Abstract
In light of the outbreak of the 2019 novel coronavirus disease (COVID-19), the international scientific community has joined forces to develop effective treatment strategies. The Angiotensin-Converting Enzyme (ACE) 2, is an essential receptor for cell fusion and engulfs the SARS coronavirus infections. ACE2 plays an important physiological role, practically in all the organs and systems. Also, ACE2 exerts protective functions in various models of pathologies with acute and chronic inflammation. While ACE2 downregulation by SARS-CoV-2 spike protein leads to an overactivation of Angiotensin (Ang) II/AT1R axis and the deleterious effects of Ang II may explain the multiorgan dysfunction seen in patients. Specifically, the role of Ang II leading to the appearance of Macrophage Activation Syndrome (MAS) and the cytokine storm in COVID-19 is discussed below. In this review, we summarized the latest research progress in the strategies of treatments that mainly focus on reducing the Ang II-induced deleterious effects rather than attenuating the virus replication.
Keywords: COVID-19, SARS, Coronavirus, Macrophage Activation Syndrome (MAS), ACE2, RAS system
Graphical abstract
ACE2 Downregulation in severe COVID-19 patient and appearance of MAS.
Highlights
-
•
Protective role of ACE2 in the organs and system
-
•
Downregulation of ACE2 expression by SARS-CoV-2 leads to Ang II-induced organ damage.
-
•
The appearance of MAS in COVID-19 patient
-
•
Suggested treatment to diminish the deleterious effect of Ang II or appearance of MAS
1. Introduction
As of early April, the death toll of Coronavirus Disease 2019 (COVID-19) pandemic caused by a coronavirus SARS-CoV-2 exceeds thousands of people. Angiotensin-Converting Enzyme 2 (ACE2) that is recognized as a protective molecule against kidney, heart, liver, and respiratory diseases [1] in the context of negative regulation of the Renin-Angiotensin System (RAS), is now also recognized as a functional receptor for SARS-CoV-2 [2,3]. The virus- ACE2 recognition is too efficient and the SARS-CoV-2 spike protein has a strong binding affinity to human ACE2 [4,5]. This virus uses ACE2 not only for its cellular entry to the host cell but also downregulates ACE2 expression [6,7], contributing to the pathogenesis of the severe acute respiratory distress syndrome (ARDS) or severe acute respiratory syndrome (SARS), see Fig. 1 [8]. The epidemiologic data suggests that there is an elevated level of ACE2 expression in young adults as compared to aged groups [9]. The less ACE2 content in aged groups may contribute to the predominance of complications in aging.
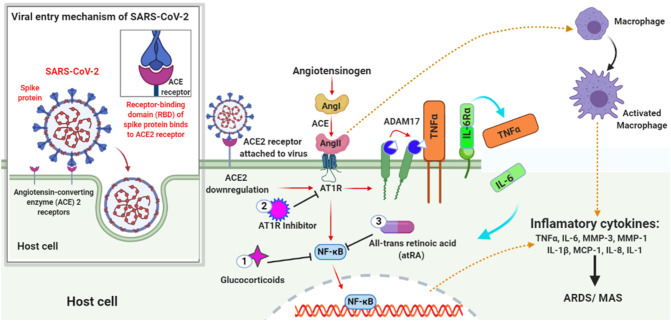
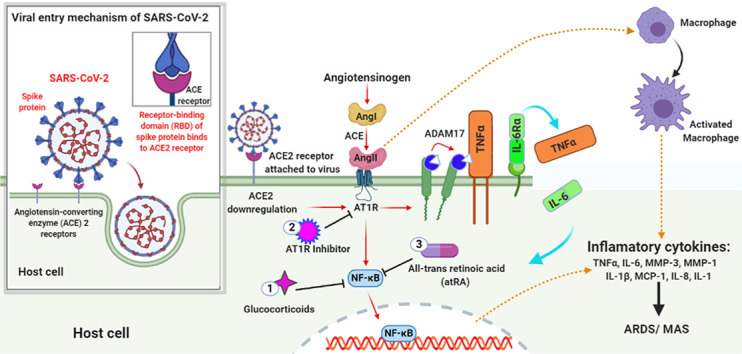
Fig. 1.
Illustration of ACE2 Downregulation in COVID-19 patient and appearance of MAS. The virus enters the host cell by interacting with the ACE2 receptor through its spike protein. Later, the virus downregulates the ACE2 expression that in turn upregulates Ang II. Ang II is a product of the RAS system obtained by the cleavage of Ang I by enzyme ACE. The upregulated Ang II interacts with its receptor AT1R and modulates the gene expression of several inflammatory cytokines via NF-κB signaling. This Ang II/AT1R interaction also influences the macrophage activation that in turn produces the inflammatory cytokines Thereby, inducing ARDS or MAS. Also, some metalloproteases like ADAM17 shed these proinflammatory cytokines and ACE2 receptors to the soluble form which aids in loss of the protective function of surface ACE2 and may increase SARS pathogenesis. The treatment with Glucocorticoids, AT1R inhibitor, and All-trans retinoic acid tends to inhibit NF-κB signaling thus reducing the cytokine storms thereby improving the severity of SARS-CoV2 infection.
The RAS system includes angiotensinogen (ANG), angiotensin (Ang) I, Ang II, renin, and the Angiotensin-Converting Enzyme (ACE). The substrate ANG is degraded to inactive Ang I by the enzyme renin, which is then cleaved by ACE to generate an octapeptide Ang II [10]. The ACE2, a recently identified member of RAS is an 805 amino-acid type-I transmembrane protein that degrades Ang II to Ang-(1–7) [ [11]], see Fig. 1. The two isotypes of Ang II receptors that belong to the G-protein coupled receptor superfamily, Ang II type 1 receptor (AT1R) and Ang II type 2 receptor (AT2R) have been identified [12]. Ang II exerts its biological functions mainly through the AT1R [13], but in some harmful pathological conditions, Ang II/AT1R is overactive, see Fig. 2 [10]. Also, Ang II may be implicated in the impairment of nitric oxide bioavailability, cell oxidative stress, and increases the retention of sodium and water by the release of aldosterone and vasopressin, see Fig. 2 [14]. ACE2 counteracts the deleterious effect of Ang II by maintaining the balance between the two axes ACE2/Ang-(1–7)/Mas receptor and ACE/Ang II/AT1R of the RAS. Thereby, reducing the bioavailability of Ang II and increasing Ang-(1–7) expression [15].
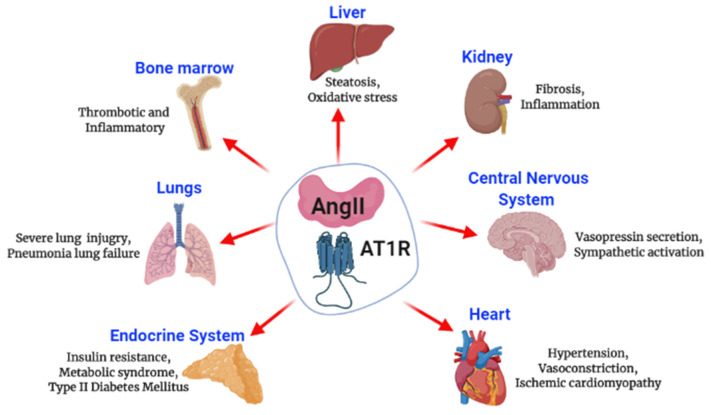
Fig. 2.
Schematic representation of Angiotensin II effects. The interaction of Ang II with AT1R mediates the harmful effects leading to multi-organ failure.
ACE2 also limits the macrophage expression of several proinflammatory cytokines in vitro, including Tumor Necrosis Factor (TNF-α) and Interleukin-6 (IL-6) [16]. But in the case of COVID-19, this virus tends to downregulate ACE2 thus enhancing macrophage expression [17], as observed in the Macrophage Activation Syndrome (MAS), see Fig. 1. Severe SARS-CoV-2 patients show similar symptoms than MAS patients like persistent fever, multiple organ failure, and the same sustained cytokine pattern [18]. ACE2 is abundantly expressed in the luminal surface of tubular epithelial cells in the kidney [19], and cardiomyocytes in the heart [20]. Some level of ACE2 is also seen in the gut and lungs [21]. This wide distribution pattern of ACE2 expression explains the multiple organ dysfunction with COVID-19 and MAS patients. Thus, we hypothesized that the infection with SARS-CoV-2 may lead to MAS through the downregulation of ACE2 and the involvement of Ang II.
Regarding the above, in this review, we have discussed the protective role of ACE2 in different organs and the deleterious effect caused by the downregulation of ACE2 in SARS-CoV-2 infection and MAS. Based on this discussion we have also suggested possible treatments for the better outcome of severe COVID-19 patients.
2. Protective role of ACE2 in different organs and system
2.1. ACE2 in different settings of pathologies in the lungs
It has been demonstrated the capacity for local Ang II generation within the parenchyma of the lungs [22]. After an acute injury, the expression of AGT mRNA, AGT protein, and the Ang II derived from it had increased [23]. Ang II is known to be proapoptotic for epithelial cells including the lung [24] and significant in vivo evidence suggests that Ang II plays a very important role in lung inflammation and fibrogenesis [25]. Thus, blocking Ang II synthesis or its functions ameliorates lung damage. In contrast, ACE2 has been positively correlated with the differentiation state of epithelia [26]. The abundant expression of ACE2 is seen in differentiated cells than in the undifferentiated cells [27]. In the respiratory tract, ACE2 is predominantly expressed in the alveolar and bronchiolar epithelium, endothelium, and smooth muscle cells of pulmonary vessels [26]. The state of cell differentiation and ACE2 expression levels are important determinants of the susceptibility of human airway epithelia to infection [28]. Kuba et al. discovered that the injection of the SARS-CoV Spike protein could decrease the expression of ACE2 in the lungs and cause acute lung injury [29].
Animal models prove ACE2 protective effects in the different settings of infections and no infections pathologies of the lungs [7,8,[29], [30], [31]]. In mice, ACE2 protects against acute lung injury triggered by acid aspiration and sepsis [29]. In this model which mimics human acute lung injury, the loss of ACE2 resulted in worsened oxygenation, massive lung edema, and increased inflammatory cell infiltration [29]. Similarly in Bleomycin-induced lung injury, ACE2 knockout mice exhibited poorer exercise capacity, worse lung function, and exacerbated lung inflammation and fibrosis compared with age-matched wild-type [32]. The genetic inactivation of ACE2 causes severe lung injury in H5N1-challenged mice [31].
