Abstract
Objectives
The aim was to characterise age- and sex-specific severe acute respiratory syndrome coronavirus disease-2 (SARS-CoV-2) RT-PCR sampling frequency and positivity rate in Greater Helsinki area in Finland during February–June 2020. We also describe the laboratory capacity building for these diagnostics.
Methods
Laboratory registry data for altogether 80,791 specimens from 70,517 individuals was analysed. The data included the date of sampling, sex, age and the SARS-CoV-2 RT-PCR test result on specimens collected between 1 February and 15 June 2020.
Results
Altogether, 4057/80,791 (5.0%) of the specimens were positive and 3915/70,517 (5.6%) of the individuals were found positive. In all, 37% of specimens were from male and 67% from female subjects. While the number of positive cases was similar in male and female subjects, the positivity rate was significantly higher in male subjects: 7.5% of male and 4.4% of female subjects tested positive. The highest incidence/100,000 was observed in those aged ≥80 years. The proportion of young adults in positive cases increased in late May 2020. Large dips in testing frequency were observed during every weekend and also during public holidays.
Conclusions
Our data suggest that men pursue SARS-CoV-2 testing less frequently than women. Consequently, a subset of coronavirus disease-2019 infections in men may have gone undetected. People sought testing less frequently on weekends and public holidays, and this may also lead to missing of positive cases. The proportion of young adults in positive cases increased towards the end of the study period, which may suggest their returning back to social behaviour with an increased risk of infection.
Keywords: COVID-19, Nucleic acid amplification, Real-time RT-PCR, SARS-CoV-2, Surveillance
The WHO has advocated for the ‘test, trace, treat’ strategy in the mitigation of coronavirus disease-2019 (COVID-19) pandemic (WHO, 2020). Rarely has sophisticated laboratory diagnostics been set up at this pace and extent. As the epidemiological situation evolved quickly, the laboratory capacity building over the first few months played a key role in the epidemic response in each country. At the same time, laboratory-based surveillance can provide high quality data for public health management. By using laboratory registry data, the aim of this study was to characterise age- and sex-specific sampling frequency and positivity rate and to characterise laboratory capacity building of severe acute respiratory syndrome coronavirus disease-2 (SARS-CoV-2) RT-PCR testing in the Greater Helsinki area in Finland during February–June 2020.
Materials and methods
The study was conducted at the Helsinki University Hospital Laboratory (HUS Diagnostic Center, HUSLAB), Finland, according to research permit HUS/157/2020 (Helsinki University Hospital, Finland).
Registry data
This study was based on the laboratory registry database of the Helsinki University Hospital Laboratory, Finland. This laboratory provides services to primary care and hospitals of Greater Helsinki, with a population of 1,685,983 (48.8% male and 51.2% female subjects; see Table 1 for age distribution) as per 31 December 2019 (Official Statistics of Finland, 2020).
Table 1.
Age distribution of the population in Greater Helsinki, Finland; the study population and the SARS-CoV-2 RT-PCR test-positive population (Official Statistics of Finland, 2020).
| Age group (years) | Age distribution, Greater Helsinki population (%) | Age distribution, study population (%) | Age distribution, test positive population (%) |
|---|---|---|---|
| 0–9 | 10.8 | 6.7 | 3.6 |
| 10–19 | 10.9 | 6.0 | 6.2 |
| 20–29 | 13.4 | 14.9 | 16.0 |
| 30–39 | 15.3 | 17.4 | 18.0 |
| 40–49 | 13.4 | 15.1 | 15.4 |
| 50–59 | 13.1 | 13.6 | 16.4 |
| 60–69 | 10.7 | 9.2 | 8.9 |
| 70–79 | 8.4 | 8.6 | 5.9 |
| ≥80 | 4.1 | 8.5 | 9.7 |
The data included the date of sampling, sex, age, and the SARS-CoV-2 RT-PCR test result on specimens from 1 February to 15 June 2020. The data were collected according to permit HUS/157/2020 (Helsinki University Hospital, Finland). The data were analysed with GraphPad Prism® according to tests and individual cases. In the case analysis, only the first test of the negative cases was counted. For the positive cases, only the first positive test was counted.
Data from the National Infectious Disease Register of the National Institute for Health and Welfare (THL) were retrieved to calculate age-specific incidence.
