboxamides: Investigation of biological activities associated with Alzheimer’s disease. Chem. Biodivers. 2020;17:e1900746. doi: 10.1002/cbdv.201900746. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X., Guo J., Lv Y., Yao C., Zhang C., Mi Z., Shi Y., Gu J., Zhou T., Bai R., et al. Rational design, synthesis and biological evaluation of novel multitargeting anti-AD iron chelators with potent MAO-B inhibitory and antioxidant activity. Bioorg. Med. Chem. Lett. 2020;28:115550. doi: 10.1016/j.bmc.2020.115550. [DOI] [PubMed] [Google Scholar]
- 48.Singh A., Sharma S., Arora S., Attri S., Kaur P., Gulati H.K., Bhagat K., Kumar N., Singh H., Singh J.V., et al. New coumarin-benzotriazole based hybrid molecules as inhibitors of acetylcholinesterase and amyloid aggregation. Bioorg. Med. Chem. Lett. 2020;30:127477. doi: 10.1016/j.bmcl.2020.127477. [DOI] [PubMed] [Google Scholar]
- 49.Shi D.H., Min W., Song M.Q., Si X.X., Li M.C., Zhang Z.Y., Liu Y.W., Liu W.W. Synthesis, characterization, crystal structure and evaluation of four carbazole-coumarin hybrids as multifunctional agents for the treatment of Alzheimer’s disease. J. Mol. Struct. 2020;1209:127897. doi: 10.1016/j.molstruc.2020.127897. [DOI] [Google Scholar]
- 50.Agbo E.N., Gildenhuys S., Choong Y.S., Mphahlele M.J., More G.K. Synthesis of furocoumarin–stilbene hybrids as potential multifunctional drugs against multiple biochemical targets associated with Alzheimer’s disease. Bioorg. Chem. 2020;101:103997. doi: 10.1016/j.bioorg.2020.103997. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Enríquez F., Viña D., Uriarte E., Laguna R., Matos M.J. 7-Amidocoumarins as multitarget agents against neurodegenerative diseases: Substitution pattern modulation. ChemMedChem. 2020;15 doi: 10.1002/cmdc.202000454. [DOI] [PubMed] [Google Scholar]
- 52.Mellado M., Mella J., González C., Viña D., Uriarte E., Matos M.J. 3-Arylcoumarins as highly potent and selective monoamine oxidase B inhibitors: Which chemical features matter? Bioorg. Chem. 2020;101:103964. doi: 10.1016/j.bioorg.2020.103964. [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Enríquez F., Viña D., Uriarte E., Fontenla J.A., Matos M.J. Discovery and optimization of 3-thiophenylcoumarins as novel agents against Parkinson’s disease: Synthesis, in vitro and in vivo studies. Bioorg. Chem. 2020;101:103986. doi: 10.1016/j.bioorg.2020.103986. [DOI] [PubMed] [Google Scholar]
- 54.Hindam M.O., Sayed R.H., Skalicka-Woźniak K., Budzyńska B., EL Sayed N.S. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a streptozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2/STAT3 and Nrf2/HO-1 signalling pathway modulation. Phytoth. Res. 2020;34:2351–2365. doi: 10.1002/ptr.6686. [DOI] [PubMed] [Google Scholar]
- 55.Kasperkiewicz K., Ponczek M.B., Owczarek J., Guga P., Budzisz E. Antagonists of vitamin K -popular coumarin drugs and new synthetic and natural coumarin derivatives. Molecules. 2020;25:1465. doi: 10.3390/molecules25061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunacd X.Y., Liubcd T., Sun J., Wan X.J. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020;10:10826–10847. doi: 10.1039/C9RA10290F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Breidenbach J., Bartz U., Gütschow M. Coumarin as a structural component of substrates and probes for serine and cysteine proteases. BBA-Proteins Proteom. 2020;1868:140445. doi: 10.1016/j.bbapap.