Abstract
Background
With the unprecedented morbidity and mortality associated with the COVID-19 pandemic, a vaccine against COVID-19 is urgently needed. We investigated CoronaVac (Sinovac Life Sciences, Beijing, China), an inactivated vaccine candidate against COVID-19, containing inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), for its safety, tolerability and immunogenicity.
Methods
In this randomised, double-blind, placebo-controlled, phase 1/2 clinical trial, healthy adults aged 18–59 years were recruited from the community in Suining County of Jiangsu province, China. Adults with SARS-CoV-2 exposure or infection history, with axillary temperature above 37·0°C, or an allergic reaction to any vaccine component were excluded. The experimental vaccine for the phase 1 trial was manufactured using a cell factory process (CellSTACK Cell Culture Chamber 10, Corning, Wujiang, China), whereas those for the phase 2 trial were produced through a bioreactor process (ReadyToProcess WAVE 25, GE, Umea, Sweden). The phase 1 trial was done in a dose-escalating manner. At screening, participants were initially separated (1:1), with no specific randomisation, into two vaccination schedule cohorts, the days 0 and 14 vaccination cohort and the days 0 and 28 vaccination cohort, and within each cohort the first 36 participants were assigned to block 1 (low dose CoronaVac [3 μg per 0·5 mL of aluminium hydroxide diluent per dose) then another 36 were assigned to block 2 (high-dose Coronavc [6 μg per 0·5 mL of aluminium hydroxide diluent per dse]). Within each block, participants were randomly assigned (2:1), using block randomisation with a block size of six, to either two doses of CoronaVac or two doses of placebo. In the phase 2 trial, at screening, participants were initially separated (1:1), with no specific randomisation, into the days 0 and 14 vaccination cohort and the days 0 and 28 vaccination cohort, and participants were randomly assigned (2:2:1), using block randomisation with a block size of five, to receive two doses of either low-dose CoronaVac, high-dose CoronaVac, or placebo. Participants, investigators, and laboratory staff were masked to treatment allocation. The primary safety endpoint was adverse reactions within 28 days after injection in all participants who were given at least one dose of study drug (safety population). The primary immunogenic outcome was seroconversion rates of neutralising antibodies to live SARS-CoV-2 at day 14 after the last dose in the days 0 and 14 cohort, and at day 28 after the last dose in the days 0 and 28 cohort in participants who completed their allocated two-dose vaccination schedule (per-protocol population). This trial is registered with ClinicalTrials.gov, NCT04352608, and is closed to accrual.
Findings
Between April 16 and April 25, 2020, 144 participants were enrolled in the phase 1 trial, and between May 3 and May 5, 2020, 600 participants were enrolled in the phase 2 trial. 743 participants received at least one dose of investigational product (n=143 for phase 1 and n=600 for phase 2; safety population). In the phase 1 trial, the incidence of adverse reactions for the days 0 and 14 cohort was seven (29%) of 24 participants in the 3 ug group, nine (38%) of 24 in the 6 μg group, and two (8%) of 24 in the placebo group, and for the days 0 and 28 cohort was three (13%) of 24 in the 3 μg group, four (17%) of 24 in the 6 μg group, and three (13%) of 23 in the placebo group. The seroconversion of neutralising antibodies on day 14 after the days 0 and 14 vaccination schedule was seen in 11 (46%) of 24 participants in the 3 μg group, 12 (50%) of 24 in the 6 μg group, and none (0%) of 24 in the placebo group; whereas at day 28 after the days 0 and 28 vaccination schedule, seroconversion was seen in 20 (83%) of 24 in the 3 μg group, 19 (79%) of 24 in the 6 μg group, and one (4%) of 24 in the placebo group. In the phase 2 trial, the incidence of adverse reactions for the days 0 and 14 cohort was 40 (33%) of 120 participants in the 3 μg group, 42 (35%) of 120 in the 6 μg group, and 13 (22%) of 60 in the placebo group, and for the days 0 and 28 cohort was 23 (19%) of 120 in the 3 μg group, 23 (19%) of 120 in the 6 μg group, and 11 (18%) of 60 for the placebo group. Seroconversion of neutralising antibodies was seen for 109 (92%) of 118 participants in the 3 μg group, 117 (98%) of 119 in the 6 μg group, and two (3%) of 60 in the placebo group at day 14 after the days 0 and 14 schedule; whereas at day 28 after the days 0 and 28 schedule, seroconversion was seen in 114 (97%) of 117 in the 3 μg group, 118 (100%) of 118 in the 6 μg group, and none (0%) of 59 in the placebo group.
