Abstract
Novel coronavirus disease 2019 (COVID-19) is a positive-sense single-stranded RNA virus which belongs to the Coronaviridae family. COVID-19 outbreak became evident after the severe acute respiratory syndrome coronavirus and the Middle East respiratory syndrome coronavirus in the twenty-first century as the start of the third deadly coronavirus. Currently, research is at an early stage, and the exact etiological dimensions of COVID-19 are unknown. Several candidate drugs and plasma therapy have been considered and evaluated for the treatment of severe COVID-19 patients. These include clinically available drugs such as chloroquine, hydroxychloroquine, and lopinavir/ritonavir. However, understanding the pathogenic mechanisms of this virus is critical for predicting interaction with humans. Based on recent evidence, we have summarized the current virus biology in terms of the possible understanding of the various pathophysiologies, molecular mechanisms, recent efficient diagnostics, and therapeutic approaches to control the disease. In addition, we briefly reviewed the biochemistry of leading candidates for novel therapies and their current status in clinical trials. As information from COVID-19 is evolving rapidly, this review will help the researcher to consider new insights and potential therapeutic approaches based on up-to-date knowledge. Finally, this review illustrates a list of alternative therapeutic solutions for a viral infection.
Keywords: Coronavirus, COVID-19, Diagnosis, Therapeutic, Molecular mechanism
Introduction
A pneumonia outbreak with uncertain etiology occurred in Wuhan, Province of Hubei, in the last week of December 2019 is severely impending for human health worldwide (Li et al. 2020b). Later on January 7, 2020, the Chinese administration announced the naming of a new form of coronavirus-2 (SARS-CoV-2) that belongs to the Coronaviridae family (Xiao et al. 2020). It is noteworthy that SARS-CoV-2 is 96% genetically similar to a bat coronavirus at the whole genome level (Zhou et al. 2020). Coronavirus disease (COVID-19) is an extreme acute respiratory syndrome with the most common symptoms of moderate infection include fever, dry cough, tiredness, sore throat or dyspnea. Patients infected with this virus suffer from other potential damage to vital organs, such as gastrointestinal, cardiac, renal, and nervous systems (Gavriatopoulou et al. 2020; Merad and Martin 2020; Rohollah et al. 2020; Bhaskar et al. 2020). Recent knowledge indicated that the SARS-CoV-2 transmission rate is higher than SRAS-CoV due to the protein structure's genetic makeup. Before COVID-19, many infectious diseases affected global populations, including plague, Spanish flu, cholera, Swine flu (H1N1), severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) (Bramanti et al. 2019; Taubenberger and Morens 2006; Chowdhury et al. 2017; Ratre et al. 2020; Luk et al. 2019).
Till now, there are no effective vaccines available to prevent the spread of COVID-19 although many clinical trials of vaccines are underway (Verma et al. 2020b). SARS-CoV-2 has now spread to more than 205 other nations, including the United States, India, Russia, Brazil, Italy, Spain, Japan, Korea, Iran and Germany. In March, the WHO announced that COVID-19 was a pandemic and a significant health issue, causing severe infections in the respiratory tract (Cucinotta and Vanelli 2020; Mahase 2020). According to the latest statistics, more than 50 million people have been infected worldwide up to 10 December 2020, and more than 10, 35, 000 were died because of this disease. Every day, the number of infected people are growing worldwide. India has 95,53,100 cases registered to date. Fortunately, the death rate is very low in youngsters. As COVID-19 is a novel infectious disease, scientists and doctors are not much aware of it. In this critical circumstance, in-depth knowledge is highly essential for the future treatment and management of the disease. In this review, we summarized the pathophysiology, molecular mechanism, diagnosis, and possible therapeutic options for this disease. Because knowledge of COVID-19 is evolving rapidly, this review may help researchers accurately understand disease.
Pathophysiology of COVID-19
Coronaviruses (CoVs) are non-segmented nucleocapsid-protein enveloped + SS-RNA viruses that are known to cause infection in mammals, including humans and other host species (Pal et al. 2020). Genomic studies have revealed that the CoVs has the most abundant RNA genome of around 30–30 kb in length with a 5′-capping site and 3′poly-A tail region that promotes host genome transcription and translation. Based on the genomic structure and target host, this virus is generally categorized into four groups, including alpha, beta, gamma, and delta. Among these forms, only alpha and beta forms of viruses known to infect mammals and typically cause upper respiratory infections (Rabi et al. 2020). Seven human coronaviruses (HCoVs) have been identified to date, such as 229E, OC43, HKU1, NL63, SARS-CoV, MERS-CoV, and SARS-CoV-2 was identified as belonging to the beta coronavirus family.
In the host genome, the pathogenic in vivo appearance of SARS-CoVs falls in different stages. The binding of the virus to the host receptors, entry of the virus into the host cells by membrane fusion or endocytosis, the release of virus particles within the host cell followed by replication and biosynthesis of viral proteins, and budding/release of viral particles are sequential steps. According to the virologists, the pathophysiology and virulence mechanism of human SARS-CoV and SARS-CoV-2 are possibly similar (82% identical) to the work of non-structural proteins (nsps) and structural proteins (sps) (Chan et al. 2020a). To date, the coronavirus genome has recorded six open reading frames (ORFs), these ORFs can serve as a template for the biosynthesis of subgenomic mRNAs. Among these, ORF1a and ORF1b encode 16 nsps that are typically crucial for virus transcription and replication. Other ORFs encode four major sps: spike (S), membrane (M), envelope (E) and nucleocapsid (N), and other vital proteins (Snijder et al. 2003; Luk et al. 2019). Spike a glycoprotein surface consists of two structural components S1 and S2. Among them, S1 binds to the angiotensin-converting enzyme 2 (ACE2) receptor that highly expressed in the host genome's lung epithelial cells. S2 also contains a transmembrane domain, cytoplasmic domain, and fusion protein that facilitates virus fusion in the envelope and host cell membranes to enable viral fusion. Scientists are, therefore looking S2 as a promising target for antiviral drug therapy (Kirchdoerfer et al. 2016; Xu et al. 2020a). Zhou et al. (2020) recently reported that the novel SARS-CoV-2 could also identify ACE-2 receptors in human cells.
Unlike other coronaviruses, the SARS-CoV-2 has a unique furin cleavage site at the S1/S2 boundary ("RPPA" sequence) that makes this virus pathogenic. Other cell surface proteases such as transmembrane protease serine 2 (TMPRSS2) and cathepsin L also facilitate the cleavage of the complex S1/S2-ACE2 and help to activate viral spike proteins for entry into the host genome (Ou et al. 2020; Hoffmann et al. 2020). In pathogenesis, the envelope proteins play an essential role by facilitating the assembly and release of coronavirus. The N-terminal N-protein binds to the single positive RNA genome and plays a crucial role in the replication and transcription cycle. Currently, two groups of antiviral drugs, such as theophylline and pyrimidone, may inhibit the binding of viral RNA to N-terminal protein (Sarma et al. 2020).
When the virus reaches the body, the host's immune system it activates a sequence of inflammatory events triggered by antigen-presenting cells (APCs). APCs add foreign antigen to cells containing CD4+-T-helper (TH1) and release interleukin-12 to activate TH1. Besides, the activated TH1 cells trigger CD8+-T-killer cells which recognise and eliminate infected host cells. TH1 cells also activate B-cells to synthesize viral-specific antibody that is unique to viruses. The infection spreads in the mucosa of the nasal and larynx, then targets lung epithelial cells, which express ACE2 receptors in abundance (Chen et al. 2020a; Bennardo et al. 2020). Activated WBCs activate positive cytokines, including IL-6. However, the IL-6 level elevates in the extreme state of the disease, which raises the aggressiveness of coronavirus.
Molecular mechanism of COVID-19
Coronaviruses are ubiquitous pathogens that cause respiratory or gastrointestinal infections. A recent phylogenetic analysis found that 2015-SARS coronavirus sequence and human 2019-SARS-CoV-2 emerged in the congregation of a bat. In addition, the Bayesian Quick Unconstrained AppRoximation (FUBAR) analysis revealed genomic mutations in the viral S glycoprotein and nucleocapsid protein (Benvenuto et al. 2020). Compared to the ability of infection with coronaviruses, SARS-CoV-2 has a higher binding affinity to human ACE2 than SARS-CoV, which may explain the frequency of SARS-CoV-2 than SARS-CoV (He et al. 2020). Diverse work has shown that this link between SARS-coronavirus and ACE2 enables a smooth entry into human cells (Struck et al. 2012). Unlike non-ACE2 expressing cells in the ACE2-expressing cells, the novel SARS-CoV-2 uses ACE2 after binding to infected host cells as an entry receptor. Chemokine ligand 2 (CCL2) is the small chemical molecules that attract immune cells including lymphocytes, basophils, and monocytes. This also penetrated the various inflammatory reactions and in the S-ACE2 signaling (Hoffmann et al. 2020), and serves as a critical cytokine. Cheung et al. confirmed the increased expression of chemotactic protein CCL2/monocyte 1 in patients with SARS-CoV and hence CCL2 can be a promising therapeutic marker (Cheung et al. 2005).