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raunio H., Pentikaeinen O., Juvonen R.O. Coumarin-based profluorescent and fluorescent substrates for determining xenobiotic-metabolizing enzyme activities in vitro. Int. J. Mol. Sci. 2020;21:4708. doi: 10.3390/ijms21134708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen W., Zheng J., Zhou Z., Zhang D. Approaches for the synthesis of o-nitrobenzyl and coumarin linkers for use in photocleavable biomaterials and bioconjugates and their biomedical applications. Acta Biomater. 2020;115:75–91. doi: 10.1016/j.actbio.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 60.Ying W., Xiaohui H., Lixun L., Luyao G., Xumin R., Yonggang W., Hongchi Z. A coumarin-containing Schiff base fluorescent probe with AIE effect for the copper (II) ion. RSC Adv. 2020;10:6109–6113. doi: 10.1039/c9ra10632d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arslan F.N., Geyik G.A., Koran K., Ozen F., Aydin D., Elmas S.N.K., Gorgulu A.O., Yilmaz I. Fluorescence “turn on-off” sensing of copper (II) ions utilizing coumarin-based chemosensor: Experimental study, theoretical calculation, mineral and drinking water analysis. J. Fluoresc. 2020;30:317–327. doi: 10.1007/s10895-020-02503-4. [DOI] [PubMed] [Google Scholar]
- 62.Hien N.K., Bay M.V., Bao N.C., Vo Q.V., Cuong N.D., Thien T.V., Nhung N.T.A., Van D.U., Nam P.C., Quang D.T. Coumarin-based dual chemosensor for colorimetric and fluorescent detection of Cu2+ in water media. ACS Omega. 2020;5:21241–21249. doi: 10.1021/acsomega.0c03097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J., Guo Y., Dong B., Sun J., Lyu J., Sun L., Hu S., Xu L., Bai X., Xu W., et al. Water-soluble coumarin oligomer based ultra-sensitive iron ion probe and applications. Sens. Actuator B-Chem. 2020;320:128361. doi: 10.1016/j.snb.2020.128361. [DOI] [Google Scholar]
- 64.Jiang X., Yang Y., Li H., Qi X., Zhou X., Deng M., Lü M., Wu J., Liang S. A Water-soluble fluorescent probe for the selective sensing of Ag+ and its application in imaging of living cells and nematodes. J. Fluoresc. 2020;30:121–129. doi: 10.1007/s10895-019-02477-y. [DOI] [PubMed] [Google Scholar]
- 65.Ngororabanga J.M.V., Tshentu Z.R., Mama N. New highly selective colorimetric and fluorometric coumarin-based chemosensor for Hg2+ J. Fluoresc. 2020;30:985–997. doi: 10.1007/s10895-020-02542-x. [DOI] [PubMed] [Google Scholar]
- 66.Pan Z., Xu Z., Chen J., Hu L., Li H., Zhang X., Gao X., Wang M., Zhang J. Coumarin thiourea-based fluorescent turn-on Hg2+ probe that can be utilized in a broad pH range 1–11. J. Fluoresc. 2020;30:505–514. doi: 10.1007/s10895-020-02517-y. [DOI] [PubMed] [Google Scholar]
- 67.Khoa Hien N., Van Bay M., Diem Tran P., Tan Khanh N., Dinh Luyen N., Vo Q.V., Ung Van D., Cam Nam P., Tuan Quang D. A coumarin derivative-Cu2+ complex-based fluorescent chemosensor for detection of biothiols. RSC Adv. 2020;10:36265–36274. doi: 10.1039/D0RA05651K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S., Cao D., Meng X., Hu Z., Li Z., Yuan C., Zhou T., Han X., Ma W. A novel fluorescent sensor for specific recognition of GSH based on the copper complex and its bioimaging in living cells. Bioorg. Chem. 2020;100:103923. doi: 10.1016/j.bioorg.2020.103923. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., Han J., Xu Y., Gao Y., Wen H., Cui H. Taking advantage of the aromatization of 7-diethylamino-4-methyl-3,4-dihydrocoumarin in the fluorescence sensing of superoxide anion. Chem. Commun. 2020;56:9827–9829. doi: 10.1039/D0CC02282A. [DOI] [PubMed] [Google Scholar]
- 70.Shi L., Yu H., Zeng X., Yang S., Gong S., Xiang H., Zhangd K., Shao G. A novel ratiometric fluorescent probe based on thienocoumarin and its application for the selective detection of hypochlorite in real water samples and in vivo. New J. Chem. 2020;44:6232–6237. doi: 10.1039/D0NJ00318B. [DOI] [Google Scholar]