Laboratory methods
The respiratory specimens were subjected to one of the following methods (gene targets): a protocol based on Corman et al. (N) (Corman et al., 2020), cobas® SARS-CoV-2 test kit on the cobas® 6800 system (orf1ab and E) (Roche Diagnostics, Basel, Switzerland), Amplidiag® COVID-19 test (orf1ab and N) (Mobidiag, Espoo, Finland) and Mobidiag Novodiag® Covid-19 assay (orf1ab and N) (Mobidiag, Espoo, Finland). The performance of these tests in our laboratory is reported elsewhere (Jokela et al., 2020, Mannonen et al., 2021).
Testing criteria
The testing criteria evolved during the study period. Until mid-March 2020, only symptomatic individuals with travel history to epidemic areas or contact with a laboratory -confirmed COVID-19 cases and those admitted to the ICU were tested. As of 15 March, symptomatic patients who were either admitted to hospital; over the age of 70; had a possible exposure or were nursing home residents were tested. A week later, symptomatic healthcare workers were included. As of 13 April, all symptomatic individuals and hospitalised risk group patients were tested.
Results
Of the 86,927 specimens sent to Helsinki University Hospital Laboratory (HUSLAB) for SARS-CoV-2 RT-PCR testing between 1 February and 15 June, 5061 were excluded as they originated outside of Greater Helsinki. Additional 1075 specimens were excluded as they repeatedly gave an invalid test result or were never analysed due to a preanalytical failure. The final data included 80,791 specimens (Figure 1 a) from 70,517 individuals. The tested individuals had a median age of 43 years, mean 45 years and a range of 0 days–106 years. Table 1 outlines the age distribution of the tested individuals and the test-positive individuals. Altogether, 4057/80,791 (5.0%) of the specimens were positive and 3915/70,517 (5.6%) of the individuals were found positive. The positive cases had a median age of 43 years, mean 45 years and range 0–100 years.
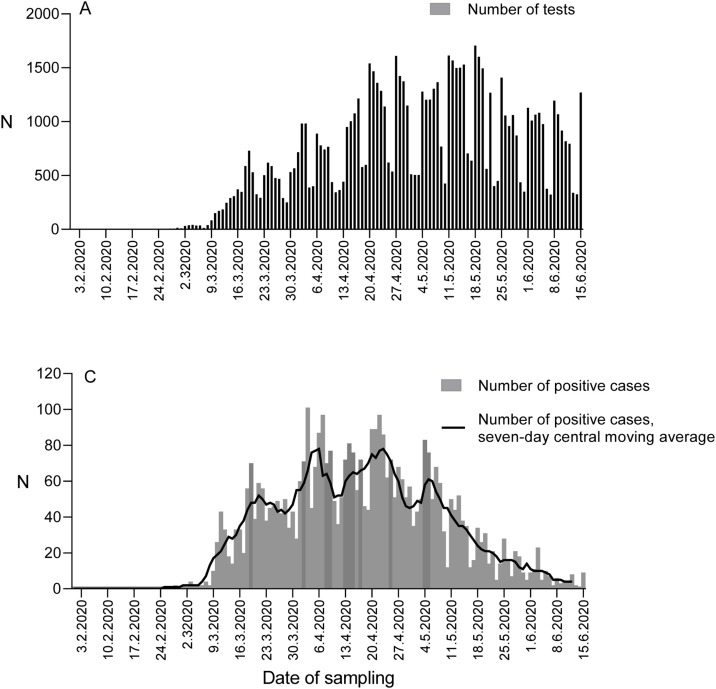
Figure 1.
Number of SARS-CoV-2 RT-PCR tests and positive cases. (A) The number of SARS-CoV-2 RT-PCR tests conducted in HUSLAB by date of sampling and positivity rate, between 1 March and 15 June 2020. (B) The number of SARS-CoV-2 positive cases (columns) with seven-day central moving average (line) by the date of sampling, between 1 March and 15 June 2020.
The first positive case in Greater Helsinki was diagnosed on 25 February (Figure 1a). This was the second COVID-19 case diagnosed in Finland, the first being a Chinese tourist in Lapland (Haveri et al., 2020). As of 9 March, both the number of tests, positive tests and cases increased rapidly (Figure 1). A peak was reached on 3 April (96 new cases) (Figure 1b), and the daily number of new cases remained high throughout April and early May.