Heurich et al. found that infected lung epithelial cells facilitate casein kinase II (CK II) which induces phosphorylation of the ACE2 receptor at the Ser-787 site, whereby SARS-CoV recognises and binds to the ACE2 receptor leading to a structural change of the ACE2 receptor. It also involved the establishment of ACE2 induced downstream signal transduction pathways, including ERK1/2 and AP-1(Heurich et al. 2014). Simmons and colleagues reported that the SARS-CoV accomplishes its entry into host cells via membrane fusion between the virus and lipid bilayer (Simmons et al. 2004). Another finding from Belouzard et al. revealed that the occurrence of a crucial proteolytic cleavage on the S protein of SARS-CoV at S2 position mediates the membrane fusion involving host and CoV pathogen (Belouzard et al. 2009). Besides CoV, the MERS-CoV required furin activation for membrane fusion (Millet and Whittaker 2014). In addition to membrane fusion, clathrin-dependent and independent endocytosis has been reported to be mediated by the SARS-CoV entry (Kuba et al. 2010). Once the virus enters the host, eventually, the viral RNA releases into the cytoplasm.
Further, it translates itself into two polyproteins and structural proteins followed by replication of the viral genome (Perlman and Netland 2009). Later, the newly formed envelope glycoproteins enter the cell organelles naming the endoplasmic reticulum (ER) or Golgi complex. The nucleocapsid contains the mixing of genomic RNA and capsid protein. Then the virus buds are sprout into the ER-Golgi intermediate compartment. Lastly, the vesicles containing the virus particles blend with the plasma membrane to discharge the virus (de Wit et al. 2016). Details related to this is graphically presented in Figs. 1 and 2.
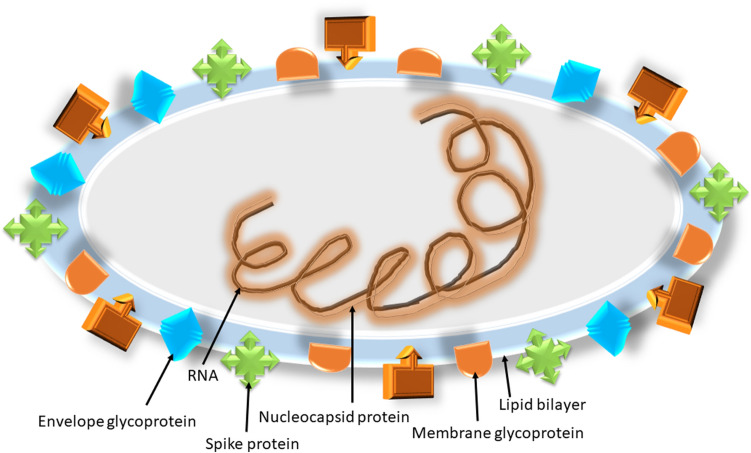
Fig. 1.
Structure of SARS-CoV 2
(Modified from Shereen et al. 2020)
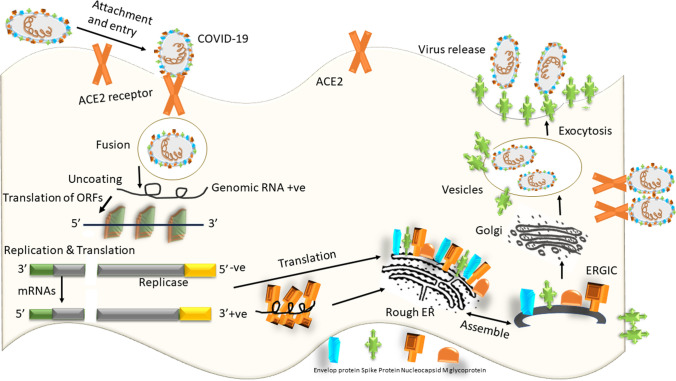
Fig. 2.
Life cycle and mechanism of infection of SARS-CoV 2 in host cells. SARS-CoV 2 begins its life cycle when spike protein (S) binds to the cellular receptor ACE2. After receptor binding, the conformation change in the S protein facilitates viral envelope fusion with the cell membrane through the endosomal pathway. Then SARS-CoV 2 releases RNA. Genome RNA gets translated into viral replicase polyproteins (pp1a and 1ab) and further cleaved into small products by viral proteinases. The polymerase generates a series of subgenomic mRNAs by discontinuous transcription and finally translated into appropriate viral proteins. Viral proteins and genome RNA are subsequently assembled into virions in the ER and Golgi body and then transported via vesicles and released out of the cell. ACE2 Angiotensin-converting enzyme 2, ER Endoplasmic reticulum, ERGIC ER–Golgi intermediate compartment
Additionally, it has been reported that the SARS-CoV is involved in the antigen-dependent presentation of MHC I molecules, but MHC II also contributes to its presentation (Liu et al. 2010). Want et al., conducted a polymorphism-based study and found that the human leukocyte antigen (HLA) polymorphisms such as HLA-B*4601, HLA-B*0703, HLA-DR B1 * 1202, and HLA Cw*0801 are associated with the susceptibility of SARS-CoV (Keicho et al. 2009). In comparison, they found that the HLA-DR0301, HLA-Cw1502, and HLA-A*0201 play a vital role in SARS infection and functioning as protective alleles (Wang et al. 2011). It has also been observed that mannose-binding lectin (MBL) gene polymorphisms are associated with antigen presentation and thus linked to the risk of infection with SARS-CoV (Tu et al. 2015).
Research has indicated that acute respiratory distress syndrome (ARDS) is the leading cause of death in COVID-19 and one of the main routes for the cytokine storm associated with ARDS. Nevertheless, lethal systemic inflammatory response leading to elevated levels of pro-inflammatory cytokines such as IFN-α, IFN-α, TNF-α, TGFβ, IL-1β, IL-6, IL-12, IL-18, IL-33 and chemokines such as CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10 (Williams and Chambers 2014; Channappanavar and Perlman 2017). Xu et al. recently reported that the peripheral blood of SARS-CoV-2 patients displayed a substantial reduction in immune defence cells such as CD4+ and CD8+ T cells. In contrast, high concentrations of HLA-DR (CD4 3.47%) and CD38 (CD8 39.4%) were also found in double-positive fractions within the same patients (Xu et al. 2020b). SARS-CoV viruses are adequate to employ many methods to prevent the survival of the immune system in host cells. Snijder et al. reported that SARS-CoV and MERS-CoV could provoke the assemblage of membrane vesicles that require for Porcine reproductive and respiratory syndrome (PRRS) and avoiding the host detection of their dsRNA (Snijder et al. 2006). These findings are valuable for the effective treatment of COVID-19.
Diagnostic approach
Many COVID-19 cases have moderate or non-specific symptoms for a correct diagnosis, while severe patients have respiratory problems, including fever, cough, tiredness, and shortness of breath, and decreased or diminished vocal fremitus on palpation (Xie et al. 2020a). Patient screening for precise diagnosis must be comfortable, low cost, quick, and the most reliable result. Studies into epidemiological history, clinical findings, and tests are essential for the clinical diagnosis of COVID-19. Imaging will be the first diagnosis. Suspected patients will undergo chest x-ray as soon as possible and an urgent CT scan based on severity (Shen et al. 2020a). The image can provide a better understanding of how the disease is progressing. Chest images may show interstitial changes in the preliminary process, and the presence of small plaques, especially in the lung periphery. This disease further deteriorates bilaterally and is primarily distributed with several infiltrative shadows in the middle and outer zones of the lung. In extreme cases, consolidation of the lung may occur (Pan et al. 2020b).