Interpretation
Taking safety, immunogenicity, and production capacity into account, the 3 μg dose of CoronaVac is the suggested dose for efficacy assessment in future phase 3 trials.
Funding
Chinese National Key Research and Development Program and Beijing Science and Technology Program.
Introduction
The on-going COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to high morbidity and mortality worldwide.1 Globally, as of Oct 28, 2020, 43·3 million laboratory-confirmed cases of SARS-CoV-2 infection have been reported, resulting in 1·15 million deaths.2
Although physical distancing, quarantine, and isolation were effective in limiting the number of people becoming infected during the pandemic in the short term, the absence of immunity in the population leave them susceptible to further waves of SARS-CoV-2 infection. Health-care workers, older people (aged >60 years), and those with underlying health conditions are at particularly high risk.3, 4 The shortage of an effective treatment for COVID-19 has led to quick action in the development of potential vaccines against the disease.
Since the outbreak began, researchers around the world have been trying to develop vaccines for COVID-19, with more than 198 vaccines currently in preclinical or clinical development.5 Frenetic efforts towards the development of a vaccine have led to several candidate vaccines, derived from multiple platforms and progressing to the clinical evaluation stage, including inactivated vaccines, live virus vaccines, recombinant protein vaccines, vectored vaccines, and DNA or RNA vaccines.6, 7, 8, 9, 10, 11, 12, 13, 14 Development of various vaccine platforms and strategies in parallel is essential because little is known of the nature of protective immune responses to COVID-19 and which vaccine strategies will be most successful is unclear.
CoronaVac (Sinovac Life Sciences, Beijing, China) is an inactivated vaccine candidate against COVID-19 that has shown good immunogenicity in mice, rats, and non-human primates with vaccine-induced neutralising antibodies to SARS-CoV-2, which could neutralise ten representative strains of SARS-CoV-2.15 Moreover, the results indicated CoronaVac provided partial or complete protection in macaques from severe interstitial pneumonia after a SARS-CoV-2 challenge, without observable antibody-dependent enhancement of infection, which support progression to clinical trials in humans.15
Research in context.
Evidence before this study
We searched PubMed and the American Medical Association website on Aug 13, 2020, for published research articles, with no language or date restrictions, using the search terms of “SARS-CoV-2”, “COVID-19”, “vaccine”, and “clinical trial”. The search results showed that the COVID-19 pandemic resulted in an unprecedented race to develop an effective vaccine. We identified preclinical data on three immunisations using two different doses of CoronaVac (3 μg and 6 μg per dose), an inactivated whole virus vaccine against COVID-19 developed by Sinovac Life Sciences (Beijing, China), providing partial or complete protection in macaques against SARS-CoV-2 challenge, without observable antibody-dependent enhancement of infection. We also identified a phase 2 clinical trial of another inactivated vaccine developed by Sinopharm (Beijing, China), which showed the incidence of adverse reactions was 19·0% within 28 days after two doses of vaccine (5 μg in 0·5 mL of diluent) in a day 0 and 21 vaccination schedule, and the seroconversion rates of the neutralising antibody detected by plaque reduction neutralisation test was 97·6% at 14 days after a day 0 and 21 vaccination schedule. The clinical study of CoronaVac can further provide safety and immunogenic evidence for the inactivated vaccine.
Added value of this study
In this first in-human study of CoronaVac, we used a phase 1/2 study design to screen the safety of two doses and two vaccination schedules in a dose-escalation study in a small cohort before expanding the study to a larger cohort to explore the immunogenicity of the vaccine in healthy adults. The immune response in the phase 2 study was substantially higher than in the phase 1 study, which might be due to the difference in preparation process of vaccine batches used in phase 1 and 2 resulting in a higher proportion of intact spike protein on the purified inactivated SARS-CoV-2 virions in the vaccine used in phase 2 than that used in phase 1.
Implications of all the available evidence
Data from this study support the approval of emergency use of CoronaVac in China, and three phase 3 clinical trials that are ongoing in Brazil, Indonesia, and Turkey.