During the epidemic peak, the daily number of tests was still increasing through rapid capacity building. The daily number of tests (by sampling date) increased from 308 tests on March 15 to 1005 tests on April 15, and further to 1530 tests on May 15 (Figure 1a). The positivity rate of the tests remained >10% until early May 2020 (Figure 1a).
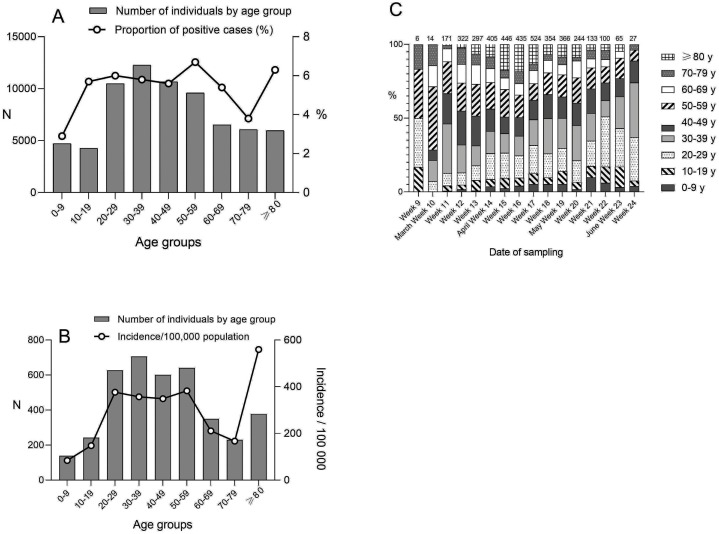
The highest proportion of positive cases was found in the age group 50–59 years (6.7%) and the lowest in the age group 0–9 years (2.9%) (Figure 2 a). The number of test-positive cases was highest in working-age adults (20–59 years) (Figure 2b). The age stratification of new positive cases over time showed a shift towards an increasing proportion of young adults (20–39 years) as of late May (Figure 2c). The incidence per 100,000 population in the Greater Helsinki area was 287/100,000 population and highest in those aged ≥80 years (560/100,000) (Figure 2b).
Figure 2.
Age characteristics of SARS-CoV-2 RT-PCR tested cases. (A) The number of individuals tested for SARS-CoV-2 RT-PCR by age group (columns) and the proportion of SARS-CoV-2 positive cases (line). (B) The number of SARS-CoV-2 positive cases by age group and the age-specific incidence/100,000 population of SARS-CoV-2 in the Greater Helsinki area (line), data from the National Infectious Diseases Registry. (C) Age stratification of SARS-CoV-2 positive cases by calendar week of 2020. The proportion (%) of each age group is shown.
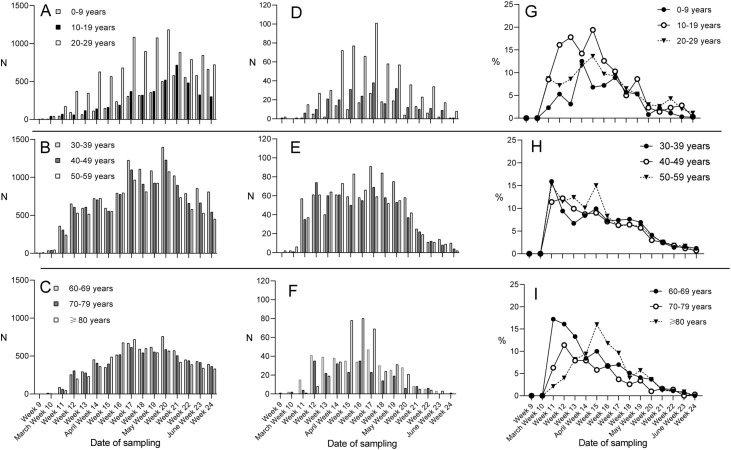
In the age group 0–29 years, the proportion of test-positive cases peaked during the weeks 13–15, while in the age group 30–79 years, the highest proportions were observed already in weeks 11 and 12 (Figure 3 ). In those aged ≥80 years, the proportion of positive cases began to increase in week 13 and peaked in week 15 (Figure 3).
Figure 3.