Laboratory assessment
In the early stage, the count of white blood cells generally appears normal or slightly low, with a smaller count of lymphocytes. But if the absolute count of lymphocytes is < 0.8/L or the counts of CD4+ and CD8+ T-cells are significantly decreased, this is a warning. But if the absolute lymphocyte count is < 0.8 × 109/L or the CD4+ and CD8+ T-cell counts are decreases substantially, that’s an alarm. In some patients, muscle enzymes, liver enzymes, and levels of myohemoglobin are elevated; in some critical cases, even an increased amount of troponin is observed. Infected patients mostly show high erythrocyte sedimentation (ESR) and C-reactive protein (CRP) levels, with normal procalcitonin levels and progressively decreased blood lymphocyte counts with elevated D-dimer concentrations. In severe patients, inflammatory factors are also increased. It is recommended that blood changes be rechecked every 3 days (Jin et al. 2020).
Detection methods based on nucleic acid are an essential tool for the diagnosis of viral agents. In particular, the polymerase chain reaction (PCR) is a "gold standard method" for virus detection due to rapid identification, high sensitivity and specificity. Molecular diagnosis methods like real-time PCR (RT-PCR) can be executed using blood, feces, and tissue samples of the upper part of the respiratory tract (nasopharyngeal and oropharyngeal), lower respiratory tract (expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage) from suspected patients with SARS-CoV-2 (Yu et al. 2020a). However, Nucleic acid detection has some drawbacks in its operation, such as the time-consuming and high risk of contamination. Nonetheless, current PCR methods appear to have functional specificity except for sensitivity, which means that negative test results did not guarantee that SARS-CoV-2 was absent. In addition, false-positive results may occur due to sample contamination, or false negative results may appear due to inadequate viral load. For people with high clinical suspicion but negative RT-PCR screening, chest CT can assist with clinical diagnosis.
A combination of CT scanning and swab examination appears to be more helpful (Xie et al. 2020b). Early detection and development of disease in patients with SARS-CoV-2 can be done with high-resolution computed tomography scans (HRCT) (Pan et al. 2020a). The CT images of patients with SARS-CoV and MERS-CoV indicate lung involvement close to SARS-CoV-2 infection; besides, HRCT scans have certain drawbacks, including other virus infections and irregular CT imaging. RT-PCR experiments can be used with the chest CT scan in combinations to boost false-positive outcomes (Chen et al. 2020b; Jiang et al. 2020). Although the detection of IgM and IgG antibody detection is highly responsive to enzyme-linked immunosorbent assay (ELISA) (Jia et al. 2020). A study found that 94.7% of SARS-CoV N-based IgG ELISA and 58.9% of S-based IgG ELISA and SARS-CoV-2 IgG/IgM are still being studied (Li et al. 2020a).
The Loop-mediated isothermal amplification (LAMP) is a novel method of amplification of DNAs and RNAs that enhances product with high sensitivity and specificity. The key benefits of this approach are low cost and less costly reagents and involving simple instruments (Shen et al. 2020b). Recently Mohamed et al. demonstrated that the LAMP methods have 10 times more sensitivity than traditional RT-PCR in COVID-19 detection without false-negative events (Mohamed et al. 2020). The iLACO (isothermal LAMP based method for COVID-19)uses 6 primers for amplifying the ORF1ab gene sequences of 11 associated viruses. Yu et al. compared both methods and found that the iLACO sensitivity and accuracy are higher than the Taqman-based qPCR detection method (Yu et al. 2020b). Zhang et al. also stated that LAMP with a colorimetric detection method has a high sensitivity to COVID-19 and that these methods can be used in the diagnosis for the bulk (Zhang et al. 2020b). Comparison of three novel RT-PCR assays targeting RNA-dependent RNA polymerase (RdRp)/helicase (Hel), spike (S), and COVID-19 nucleocapside (N) genes showed that the RdRp/Hel assay did not cross-react with SARS-CoV and MERS coronaviruses and revealed high sensitivity and specificity for COVID-19 (Chan et al. 2020b).
In-silico approach
Kellner et al. demonstrated that the CRISPR-based SHERLOCK (Specific High-Sensitivity Enzymatic Reporter UnLOCKing) technique could be a diagnostic tool for COVID-19 to enable compact, multiplexed and ultrasensitive detection of RNA or DNA from clinically relevant samples (Kellner et al. 2019). SHERLOCK assays are set up with recombinase-mediated polymerase pre-amplification of DNA or RNA and subsequent Cas13-or Cas12-mediated detection via colorimetric read-outs and fluorescence. Cas13a can detect new coronavirus in the given sample. By using synthetic RNA fragments of the SARS-CoV-2 virus, target sequences of SARS-CoV-2 can be detected between 20 and 200 aM (10–100 copies per microliter of input). To improve the precision of the detection, scientists have implemented sequences unique to the current coronavirus to avoid interaction with the genomes of respiratory viruses. While the test has better readability without any difficulty, the testing of multiple patient samples also needs confirmation.
Till date, therapeutic approaches are still under trial. Cava et al. defined new functions and pathways of ACE2-correlated genes using gene expression profiles from the public datasets. Researchers found a network of 193 genes, 22 interactions and 36 possible drugs, including nimesulide, fluticasone propionate, thiabendazole and photofrin. Among all the potentially active drugs, didanosine is a real antiviral drug, while the others are mostly anti-inflammatory (Cava et al. 2020). Since sialic acids linked to glycoproteins and gangliosides and are used as invasive receptors by a wide range of viruses (Matrosovich et al. 2015). Several modeling approaches have been used to decipher protein molecular mechanisms sugar interactions that account for the interaction of viruses, membranes and amyloid proteins with cell surface glycolipids (Yahi and Fantini 2014; Flores et al. 2019).
The molecular mechanisms underlying the antiviral mechanisms of chloroquine against SARS-CoV-2 infection was unraveled using novel in-silico strategy (Fantini et al. 2020). EK1C4 is an inhalation formulation for SARS-CoV-2 patients to reduce viral loads in the lungs. Recently, Xia et al. stated that EK1C4 might help to prevent SARS-CoV-2 infection due to the high retention of its target. Peptide drugs are usually safer than chemical drugs, and EK1C4 is considered to be harmless for humans (Xia et al. 2020). While no successful drugs available against COVID-19, Joshi et al. conducted a nucleocapsid virtual screening of 318 phytochemicals against molecular targets such as main protease (Mpro) and ACE2. This study identified 11 plants for their antiviral, antibacterial and antifungal activity based on low binding energy compared to the reference molecule (Joshi et al. 2020). Sekiou et al. compared the efficacy of hydroxychloroquine, Quercetin, Hispidulin, Cirsimaritin, Sulfasalazine, Artemisin and Curcumin natural compounds against COVID-19 using AutoDock indicated that these compounds have better potential inhibition than hydroxychloroquine (Omar et al. 2020).
Current therapeutic option
Coronavirus belongs to a large family of viruses found in various types of animals, including caterpillars, bats, and camels. The newly discovered strain of this virus has been established as the cause of respiratory disease in humans. Occasionally, animal coronavirus has jumped to species and eventually infected humans so that it can appear more between humans, such as MARS-CoV, SARS-CoV, and now SARS-CoV2. Some countries are likely to have distributed COVID-19 in the early stages, before any inaccurate measurements. Nevertheless, Anderson and his colleagues suggest that while governments would not be able to reduce the death rate of COVID-19 infections, the remission of this pandemic may have an immense economic effect on a viral spread (Anderson et al. 2020). To date, there are no definite treatments available to cure it. However, medical professionals are still looking for the best way to prevent these infections. People are now seeking alternative remedies to prevent their infections as a pandemic, which means that they could be globally prevalent.
Antivirus therapy
Drug therapy that is already approved for the treatment of other infectious diseases, including Ebola, Aids, HIV, malaria, is beneficial for the treatment of COVID-19. Apart from this antibodies-based protein processed using recombinant technology and injected into the patient body can binds to SARS-CoV-2. This method can be a promising alternative that minimize the inflammation caused due the virus. Other therapeutic strategies, such as corticosteroids for viral therapy, are not recommended. Several drug therapies have been suggested based on current published evidence, such as lopinavir with a combination of ritonavir (400 + 100 mg), chloroquine (500 mg), and hydroxychloroquine (200 mg) per 12 h (Cascella et al. 2020). Recently, several preclinical studies have indicated that remdesivir has an inhibitory activity of RNA polymerase in vitro against RNA viruses, including Ebola, MERS-CoV and HCoV infections (Gordon et al. 2020; de Wit et al. 2020). Nowadays, in the USA, this drug has been re-used for the treatment of COVID-19. Recently, the new antiviral medication favipiravir (FabiFlu) has been approved in India to combat mild to moderate cases of COVID-19 (https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/coronavirus-vaccine-roundup-from-imperial-college-human-trials-to-treatment-here-are-all-the-fresh-updates-around-covid-19/photostory/76644042.cms). Traditional antiviral medications with a different formulations used in covid-19 therapy have been shown in Table 1.