- 71.Nguyen V.N., Heo S., Kim S., Swamy K.M.K., Ha J., Park S., Yoon J. A thiocoumarin-based turn-on fluorescent probe for hypochlorite detection and its application to live-cell imaging. Sens. Actuator B-Chem. 2020;317:128213. doi: 10.1016/j.snb.2020.128213. [DOI] [Google Scholar]
- 72.Tang X.J., Wu Y., Zhao R., Kou X., Dong Z., Zhou W., Zhang Z., Tan W., Fang X. Photorelease of pyridines using a metal-free photoremovable protecting group. Angew. Chem. Int. Edit. 2020;59:18386–18389. doi: 10.1002/anie.202005310. [DOI] [PubMed] [Google Scholar]
- 73.Sarkar T., Bhattacharyya A., Banerjee S., Hussain A. LMCT transition-based red-light photochemotherapy using a tumour-selective ferrocenyl iron (III) coumarin conjugate. Chem. Commun. 2020;56:7981–7984. doi: 10.1039/D0CC03240A. [DOI] [PubMed] [Google Scholar]
- 74.Xia Z., Chen D., Song S., van der Vlag R., van der Wouden P.E., van der Merkerk R., Cool R.H., Hirsch A.K.H., Melgert B.N., Quax W.J., et al. 7-Hydroxycoumarins are affinity-based fluorescent probes for competitive binding studies of macrophage migration inhibitory factor. J. Med. Chem. 2020;63:11920–11933. doi: 10.1021/acs.jmedchem.0c01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han X., Zhai Z., Yang X., Zhang D., Tang J., Zhu J., Zhu X., Ye Y. A FRET-based ratiometric fluorescent probe to detect cysteine metabolism in mitochondria. Org. Biomol. Chem. 2020;18:1487–1492. doi: 10.1039/D0OB00002G. [DOI] [PubMed] [Google Scholar]
- 76.Wu X., Wang Q., Dickie D., Pu L. Mechanistic study on a BINOL–coumarin-based probe for enantioselective fluorescent recognition of amino acids. J. Org. Chem. 2020;85:6352–6358. doi: 10.1021/acs.joc.0c00074. [DOI] [PubMed] [Google Scholar]
- 77.Kim J., Kim Y., Abdelazem A.Z., Kim H.J., Choo H., Kim H.S., Kim J.O., Park Y.J., Min S.J. Development of carbapenem-based fluorogenic probes for the clinical screening of carbapenemase-producing bacteria. Bioorg. Chem. 2020;94:103405. doi: 10.1016/j.bioorg.2019.103405. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Xu W., Xue S., Yua T., Xie H. A minor structure modification serendipitously leads to a highly carbapenemase-specific fluorogenic probe. Org. Biomol. Chem. 2020;18:4029–4033. doi: 10.1039/D0OB00114G. [DOI] [PubMed] [Google Scholar]
- 79.Kumar A., Baccoli R., Fais A., Cincotti A., Pilia L., Gatto G. Substitution effects on the optoelectronic properties of coumarin derivatives. Appl. Sci. 2020;10:144. doi: 10.3390/app10010144. [DOI] [Google Scholar]
- 80.Cuevas J.M., Seoane-Rivero R., Navarro R., Marcos-Fernández A. Coumarins into polyurethanes for smart and functional materials. Polymers. 2020;12:630. doi: 10.3390/polym12030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lončarić M., Gašo-Sokač D., Jokić S., Molnar M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules. 2020;10:151. doi: 10.3390/biom10010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molnar M., Lončarić M., Kovač M. Green chemistry approaches to the synthesis of coumarin derivatives. Curr. Org. Chem. 2020;24:4–43. doi: 10.2174/1385272824666200120144305. [DOI] [Google Scholar]
- 83.Dong-wei C., Yuan Z., Xiao-Yi D., Yu Z., Guo-hui L., Xue-song F. Progress in pretreatment and analytical methods of coumarins: An update since 2012—A review. Crit. Rev. Anal. Chem. 2020 doi: 10.1080/10408347.2020.1750338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented is original and not inappropriately selected, manipulated, enhanced, or fabricated.