Age-specific number of SARS-CoV-2 RT-PCR tests and positive cases according to calendar week. (A–C) The age-specific number of individuals tested for SARS-CoV-2 RT-PCR by calendar week of 2020. (D–F) The age-specific number of SARS-CoV-2-positive cases by calendar week of 2020. (G–I) The age-specific proportion (%) of SARS-CoV-2-positive cases by calendar week of 2020. The age range for all tested male subjects was 1 d–102 years (mean 43.1 and median 41 years) and for positive male subjects 2 months–97 years (mean 43.5 and median 41 years). The age range for all tested female subjects was 0 d–106 years (mean 45.5 and median 43 years) and for positive female subjects was 1 month–100 years (mean 47.7 and median 45 years).
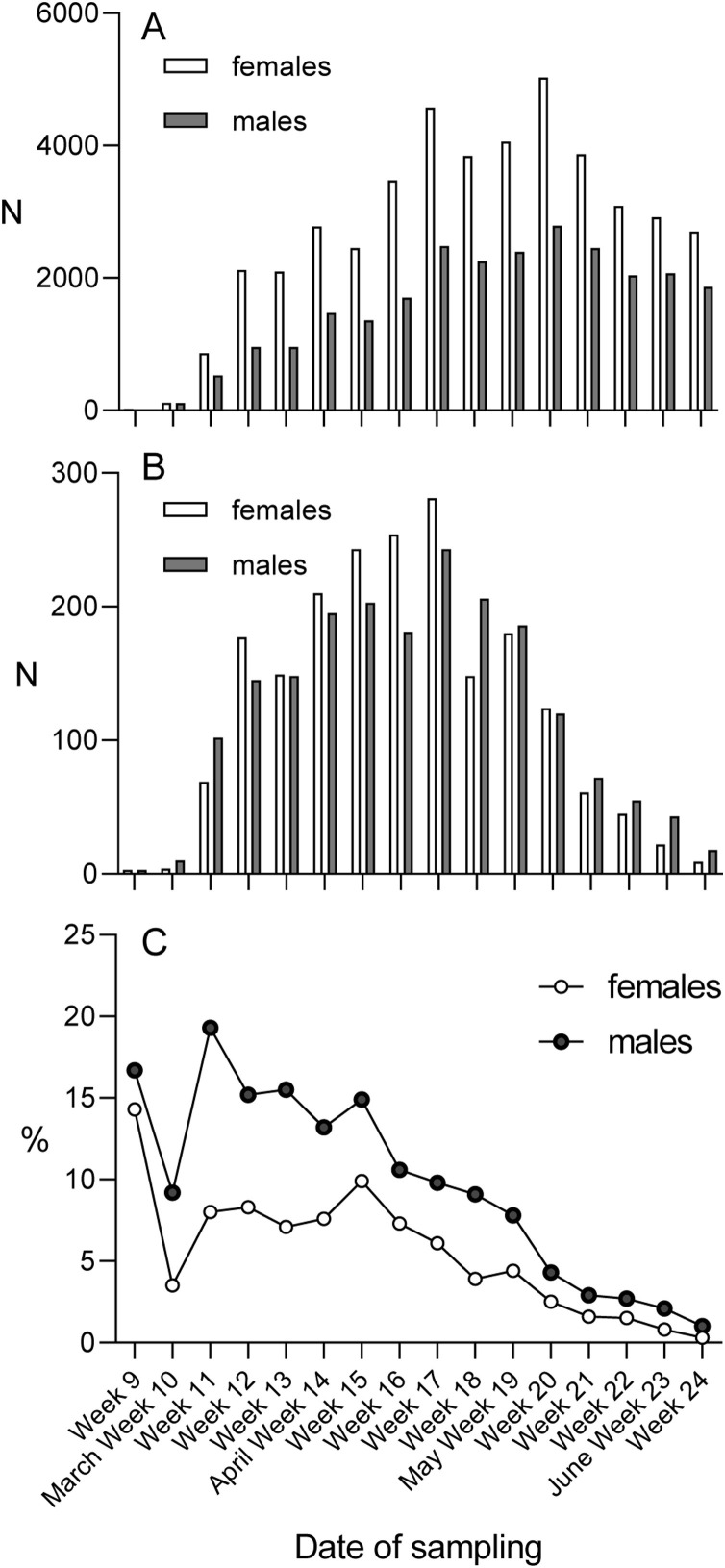
Of the 80,791 specimens included in the study, 29,885 specimens were from 25,948 (36.8%) individual males and 50,906 specimens from 44,569 (63.2%) individual females. In contrast, of the positive cases, 1935/3915 (49.4%) were male and 1980/3915 (50.6%) were female subjects. Thus, 7.5% (1935/25,948) of male and 4.4% (1980/44,569) of female subjects tested were positive. There was a statistically significant difference in these proportions (z = 16.8595 and p < 0.01, calculated with Z-test). The difference appeared to decrease towards the end of the study period (Figure 4 ).