Table 1.
List of potential alternative drugs for treatment of COVID-19
| S.N. | Therapeutic drug | Common name | Target | Effective concentration EC 50 | References |
|---|---|---|---|---|---|
| 1 | Favipiravir (T-705) | Avigan | Influenza | 61.88 μM | Sui et al. (2004) |
| 2 | Remdesivir (GS5734) | Ebola | 0.77 μM | Sui et al. (2004) | |
| 3 | Chloroquine /hydroxychloroquine | Aralen/plaquenil | Malaria | 1.13 μM | Sui et al. (2004) |
| 4 | Lopinavir/ritonavir | Kaletra | HIV | ||
| 5 | Pegylated interferon with ribavirin | Virazole | HBV,HCV | 109 μM | Sui et al. (2004) |
| 6 | Teicoplanin | – | MERS-CoV and SARS-CoV | 1.66 μM | Zhai et al. (2020) |
| 7 | Arbidol tablets | – | SARS-CoV 2 | Under clinical trial | |
| 8 | Methylprednisolone | – | Severe novel coronavirus pneumonia: | Under clinical trial | |
| 9 | Nelfinavir (HIV-protease) | – | SARS-CoV | Yamamoto et al. (2004) |
Plasma therapy
Among the various treatment strategies, convalescent plasma therapy (CPT) is commonly prescribed for the treatment of COVID-19. Plasma, which is only a short-term solution for susceptible people after two weeks of healing, should be used to ensure high neutralisation of antibodies. Now, we need a good design of clinical trials and a well-managed plasma therapy program to prevent COVID-19 infection (Verma et al. 2020a). Convalescent plasma transfusion from the recovered infected patient to newly infected patients can kick-start their immune system. Rajendran et al. found that, firstly, these treatments could reduce the mortality rate in critically ill patients. Second, it improved the neutralization of antibodies in newly infected patients. Thirdly, decreased flu-like symptoms, and other beneficial effects have been shown following administration of plasma therapy (Rajendran et al. 2020). Since this is just part of the treatment, there is no full solution for patients and their reaction can vary from individual to individual receiving plasma transfusion therapy (Table 2).
Table 2.
Ongoing clinical trials of COVID-19 patients treated with convalescent plasma patients listed in World Health Organization International Clinical Trial Registry Platform (ICTRP) database 76
| Trial ID | Scientific title | Sponsor | Recruitment status | Interventions | Inclusion age | Phase | Date enrollment | Study type | Enrollment | Countries |
|---|---|---|---|---|---|---|---|---|---|---|
| ChiCTR 2000029850 | Efficacy and safety of convalescent plasma treatment for severe patients with novel coronavirus pneumonia (COVID-19): a prospective cohort study | Zhejiang University School of Medicine | Recruiting | Convalescent plasma | 18 years and older | N/A | 15-02-2020 | Interventional | 20 | China |
| ChiCTR 2000030179 | Experimental study of novel coronavirus pneumonia rehabilitation plasma therapy severe novel coronavirus pneumonia (COVID-19) | Hospital of Nanchang University | Recruiting | Convalescent plasma | 18 years and older | N/A | 24-02-2020 | Interventional | 100 | China |
| ChiCTR 2000030046 | A single-arm trial to evaluate the efficacy and safety of anti-2019-nCoV inactivated convalescent plasma in the treatment of novel coronavirus pneumonia patient (COVID-19) | Hospital of Jiangxia District, Wuhan (Union JiangnanHospital) | Recruiting | Convalescent plasma | 18 years and older | N/A | 07-02-2020 | Interventional | 10 | China |
| ChiCTR 2000030010 | A randomized, double-blind, parallel-controlled, trial to evaluate the efficacy and safety of anti-SARS-CoV-2 virus inactivated plasma in the treatment of severe novel coronavirus pneumonia patients (COVID-19) | Wuhan Jinyintan Hospital (Wuhan Infectious Diseases Hospital) | Not recruiting | Convalescent plasma | 18 years and older | N/A | 19-02-2020 | Interventional | 100 | China |
| ChiCTR 2000030039 | Clinical study for infusing convalescent plasma to treat patients with new coronavirus pneumonia (COVID-19) | Affiliated Hospital of Xuzhou Medical University | Recruiting | Convalescent plasma | 18 years and older | N/A | 31-05-2020 | Interventional | 90 | China |
| ChiCTR 2000030627 | Study on the application of convalescent plasma therapy in severe COVID-19 | The First Affiliated Hospital of Zhengzhou University | Recruiting | Convalescent plasma | 18 years and older | N/A | 01-02-2020 | Interventional | 30 | China |
| ChiCTR 2000029757 | Convalescent plasma for the treatment of severe and critical novel coronavirus pneumonia (COVID-19): a prospective randomized controlled trial | China–Japan friendship hospital | Recruiting | Convalescent plasma | 18 years and older | N/A | 14-02-2020 | Interventional | 200 | China |
| NCT 04292340 | The Efficacy and Safety of Anti-SARS-CoV-2 Inactivated Convalescent Plasma in the Treatment of Novel Coronavirus Pneumonia Patient (COVID-19): An Observational Study | Shanghai Public Health Clinical Center | Recruiting | Convalescent plasma | 18 years and older |
Phase 2 Phase 3 |
01-02-2020 | Observational | 15 | China |
| ChiCTR 2000030312 | A single-center, open-label and single-arm trial to evaluate the efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of novel coronavirus pneumonia (COVID-19) patient | First people's hospital of Jiangxi district, Wuhan | Not recruiting | Convalescent plasma | 18 years and older | N/A | 29-02-2020 | Interventional | 24 | China |
| ChiCTR 2000030702 | Convalescent plasma for the treatment of common COVID-19: a prospective randomized controlled trial | China-Japan friendship hospital | Recruiting | Convalescent plasma | 18 years and older | N/A | 15-02-2020 | Interventional | 50 | China |
| ChiCTR 2000030381 | A randomized, open-label, controlled and single-center trial to evaluate the efficacy and safety of anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of novel coronavirus pneumonia (COVID-19) patient | First people's hospital of Jiangxi district, Wuhan | Not recruiting | Convalescent plasma | 18 years and older | N/A | 29-02-2020 | Interventional | 40 | China |
| ChiCTR 2000029818 | Clinical Study for Umbilical Cord Blood Plasma in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19) | Guangzhou reborn health management consultation co., LTD | Not recruiting | Umbilical cord blood plasma | 18 years and older | N/A | 20-02-2020 | Interventional | 60 | China |
| ChiCTR 2000030929 | A randomized, double-blind, parallel-controlled trial to evaluate the efficacy and safety of anti-SARS-CoV-2 virus inactivated plasma in the treatment of severe novel coronavirus pneumonia (COVID-19) | Renmin Hospital of Wuhan University | Not recruiting | Convalescent plasma | 18 years and older | N/A | 17-03-2020 | Interventional | 60 | China |
| NCT 04323800 | Convalescent Plasma to Stem Coronavirus: A Randomized, Blinded Phase 2 Study Comparing the Efficacy and Safety Human Coronavirus Immune Plasma (HCIP) vs. Control (SARS-CoV-2 Non-immune Plasma) Among Adults Exposed to COVID-19 | Johns Hopkins University | Not recruiting | Convalescent plasma | 18 years and older | Phase 2 | 01-04-2020 | Interventional | N/A | United States |
| NCT 04321421 | Plasma From Donors Recovered From New Coronavirus 2019 As Therapy For Critical Patients With Covid-19 | Foundation IRCCS San Matteo Hospital | Not recruiting | Convalescent plasma | 18 years and older | N/A | 17-03-2020 | Interventional | N/A | Italy |
| NCT 04325672 | Convalescent Plasma to Limit Coronavirus Associated Complications: An Open-Label, Phase 2A Study of High-Titer Anti-SARS-CoV-2 Plasma in Hospitalized Patients With COVID-19 | Mayo Clinic | Not recruiting | Convalescent plasma | 18 years and older | Phase 2 | 01-04-2020 | Interventional | NA | United States |
| NCT 04342182 | Convalescent Plasma Therapy From Recovered Patients to Treat Severe SARS-CoV-2 Disease (CONCOVID Study) | Erasmus Medical Center|Maasstad Hospital | Recruiting | Convalescent plasma | 18 years and older |
Phase 2 Phase 3 |
01-04-2020 | Randomized | 426 | Netherlands |
| NCT 04334876 | Rapid SARS-CoV-2 IgG Antibody Testing in High Risk Healthcare Workers | Indiana University | Not yet recruiting | Diagnostic test: SARS-CoV-2 IgG Antibody Testing Kit | 18 years and older | N/A | 01-04-2020 | Observational | 340 | United States |