A soluble and catalytically active form of ACE2 has also been described in the lung [26]. ACE2 is released from the surface of epithelia into the airway surface liquid via cleavage by TACE (ADAM17) and other sheddases [33,34]. In response to stimuli, many membrane proteins undergo either shedding or internalization [33] Haga et al. reported that the SARS-CoV Spike protein once binds to ACE2, induces ACE2 shedding by further activating cellular ADAM17 [35,36]. While some authors suggested that increasing soluble ACE2 may be a negative feedback mechanism to control viral infection [26,33], enhanced ACE2 shedding resulting from RAS overactivation, and subsequent ADAM17 upregulation drives pathogenesis in several models of cardiovascular diseases [37,38]. Ang II accumulation by the loss of membrane ACE2, also activates ADAM17, creating a vicious circle of membrane shedding of ACE2, RAS overactivation, and inflammation [39].
In the respiratory system, the administration of recombinant ACE2 has been shown to have a beneficial effect on improving lung pathologies and patient's survival rate in ARDS and SARS induced by viruses [30,40,41]. Importantly, the depletion of ACE2 at the cell surface with a loss of ACE2-mediated tissue protection is a critical pathological outcome of SARS-CoV-2 infection. As ACE2 also has nuclear effects [42,43], the efficacy of the recombinant human ACE2 administration in patients with COVID-19 will require careful experimentation in appropriate models together with well-controlled clinical trials. For example, ACE2 regulates alveolar epithelial cell's survival by inhibiting both JNK phosphorylation and apoptosis [44]. On the other hand, the use of ACE inhibitors or AT1R-selective antagonists exerted inhibitory effects on bleomycin-, γ irradiation-, amiodarone- and paraquat-induced pulmonary damage in rats, and hyperoxia-induced chronic lung disease in neonatal rats [[45], [46], [47], [48]].
Moreover, the lung epithelial stem/progenitor cell express ACE2 [49]. A subset of putative stem/progenitor cells has been reported as the major target for SARS coronavirus in the human lung [28,50]. The effects of the infection of these cells may result in cell death, infiltration of the immune cells, including macrophages and the production of proinflammatory cytokines. Recently, an antitrypanosomal drug that enhances the enzymatic activity of ACE2 improved the angiogenic progenitor cell functions in the lung [51]. The exogenous administration of ACE2 could induce MSCs proliferation and differentiation and participate in healing injured lung from inflammatory lung disease [26,52]. Thus, the downregulation of ACE2 during SARS-CoV-2 infection decreases the lung's ability to recover from the acute injury and may cause severe pneumonia lung failure, as clinically observed, see Fig. 2.
2.2. ACE2 protects the liver from acute and chronic inflammation
Normal liver tissue expresses a low amount of ACE2 [53] but in the case of a liver injury, ACE2 is upregulated at the gene and protein level, accompanied by an increase in ACE2 activity, possibly in response to increasing hepatocellular hypoxia [54]. In a chronic liver injury model, the loss of ACE2 activity worsens liver fibrosis [55] and liver steatosis [56]. In Multiple Drug-Resistant Gene 2-Knockout Mice, ACE2 therapy was seen to inhibit the Chronic Biliary Fibrosis [57]. ACE2 mice knockout study demonstrated that the marker genes for oxidative stress signaling (Gpx1, catalase, and SOD2) and pro-inflammatory cytokines like TNF-α, monocyte chemotactic protein-1 (MCP-1) and Interleukin-8 (IL-8) were significantly increased resulting in aggravation of oxidative stress and inflammation in the liver, see Fig. 2 [56]. ACE2/Ang-(1–7)/Mas axis acts through the Akt/PI3K/IRS-1/JNK insulin signaling pathway leading to improved liver insulin resistance [58]. All these researches suggest that ACE2 may play a role in liver disease pathogenesis.
2.3. ACE2 is physiologically renoprotective
In humans, kidneys exhibit ACE and ACE2 colocalization in apical brush borders of the proximal tubules and glomeruli [59]. In renal endothelial cells, Ang II can increase TNF-α expression and produce kidney injury in immune-complex nephritis, lupus nephritis, anti-glomerular basement membrane, and puromycin nephrosis [60]. TNF-α from endothelial cells also has paracrine effects in target cells on the endothelial surface and other neighboring cells [60]. Also, different in vitro and in vivo models have been shown that Ang II increases IL-6, Transforming Growth Factor Beta (TGF-β), connective tissue growth factor (CTGF), parathyroid hormone-related protein (PTHrP) and other growth factors [61,62]. Several chemokines induced by Ang II have been associated with the high inflammatory cell recruitment observed in renal pathologies, see Fig. 2 [[63], [64], [65], [66]]. In contrast, ACE2 protects against inflammation and fibrosis by limiting the induction of renal TGF-β expression [64]. Thus, counteracting the ACE action and protects the renal system [65].
2.4. ACE2 in cardiovascular function
ACE2 is widely distributed in cardiovascular tissue and various cellular compartments including the coronary microcirculation, cardiofibroblasts, and cardiomyocytes [66]. The upregulation of ACE2 is seen in the case of heart failure and ischemic cardiomyopathy [67] where it depends on Ang II as the substrate for Ang-(1–7) generation [68]. The deletion of the ACE2 gene in mice resulted in abnormal heart function [69,70], increased Ang II levels, upregulation of hypoxia-induced gene, leading to severe cardiac contractility defect [71]. Also, ACE2 negatively regulates RAS to control blood pressure [71] and confers endothelial protection [72]. Moreover, ACE2 tends to diminishes Ang II-induced oxidative stress and inflammation through AT1R downstream phosphatidylinositol-3-kinase (PI3K) signaling [73]. Overexpression of ACE2 protects the heart against myocardial injuries induced by Ang II infusion in rats [74]. The vascular endothelial dysfunction observed in aortic rings from rats with myocardial infarction was also reversed by the chronic infusion of Ang-(1–7) [75]. Relatively, a study revealed that ACE2 shedding contributes to the development of neurogenic hypertension [76]. Suggesting that ACE2 is an essential regulator of heart function.
2.5. ACE2 in the central nervous system
ACE2 tends to be localized in the cytoplasm of neuronal cells in the brain where its expression is involved in the control of cardiovascular function [77]. ACE2/Ang-(1–7)/Mas exerts neuroprotective functions in endothelin-1-induced ischaemic stroke in rodent models [78]. Moreover, an in vivo study with Mas receptor deficiency showed an increase in the macrophage infiltration and proinflammatory genes expression in the spleen and spinal cord, worsening the experimental course of autoimmune encephalomyelitis [79]. ACE2 tends to drive neoantigen-specific immune responses by effecting dendritic cell function to enhance their ability to induce FoxP3+ and IL-17A+ effector T cell thereby, controlling the immune response [80]. Furthermore, ACE2 overexpression in the brain attenuates neurogenic hypertension by inhibiting cyclooxygenase mediated inflammation [81,82]. Some research also proved that ACE2 mediates the reduction of oxidative stress in the brain and improve the autonomic function [83]. Besides, ACE may be detrimental to Alzheimer's disease and the use of an AT1R inhibitor ameliorates the cognitive impairment thus showing a beneficial effect on Alzheimer's disease [15,84].
2.6. ACE2 in intestinal immunity
ACE2 regulates intestinal epithelial immunity by controlling amino acid homeostasis, prevents the alteration of antimicrobial peptide expression, and maintains the ecology of the gut microbiome [85]. ACE2 expression in colonic epithelial cells is positively associated with Natural Killer (NK) cells and T cell-mediated cytotoxicity along with type I immunity and negatively associated with phagocytosis and complement activation [86]. In ACE2 knockout mice, Dextran Sodium Sulphate (DSS) and Trinitrobenzene Sulphonic Acid (TNBS)-induced colitis challenges resulted in infiltration of inflammatory cells, significant shortening of the colon length, intestinal bleeding, crypt damage, weight loss, and severe diarrhea [87]. Also, ACE2 is a key regulator of dietary amino acid homeostasis in colitis [85]. Amino acids and nicotinamide can activate the mammalian target of rapamycin (mTOR), which is involved in cell proliferation, survival, redox sensor, longevity, and cellular senescence, protein synthesis and transcription [85]. The deficiency of ACE2 causes a critical impairment of nicotinamide and tryptophan which increases the susceptibility to intestinal inflammation and decreases the regenerative responses [85,88].
2.7. ACE2 in the endocrine system
Recent evidence suggests that enhanced circulating levels of Ang II are involved in the development of insulin resistance, type 2 Diabetes Mellitus (DM), and metabolic syndrome, see Fig. 2 [89,90]. In a rat model of DM, the expressions of ACE2, Mas receptor, and Ang-(1–7) levels in enterocytes are considerably higher compared with controls, and Ang-(1–7) decreased the glucose uptake [91]. Moreover, the evidence showed that ACE2 can attenuate fibrosis, increase islet insulin content, and stimulate beta-cell proliferation in the pancreas probably by increasing the intracellular calcium influx and restored impaired mitochondrial oxidation [92]. The involvement of Ang II/AT1R signaling leads to cell apoptosis and ROS generation due to hyperglycemia [93].
A high grade of inflammation is also important in DM pathogenesis. Ang II-induced CXCL16 endothelial expression is through the AT1R and RhoA/p38-MAPK/NF-κB activation [94]. Ang II activates the Rho/Rho-associated protein kinase (ROCK) pathway more than in NF-κB activation and subsequent IL-6 expression [95]. The circulating CXCR6-expressing platelets, neutrophil, monocyte, and CD8T lymphocytes are elevated in patients with metabolic syndrome [94]. Interestingly, the ATIR blockade improved the CXCL16 angiogenic properties and decreased the monocyte and lymphocyte cellularity along with its activation [94]. Thus, the downregulation of ACE2 may have important metabolic repercussions in patients who suffered from DM and SARS-CoV-2 and may explain why these patients are more susceptible to develop complications.