Methods
Study design and participants
In this single-centre, double-blind, randomised, placebo-controlled, phase 1/2 clinical trial, participants were recruited from the community to assess two two-dose regimens of CoronaVac. The study was run at Jiangsu Provincial Center for Disease Control and Prevention (CDC) in Suining County, Jiangsu province, China. The phase 1 trial was dose-escalation study. In phase 1, participants were recruited and allocated sequentially (1:1), with no specific randomisation, to one of two vaccination schedules, with either a 14-day interval (the day 0 and 14 vaccination cohort) or a 28-day interval (the day 0 and 28 vaccination cohort) between doses. Within each cohort, the first 36 participants (block 1) were randomly assigned to either the low dose vaccine or placebo, and then after 7 days of follow-up for safety after the first dose, another 36 (block 2) were randomly assigned to either high-dose vaccine or placebo. Phase 2 was initiated after all participants in phase 1 has finished a 7-day safety observation period after the first dose. As in phase 1, participants were recruited and allocated (1:1) with no specific randomisation to one of the two vaccination-schedule cohorts, and then randomly assigned within each cohort to either low-dose vaccine, high-dose vaccine, or placebo.
Participants were eligible if they were healthy and aged 18–59 years. The key exclusion criteria were high-risk epidemiology history within 14 days before enrolment (eg, travel or residence history in Wuhan city and surrounding areas or other communities with case reports; contact history with someone infected with SARS-CoV-2); SARS-CoV-2 specific IgG or IgM positive in serum; positive PCR test for SARS-CoV-2 from a pharyngeal or anal swab sample; axillary temperature of more than 37·0°C; and known allergy to any vaccine component. A complete list of exclusion criteria is in the protocol.
Written informed consent was obtained from each participant before enrolment. The clinical trial protocol and informed consent form were approved by the Jiangsu Ethics Committee (JSJK2020-A021–02). This study was conducted in accordance with the requirements of Good Clinical Practice of China and the International Conference on Harmonisation.
Randomisation and masking
In both phase 1 and 2, no specific randomisation was used when allocating participants to the vaccinations schedule cohorts. In phase 1, participants in blocks 1 and 2 in each schedule cohort were randomly assigned (2:1) to either CoronaVac or placebo, and in phase 2, participants in each schedule cohort were randomly assigned (2:2:1) to either low-dose CoronaVac, high-dose CoronaVac, or placebo. The randomisation codes for each vaccination schedule cohort were generated individually, using block randomisation with a block size of six in phase 1 and a block size of five in phase 2, using SAS software (version 9.4). The randomisation code was assigned to each participant in sequence in the order of enrolment, and then the participants received the investigational products labelled with the same code. The vaccine and the placebo are identical in appearance. All participants, investigators, and laboratory staff were masked to treatment allocation.
Procedures
The phase 1 clinical trial was run in a dose-escalation manner. First, participants in block 1 were given the low dose of vaccine, and only after a successful safety observation 7 days after the first dose was the trial able to proceed and participants in block 2 be given the high dose of vaccine. The criteria that had to be met from the 7-day safety observation were that no life-threatening adverse events occur, no more than 15% of vaccinated participants report severe adverse events, and no other safety concerns in the opinion of the data monitoring committee (DMC) occur. The same conditions needed to be met 7 days after the first dose in block 2 of the phase 1 trial before the study could proceed to the phase 2 trial.
CoronaVac is an inactivated vaccine candidate against COVID-19, created from African green monkey kidney cells (Vero cells) that have been inoculated with SARS-CoV-2 (CN02 strain). At the end of the incubation period, the virus was harvested, inactivated with β-propiolactone, concentrated, purified, and finally absorbed onto aluminium hydroxide. The aluminium hydroxide complex was then diluted in a sodium chloride, phosphate-buffered saline, and water solution before being sterilised and filtered ready for injection. The placebo is just the aluminium hydroxide diluent solution with no virus. Both the vaccine and placebo were prepared in a Good Manufacturing Practice-accredited facility of Sinovac Life Sciences (Beijing, China) that is periodically inspected by the Chinese National Medical Products Administration committee for compliance. Vaccine of 3 μg and 6 μg in 0·5 mL of aluminium hydroxide diluent per dose and placebo in ready-to-use syringes were administered intramuscularly according to the dosing schedule of either day 0 and day 14, or day 0 and day 28, depending on the cohort. These vaccine doses had been found to be sufficient for protection against SARS-CoV-2 challenge in macaques.15 Cultivation technology by cell factory system (CellSTACK Cell Culture Chamber 10, Corning, Wujiang, China) was used in the preparation of the vaccine used in the phase 1 trial. However, for the phase 2 trial, we used a highly automated bioreactor (ReadyToProcess WAVE 25, GE, Umea, Sweden) to produce the vaccine to increase vaccine production capacity. After the immunogenicity results of the trial were obtained, we discovered that the change in manufacture of the vaccine optimised the cell culture and resulted in higher intact spike protein content of the vaccine batch for the phase 2 trial, which was unexpected. However, we were not aware of this antigen-level difference between the vaccine batches for the phase 1 and 2 trials when we obtained the ethical approval for the trials.