| NCT 04338360 | Expanded Access to Convalescent Plasma for the Treatment of Patients With COVID-19 | Mayo Clinic | Available | Convalescent plasma | 18 years and older | N/A | N/A | N/A | N/A | United States |
| NCT 04333355 | Safety in Convalescent Plasma Transfusion to COVID-19 | Hospital San Jose Tec de Monterrey|Tecnologico de Monterrey | Not yet recruiting | Convalescent plasma | 18 years and older | Phase 1 | 15-04-2020 | Interventional | 20 | Mexico |
| NCT 04332835 | Convalescent Plasma for Patients With COVID-19: A Randomized, Open Label, Parallel, Controlled Clinical Study (CP-COVID-19) | Universidad del Rosario | Not yet recruiting | Plasma, hydroxychloroquine, azithromycin | 18 years and older | Phase 2 Phase 3 | 01-04-2020 | Interventional | 80 | Universidad del Rosario |
| NCT 04325672 | Convalescent Plasma to Limit Coronavirus Associated Complications: An Open label, Phase 2A Study of High-Titer Anti-SARS-CoV-2 plasma in hospitalized patients with COVID-19 | Johns Hopkins via a national IND | Not yet recruiting (stop) | Convalescent plasma | 18 years and older | 2A open label | 27-03-2020 | Interventional | 5 | United States |
| NCT 04323800 | Convalescent Plasma to Stem Coronavirus: A Randomized Controlled Double Blinded Phase 2 Study Comparing the Efficacy and Safety of Human Coronavirus Immune Plasma (HCIP) vs. control (SARS-CoV-2 non-immune plasma) among Adults Exposed to COVID-19 | Johns Hopkins University | NA | Convalescent plasma | 18 years and older | Phase 2 | 01-04-2020 | Interventional | 150 | United States |
| NCT 04332380 | Convalescent Plasma for Patients With COVID-19: A Pilot Study (CP-COVID-19) | Universidad del Rosario | Not yet recruiting | Convalescent plasma | 18 years and older | Phase 2 | 01-04-2020 | Interventional | 10 | Spain |
| NCT 04327349 | Investigating Effect of Convalescent Plasma on COVID-19 Patients Outcome: A Clinical Trial | Mazandaran University of Medical Sciences | Enrolling by invitation | Convalescent PLASMA | Age 30–70 years | N/A | 28-03-2020 | Interventional | 30 | Iran |
| NCT 04353206 | Convalescent Plasma in ICU Patients With COVID-19-induced Respiratory Failure | Noah Merin | Recruiting | Convalescent plasma | 18 years and older | Early phase 1 | 14-05-2020 | Interventional | 60 | United States |
| NCT 04373460 | Convalescent Plasma to Limit SARS-CoV-2 Associated Complications (CSSC-004) | Johns Hopkins University | Not yet recruiting | Convalescent plasma | 18 years and older | Phase 2 | 19-05-2020 | Interventional | 1344 | United States |
NA: not available
Antibodies
Based on the recently available study, the case fatality rate (CFR) of COVID-19 is unknown; more detailed studies are needed for identifying the actual number of infected individuals in any population. Nonetheless, we are oblivious to any completed large-scale serological survey of COVID-19 antibody detection. However, Immunotherapy may be a safer choice before the development of the vaccine that helps in developing and innate immunity to the virus. In addition, herd immunity is an alternative idea for combating COVID-19 by improving natural immunity in underdeveloped countries. In Sweden, the approach to herd immunity has been updated with significant criticism (Kwok et al. 2020). Furthermore, the monoclonal antibody will be a potential therapeutic intervention for treatment against COVID 19. The monoclonal antibodies developed against the virus are listed in Table 3.
Table 3.
List of neutralizing monoclonal antibody and protease inhibitors
| S.N. | Name | Experimental model | Mechanism | References |
|---|---|---|---|---|
| 1 | Anti-ACE2 | Mouse | Target the spike protein of SARS-CoV-2 that inhibits virus binding cell receptors | Shanmugaraj et al. (2020) |
| 2 | CR3014 | Ferret and in vitro | Binding to the 318–510 amino acid residues with high affinity on S1 fragment of SARS-CoV andblock the interaction of S1 subunit protein with cellular receptor ACE2 in vitro and in vivo | van den Brink et al. (2005), ter Meulen et al. (2006), Jan ter Meulen et al. (2004) |
| 3 | CR3022 | In vitro | Binding to the 318–510 amino acid residues on S1 fragment of SARS-CoVand block the interaction of S1 subunit protein with cellular receptor ACE2 | ter Meulen et al. (2006) |
| 4 | F26G18 | In vitro | Binding to the linear epitope460-476amino acid residues on S1 fragment of SARS-CoVand block the interaction of S1 subunit protein with cellular receptor ACE2 | Berry et al. (2010) |
| 5 | F26G19 | In vitro | Binding to the conformational epitope (amino acid residues 359–362, 391–392, 424–427, and 486–492) on S1 fragment of SARS-CoVand block the interaction of S1 subunit protein with cellular receptor ACE2 | Berry et al. (2010) |
| 6 | m396 | In vitro | Binding to the conformational epitope amino acid residues 482–491 on S1 fragment of SARS-CoV and block the interaction of S subunit protein using CDR loops H1, H2, H3, and L3 with cellular receptor ACE2 | Berry et al. (2010), Zhu et al. (2007) |
| 7 | 1A9 | In vitro | Binding to the Heptad repeat loops, including heptad repeat one and heptad repeat one domain on S2 fragmentof SARS-CoV and block the interaction of S2 subunit protein with cellular receptorACE2 | Ng et al. (2014), Lip et al. (2006) |
| 8 | 201 | Mouse Syrian Hamster and in vitro | Binding to the 490–510 amino acid residues on S1 fragment of SARS-CoV and block the interaction of S1 subunit protein with cellular receptor ACE2 in vitro and in vivo | Greenough et al. (2005), Coughlin and Prabhakar (2012) |
| 9 | 4D4 | in vitro | Binding to the 12–261 amino acid residues of SARS-CoV and N-terminal of RBD and Inhibit the post-interaction in the viral permeation | Coughlin and Prabhakar (2012), Elshabrawy et al. (2012) |
| 10 | S230 | in vitro | Binding to epitopes partially overlapping with receptor binding motifs on B domain of SARS-CoVand block the interaction of S1 subunit protein with cellular receptor ACE2 in vitro | Walls et al. (2019) |
Vitamin
Recently researchers have been found that vitamin plays a vital role in clinical therapies. For instance, vitamin D boosts cellular immunity by reducing the cytokine storm in COVID-19 by inducing pro and anti-inflammatory factors, and it also acts as a modulator of adaptive immunity (Cantorna 2010; Rondanelli et al. 2018; Grant et al. 2020). For infected patient’s vitamin D might be a useful medication to prevent infections. Many reviews are suggesting that vitamin D loading doses of 200,000–300,000 IU capsules reduces the risk and severity of COVID-19 (Gasmi et al. 2020). Some recommended vitamin with their doses listed in Table 4.
Table 4.
List of important recommended vitamins and their dosages for treatment of COVID-19
| S.N. | Vitamin name | Age group | Recommended dose | Disease | Target | References |
|---|---|---|---|---|---|---|
| 1 | 25-hydroxyvitamin D | 0–95 year | Daily or weekly 2000 IU/day (50 µg/day) | Acute respiratory tract infection | Reduce inflammation | Martineau et al. (2017), Autier et al. (2017), Martineau et al. (2019), Rejnmark et al. (2017), Bergman et al. (2013), Charan et al. (2012) |
| 2 | Vitamin-E (Tocopherol) | All age | One-year daily supplement | Respiratory tract infection | Enhance T-cell mediated immunity | Wu and Meydani (2014) |
| 3 | Vitamin-C (Ascorbic acid) | 1–3 year to adult | Least 200 mg/day-2 g/day | Upper and lower respiratory tract infection | Monsen (2000) |
Summary of meta-analysis
To date, several research groups are trying to resolve the present pandemic problem. In this perspective, many reaserchers have summarised reliable medical evidence by the meta-analysis of all published clinical trials against COVID-19, among them ten meta-analyses we have included with either therapeutic target as vaccines or placebo infections, chloroquine derives hydroxy-chloroquine, dexamethasone, lopinavir-ritonavir, remdesivir with placebo, hydroxy-chloroquine with azithromycin, tocilizumab, convalescent plasma. The results of all meta-analysis were inconsistent (Table 5).