2.8. Role of ACE2 in bone marrow
Transcriptomic molecular studies demonstrated that the hematopoietic bone marrow (BM) stromal niche contains local RAS, AT1R, AT2R, and the inhibitory natural stem cell regulator tetrapeptide N-Acetyl-Ser-Asp-Lys-Pro (AcSDKP) [96]. Many biological functions like proliferation, migration, angiogenesis, and fibrosis in BM cells are mediated by RAS [97]. ACE is a regulator of hematopoiesis, especially, the role of Ang II in the proliferation of all lineages in BM has been extensively proven [98]. Ang II/AT1R is also involved in Myeloid differentiation and development. Importantly, neutrophils level decreased by more than 30% in ACE-knockout mice [99]. Also, acute ACE inhibition showed an increase in the AcSDKP level in plasma [100] AcSDKP substantially inhibits cell cycle entry of normal hematopoietic stem cells (HSCs) and protects hemopoiesis against damage caused by cycle-active cytotoxic agents [101] AcSDKP can also inhibit the proliferation of lymphocytes, stimulate angiogenesis and have antifibrotic effects in vivo [15].
Ang II Receptor-Associated Protein amplifies the thrombopoietin receptor Mpl which is involved in megakaryocyte growth and thrombocyte development, controls the hematopoietic stem cells homeostasis and self-renewal [102]. Ang II/AT1R induce platelet activation and production indicators developing more thrombotic and inflammatory effects, see Fig. 2 [103]. Studies demonstrated that Ang II increases rolling thrombocytes, adhered thrombocytes on the leukocytes and the endothelial cells, rolling leukocytes, and adhered leukocytes, as well as an escalation in thrombocyte-leukocyte-endothelial cell relations [98,103]. Therefore, in pathological conditions, Ang II/AT1R overactivation may lead to thrombotic complications.
Ang II was found to control the CD115 in HSCs. CD115 influences the differentiation and function of macrophage [104]. Ang II also controls monocytic cells over BM stromal cell-derived TNF-α to increase macrophage colony-stimulating factor (M-CSF)-induced management of monocytic cells [98,105]. Similar to these findings, another study demonstrated that the deficiency of ACE2 in BM-Derived Cells increases the expression of TNF-α in Adipose Stromal Cells [106]. It suggested that ACE2 expression in BM cells control the inflammation in adipose tissue. ACE2 deficiency in BM-derived cells also promotes atherosclerosis through the regulation of Ang II/Ang-(1–7) peptides, [107].
3. An excessive inflammatory response is deleterious: Macrophage Activation Syndrome
MAS or secondary Hemophagocytic lymphohistiocytosis (HLH) is a poorly recognized syndrome characterized by a fulminant cytokine storm, multiple organ dysfunction, and a high mortality rate [108]. MAS can occur during an autoimmune, tumor, and even an infectious disease [109,110]. Viral infections have especially been linked to this syndrome in adults [111,112]. An inappropriate immune stimulation and a self-perpetuating excessive inflammatory response are key facts within the pathogenesis of MAS [110,113], and the over-activation of tissue macrophages for the release of a storm of cytokines is a dominant feature observed both in MAS and severe COVID-19 patients [[114], [115], [116], [117]].
Persistent fever is the most common clinical manifestation seen in MAS [118]. Hepatobiliary dysfunction with hepatosplenomegaly, fibrinolytic consumptive coagulopathy, hyperferritinemia, and hemophagocytosis in the BM, are other common clinical and laboratory features [119]. Neurological dysfunction and acute kidney injury may also be present and could be considered as poor prognostic indicators [120,121]. The early detection of MAS with the laboratory tests including soluble interleukin 2 receptor alpha chain (sCD25) and soluble CD163 (sCD163) [122] has a profound impact on a patient's outcome.
4. Downregulation of ACE2 in COVID-19 and MAS
A direct link between ACE2 in the progression of MAS and COVID-19 has not been described yet. However, the excess of Ang II associated with ACE2 dysregulation in COVID-19 [123] leading to the cytokine storm, also seen in MAS [119] could explain the appearance of MAS in the course of SARS-CoV-2 viral infection, see Fig. 1. The rationale for this concept stems from the following evidence: (1) we have previously described how the overexpression of Ang II exert deleterious effects in almost all organs and system. Ang II is a well-established pro-inflammatory peptide that closely interacts with the immune system and can modulate the regional cytokine milieu [[124], [125], [126], [127]]. (2) Macrophages expressed almost all components of the RAS system [23,128,129]. (3) Furthermore, the monocyte mediated inflammation is directed by Ang II-induced cytoskeleton rearrangement and monocyte migration to the inflammation site [130,131]. (4) Macrophage maturation and differentiation also require Ang II/AT1R signaling [128]. (5) Ang II plays a role in macrophage function per se and the neighboring cells through autocrine/paracrine mechanisms [23,131,132]. (6) An excessive inflammatory response by the over-activation of tissue macrophages is involved both in MAS [110,113] and severe COVID-19 patients [[114], [115], [116], [117]]. The regulation of these cytokines along with monocyte/macrophage with Ang II is discussed below. Also, the role of the metallopeptidase domain 17 (ADAM17) in Ang II-macrophage inflammation will be analyzed.
The increased oxidative stress, inflammation, and apoptosis seem to be a common mechanism of Ang II worsening the course of several inflammatory diseases [69,79,133]. In COVID-19 patients, the increased serum levels of several cytokines and chemokines have been associated with the disease severity and death [[134], [135], [136], [137]]. In vitro studies showed the involvement of macrophages, T cells, NK cells proliferation [138], dendritic cell migration, CCR7 expression in Ang II-induced renal damage [139]. Ang II also triggers vascular damage via AT1R by upregulating the connective tissue growth factor (CTGF), a mediator of TGF-β [140], inducing adhesion molecules, recruiting inflammatory cells, and modulating the IL-1β, IL-18, IFNγ, TNF-α, and IL-6 cytokine expression [60,63,119,141], see Fig. 1. The TNF-α induces macrophage polarization toward the M1 phenotype creating a vicious circle [142]. Although TNF-α is produced by various cell types, the primary source of this cytokine is monocytes/macrophages [143], and in these cells, Ang II upregulates IL-6 and TNF-α gene expression being NF-κB the potential mediator of these Ang II-induced inflammatory process [63,144,145]. Moreover, TNF-α and IFNγ induce the expression of CXCL16 on dendritic cells, B cells, and macrophages [146,147]. Thus, in both the cases with MAS and COVID-19, Ang II may initiate events leading to innate and acquired immune response.
It has been described that a pool of monocytes resides in clusters of ~50 cells in the resting spleen [124,130]. Upon Ang II-AT1R interaction, splenic monocytes increase their motility and intravasate into nearby splenic veins [124]. But in such cases, ACE2 activity is increased suggesting their protective role during inflammation [148]. In mice, this “emergency reservoir” releases up to 1 million monocytes within 24 h after myocardial infarction, which is subsequently recruited into the infarct mainly via interaction of the chemokine MCP-1 with its cognate receptor CCR2 [130,149]. Ang II can promote CCL2 generation and release, inducing mononuclear leukocyte interactions with the endothelium [150]. Corroborating to the above, CCL2, the most potent chemokines at recruitment of CCR2 monocytes were seen to be upregulated in the bronchoalveolar fluid in severe COVID-19 patients [151]. Besides, Ang II increases the production of IL-8, Fractalkine, RANTES, and IP-10, among other chemotactic factors [63,[152], [153], [154]], and promotes CXCL16 endothelial expression through AT1R via RhoA/p38-MAPK/NFκB activation [94,155].
In animal models that are characterized by macrophage-mediated inflammation, the deletion of the receptor Mas enhances the migratory capacity of macrophages and induces the M1 phenotype [79]. Similar results have been reported with the specific deletion of ACE2 [156]. Mas deficiency especially affects CD11b+ macrophages interfering with the cytokine expression and activation capacities of different macrophage subtypes and may drive proinflammatory M(LPS + IFNγ)-like responses [79]. ACE inhibitors or AT1R antagonists can modulate cellular adhesion and chemotaxis [131,[157], [158], [159]]. For example, the blockade of AT1R improve CXCL16 angiogenic properties and decreased the monocyte and lymphocyte cellularity and activation [94]. In addition to CXCL16, The beneficial effects of a selective AT2R agonist were associated with the decreased recruitment or infiltration of macrophages in the lungs, reduced lung inflammation [160], diminished pulmonary collagen accumulation and improved cardiopulmonary complications through the downregulation of CCL2, IL-6, and TLR4 [161].
Macrophage maturation also requires Ang II/AT1R signaling. Ang II controls the c-Fms in HSCs and monocytic cells over local TNF-α to increase M-CSF-induced management of monocytic cells [124]. During the process, monocytes expressed the whole components for Ang II generation and increased the production of Ang II [162]. Moreover, T lymphocytes contain a functional NADPH oxidase and an AT1R [163]. Ang II via NADPH oxidases stimulates T cell proliferation and activates it to produce TNF-α, IFNγ, and TH1 generation [164]. Thus, ACE2/Mas deficiency affects macrophage phenotypes and functions and leads to an increase in oxidative stress and impaired endothelial function [79].
Evidence is accumulating that ADAM17 is an important regulator of the acute inflammatory response [[165], [166], [167], [168], [169]]. In addition to ACE2, it is a primary sheddase of relevant inflammatory factors, including TNF-α, its two receptors TNFR-I (CD120a) and TNFR-II(CD120b), IL-6R, ligands of ErbB (e.g. TGFα and amphiregulin), and the L-selectin (CD62L) and ICAM-1 adhesion molecules [[170], [171], [172]]. ADAM17 also regulates leukocyte rolling along activated endothelium and leukocyte transmigration by shedding L-selectin, CX3CL1, ICAM-1, and VCAM-1 as well as JAM-A [173,174]. Macrophage ADAM17 is an essential component for activation and the pro-inflammatory phenotype [[175], [176], [177], [178]]. Interestingly, the relationship between Ang II and ADAM17 pathogenic effects has been well studied [34,[179], [180], [181]]. Ang II-mediated proteolytic loss of ACE2 is associated with elevated ADAM17 activity prevented by AT1R blockade [39], while Ang-(1–7)/Mas signaling inhibits LPS-induced alveolar epithelial cell apoptosis by inhibiting LPS-induced shedding activity of ADAM17 [182]. Loss of ADAM17 suppressed Ang II-mediated migration and proliferation in VSMCs [183]. AT1R, promotes ADAM17-mediated ACE2 shedding in the brain of hypertensive patients, leading to a loss in compensatory activity during neurogenic hypertension [184].