For the first 7 days after each dose, participants were required to record the injection-site adverse events (eg, pain, redness, swelling), or systemic adverse events (eg, allergic reaction, cough, fever) on paper diary cards. From day 8 to day 28 after each dose (and day 8 to day 14 for the first dose of the days 0 and 14 vaccination cohort), safety data were collected by spontaneous report from the participants combined with the regular visit (which occurred on day 8 and day 28 after each dose, and on day 8 and day 14 for the first dose in the days 0 and 14 vaccination schedule cohort). Serious adverse events were collected through the trial and will be collected until 6 months after the last dose. The reported adverse events were graded according to the China National Medical Products Administration guidelines.16 The causal association between adverse events and vaccination was determined by the investigators.
In the phase 1 trial, blood and urine samples were taken on day 3 after each dose and tested to investigate any abnormal changes of the haematology and biochemistry indexes. 7 days after each dose, blood and urine samples were taken to measure serum inflammatory factors including IL-2, IL-6, and TNF-α using the solid phase sandwich ELISA method to explore the underlying pathological immune responses. Blood samples were collected at days 0 (baseline), 7, 14, 21, 28, and 42 from participants in the day 0 and 14 vaccination cohort, and days 0, 28, 35, 42, and 56 from participants in the days 0 and 28 vaccination schedule cohort, to determine the levels of neutralising antibodies, receptor-binding domain (RBD)-specific IgG, S-specific IgG, and IgM. Additionally, T-cell responses were determined via IFN-γ detection on day 14 after each dose.
In the phase 2 trial, blood samples were collected on day 0, 28, and 56 from participants in the days 0 and 14 cohort, and on day 56 from participants in the days 0 and 28 cohort, to determine the levels of neutralising antibodies and RBD-specific IgG.
The neutralising antibodies to live SARS-CoV-2 (virus strain SARS-CoV-2/human/CHN/CN1/2020, GenBank number MT407649.1) were quantified using a micro cytopathogenic effect assay17 with a minimum four-fold dilution, and neutralising antibodies to pseudovirus18 were quantified with a minimum ten-fold dilution. The S-specific IgG and IgM were detected using the chemiluminescence qualitative kit (Auto Biotechnology, Zhengzhou, China). These antibody detection tests were done by the National Institute for Food and Drug Control (Beijing, China).
Additionally, antibody titres for RBD-specific IgG were quantified using the in-house ELISA kit from Sinovac, with a minimum 160-fold dilution. T-cell response was determined with the ELISpot method using a commercial kit (Human IFN γ ELISpotPRO [3420-2AST-10, AID]; Mabtech, Stockholm, Sweden). Further information on all methods is in the appendix 2 (pp 1–3). Additionally, in a post-hoc analysis, we tested serum samples from 117 convalescent patients who had previously had COVID-19 collected in the hospitals for neutralising antibodies to live SARS-CoV-2 using the same method as for the detection of serum neutralising antibodies to live SARS-CoV-2 in the phase 1 and 2 trials, to give a comparison of the vaccine-induced and infection-induced humoral immunity. Written informed consent was obtained from all these convalescent patients.
Outcomes
The primary safety endpoint was any adverse reactions within 28 days after each dose of study drug. Secondary safety endpoints were any abnormal changes in laboratory measurements at day 3 and in serum inflammatory factors 7 days after each dose of study drug. The secondary safety endpoints were prespecified only in the phase 1 trial.