Figure 4.
Sex-specific number of SARS-CoV-2 RT-PCR tests and positive cases according to calendar week. (A) The sex-specific number of SARS-CoV-2 individuals tested for SARS-CoV RT-PCR by calendar week of 2020. (B) The sex-specific number of SARS-CoV-2 positive cases by calendar week of 2020. (C) The sex-specific proportion (%) of SARS-CoV-2 positive cases by calendar week of 2020.
Discussion
By 30 June 2020, 80.2% of all tests performed and 77.3% of new positive cases detected within the Hospital District of Helsinki and Uusimaa were analysed in HUSLAB (THL, 2020), rendering our surveillance data representative of Greater Helsinki. In Finland, COVID-19 has mostly affected the Greater Helsinki area, which represents 30% of the Finnish population (Official Statistics of Finland, 2020). Indeed, 73% of the positive cases in Finland, by the end of June 2020, have been detected in Greater Helsinki (THL, 2020).
The number of test-positive cases was similar in male and female subjects, but the positivity rate in males was significantly higher than in females. This may, to a small degree, be explained by the overrepresentation of healthcare workers and female predominance in the elderly. However, our data suggest that men pursued SARS-CoV-2 testing much less frequently than women. Consequently, a subset of COVID-19 infections in men may have gone undetected. Abundant evidence shows that women generally seek more healthcare services than men (Deveugele et al., 2002, Thompson et al., 2016).
As of 18 March, several restrictions were implemented in Finland. Schools were closed; public gatherings were restricted to 10 people; museums, theatres, libraries and public sports facilities were closed; visits to nursing homes were forbidden and public sector employees switched to remote work whenever possible. As of 4 April, restaurants were also closed. Schools were reopened on 14 May and restaurants and museums on 1 June (with certain prerequisites) (Prime Minister’s Office, 2020). The proportion of young adults who tested positive appeared to increase towards the end of the study period, which may suggest their returning back to social behaviour with risk of infection. However, considering the incubation period (Lauer et al., 2020), it does not appear likely that relaxing restrictions played a role with this observation. Extension of testing criteria into mild symptoms may have also contributed.
The high incidence rate observed in the elderly is in line with earlier reports (Natale et al., 2020). Vigilance in the infection control in long-term care facilities remains critically important in the mitigation of the pandemic.
Regarding limitations, our data did not include all tests analysed in Greater Helsinki. No clinical data were available. A bias may have been introduced by the restricted sampling criteria during March 2020. During the first weeks, control swabs were recommended for the positive cases — a policy since abandoned. Furthermore, during the first two weeks of April, less preferred oropharyngeal swabs were used due to the global shortage of nasopharyngeal swabs, which may have temporarily influenced the overall test sensitivity.
Large dips in testing frequency were observed on every weekend and also during public holidays (Figure 1). Simultaneously, the number of new positive cases dropped each time, and the epicurve (Figure 1b) may suggest that this testing deficit was not fully compensated during the following weekdays.
In a response to need for large-scale testing, HUSLAB switched from two-shift work into three-shift work on 23 March, and personnel were reallocated. Following a rapid training period, personnel from other laboratories were allocated for SARS-CoV-2 analytical testing. A laboratory-developed test (Corman et al., 2020) was ready for use in mid-January and testing on the Cobas® 6800 system in late March. Because of constant global shortages of reagents and plasticware, the need to deploy several independent methods was evident to secure laboratory services and capacity building. The Amplidiag® COVID-19 tests were deployed as of mid-April and the Novodiag® sample-to-answer test as of mid-May.
In preparation for the potential next epidemic waves of COVID-19 pandemic, the difference in healthcare seeking behaviours between men and women needs to be accounted for to facilitate high universal testing frequency. This is particularly relevant as men are at higher risk of fatal infection and more likely to be hospitalised and admitted to intensive care units due to COVID-19 (Garg et al., 2020, Grasselli et al., 2020, Mikami et al., 2020, Yu et al., 2020). In addition, advocating and maintaining social behaviours that reduce the risk of infection remains a key measure of mitigation in all age groups.