5. Immune suppression therapy reduces Ang II-dependent inflammation and MAS
The above discussion suggests that the decrease of ACE2 is a major factor contributing to the pathogenesis of a variety of pathologies in the course of chronic or acute inflammation by permitting Ang II accumulation. In COVID-19 patients appeared to have elevated levels of plasma Ang II, which were in turn correlated with total viral load and degree of lung injury [185]. Accordingly, therapies aimed at increasing ACE2 expression might attenuate inflammation and can be used as a novel therapeutic tool. Since the downregulation of ACE2 appears to be a shared phenomenon in ARDS during viral or bacterial infection [7,24,25,29,32,186], the administration of the recombinant ACE2 or AT1R blockers can ameliorate coronavirus SARS-CoV-2 lung complications as well as have been reported for syncytial virus and H5N1 virus infection-induced lung injury [30,31,40].
AT1R blockers are among the most common medications used for the treatment of cardiovascular diseases with a high-security profile [187]. As mentioned earlier, in adults AT1R is expressed in cells of the immune system and in particular on macrophages, T, and B lymphocytes [141,162]. Ang II exerts pro-inflammatory responses mostly binding to AT1R [[188], [189], [190], [191]], and the treatment with one AT1R blocker efficiently prevented Ang II-inducing inflammation [[192], [193], [194], [195]]. AT1R inhibitors suppressed the expression of Ang II, IL6, and directly blocked AT1R, thus avoiding STAT3 activation [196]. Additionally, they suppress TNF-α synthesis in vitro and in vivo, see Fig. 1 [197] and are associated with a lower level of plasma TNF-α [198]. AT1R blockade produces a significant decrease in IFN-γ producing peripheral blood lymphocytes both a protein and IFN-γ mRNA levels [199]. Moreover, AT1R blockade attenuated end-organ damage [200].
Accumulating evidence suggests that AT1R inhibitors have potent anti-inflammatory actions not usually associated with the activation of the RAS system [[201], [202], [203], [204], [205]]. These drugs also can exhibit antioxidant effects [[205], [206], [207]]. Candesartan suppressed TNF-induced chemokine expression and NFκB activation, decreased reactive oxygen generation, and reinstated redox homeostasis [208]. redox-sensitive NF-kB-mediated inflammation has been described. The same drug diminished the TLR signaling pathway and the downstream effectors TNF-α, IL-1β, IL-6, and NF-Kb [203]. Amelioration of renal tissue inflammation with AT1R blockade was associated with a significant reduction of MCP-1 [189]. The peroxisome proliferator-activated receptor-gamma (PPARγ) also is involved in the anti-inflammatory effects of AT1R antagonists [204,209,210]. Importantly, a study demonstrated that the treatment with one ATR1 inhibitor induced the expression of FoxP3 in CD4+CD25+ T cells [211]. Therefore, the AT1R inhibitor administration has a profound impact on the immune response and the inhibition of monocyte mobilization from their reservoirs represents a powerful anti-inflammatory action that may have therapeutic implications [212].
With regards to the activation of pulmonary RAS influencing the pathogenesis of ARDS and SARS, three reports recommended the use of AT1R inhibitor or blocker to improve the quality of life and survival outcomes [[213], [214], [215]]. The chronic AT1R blockade also results in ACE2 upregulation in both animal models and humans [[216], [217], [218], [219], [220], [221]]. Unless ACE, ACE2 activity is unaffected by these drugs [222]. The AT1R blocker has been suggested by some researchers and reported to ameliorate coronavirus SARS-CoV-2 lung complications [7,223,224]. Human recombinant ACE2 also is a negative regulator of Ang II-induced deleterious effects [[225], [226], [227]]. It increased Ang 1–7 while lowered Ang II levels and reduced NADPH oxidase activity [228]. ACE2 administration also suppresses pulmonary arterial pressure and resistance improving lung compliance during acute hypoxia [229]. Moreover, the administration of recombinant ACE2 was well tolerated by healthy human subjects [41]. The neutralization of the 2019 novel coronavirus by the administration of ACE2 protein has been proposed as a therapeutic modality [230,231]. Recombinant ACE2 also remarkably rescued Ang II-induced hypertension, pathological hypertrophy, oxidant injury, and cardiac dysfunction [232,233]. Nevertheless, the efficacy of the recombinant ACE2 protein or AT1R blockers on lung diseases should be further tested in clinical settings.
One additional potential strategy for COVID-19 could be to suppress the rate of cleavage of ACE2 from the surface of lung epithelial cells leading to retention of the enzymatic activity and reduced Ang II. The evidence also suggests that the disruption of ADAM17 expression by siRNA reduces the ACE2 shedding [34]. Furthermore, ongoing research suggests that ADAM17 inhibitors had efficacy in some inflammatory conditions [[234], [235], [236]]. Preclinical trials using inhibitors of ADAM17 showed effectivity in mouse models of arthritis [237,238]. Such strategies in COVID-19 would need to account for the fact that ACE2 is cleaved at more than one site and that multiple enzymes appear to serve as sheddases in this process [225]. In addition, ADAM17 is involved in the regulation of multiple cellular processes, both pathological and normal [[239], [240], [241]]. Therefore, one of the biggest challenges in developing agents inhibiting ADAM17 is to attain selective inhibition of pathological processes or ACE2 shedding while sparing normal processes to avoid adverse effects. A member of the rhomboid family, iRhom2 is predominantly expressed in immune cells and iRhom2 is needed for transport of ADAM17 to the cell surface [[242], [243], [244]]. The inhibition of iRhom2 would lead to a selective deficiency of ADAM17 in immune cells with no effects on epithelia, representing a new perspective of ADAM17 blockade.
Similar to the AT1R blockade, the inhibition of NF-κB markedly attenuated the Ang II-induced inflammatory damage [245]. Upon infection, the role of Glucocorticoids (GCs) is to desensitize inflammation and to avoid an overshooting immune response, which may be detrimental for the organism. The GC's anti-inflammatory responses are in part, the result of interfering with the NF-κB signaling pathway, see Fig. 1 [246,247]. NF-kB signaling is essential for M1 macrophage polarization [248]. Thus, blocking such signaling can induce re-polarization from M1 to M2 [ [249]]. Studies have demonstrated that Dexamethasone can reduce NF-κB activity [139], and can efficiently down-regulates the TNF-α-induced IL-1β, and NF-κB -driven transcriptional expression of matrix metalloproteinases like MMP-1 and MMP-3, TNF-α, IL-6, IL-8, IL-1 and MCP1 [250,251]. An increasing number of GCs receptor-induced genes are now recognized as contributing to the anti-inflammatory effects of GCs [252,253]. Moreover, the GCs target like airway epithelium engage in cross-talk with immune cells [254], then they act via the GCs receptor in airway epithelial cells to repress inflammatory responses [255]. Taking into account that Ang II upregulation may initiate events leading to innate and acquired immune response resulting in cytokine storm in both MAS and COVID-19 patients, the use of GCs could protect against Ang II-induced end-organ damage in both pathologies. Central management of MAS is its early recognition, followed by prompt treatment. Interestingly, the commonest initial treatment for MAS is also, corticosteroids [256]. Collectively, the above information incriminates the use of GCs as a valuable tool in patients complicated with SARS-CoV-2.
A problem to consider with the use of GCs would be whether they increase CoV-2 virulence. In several infectious conditions, including mononucleosis, pneumococcal pneumonia, tuberculosis, typhoid fever, tetanus, and pneumocystis pneumonia, GCs administration improved patients' survival [257]. Some studies demonstrated that the use of these drugs did not increase the viral yield in Herpes Simplex Virus type 1 [258,259], and HIV-1 [260]. Direct repression of HIV transcription by GCs have been described in various works [261,262]. Also, meta-analyses report lower reintubation rates in neonates, children, and adults that received corticosteroids despite the infectious disease [263,264]. Thus, we suggest that GCs are strong candidates as a therapeutic agent for limiting coronavirus SARS-CoV-2 inflammatory complications and death.
Mesenchymal stem cell (MSC)-based gene therapy is a novel therapeutic approach for several diseases that currently have limited treatment options [265,266]. Importantly, MSCs can also act as a vehicle for delivering a protective gene by overexpressing a transgene at the injured site also promoting local tissue repair [267]. ACE2/Mas receptor is expressed in MSCs and Ang-(1–7) supports migratory function and stimulates vascular repair-relevant functions [268]. In an animal model of acute lung injury (ALI) and ARDS induced by lipopolysaccharide (LPS), MSCs treatment significantly reduced LPS-induced pulmonary inflammation [266]. Furthermore, the administration of MSCs overexpressing ACE2 resulted in a further improvement in the inflammatory response and pulmonary endothelial function of LPS-induced ALI mice [267] A recent in vivo study has demonstrated that MSCs can differentiate into lung epithelial cells [269,270] Also, MSCs may also exhibit immunosuppressive properties [266] A very recent study shown that the intravenous injection of MSCs significantly improved the inflammation situation and improves the outcome of patients with COVID-19 pneumonia [271]. Thus, based on its anti-inflammatory and repair properties we suggest implicating the MSCs based treatment for COVID-19 patients.
Another promising therapeutic molecule could be All-trans retinoic acid (atRA), the active metabolite of Vitamin A. It has been described that All-trans retinoic acid (atRA) suppresses AT1R at both mRNA and protein levels [ [272,273]]. Considering the above discussion about the involvement of Ang II/AT1R signaling in diabetes, atRA has proved to prevent the deleterious effects caused by hyperglycemia and Ang II [93]. As mentioned before about the NF-κB signaling, atRA tends to inhibit several inflammatory reactions by suppressing NF-κB-mediated gene expression of IL-6, IL-1β, TNF-α, and MCP-1 in vitro and in vivo see Fig. 1 [273,274]. Furthermore, Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis by inhibiting NF-κB signaling [275]. Thus, patients with DM may have an additional benefit with the use of this drug. Accordingly, the treatment with atRA showed an increase in gene and protein expressions of ACE2 in hypertensive rats [276]. All these results suggest that atRA could be an attractive candidate for the potential treatment of patients with coronavirus SARS-CoV-2.