The primary immunogenic endpoint was the seroconversion of neutralising antibodies to live SARS-CoV-2 at day 14 after the last dose in the days 0 and 14 vaccination cohort, or day 28 after the last dose in the days 0 and 28 vaccination cohort. Secondary immunogenic endpoints were geometric mean titres (GMTs) of neutralising antibodies to live SARS-CoV-2, RBD-specific IgG, S-specific IgG, and IgM. Exploratory endpoints were T-cell responses and, post hoc, GMTs of neutralising antibodies to psuedovirus. Seroconversion of antibodies was defined as a change from seronegative at baseline to seropositive or a four-fold titre increase if the participant was seropositive at baseline. The positive cutoff of the neutralising antibodies to live SARS-CoV-2 was 1/8, neutralising antibodies to pseudovirus was 1/30, and RBD-specific IgG was 1/160. Regarding the ELISpot measured T-cell response, the results were expressed as the number of spot-forming cells (SFCs) per 100 000 cells.
Other secondary endpoints are listed in the appendix 2 (p 4), including 6 month outcomes that are not available yet, which will be reported elsewhere.
Statistical analysis
We assessed the safety endpoints in the safety population, which included all participants who received at least one dose of study drug. We assessed immunogenic endpoints in the per-protocol population, which included all participants who completed their assigned two-dose vaccination schedule and with available antibody results.
We did not determine the sample size on the basis of a statistical power calculation, but followed the requirement of the National Medical Products Administration in China—ie, recruitment of at least of 20–30 participants in phase 1 and 500 participants in phase 2.
We used the Pearson χ2 test or Fisher's exact test for the analysis of categorical outcomes. We calculated 95% CIs for all categorical outcomes using the Clopper-Pearson method. We calculated GMTs and corresponding 95% CIs on the basis of standard normal distribution of the log-transformed antibody titre. We used the ANOVA method to compare the log-transformed antibody titre. When the comparison among all three groups showed significant difference, we then did pairwise comparisons. Hypothesis testing was two-sided and we considered p values of less than 0·05 to be significant.
An independent data monitoring committee consisted of one independent statistician, one clinician, and one epidemiologist was established before commencement of the study. Safety data were assessed and reviewed by the committee to ensure the suspension criteria of the dose-escalation part of phase 1 were not met and allow the further proceeding of the clinical trial.
We used SAS (version 9.3) for all analyses. This trial is registered with ClinicalTrials.gov, NCT04352608.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All the authors have full access to all the data in the study and the corresponding authors had final responsibility for the decision to submit for publication.
Results
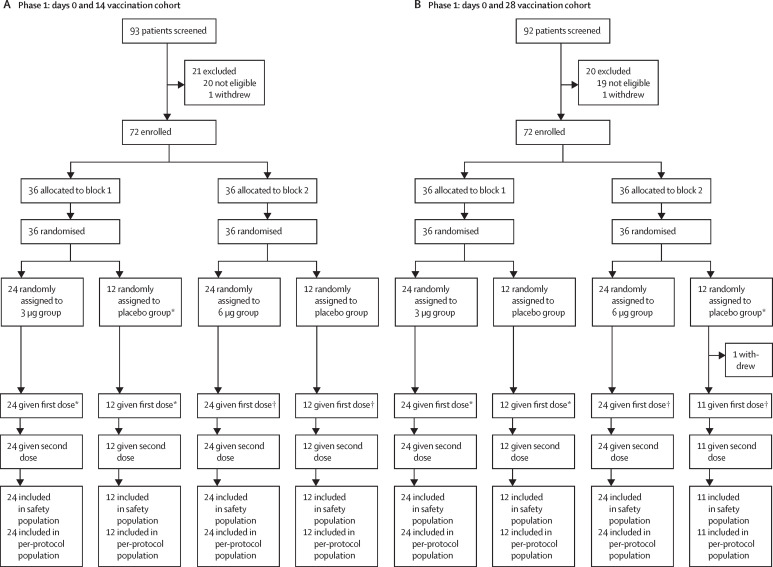
Between April 16 and April 25, 2020, 185 individuals were screened and 144 participants were enrolled in the phase 1 trial, and between May 3 and May 5, 2020, 662 individuals were screened and 600 participants were enrolled in the phase 2 trial. 743 participants received at least one dose of the investigational product (143 for phase 1 and 600 for phase 2) and were included in the safety population (figure 1 ). 143 participants in phase 1 and 591 participants in phase 2 were eligible for the immunogenic evaluation (per-protocol population; figure 1). Baseline demographic characteristics of the participants in the safety population at enrolment were similar among the treatment groups in terms of sex, nationality, and mean age (table 1 ).