Conflict of interest
None of the authors have any conflicts of interest.
Funding statement
Funded by Helsinki University Hospital, HUSLAB, Helsinki, Finland.
Ethical approval
No ethical permission was required.
CRediT authorship contribution statement
H. Jarva: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing - original draft, Writing - review & editing. M. Lappalainen: Conceptualization, Investigation, Methodology, Resources, Validation, Writing - review & editing. O. Luomala: Data curation, Formal analysis, Investigation, Writing - review & editing. P. Jokela: Investigation, Methodology, Writing - review & editing. A.E. Jääskeläinen: Investigation, Methodology, Writing - review & editing. A.J. Jääskeläinen: Investigation, Writing - review & editing. H. Kallio-Kokko: Investigation, Methodology, Writing - review & editing. E. Kekäläinen: Conceptualization, Investigation, Writing - review & editing. L. Mannonen: Investigation, Methodology, Writing - review & editing. H. Soini: Investigation, Writing - review & editing. S. Suuronen: Investigation, Writing - review & editing. A. Toivonen: Investigation, Writing - review & editing. C. Savolainen-Kopra: Investigation, Writing - review & editing. R. Loginov: Investigation, Methodology, Writing - review & editing. S. Kurkela: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing - original draft, Writing - review & editing.
Acknowledgments
We would like to thank the staff at the Department of Virology and Immunology working with SARS-CoV-2 diagnostics.
References
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveugele M., Derese A., van den Brink-Muinen A., Bensing J., De Maeseneer J. Consultation length in general practice: cross sectional study in six European countries. BMJ. 2002;325(7362):472. doi: 10.1136/bmj.325.7362.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25(11):2000266. doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela P., Jääskeläinen A.E., Jarva H., Holma T., Ahava M.J., Mannonen L. SARS-CoV-2 sample-to-answer nucleic acid testing in a tertiary care emergency department: evaluation and utility. J Clin Virol. 2020;131(October):104614. doi: 10.1016/j.jcv.2020.104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannonen L., Kallio-Kokko H., Loginov R., Jääskeläinen A., Jokela P., Antikainen J. Comparison of two commercial platforms and a laboratory developed test for detection of SARS-CoV RNA. The Journal of Molecular Diagnostics. 2021 doi: 10.1016/j.jmoldx.2021.01.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami T., Miyashita H., Yamada T., Harrington M., Steinberg D., Dunn A. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2020;(June):1–10. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale F., Ghio D., Tarchi D., Goujon A., Conte A. 2020. COVID-10 cases and case fatality rate by age.https://ec.europa.eu/knowledge4policy/publication/covid-19-cases-case-fatality-rate-age_en Retrieved from: [Google Scholar]
- Official Statistics of Finland (OSF) Statistics Finland; Helsinki: 2020. Population structure [e-publication] ISSN=1797-5395. [Referred: 28.6.2020]. Access method: http://www.stat.fi/til/vaerak/index_en.html. [Accessed 27 June 2020] [Google Scholar]
- Prime Minister’s Office in Finland . 2020. Government decides on plan for hybrid strategy to manage coronavirus crisis and for gradual lifting of restrictions.https://vnk.fi/-/hallitus-linjasi-suunnitelmasta-koronakriisin-hallinnan-hybridistrategiaksi-ja-rajoitusten-vaiheittaisesta-purkamisesta?languageId=en_US Retrieved from: [Google Scholar]
- THL, National Institute for Health and Welfare . 2020. National infectious disease register.https://sampo.thl.fi/pivot/prod/fi/epirapo/covid19case/fact_epirapo_covid19case Retrieved from: [Google Scholar]
- Thompson A.E., Anisimowicz Y., Miedema B., Hogg W., Wodchis W.P., Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract. 2016;17:38. doi: 10.1186/s12875-016-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. World Health Organization (14 April 2020). Strategic preparedness and response plan.https://www.who.int/publications/i/item/strategic-preparedness-and-response-plan-for-the-new-coronavirus Retrieved from: [Google Scholar]
- Yu C., Lei Q., Li W., Wang X., Liu W., Fan X. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. 2020;59:168–175. doi: 10.1016/j.amepre.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]