The other drugs to be considered to treat SARS-CoV-2 infection would be the one that could modulate the levels of IL-1 and IL-6. Anakinra, a recombinant interleukin-1 (IL-1) receptor antagonist, has been used to treat a variety of autoinflammatory diseases [277]. Recently, a continuous intravenous Anakinra in a rapidly escalating dose regimen results in rapid serologic and clinical improvement in patients with MAS [278,279]. Moreover, the data from a phase 3 randomized controlled trial of Anakinra in sepsis, showed higher survival outcomes in patients with hyper-inflammation, without increased adverse events [280]. IL-6 antibody blocker and TNF-α-inhibiting agents have been reported to be effective in some MAS patients [281]. Tocilizumab is a recombinant humanized monoclonal antibody against the IL-6 receptor. Currently, a small sample clinical trial in China (ID: ChiCTR2000029765) has shown good efficacy in tocilizumab for SARS-CoV-2 [282].
6. Conclusion
ACE2 is a RAS component, widely distributed in almost all the organs. It plays a protective role mostly by counteracting the harmful effect of Ang II-induced inflammation. ACE2 being the receptor for SARS-CoV-2 and its wide distribution explains why some COVID-19 patients suffer from a variety of symptoms and potential complications. Ang II has important physiological effects in the immune response, particularly on the activation and the recruitment of monocytes/macrophages. Thus, the appearance of MAS during SARS-CoV-2 infection is an expected phenomenon that must be promptly identified and treated appropriately. We suggest strategies of treatments that mainly focus on reducing the Ang II-induced deleterious effects rather than attenuating virus replication. Thus, we aim that this review will contribute to the development of novel strategies to prevent and control the COVID-19 pandemic.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgements
No funding was received for this work. One of the authors Nehla Banu acknowledge CONACYT for PhD scholarship. Also, author Sandeep Surendra Panikar acknowledges DGAPA postdoctoral fellowship from UNAM.
References
- 1.Cheng H. Organ-protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020:1–5. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheblawi MM. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan Y. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.South A.M. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glowacka I. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010;84(2):1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuba K. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuba K. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie X. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kittana N. Angiotensin-converting enzyme 2–Angiotensin 1-7/1-9 system: novel promising targets for heart failure treatment. Fundamental & Clinical Pharmacology. 2018;32(1):14–25. doi: 10.1111/fcp.12318. [DOI] [PubMed] [Google Scholar]
- 11.Donoghue M. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 12.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Phys. Cell Phys. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg B. An ACE in the hole alternative pathways of the renin angiotensin system and their potential role in cardiac remodeling. J. Am. Coll. Cardiol. 2008;52(9):755–757. doi: 10.1016/j.jacc.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi T. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension. 2008;51(3):734–741. doi: 10.1161/HYPERTENSIONAHA.107.104299. [DOI] [PubMed] [Google Scholar]
- 15.Guang C. Three key proteases--angiotensin-I-converting enzyme (ACE), ACE2 and renin—within and beyond the renin-angiotensin system. Archives of Cardiovascular Diseases. 2012;105(6–7):373–385. doi: 10.1016/j.acvd.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel V.B. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes. 2016;65(1):85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGonagle D. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danilczyk U., Penninger J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006;98(4):463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 20.Kuba K. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ. J. 2013;77(2):301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- 21.Hamming I. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.X L. Extravascular sources of lung angiotensin peptide synthesis in idiopathic pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2006;(5):291. doi: 10.1152/ajplung.00432.2005. [DOI] [PubMed] [Google Scholar]
- 23.BD U. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr. Pharm. Des. 2007;13(12) doi: 10.2174/138161207780618885. [DOI] [PubMed] [Google Scholar]
- 24.R W. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am. J. Phys. 1999;276(5) doi: 10.1152/ajplung.1999.276.5.L885. [DOI] [PubMed] [Google Scholar]
- 25.Uhal B.D. Angiotensin signalling in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2012;44(3):465–468. doi: 10.1016/j.biocel.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46(3):239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 27.Jia H.P. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.B M. MicroRNome analysis unravels the molecular basis of SARS infection in bronchoalveolar stem cells. PLoS One. 2009;(11):4. doi: 10.1371/journal.pone.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai Y. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu H. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6:19840. doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Z. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GJ R.-P. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J. Mol. Med. 2012;90(6) doi: 10.1007/s00109-012-0859-2. (Berlin, Germany) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia H.P. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DW L. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;(34):280. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.H S. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008;105(22) doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.S H. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antivir. Res. 2010;85(3) doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.S E. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J. Am. Coll. Cardiol. 2008;(9):52. doi: 10.1016/j.jacc.2008.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.M S. Cell-specific functions of ADAM17 regulate the progression of thoracic aortic aneurysm. Circ. Res. 2018;(3):123. doi: 10.1161/CIRCRESAHA.118.313181. [DOI] [PubMed] [Google Scholar]
- 39.VB P. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J. Mol. Cell. Cardiol. 2014:66. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 40.A K A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Critical Care (London, England) 2017;21(1) doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.M H. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 2013;52(9) doi: 10.1007/s40262-013-0072-7. [DOI] [PubMed] [Google Scholar]
- 42.Gwathmey T.M. Angiotensin-(1-7)-ACE2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension. 2010;55(1):166. doi: 10.1161/HYPERTENSIONAHA.109.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gwathmey T.M. Review: novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012:R518–R530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uhal B.D. 2011. Regulation of Alveolar Epithelial Cell Survival by the ACE-2/Angiotensin 1–7/Mas Axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A M. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr. Pharm. Des. 2007;13(13) doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 46.A M.-K. Lisinopril ameliorates paraquat-induced lung fibrosis. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2006:367(1–2. doi: 10.1016/j.cca.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Y M. Perindopril and losartan attenuate bleomycin A5-induced pulmonary fibrosis in rats. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2008;28(6) [PubMed] [Google Scholar]
- 48.N C. Effect of losartan on lung fibrosis in neonatal rats with hyperoxia-induced chronic lung disease. Zhongguo dang dai er ke za zhi = Chinese Journal of Contemporary Pediatrics. 2007;9(6) [PubMed] [Google Scholar]
- 49.Ling T.-Y. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 2006;103(25):9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y. A novel subset of putative stem/progenitor CD34+Oct-4+ cells is the major target for SARS coronavirus in human lung. J. Exp. Med. 2007:2529–2536. doi: 10.1084/jem.20070462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.V S. Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am. J. Respir. Crit. Care Med. 2013;(6):187. doi: 10.1164/rccm.201205-0880OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.F L. The functional study of human umbilical cord mesenchymal stem cells harbouring angiotensin-converting enzyme 2 in rat acute lung ischemia-reperfusion injury model. Cell Biochem. Funct. 2014;32(7) doi: 10.1002/cbf.3054. [DOI] [PubMed] [Google Scholar]
- 53.Harmer D. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 54.Paizis G. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54(12):1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osterreicher C.H. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology (Baltimore, Md.) 2009;50(3):929–938. doi: 10.1002/hep.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci. Rep. 2016;6:21592. doi: 10.1038/srep21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajapaksha I.G. Liver-targeted angiotensin converting enzyme 2 therapy inhibits chronic biliary fibrosis in multiple drug-resistant gene 2-knockout mice. Hepatology Communications. 2019;3(12):1656–1673. doi: 10.1002/hep4.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao X. The ACE2/Ang-(1–7)/Mas axis can inhibit hepatic insulin resistance. Mol. Cell. Endocrinol. 2014;393(1):30–38. doi: 10.1016/j.mce.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 59.Mizuiri S. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 2008;51(4):613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 60.Niimi R. Suppression of endotoxin-induced renal tumor necrosis factor-alpha and interleukin-6 mRNA by renin-angiotensin system inhibitors. Jpn. J. Pharmacol. 2002;88(2):139–145. doi: 10.1254/jjp.88.139. [DOI] [PubMed] [Google Scholar]
- 61.Mezzano Sergio A. Angiotensin II and renal fibrosis. Hypertension. 2001;38(3):635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 62.Lorenzo O. Angiotensin II increases parathyroid hormone-related protein (PTHrP) and the type 1 PTH/PTHrP receptor in the kidney. J. Am. Soc. Nephrol. 2002;13(6):1595. doi: 10.1097/01.asn.0000015622.33198.bf. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz-Ortega M. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. 2002;62:S12–S22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu Z. Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab. Investig. 2012;92(5):650–661. doi: 10.1038/labinvest.2012.2. [DOI] [PubMed] [Google Scholar]
- 65.Iwai M., Horiuchi M. Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis. Hypertens. Res. 2009;32(7):533–536. doi: 10.1038/hr.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallagher P.E. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008;295(6):H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goulter A.B. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zisman L.S. Angiotensin-(1-7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation. 2003;108(14):1679–1681. doi: 10.1161/01.CIR.0000094733.61689.D4. [DOI] [PubMed] [Google Scholar]
- 69.Oudit G.Y. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007;75(1):29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Gurley S.B. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J. Clin. Invest. 2006;116(8):2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crackower M.A. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 72.Lovren F. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295(4):H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 73.Satou R. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr. Hypertens. Rep. 2018;20(12):100. doi: 10.1007/s11906-018-0900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huentelman M.J. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp. Physiol. 2005;90(5):783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 75.Loot A.E. Angiotensin-(1-7) attenuates the development of heart failure after myocardial infarction in rats. Circulation. 2002;105(13):1548–1550. doi: 10.1161/01.cir.0000013847.07035.b9. [DOI] [PubMed] [Google Scholar]