Figure 1.
Study profile
*7 days after first dose, safety observation was done, and safety criteria were met, as determined by the data monitoring committee, participants in block 2 were then given their first dose of vaccine. †7 days after first dose of study drug in block 2, if safety criteria were met as determined by the data monitoring committee, participants enrolled in phase 2 were started on study treatment. ‡A participant in the 6 μg group was mistakenly given placebo rather than vaccine at the second dose; therefore, this participant was included in the 6 μg group dataset in the overall safety evaluation but not in the immunogenicity analysis. §Two participants did not have available antibody results, and so were not included in the immunogenicity analysis. ¶One participant did not have available antibody results, and so was not included in the immunogenicity analysis.
Table 1.
Baseline demographic characteristics for the safety population, phases 1 and 2 combined
| 3 μg group | 6 μg group | Placebo group | Overall | ||
|---|---|---|---|---|---|
| Days 0 and 14 vaccination cohorts, pooled | |||||
| Participants | 144 | 144 | 84 | 372 | |
| Sex | |||||
| Female | 77 (53%) | 86 (60%) | 44 (52%) | 207 (56%) | |
| Male | 67 (47%) | 58 (40%) | 40 (48%) | 165 (44%) | |
| Han nationality | 144 (100%) | 144 (100%) | 84 (100%) | 372 (100%) | |
| Age, years | 42·4 (10·2) | 42·8 (9·0) | 42·4 (8·8) | 42·6 (9·4) | |
| Days 0 and 28 vaccination cohorts, pooled | |||||
| Participants | 144 | 144 | 83 | 371 | |
| Sex | |||||
| Female | 75 (52%) | 70 (49%) | 45 (54%) | 190 (51%) | |
| Male | 69 (48%) | 74 (51%) | 38 (46%) | 181 (49%) | |
| Han nationality | 144 (100%) | 144 (100%) | 83 (100%) | 371 (100%) | |
| Age, years | 41·8 (9·4) | 41·2 (10·2) | 44·1 (9·1) | 42·1 (9·7) | |
Data are n, n (%), or mean (SD).
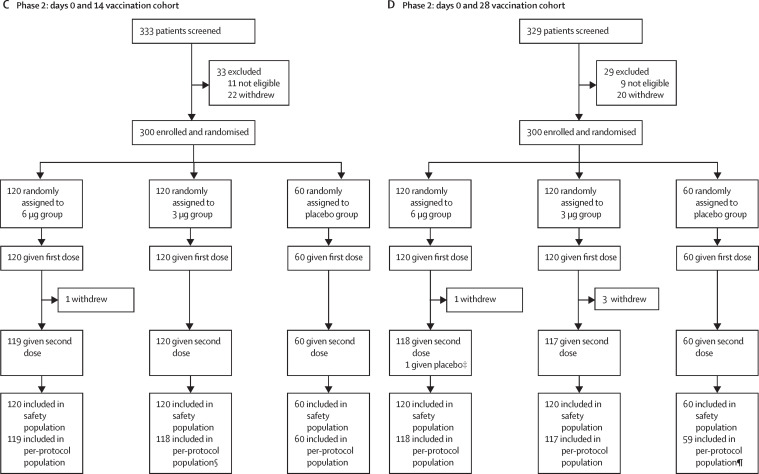
In the phase 1 trial, the overall incidence of adverse reactions was seven (29%) of 24 participants in the 3 μg group, nine (38%) of 24 in the 6 μg group, and two (8%) of 24 in the placebo group in the days 0 and 14 vaccination cohort; and three (13%) of 24 in the 3 μg group, four (17%) of 24 in the 6 μg group, and three (13%) of 23 in the placebo group in the days 0 and 28 vaccination cohort, with no significant difference seen among the three groups for both vaccination schedules (figure 2 ; appendix 2 pp 5–6). The most common symptom was injection-site pain, which was reported by four (17%) participants in the 3 μg group, five (21%) in the 6 μg, and one (4%) in the placebo group in the days 0 and 14 vaccination cohort and three (13%) in the 3 μg group, three (13%) in the 6 μg group, and three (13%) in the placebo group in the days 0 and 28 vaccination cohort. Most adverse reactions were mild (grade 1) in severity and participants recovered within 48 h. Only one case of acute hypersensitivity with manifestation of urticaria 48 h after the first dose of study drug was reported in the 6 μg group (one [4%] of 24) in the days 0 and 14 vaccination cohort, which was graded as severe and considered to be possibly related to vaccination. The participant was given chlorphenamine and dexamethasone and recovered within 3 days, and no similar reaction was observed after the second dose of vaccine. No vaccine-related serious adverse events were noted within 28 days of vaccination (figure 2; appendix 2 pp 4–5). Additionally, ten (7%) of 143 participants in phase 1 had a clinically significant increase of laboratory indicators at day 3 after vaccination (appendix 2 pp 15–16), but none was considered to be related to the vaccination. No significant increases in inflammatory factors in serum were detected at day 7 after each dose (appendix 2 pp 17–18).