Table 5.
Summary of meta-analysis conducted by several groups for the treatment of COVID-19
| S.N. | Therapeutic drug | Common name | Target | Comments/significance | References |
|---|---|---|---|---|---|
| 1 | Chloroquine derives hydroxy-chloroquine | Aralen/plaquenil | SARS-CoV 2 | More useful to improve clinical and virological outcomes. But reduce mortality | Million et al. (2020), Pathak et al. (2020), Siemieniuk et al. (2020) |
| 2 | Dexamethasone | Decadron | SARS-CoV 2 | Risk of mortality and benefits for severing clinical condition patients | Abeldano Zuniga et al. (2020), Siemieniuk et al. (2020) |
| 3 | Lopinavir-Ritonavir | Kaletra | HIV, SARS-CoV 2 | Risk of mortality, adverse events | Abeldano Zuniga et al. (2020), Siemieniuk et al. (2020) |
| 4 | Remdesivir with placebo | Veklury | Ebola, COVID-19 | Risk of mortality | Abeldano Zuniga et al. (2020), Siemieniuk et al. (2020) |
| 5 | Hydroxy-chloroquine with azithromycin | Aralen/plaquenil and zitromax | SARS-CoV 2 | Not alter the rate of risk of virologic cure and risk of mortality, Not different from hydroxy-chloroquine alone | Ayele Mega et al. (2020), Chen et al. (2020c), (Tang et al. (2020), Fiolet et al. (2020), Abeldano Zuniga et al. (2020), Siemieniuk et al. (2020) |
| 6 | Tolicizumab | Actemra | Lower mortality was 12% | Malgie et al. (2020) | |
| 7 | Convalescent Plasma | NA | SARS-CoV 2 | Mortality and virological clearance | Abeldano Zuniga et al. (2020), Siemieniuk et al. (2020) |
| 8 | Vaccine vs placebo | NA | SARS-CoV 2 | Significantly increase IgG level than placebo, total local events also changed (P > 0.05) | Yuan et al. (2020) |
Other treatments
In the current scenario, most of the studies using western medicine for the treatment of COVID-19 but Chinese traditional medicine also has a potential activity for COVID-19 (Zhang et al. 2020a). According to the latest clinical guidelines for the treatment of SARS or MERS patients are same, and both western medicine and traditional Chinese medicine are used for the treatment of COVID-19 in China (Li and De Clercq 2020; Li and Peng 2013). Due to the genomic homology, clinical evidence shows the safety and efficacy of traditional Chinese medicine in the treatment of COVID-19. Some of them include herbal folk therapies and herbal teas. It also suggested that some of them might not be safe to consume (Coghlan et al. 2015). According to the Ministry of AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homeopathy), Government of India, herbal medicines may helpful to combat the corona outbreak and at least reduce the infection. An autonomous body of AYUSH, CCRAS (Central Council for Ayurvedic Sciences Research), had developed AYUSH-64 for effective treatment without malaria. Scientific experts recommending that there is no adequate evidence for the use of Arsenicum Album in the fight against the symptoms of COVID-19. Besides, homoeopathy derived arsenic was recommended to take on an empty stomach. Due to the immunity boosting properties, anti-inflammatory ayurvedic drugs were also used against coronavirus. An evidence-based study shows that the intake of the antimalarial ayurvedic drugs (Ayush 64) developed by the Indian Ministry has a beneficial effect on COVID-19 (CCMI 2020).
Conclusion
The advancement of COVID-19 infection depends upon the interface between the load of the virus and the human immune system. As the global demand for diagnosis and therapy continues to rise, it is essential to quickly develop various algorithms to identify and contain the virus successfully. The pathogenic effects of the virus tend to be mediated by binding to human receptor ACE2. If the virus has a high load in an individual, the level of infection could increase the number of deaths. Conversely, due to the undetectable virulence load, moderate and lower viral load displays false-negative effects. This load of virulence is determined by gender, age, nutritional status, dietary habits, co-morbidity, and physical condition. These parameters play a contributing role in the severity of the disease and the re-infection of individuals. From this perspective, it is essential to develop new, fast, reliable, feasible, compact and straight forward technologies for the detection of COVID-19. The worldwide collaboration of healthcare professionals to engage patients in clinical trials is essential. A multicentre mega-trial is vital for comparing the clinical outcome of patients treated with different combination drugs. There is no doubt that researchers are directed to gain insight into this disease and to develop an effective therapeutic option. As far as management is concerned, the first step that can be taken is to get the right advice and clear people's fears. Besides, much-fabricated knowledge about COVID-19 is being disseminated, which has triggered fear in society. Clear guidelines should be implemented at the group level, which will help patients heal as quickly as possible. Meantime, research will continue, and CP therapy or neutralizing antibodies are considered for treatment until the vaccine arrives.
Abbreviations
- CoVs
Coronaviruses
- HCoVs
Human coronaviruses
- SARS-CoV
Severe acute respiratory syndrome
- MERS-CoV
Middle East Respiratory Syndrome
- ORFs
Open reading frames
- ACE2
Angiotensin-converting enzyme 2
- APCs
Antigen-presenting cells
- TH1
T-helper
- ER
Endoplasmic reticulum
- HLA
Human leukocyte antigen
- MBL
Mannose-binding lectin
- ARDS
Acute respiratory distress syndrome
- PRRS
Porcine reproductive and respiratory syndrome
- ESR
Erythrocyte sedimentation
- CRP
C-reactive protein
- PCR
Polymerase chain
- RT-PCR
Real-time reverse transcription PCR
- HRCT
High-resolution computed tomography scans
- ELISA
Enzyme-linked immunosorbent assay
- LAMP
Loop-mediated isothermal amplification
- RdRp
RNA-dependent RNA polymerase
- CCRAS
Central Council for Ayurvedic Sciences Research
Author contributions
The authors HKV conceived the study, RKR, NK, AB retrieved the data and HKV, RKR, NK, AB, and BLVKS wrote the manuscript and approved the final version and made manuscript technically sound.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All the authors have read the manuscript and have approved this submission.
Footnotes
Yashwant Kumar Ratre and Namrata Kahar are contributed equally to this work.
Contributor Information
Yashwant Kumar Ratre, Email: yashwntratre@gmail.com.
Namrata Kahar, Email: namratakahar3@gmail.com.
L. V. K. S. Bhaskar, Email: lvksbhaskar@gmail.com
Antaripa Bhattacharya, Email: antaripa1210@gmail.com.
Henu Kumar Verma, Email: henu.verma@yahoo.com, Email: henu.verma@ieos.cnr.it.