- 76.Xia H. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ. Res. 2013;113(9):1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doobay M.F. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mecca A.P. Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke. Exp. Physiol. 2011;96(10):1084–1096. doi: 10.1113/expphysiol.2011.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hammer A. Role of the receptor Mas in macrophage-mediated inflammation in vivo. Proc. Natl. Acad. Sci. 2016;113(49) doi: 10.1073/pnas.1612668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kovacs A. ACE2 drives dendritic cell function and neuroantigen specific immune responses. Brain Behav. Immun. 2013;29:S19. [Google Scholar]
- 81.Feng Y. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ. Res. 2010;106(2):373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sriramula S. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension. 2015;65(3):577–586. doi: 10.1161/HYPERTENSIONAHA.114.04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia H. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamada K. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer's disease. Brain Res. 2010;1352:176–186. doi: 10.1016/j.brainres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Hashimoto T. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J. 2020. ACE2 Expression by Colonic Epithelial Cells Is Associated With Viral Infection, Immunity and Energy Metabolism. [Google Scholar]
- 87.Cooper H.S. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 88.Ghosh H.S. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5(2):e9199–e. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Das U.N. Renin-angiotensin-aldosterone system in insulin resistance and metabolic syndrome. J Transl Int Med. 2016;4(2):66–72. doi: 10.1515/jtim-2016-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabandugama P.K. The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin North Am. 2017;101(1):129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong T.P. Upregulation of ACE2-ANG-(1–7)-Mas axis in jejunal enterocytes of type 1 diabetic rats: implications for glucose transport. American Journal of Physiology-Endocrinology and Metabolism. 2012;303(5):E669–E681. doi: 10.1152/ajpendo.00562.2011. [DOI] [PubMed] [Google Scholar]
- 92.Shi T.T. Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic β-cells. Biochem. Biophys. Res. Commun. 2018;495(1):860–866. doi: 10.1016/j.bbrc.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 93.Guleria R.S. Retinoic acid receptor-mediated signaling protects cardiomyocytes from hyperglycemia induced apoptosis: role of the renin-angiotensin system. J. Cell. Physiol. 2011;226(5):1292–1307. doi: 10.1002/jcp.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collado A. Functional role of endothelial CXCL16/CXCR6-platelet-leucocyte axis in angiotensin II-associated metabolic disorders. Cardiovasc. Res. 2018;114(13):1764–1775. doi: 10.1093/cvr/cvy135. [DOI] [PubMed] [Google Scholar]
- 95.Kawai T. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017;125(Pt A):4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nehme A. Atlas of tissue renin-angiotensin-aldosterone system in human: a transcriptomic meta-analysis. Sci. Rep. 2015;5 doi: 10.1038/srep10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi R.Z. Angiotensin II induces vascular endothelial growth factor synthesis in mesenchymal stem cells. Exp. Cell Res. 2009;315(1):10–15. doi: 10.1016/j.yexcr.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 98.Haznedaroglu I.C., Malkan U.Y. Local bone marrow renin-angiotensin system in the genesis of leukemia and other malignancies. Eur. Rev. Med. Pharmacol. Sci. 2016;20(19):4089–4111. [PubMed] [Google Scholar]
- 99.Lin C. Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2011;25(4):1145–1155. doi: 10.1096/fj.10-169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azizi M. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J. Clin. Invest. 1996;97(3):839–844. doi: 10.1172/JCI118484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Genevay M.C. The synthetic tetrapeptide AcSDKP protects cells that reconstitute long-term bone marrow stromal cultures from the effects of mafosfamide (Asta Z 7654) Exp. Hematol. 1996;24(1):77–81. [PubMed] [Google Scholar]
- 102.Haznedaroglu I.C. Thrombopoietin as a drug: biologic expectations, clinical realities, and future directions. Clin. Appl. Thromb. Hemost. 2002;8(3):193–212. doi: 10.1177/107602960200800301. [DOI] [PubMed] [Google Scholar]
- 103.Ishikawa M. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2007;292(5):H2306–H2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- 104.Jeannin P. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 2018;285(4):680–699. doi: 10.1111/febs.14343. [DOI] [PubMed] [Google Scholar]
- 105.Kim S. Angiotensin II regulation of proliferation, differentiation, and engraftment of hematopoietic stem cells. Hypertension. 2016;67(3):574–584. doi: 10.1161/HYPERTENSIONAHA.115.06474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thatcher S. Deficiency of ACE2 in bone-marrow-derived cells increases expression of TNF-α in adipose stromal cells and augments glucose intolerance in obese C57BL/6 mice. Int. J. Hypertens. 2012;2012 doi: 10.1155/2012/762094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thatcher Sean E. Angiotensin-converting enzyme 2 deficiency in whole body or bone marrow–derived cells increases atherosclerosis in low-density lipoprotein receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 2011;31(4):758–765. doi: 10.1161/ATVBAHA.110.221614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bracaglia C. Macrophage Activation Syndrome: different mechanisms leading to a one clinical syndrome. Pediatric Rheumatology Online Journal. 2017;15(1):5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ravelli A. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J. Pediatr. 2005;146(5):598–604. doi: 10.1016/j.jpeds.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 110.Carter S.J. Macrophage activation syndrome in adults: recent advances in pathophysiology, diagnosis and treatment. Rheumatology. 2018;58(1):5–17. doi: 10.1093/rheumatology/key006. [DOI] [PubMed] [Google Scholar]
- 111.Ramos-Casals M. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 112.Dunmire S.K. Primary EBV infection induces an expression profile distinct from other viruses but similar to hemophagocytic syndromes. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weiss E.S. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131(13):1442–1455. doi: 10.1182/blood-2017-12-820852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syndrome: a distinct entity leading to early death in sepsis. Front. Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.P M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395(10229) doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poyiadji N. 2020. COVID-19–Associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lerkvaleekul B., Vilaiyuk S. Macrophage activation syndrome: early diagnosis is key. Open access Rheumatology: Research and Reviews. 2018;10:117–128. doi: 10.2147/OARRR.S151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crayne C.B. The immunology of macrophage activation syndrome. Front. Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aulagnon F. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am. J. Kidney Dis. 2015;65(6):851–859. doi: 10.1053/j.ajkd.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Kim M.M. Central nervous system (CNS) involvement is a critical prognostic factor for hemophagocytic lymphohistiocytosis. Korean J Hematol. 2012;47(4):273–280. doi: 10.5045/kjh.2012.47.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sakumura N. Soluble CD163, a unique biomarker to evaluate the disease activity, exhibits macrophage activation in systemic juvenile idiopathic arthritis. Cytokine. 2018;110:459–465. doi: 10.1016/j.cyto.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 123.Zhang H. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leuschner F. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 2010;107(11):1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Carvalho Santuchi M. Angiotensin-(1-7) and alamandine promote anti-inflammatory response in macrophages in vitro and in vivo. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/2401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xie P. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Renal Physiol. 2014:F1260–F1274. doi: 10.1152/ajprenal.00069.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agarwal D. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3β in neuronal culture. Br. J. Pharmacol. 2013;169(4):860–874. doi: 10.1111/bph.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.A O. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J. Hypertens. 1999;17(4) doi: 10.1097/00004872-199917040-00012. [DOI] [PubMed] [Google Scholar]
- 129.Potter D.D. 1998. Evidence that Macrophages in Atherosclerotic Lesions Contain Angiotensin II. [DOI] [PubMed] [Google Scholar]
- 130.Swirski F.K. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.K U. Angiotensin II induces migration and Pyk2/paxillin phosphorylation of human monocytes. Hypertension (Dallas, Tex.: 1979) 2001;37(2 Pt 2) doi: 10.1161/01.hyp.37.2.587. [DOI] [PubMed] [Google Scholar]
- 132.Ziai F. Renal allograft protection with losartan in Fisher→Lewis rats: hemodynamics, macrophages, and cytokines. Kidney Int. 2000;57(6):2618–2625. doi: 10.1046/j.1523-1755.2000.00122.x. [DOI] [PubMed] [Google Scholar]
- 133.J S. Mas receptor deficiency augments angiotensin II-induced atherosclerosis and aortic aneurysm ruptures in hypercholesterolemic male mice. J. Vasc. Surg. 2019;70(5) doi: 10.1016/j.jvs.2018.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.H C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223) doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.G C. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5) doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gong J. 2020. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients with COVID-19 Pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Q C. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jurewicz M. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II–induced inflammation. J. Am. Soc. Nephrol. 2007;18(4):1093. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 139.Muller D.N. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am. J. Pathol. 2002;161(5):1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rupérez M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108(12):1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 141.Jia L. Angiotensin II induces inflammation leading to cardiac remodeling. Frontiers in Bioscience (Landmark Edition) 2012;17:221–231. doi: 10.2741/3923. [DOI] [PubMed] [Google Scholar]
- 142.Kratochvill F. TNF counterbalances the emergence of M2 tumor macrophages. Cell Rep. 2015;12(11):1902–1914. doi: 10.1016/j.celrep.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.A N. Effect of beta(2)-adrenoceptor activation and angiotensin II on tumour necrosis factor and interleukin 6 gene transcription in the rat renal resident macrophage cells. Cytokine. 1999;11(10) doi: 10.1006/cyto.1999.0488. [DOI] [PubMed] [Google Scholar]
- 145.Ferreri N.R. Angiotensin II Induces TNF Production by the Thick Ascending Limb: Functional Implications. 1998. [DOI] [PubMed]
- 146.Abel S. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J. Immunol. 2004;172(10):6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 147.Veinotte L. CXCL16-positive dendritic cells enhance invariant natural killer T cell-dependent IFNγ production and tumor control. Oncoimmunology. 2016;5(6) doi: 10.1080/2162402X.2016.1160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Keidar S. ACE2 activity is increased in monocyte-derived macrophages from prehypertensive subjects. Nephrol. Dial. Transplant. 2007;22(2):597–601. doi: 10.1093/ndt/gfl632. [DOI] [PubMed] [Google Scholar]
- 149.Dewald O. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 2005;96(8):881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 150.Mateo T. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J. Immunol. 2006;176(9):5577. doi: 10.4049/jimmunol.176.9.5577. [DOI] [PubMed] [Google Scholar]
- 151.Zhou Z, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. [DOI] [PMC free article] [PubMed]
- 152.G W. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J. Clin. Invest. 1997;100(5) doi: 10.1172/JCI119615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.M R.-O. Proinflammatory actions of angiotensins. Curr. Opin. Nephrol. Hypertens. 2001;10(3) doi: 10.1097/00041552-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 154.Ito Y. Fractalkine expression and the recruitment of CX3CR1+ cells in the prolonged mesangial proliferative glomerulonephritis. Kidney Int. 2002;61(6):2044–2057. doi: 10.1046/j.1523-1755.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 155.Guo R.-W. Angiotensin II induces NF-κB activation in HUVEC via the p38MAPK pathway. Peptides. 2006;27(12):3269–3275. doi: 10.1016/j.peptides.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 156.MC T. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ. Res. 2010;107(7) doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- 157.H A. Angiotensin II AT1 receptor blockade decreases brain artery inflammation in a stress-prone rat strain. Ann. N. Y. Acad. Sci. 2004;1018 doi: 10.1196/annals.1296.043. [DOI] [PubMed] [Google Scholar]
- 158.A R. Effect of angiotensin II antagonism on the regression of kidney disease in the rat. Kidney Int. 2002;62(3) doi: 10.1046/j.1523-1755.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- 159.WB S. Angiotensin II AT1-receptor blockade inhibits monocyte activation and adherence in transgenic (mRen2)27 rats. J. Cardiovasc. Pharmacol. 1999;33(3) doi: 10.1097/00005344-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 160.Menk M. Angiotensin II type 2 receptor agonist compound 21 attenuates pulmonary inflammation in a model of acute lung injury. J. Inflamm. Res. 2018;11:169–178. doi: 10.2147/JIR.S160573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rathinasabapathy A. vol. 9. 2018. The Selective Angiotensin II Type 2 Receptor Agonist, Compound 21, Attenuates the Progression of Lung Fibrosis and Pulmonary Hypertension in an Experimental Model of Bleomycin-Induced Lung Injury. (180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yanagitani Y. 1999. Angiotensin II Type 1 Receptor–Mediated Peroxide Production in Human Macrophages. [DOI] [PubMed] [Google Scholar]
- 163.NE H. Regulation of T-cell function by endogenously produced angiotensin II. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2009;296(2) doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jackson S.H. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004;5(8):818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 165.A C. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296(6) doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 166.J P. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J. Biol. Chem. 2010;285(1) doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.D D. Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide. EMBO Molecular Medicine. 2012;4(5) doi: 10.1002/emmm.201200217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.A C. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J. Exp. Med. 2010;207(8) doi: 10.1084/jem.20092366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.M C. The lack of ADAM17 activity during embryonic development causes hemorrhage and impairs vessel formation. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.J S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8) doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 171.Y L. ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood. 2006;108(7) doi: 10.1182/blood-2006-02-005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.JH B. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 2007;82(1) doi: 10.1189/jlb.0307193. [DOI] [PubMed] [Google Scholar]
- 173.RR K. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood. 2009;113(19) doi: 10.1182/blood-2008-04-152330. [DOI] [PubMed] [Google Scholar]
- 174.N S. Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1. Cellular and Molecular Life Sciences: CMLS. 2010;67(24) doi: 10.1007/s00018-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Driscoll W.S. 2013. Macrophage ADAM17 Deficiency Augments CD36-Dependent Apoptotic Cell Uptake and the Linked Anti-Inflammatory Phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.J T. Neutrophil and macrophage cell surface colony-stimulating factor 1 shed by ADAM17 drives mouse macrophage proliferation in acute and chronic inflammation. Mol. Cell. Biol. 2018;38(17) doi: 10.1128/MCB.00103-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.NM N. Ron receptor tyrosine kinase negatively regulates TNFalpha production in alveolar macrophages by inhibiting NF-kappaB activity and Adam17 production. Shock (Augusta, Ga.) 2010;33(2) doi: 10.1097/SHK.0b013e3181ae8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.N O. ADAM-9, ADAM-15, and ADAM-17 are upregulated in macrophages in advanced human atherosclerotic plaques in aorta and carotid and femoral arteries—Tampere vascular study. Ann. Med. 2009;41(4) doi: 10.1080/07853890802649738. [DOI] [PubMed] [Google Scholar]
- 179.Ohtsu H. 2006. ADAM17 Mediates Epidermal Growth Factor Receptor Transactivation and Vascular Smooth Muscle Cell Hypertrophy Induced by Angiotensin II. [DOI] [PubMed] [Google Scholar]
- 180.SY Z. Upregulation of Nox4 promotes angiotensin II-induced epidermal growth factor receptor activation and subsequent cardiac hypertrophy by increasing ADAM17 expression. The Canadian Journal of Cardiology. 2013;29(10) doi: 10.1016/j.cjca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 181.Oikawa H. 2020. A Disintegrin and Metalloproteinase 17 (ADAM17) Mediates Epidermal Growth Factor Receptor Transactivation by Angiotensin II on Hepatic Stellate Cells. undefined. [DOI] [PubMed] [Google Scholar]
- 182.X M. Angiotensin-(1-7)/Mas signaling inhibits lipopolysaccharide-induced ADAM17 shedding activity and apoptosis in alveolar epithelial cells. Pharmacology. 2016;97(1–2) doi: 10.1159/000441606. [DOI] [PubMed] [Google Scholar]
- 183.M S. Loss of smooth muscle cell disintegrin and metalloproteinase 17 transiently suppresses angiotensin II-induced hypertension and end-organ damage. J. Mol. Cell. Cardiol. 2017:103. doi: 10.1016/j.yjmcc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 184.Xu J. 2017. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Y L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3) doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu Q. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discovery. 2017;3 doi: 10.1038/celldisc.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chamsi-Pasha M.A.R. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Current Heart Failure Reports. 2014;11(1):58–63. doi: 10.1007/s11897-013-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xianwei W. Cross-Talk Between Inflammation and Angiotensin II: Studies Based on Direct Transfection of Cardiomyocytes With AT1R and AT2R cDNA. 2012. [DOI] [PubMed]
- 189.ND V. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J. Pharmacol. Exp. Ther. 2007;323(1) doi: 10.1124/jpet.107.123638. [DOI] [PubMed] [Google Scholar]
- 190.J R. Role of angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015:124. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.P M. Effect of asiatic acid on the Ang II-AT 1 R-NADPH oxidase-NF-κB pathway in renovascular hypertensive rats. Naunyn Schmiedeberg's Arch. Pharmacol. 2017;390(10) doi: 10.1007/s00210-017-1408-x. [DOI] [PubMed] [Google Scholar]
- 192.Nagai N. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest. Ophthalmol. Vis. Sci. 2020;46(8):2925–2931. doi: 10.1167/iovs.04-1476. [DOI] [PubMed] [Google Scholar]
- 193.Liu T.J. AT1R blocker losartan attenuates intestinal epithelial cell apoptosis in a mouse model of Crohn's disease. Mol. Med. Rep. 2020;13(2):1156–1162. doi: 10.3892/mmr.2015.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Y C. Inhibition of angiotension II type 1 receptor reduced human endothelial inflammation induced by low shear stress. Exp. Cell Res. 2017;360(2) doi: 10.1016/j.yexcr.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 195.Lanz T.V. 2010. Angiotensin II Sustains Brain Inflammation in Mice Via TGF-β. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kurihara T. Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest. Ophthalmol. Vis. Sci. 2020;47(12):5545–5552. doi: 10.1167/iovs.06-0478. [DOI] [PubMed] [Google Scholar]
- 197.Fukuzawa M. Angiotensin converting enzyme inhibitors suppress production of tumor necrosis factor-alpha in vitro and in vivo. Immunopharmacology. 1997;36(1):49–55. doi: 10.1016/s0162-3109(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 198.Stenvinkel P. Do ACE-inhibitors suppress tumour necrosis factor-α production in advanced chronic renal failure? J. Intern. Med. 1999;246(5):503–507. doi: 10.1046/j.1365-2796.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 199.Weidanz J.O.N.A. AT1R blockade reduces IFN-γ production in lymphocytes in vivo and in vitro. Kidney Int. 2005;67(6):2134–2142. doi: 10.1111/j.1523-1755.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 200.Müller D.N. Angiotensin II (AT(1)) receptor blockade reduces vascular tissue factor in angiotensin II-induced cardiac vasculopathy. Am. J. Pathol. 2000;157(1):111–122. doi: 10.1016/S0002-9440(10)64523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.RE S. Additional antiproteinuric effect of ultrahigh dose candesartan: a double-blind, randomized, prospective study. Journal of the American Society of Nephrology: JASN. 2005;16(10) doi: 10.1681/ASN.2005020138. [DOI] [PubMed] [Google Scholar]
- 202.SO T. Candesartan prevents long-term impairment of renal function in response to neonatal partial unilateral ureteral obstruction. American Journal of Physiology. Renal Physiology. 2007;292(2) doi: 10.1152/ajprenal.00241.2006. [DOI] [PubMed] [Google Scholar]
- 203.W B. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur. J. Pharmacol. 2014:724. doi: 10.1016/j.ejphar.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 204.PB S. Telmisartan attenuated LPS-induced Neuroinflammation in human IMR-32 neuronal cell line via SARM in AT1R independent mechanism. Life Sci. 2015:130. doi: 10.1016/j.lfs.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 205.T P. Telmisartan directly ameliorates the neuronal inflammatory response to IL-1β partly through the JNK/c-Jun and NADPH oxidase pathways. J. Neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.T S. Candesartan reduces superoxide production after global cerebral ischemia. Neuroreport. 2005;16(4) doi: 10.1097/00001756-200503150-00004. [DOI] [PubMed] [Google Scholar]
- 207.T N. Role of renin-angiotensin system and nuclear factor-kappaB in the obstructed kidney of rats with unilateral ureteral obstruction. Jpn. J. Pharmacol. 2002;90(4) doi: 10.1254/jjp.90.361. [DOI] [PubMed] [Google Scholar]
- 208.Chen S. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74(9):1128–1138. doi: 10.1038/ki.2008.380. [DOI] [PubMed] [Google Scholar]
- 209.T P. Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor-γ activation in human monocytes. J. Hypertens. 2012;30(1) doi: 10.1097/HJH.0b013e32834dde5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.J A. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertension Research: Official Journal of the Japanese Society of Hypertension. 2010;33(8) doi: 10.1038/hr.2010.79. [DOI] [PubMed] [Google Scholar]
- 211.P M. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(35) doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gao X.M. Inhibition of the Renin-Angiotensin System Post Myocardial Infarction Prevents Inflammation-Associated Acute Cardiac Rupture. Cardiovasc. Drugs Ther. 2017;31(2):145–156. doi: 10.1007/s10557-017-6717-2. [DOI] [PubMed] [Google Scholar]