References
- Abeldano Zuniga RA, Coca S, Abeldano G, Gonzalez Villoria RAM (2020) Clinical effectiveness of drugs in hospitalized patients with COVID-19 infection: a systematic review and meta-analysis. medRxiv. 10.1101/2020.09.11.20193011 [DOI] [PMC free article] [PubMed]
- Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Mullie P, Macacu A, Dragomir M, Boniol M, Coppens K, Pizot C. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5(12):986–1004. doi: 10.1016/S2213-8587(17)30357-1. [DOI] [PubMed] [Google Scholar]
- Ayele Mega T, Feyissa TM, Dessalegn Bosho D, Kumela Goro K, Zeleke Negera G. The outcome of hydroxychloroquine in patients treated for COVID-19: systematic review and meta-analysis. Can Respir J. 2020;2020:4312519. doi: 10.1155/2020/4312519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci. 2009;106(14):5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennardo F, Buffone C, Giudice A. New therapeutic opportunities for COVID-19 patients with tocilizumab: possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020 doi: 10.1016/j.oraloncology.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92(4):455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman P, Lindh AU, Bjorkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2013;8(6):e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JD, Hay K, Rini JM, Yu M, Wang L, Plummer FA, Corbett CR, Andonov A. Neutralizing epitopes of the SARS-CoV S-protein cluster independent of repertoire, antigen structure or mAb technology. mAbs. 2010;2(1):53–66. doi: 10.4161/mabs.2.1.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar LVKS, Roshan B, Nasri H. The fuzzy connection between SARS-CoV-2 infection and loss of renal function. Am J Nephrol. 2020;51(7):572–573. doi: 10.1159/000508087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramanti B, Dean KR, Walloe L, Chr Stenseth N. The third plague pandemic in Europe. Proc Biol Sci. 2019;286(1901):20182429. doi: 10.1098/rspb.2018.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc. 2010;69(3):286–289. doi: 10.1017/S0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment Coronavirus (COVID-19) Treasure Island: StatPearls; 2020. [PubMed] [Google Scholar]
- Cava C, Bertoli G, Castiglioni I. In silico discovery of candidate drugs against Covid-19. Viruses. 2020 doi: 10.3390/v12040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCMI (2020) Central council of indian medicine telemedicine practice guidelines for Ayurveda, Siddha and Unani Practitioners
- Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung KH, Fung AY, Ng AC, Zou Z, Tsoi HW, Choi GK, Tam AR, Cheng VC, Chan KH, Tsang OT, Yuen KY. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020 doi: 10.1128/jcm.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother. 2012;3(4):300–303. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fan H, Cai J, Li Y, Wu B, Hou Y, Xu S, Zhou F, Liu Y, Xuan W, Hu H, Sun J. High-resolution computed tomography manifestations of COVID-19 infections in patients of different ages. Eur J Radiol. 2020;126:108972. doi: 10.1016/j.ejrad.2020.108972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhang XR, Ju ZY, He WF. Advances in the research of cytokine storm mechanism induced by Coronavirus Disease 2019 and the corresponding immunotherapies. Chin J Burns. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R, Hu B, Zhang Z (2020c) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 10.1101/2020.03.22.20040758
- Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, Chan KH, Yuen KY, Gordon S, Guan Y, Peiris JS. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury FR, Nur Z, Hassan N, von Seidlein L, Dunachie S. Pandemics, pathogenicity and changing molecular epidemiology of cholera in the era of global warming. Ann Clin Microbiol Antimicrob. 2017;16(1):10. doi: 10.1186/s12941-017-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan ML, Maker G, Crighton E, Haile J, Murray DC, White NE, Byard RW, Bellgard MI, Mullaney I, Trengove R, Allcock RJ, Nash C, Hoban C, Jarrett K, Edwards R, Musgrave IF, Bunce M. Combined DNA, toxicological and heavy metal analyses provides an auditing toolkit to improve pharmacovigilance of traditional Chinese medicine (TCM) Sci Rep. 2015;5:17475. doi: 10.1038/srep17475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin MM, Prabhakar BS. Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential. Rev Med Virol. 2012;22(1):2–17. doi: 10.1002/rmv.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshabrawy HA, Coughlin MM, Baker SC, Prabhakar BS. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS ONE. 2012;7(11):e50366. doi: 10.1371/journal.pone.0050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Ramirez-Franco J, Desplantes R, Debreux K, Ferracci G, Wernert F, Blanchard MP, Maulet Y, Youssouf F, Sangiardi M, Iborra C, Popoff MR, Seagar M, Fantini J, Lévêque C, El Far O. Gangliosides interact with synaptotagmin to form the high-affinity receptor complex for botulinum neurotoxin B. Proc Natl Acad Sci USA. 2019;116(36):18098–18108. doi: 10.1073/pnas.1908051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi A, Noor S, Tippairote T, Dadar M, Menzel A, Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin Immunol. 2020;215:108409–108409. doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis-Stathopoulos I, Psaltopoulou T, Kastritis E, Terpos E, Dimopoulos MA. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020 doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough TC, Babcock GJ, Roberts A, Hernandez HJ, Thomas WD, Jr, Coccia JA, Graziano RF, Srinivasan M, Lowy I, Finberg RW, Subbarao K, Vogel L, Somasundaran M, Luzuriaga K, Sullivan JL, Ambrosino DM. Development and characterization of a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody that provides effective immunoprophylaxis in mice. J Infect Dis. 2005;191(4):507–514. doi: 10.1086/427242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Tao H, Yan Y, Huang SY, Xiao Y. Molecular mechanism of evolution and human infection with SARS-CoV-2. Viruses. 2020 doi: 10.3390/v12040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan ter Meulen M, Alexander BH, Bakker P, Edward N, van den Brink P, Gerrit J, Weverling M, Byron EE, Martina P, Bart L, Haagmans P, Thijs KP, de Kruif PJ, Wolfgang PM, Willy Spaan P, Hans R, Gelderblom M, Jaap GM, Albert DME, Osterhaus P. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363(9427):2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Zhang P, Tian Y, Wang J, Zeng H, Wang J, Jiao L, Chen Z, Zhang L, He H, He K, Liu Y (2020) Clinical significance of IgM and IgG test for diagnosis of highly suspected COVID-19 infection. medRxiv. 10.1101/2020.02.28.20029025
- Jiang Y, Guo D, Li C, Chen T, Li R. High-resolution CT features of the COVID-19 infection in Nanchong City: initial and follow-up changes among different clinical types. Radiol Infect Dis. 2020 doi: 10.1016/j.jrid.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang XH. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi T, Sharma P, Mathpal S, Pundir H, Bhatt V, Chandra S. In silico screening of natural compounds against COVID-19 by targeting Mpro and ACE2 using molecular docking. Eur Rev Med Pharmacol Sci. 2020;24(8):4529–4536. doi: 10.26355/eurrev_202004_21036. [DOI] [PubMed] [Google Scholar]
- Keicho N, Itoyama S, Kashiwase K, Phi NC, Long HT, Ha LD, Ban VV, Hoa BK, Hang NT, Hijikata M, Sakurada S, Satake M, Tokunaga K, Sasazuki T, Quy T. Association of human leukocyte antigen class II alleles with severe acute respiratory syndrome in the Vietnamese population. Hum Immunol. 2009;70(7):527–531. doi: 10.1016/j.humimm.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14(10):2986–3012. doi: 10.1038/s41596-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, Corbett KS, Graham BS, McLellan JS, Ward AB. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok KO, Lai F, Wei WI, Wong SYS, Tang JWT. Herd immunity—estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Li T, Peng T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir Res. 2013;97(1):1–9. doi: 10.1016/j.antiviral.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, Shen B, Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip KM, Shen S, Yang X, Keng CT, Zhang A, Oh HL, Li ZH, Hwang LA, Chou CF, Fielding BC, Tan TH, Mayrhofer J, Falkner FG, Fu J, Lim SG, Hong W, Tan YJ. Monoclonal antibodies targeting the HR2 domain and the region immediately upstream of the HR2 of the S protein neutralize in vitro infection of severe acute respiratory syndrome coronavirus. J Virol. 2006;80(2):941–950. doi: 10.1128/JVI.80.2.941-950.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wu P, Gao F, Qi J, Kawana-Tachikawa A, Xie J, Vavricka CJ, Iwamoto A, Li T, Gao GF. Novel immunodominant peptide presentation strategy: a featured HLA-A*2402-restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J Virol. 2010;84(22):11849–11857. doi: 10.1128/JVI.01464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk HKH, Li X, Fung J, Lau SKP, Woo PCY. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect Genet Evolut. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- Malgie J, Schoones JW, Pijls BG. Decreased mortality in COVID-19 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA., Jr Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Janssens W, Jensen ME, Kerley CP, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Trilok Kumar G, Urashima M, Camargo CA, Griffiths CJ, Hooper RL. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2):1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M, Herrler G, Klenk HD. Sialic acid receptors of viruses. Top Curr Chem. 2015;367:1–28. doi: 10.1007/128_2013_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet JK, Whittaker GR. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci USA. 2014;111(42):15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M, Gautret P, Colson P, Roussel Y, Dubourg G, Chabriere E, Honore S, Rolain JM, Fenollar F, Fournier PE, Lagier JC, Parola P, Brouqui P, Raoult D. Clinical efficacy of chloroquine derivatives in COVID-19 infection: comparative meta-analysis between the big data and the real world. New Microbes New Infect. 2020;38:100709. doi: 10.1016/j.nmni.2020.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed E-T, Haim HB, Jinzhao S. A single and two-stage, closed-tube, molecular test for the 2019 novel Coronavirus (COVID-19) at home, clinic, and points of entry. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11860137.v1. [DOI] [Google Scholar]
- Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- Ng OW, Keng CT, Leung CS, Peiris JS, Poon LL, Tan YJ. Substitution at aspartic acid 1128 in the SARS coronavirus spike glycoprotein mediates escape from a S2 domain-targeting neutralizing monoclonal antibody. PLoS ONE. 2014;9(7):e102415. doi: 10.1371/journal.pone.0102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar S, Ismail B, Bouslama Z, Djemel A (2020) In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and angiotensin converting enzyme 2 (ACE2) from natural products: quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against COVID-19 main protease active site and ACE2. ChemRxiv. 10.26434/chemrxiv.12181404.v1
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an update. Cureus. 2020;12(3):e7423–e7423. doi: 10.7759/cureus.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020 doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak DSK, Salunke DAA, Thivari DP, Pandey A, Nandy DK, Harish VKRD, Pandey DS, Chawla DJ, Mujawar DJ, Dhanwate DA, Menon DV. No benefit of hydroxychloroquine in COVID-19: results of systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. 2020;14(6):1673–1680. doi: 10.1016/j.dsx.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and Coronavirus disease 2019: what we know so far. Pathogens (Basel, Switzerland) 2020 doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020 doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratre YK, Vishvakarma NK, Bhaskar LVKS, Verma HK. Dynamic propagation and impact of pandemic influenza A (2009 H1N1) in children: a detailed review. Curr Microbiol. 2020 doi: 10.1007/s00284-020-02213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejnmark L, Bislev LS, Cashman KD, Eiriksdottir G, Gaksch M, Grubler M, Grimnes G, Gudnason V, Lips P, Pilz S, van Schoor NM, Kiely M, Jorde R. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS ONE. 2017;12(7):e0180512. doi: 10.1371/journal.pone.0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohollah V, Baradaran A, Mirzazadeh A, Bhaskar LV. Coronavirus-nephropathy; renal involvement in COVID-19. J Renal Inj Prev. 2020;9(2):e18. doi: 10.34172/jrip.2020.18. [DOI] [Google Scholar]
- Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, Allegrini P, Faliva MA, Peroni G, Nichetti M, Perna S. Self-care for common colds: the pivotal role of Vitamin D, Vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds-practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Altern Med eCAM. 2018;2018:5813095. doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P, Shekhar N, Prajapat M, Avti P, Kaur H, Kumar S, Singh S, Kumar H, Prakash A, Dhibar DP, Medhi B. In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain) J Biomol Struct Dyn. 2020 doi: 10.1080/07391102.2020.1753580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/ap-200220-0773. [DOI] [PubMed] [Google Scholar]
- Shen M, Zhou Y, Ye J, Abdullah Al-Maskri AA, Kang Y, Zeng S, Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, Zheng Y, Xu B, Xie Z, Lin L, Shang Y, Lu X, Shu S, Bai Y, Deng J, Lu M, Ye L, Wang X, Wang Y, Gao L. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020 doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo-Hernandez H, Rochwerg B, Lamontagne F, Han MA, Liu Q, Agarwal A, Agoritsas T, Chu DK, Couban R, Darzi A, Devji T, Fang B, Fang C, Flottorp SA, Foroutan F, Heels-Ansdell D, Honarmand K, Hou L, Hou X, Ibrahim Q, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Qasim A, Rada G, Riaz IB, Sadeghirad B, Sekercioglu N, Sheng L, Sreekanta A, Switzer C, Tendal B, Thabane L, Tomlinson G, Turner T, Vandvik PO, Vernooij RW, Viteri-Garcia A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci USA. 2004;101(12):4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder EJ, Bredenbeek PJ, Dobbe JC, Thiel V, Ziebuhr J, Poon LL, Guan Y, Rozanov M, Spaan WJ, Gorbalenya AE. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331(5):991–1004. doi: 10.1016/s0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, Mommaas AM. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck AW, Axmann M, Pfefferle S, Drosten C, Meyer B. A hexapeptide of the receptor-binding domain of SARS coronavirus spike protein blocks viral entry into host cells via the human receptor ACE2. Antivir Res. 2012;94(3):288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Li W, Murakami A, Tamin A, Matthews LJ, Wong SK, Moore MJ, Tallarico AS, Olurinde M, Choe H, Anderson LJ, Bellini WJ, Farzan M, Marasco WA. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA. 2004;101(8):2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J, van den Brink EN, Poon LL, Marissen WE, Leung CS, Cox F, Cheung CY, Bakker AQ, Bogaards JA, van Deventer E, Preiser W, Doerr HW, Chow VT, de Kruif J, Peiris JS, Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3(7):e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu X, Chong WP, Zhai Y, Zhang H, Zhang F, Wang S, Liu W, Wei M, Siu NH, Yang H, Yang W, Cao W, Lau YL, He F, Zhou G. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect. 2015;71(1):101–109. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink EN, Ter Meulen J, Cox F, Jongeneelen MA, Thijsse A, Throsby M, Marissen WE, Rood PM, Bakker AB, Gelderblom HR, Martina BE, Osterhaus AD, Preiser W, Doerr HW, de Kruif J, Goudsmit J. Molecular and biological characterization of human monoclonal antibodies binding to the spike and nucleocapsid proteins of severe acute respiratory syndrome coronavirus. J Virol. 2005;79(3):1635–1644. doi: 10.1128/JVI.79.3.1635-1644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma HK, Merchant N, Verma MK, Kuru CI, Singh AN, Ulucan F, Verma P, Bhattacharya A, Bhaskar L. Current updates on the European and WHO registered clinical trials of coronavirus disease 2019 (COVID-19) Biomedical journal. 2020;43(5):424–433. doi: 10.1016/j.bj.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma HK, Farran B, Bhaskar LVKS. Convalescent plasma transfusion a promising therapy for coronavirus diseases 2019 (COVID-19): current updates. Antibody Ther. 2020 doi: 10.1093/abt/tbaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Xiong X, Park YJ, Tortorici MA, Snijder J, Quispe J, Cameroni E, Gopal R, Dai M, Lanzavecchia A, Zambon M, Rey FA, Corti D, Veesler D. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5):1026–10391015. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SF, Chen KH, Chen M, Li WY, Chen YJ, Tsao CH, Yen MY, Huang JC, Chen YM. Human-leukocyte antigen class I Cw 1502 and class II DR 0301 genotypes are associated with resistance to severe acute respiratory syndrome (SARS) infection. Viral Immunol. 2011;24(5):421–426. doi: 10.1089/vim.2011.0024. [DOI] [PubMed] [Google Scholar]
- Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306(3):L217–230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283–289. doi: 10.2174/1871530314666140922143950. [DOI] [PubMed] [Google Scholar]
- Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Li X, Liu S, Sang Y, Gao S-J, Gao F. HIV-1 did not contribute to the 2019-nCoV genome. Emerg Microbes Infect. 2020;9(1):378–381. doi: 10.1080/22221751.2020.1727299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR Testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of Coronavirus Disease 2019 in China. JAMA Netw Open. 2020;3(4):e205619. doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N, Fantini J. Deciphering the glycolipid code of Alzheimer's and Parkinson's amyloid proteins allowed the creation of a universal ganglioside-binding peptide. PLoS ONE. 2014;9(8):e104751. doi: 10.1371/journal.pone.0104751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Yang R, Yoshinaka Y, Amari S, Nakano T, Cinatl J, Rabenau H, Doerr HW, Hunsmann G, Otaka A, Tamamura H, Fujii N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318(3):719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22(2):74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wu S, Hao X, Li X, Liu X, Ye S, Han H, Dong X, Li X, Li J, Liu J, Liu N, Zhang W, Pelechano V, Chen W-H, Yin X (2020b) Rapid colorimetric detection of COVID-19 coronavirus using a reverse tran-scriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic plat-form: iLACO. medRxiv. 10.1101/2020.02.20.20025874
- Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC (2020) Safety, tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 10.1101/2020.11.03.20224998
- Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Odiwuor N, Xiong J, Sun L, Nyaruaba RO, Wei H, Tanner NA (2020b) Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. medRxiv. 10.1101/2020.02.26.20028373
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chakraborti S, He Y, Roberts A, Sheahan T, Xiao X, Hensley LE, Prabakaran P, Rockx B, Sidorov IA, Corti D, Vogel L, Feng Y, Kim JO, Wang LF, Baric R, Lanzavecchia A, Curtis KM, Nabel GJ, Subbarao K, Jiang S, Dimitrov DS. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc Natl Acad Sci USA. 2007;104(29):12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]