- 213.Sun M.L. Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E014. doi: 10.3760/cma.j.issn.1001-0939.2020.0014. [DOI] [PubMed] [Google Scholar]
- 214.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020:1–7. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ishiyama Y. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 217.Ishiyama Y. 2004. Upregulation of Angiotensin-Converting Enzyme 2 After Myocardial Infarction by Blockade of Angiotensin II Receptors. [DOI] [PubMed] [Google Scholar]
- 218.Patel V.B. 2012. Cardioprotective Effects Mediated by Angiotensin II Type 1 Receptor Blockade and Enhancing Angiotensin 1–7 in Experimental Heart Failure in Angiotensin-Converting Enzyme 2–Null Mice. [DOI] [PubMed] [Google Scholar]
- 219.Upregulation of Angiotensin-Converting Enzyme (ACE) 2 in Hepatic Fibrosis by ACE Inhibitors - Huang - 2010 - Clinical and Experimental Pharmacology and Physiology. Wiley Online Library; 2020. [DOI] [PubMed] [Google Scholar]
- 220.S K. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ. Res. 2005;97(9) doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 221.CS L. Regulation of angiotensin converting enzyme II by angiotensin peptides in human cardiofibroblasts. Peptides. 2010;31(7) doi: 10.1016/j.peptides.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 222.Turner A.J., Hooper N.M. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol. Sci. 2002;23(4):177–183. doi: 10.1016/s0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- 223.Vaduganathan M. Renin–Angiotensin–Aldosterone System Inhibitors in Patients With Covid-19. 2020. [DOI] [PMC free article] [PubMed]
- 224.J G. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Oudit G.Y., Penninger J.M. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Current Heart Failure Reports. 2011;8(3):176–183. doi: 10.1007/s11897-011-0063-7. [DOI] [PubMed] [Google Scholar]
- 226.EH B. Murine recombinant angiotensin-converting enzyme 2 attenuates kidney injury in experimental Alport syndrome. Kidney Int. 2017;91(6) doi: 10.1016/j.kint.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 227.Treml B. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit. Care Med. 2010;38(2):596–601. doi: 10.1097/CCM.0b013e3181c03009. [DOI] [PubMed] [Google Scholar]
- 228.Oudit G.Y. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.A K. Recombinant angiotensin-converting enzyme 2 suppresses pulmonary vasoconstriction in acute hypoxia. Wilderness & Environmental Medicine. 2012;23(1) doi: 10.1016/j.wem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 230.RL K. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020:9. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lei C. 2020. Potent Neutralization of 2019 Novel Coronavirus by Recombinant ACE2-Ig. [Google Scholar]
- 232.Zhong J. 2010. Angiotensin-Converting Enzyme 2 Suppresses Pathological Hypertrophy, Myocardial Fibrosis, and Cardiac Dysfunction. [DOI] [PubMed] [Google Scholar]
- 233.Z J. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension (Dallas, Tex.: 1979) 2011;57(2) doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- 234.JG C. Inhibition of tumor necrosis factor-alpha (TNF-alpha) production and arthritis in the rat by GW3333, a dual inhibitor of TNF-alpha-converting enzyme and matrix metalloproteinases. J. Pharmacol. Exp. Ther. 2001;298(3) [PubMed] [Google Scholar]
- 235.Wong E. Harnessing the natural inhibitory domain to control TNFα converting enzyme (TACE) activity in vivo. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep35598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Moss M.L. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2008;4(6):300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 237.ML M. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2008;4(6) doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 238.A J., E C. ADAM17 as a therapeutic target in multiple diseases. Curr. Pharm. Des. 2009;15(20) doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 239.Moss M.L., Minond D. Recent advances in ADAM17 research: a promising target for cancer and inflammation. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/9673537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.S U. ADAM17 regulates IL-1 signaling by selectively releasing IL-1 receptor type 2 from the cell surface. Cytokine. 2015;71(2) doi: 10.1016/j.cyto.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 241.Y O. TACE cleaves neogenin to desensitize cortical neurons to the repulsive guidance molecule. Neurosci. Res. 2011;71(1) doi: 10.1016/j.neures.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 242.Adrain C. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335(6065):225–228. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.McIlwain D.R. iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science. 2012;335(6065):229–232. doi: 10.1126/science.1214448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.R-J S. ADAM17, shedding, TACE as therapeutic targets. Pharmacol. Res. 2013:71. doi: 10.1016/j.phrs.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 245.Muller D.N. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35(1):193–201. doi: 10.1161/01.hyp.35.1.193. Pt 2. [DOI] [PubMed] [Google Scholar]
- 246.Nissen R.M., Yamamoto K.R. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14(18):2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.De Bosscher K. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr. Rev. 2003;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 248.Jin X. Advanced glycation end products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κB pathway. Biomed. Res. Int. 2015;2015:732450. doi: 10.1155/2015/732450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jang S.E. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J. Appl. Microbiol. 2013;115(3):888–896. doi: 10.1111/jam.12273. [DOI] [PubMed] [Google Scholar]
- 250.Gossye V. Differential mechanism of NF-kappaB inhibition by two glucocorticoid receptor modulators in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009;60(11):3241–3250. doi: 10.1002/art.24963. [DOI] [PubMed] [Google Scholar]
- 251.Barnes P.J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 252.Vandevyver S. New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 2013;154(3):993–1007. doi: 10.1210/en.2012-2045. [DOI] [PubMed] [Google Scholar]
- 253.Vandevyver S. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J. Clin. Invest. 2012;122(6):2130–2140. doi: 10.1172/JCI60006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Walsh G.M. Corticosteroids, eosinophils and bronchial epithelial cells: new insights into the resolution of inflammation in asthma. J. Endocrinol. 2003;178(1):37–43. doi: 10.1677/joe.0.1780037. [DOI] [PubMed] [Google Scholar]
- 255.Zhang N. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J. Immunol. 2007;179(1):578–589. doi: 10.4049/jimmunol.179.1.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sen E.S. Macrophage activation syndrome. Indian J. Pediatr. 2016;83(3):248–253. doi: 10.1007/s12098-015-1877-1. [DOI] [PubMed] [Google Scholar]
- 257.Petta I. The interactome of the glucocorticoid receptor and its influence on the actions of glucocorticoids in combatting inflammatory and infectious diseases. Microbiol. Mol. Biol. Rev. 2016;80(2):495–522. doi: 10.1128/MMBR.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Notter M.F.D., Docherty J.J. Steroid hormone alteration of herpes simplex virus type 1 replication. J. Med. Virol. 1978;2(3):247–252. doi: 10.1002/jmv.1890020308. [DOI] [PubMed] [Google Scholar]
- 259.Erlandsson A.C. Herpes simplex virus type 1 infection and glucocorticoid treatment regulate viral yield, glucocorticoid receptor and NF-kappaB levels. J. Endocrinol. 2002;175(1):165–176. doi: 10.1677/joe.0.1750165. [DOI] [PubMed] [Google Scholar]
- 260.Kino T. Glucocorticoids suppress human immunodeficiency virus type-1 long terminal repeat activity in a cell type-specific, glucocorticoid receptor-mediated fashion: direct protective effects at variance with clinical phenomenology. J. Steroid Biochem. Mol. Biol. 2000;75(4–5):283–290. doi: 10.1016/s0960-0760(00)00187-4. [DOI] [PubMed] [Google Scholar]
- 261.Hanley T.M., Viglianti G.A. Nuclear receptor signaling inhibits HIV-1 replication in macrophages through multiple trans-repression mechanisms. J. Virol. 2011;85(20):10834–10850. doi: 10.1128/JVI.00789-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Alvarez-Carbonell D. The glucocorticoid receptor is a critical regulator of HIV latency in human microglial cells. J. NeuroImmune Pharmacol. 2019;14(1):94–109. doi: 10.1007/s11481-018-9798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Markovitz B.P. Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Cochrane Database Syst. Rev. 2008;2:Cd001000. doi: 10.1002/14651858.CD001000.pub2. [DOI] [PubMed] [Google Scholar]
- 264.McCaffrey J. Corticosteroids to prevent extubation failure: a systematic review and meta-analysis. Intensive Care Med. 2009;35(6):977–986. doi: 10.1007/s00134-009-1473-9. [DOI] [PubMed] [Google Scholar]
- 265.Liu Q. Mesenchymal stem cells modified with angiotensin-converting enzyme 2 are superior for amelioration of glomerular fibrosis in diabetic nephropathy. Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108093. [DOI] [PubMed] [Google Scholar]
- 266.Mei S.H.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4(9):e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.He H. Mesenchymal stem cells overexpressing angiotensin-converting enzyme 2 rescue lipopolysaccharide-induced lung injury. Cell Transplant. 2015;24(9):1699–1715. doi: 10.3727/096368914X685087. [DOI] [PubMed] [Google Scholar]
- 268.Singh N. ACE2/Ang-(1-7)/Mas axis stimulates vascular repair-relevant functions of CD34+ cells. Am. J. Physiol. Heart Circ. Physiol. 2015;309(10):H1697–H1707. doi: 10.1152/ajpheart.00854.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ortiz L.A. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. U. S. A. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Grove J.E. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am. J. Respir. Cell Mol. Biol. 2002;27(6):645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- 271.Leng Z. Transplantation of ACE2(−) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Takeda K. Downregulation of angiotensin II type 1 receptor by all-trans retinoic acid in vascular smooth muscle cells. Hypertension. 2000;35(1):297–302. doi: 10.1161/01.hyp.35.1.297. Pt 2. [DOI] [PubMed] [Google Scholar]
- 273.Pan J. Molecular mechanisms of retinoid receptors in diabetes-induced cardiac remodeling. J. Clin. Med. 2014;3(2):566–594. doi: 10.3390/jcm3020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Datta P.K., Lianos E.A. Retinoic acids inhibit inducible nitric oxide synthase expression in mesangial cells. Kidney Int. 1999;56(2):486–493. doi: 10.1046/j.1523-1755.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 275.Nizamutdinova I.T. Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-κB signaling pathway. J. Cell. Physiol. 2013;228(2):380–392. doi: 10.1002/jcp.24142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhong J.C. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44(6):907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- 277.Jang Y. Cerebral autoinflammatory disease treated with anakinra. Annals of Clinical and Translational Neurology. 2018;5(11):1428–1433. doi: 10.1002/acn3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Adam Monteagudo L. Continuous intravenous anakinra infusion to calm the Cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020;2(5):276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sönmez H.E. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin. Rheumatol. 2018;37(12):3329–3335. doi: 10.1007/s10067-018-4095-1. [DOI] [PubMed] [Google Scholar]
- 280.Shakoory B. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit. Care Med. 2016;44(2):275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Schulert G.S., Grom A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu. Rev. Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McCreary E., Pogue J. COVID-19 treatment: a review of early and emerging options. Open Forum Infectious Diseases. 2020:7. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]