Figure 2.
Incidence of adverse reactions reported within 28 days after second dose of study drug, in the days 0 and 14 vaccination cohort in phase 1 (A) and phase 2 (C) and in the days 0 and 28 vaccination cohort in phase 1 (B) and phase 2 (D)
Adverse reactions refer to the adverse events related to the vaccination. Rare injection-site symptoms reported only in the days 0 and 14 vaccination cohort are not shown in the figure and are listed in appendix 2 along with all adverse reactions after the first and second dose (pp 4–13). *The p value of comparison among three groups is significant for the incidence of any injection-site symptoms (p=0·02) and injection-site pain (p=0·04).
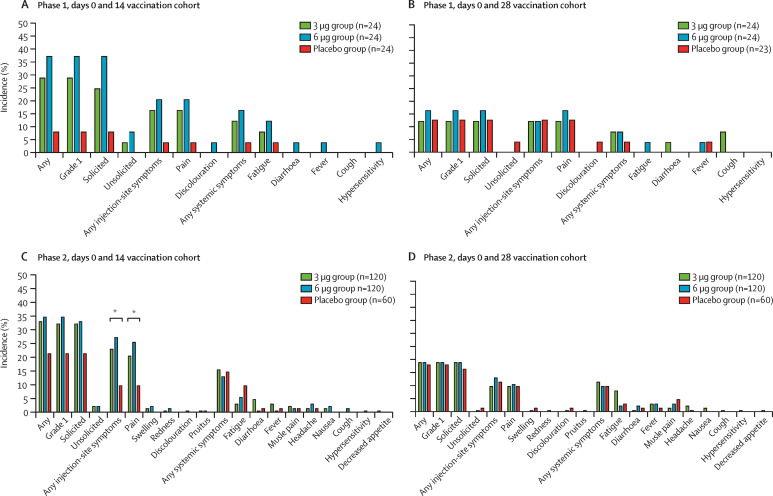
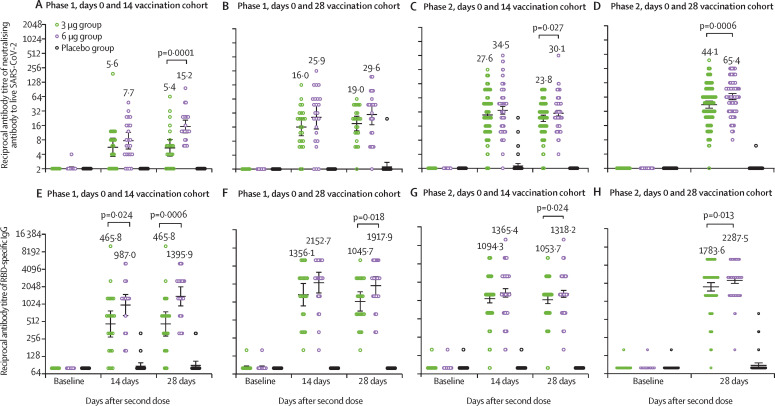
At baseline, none of the participants in the phase 1 trial had any detectable neutralising antibodies to live SARS-CoV-2. The seroconversion rates of neutralising antibodies were 11 (46%) of 24 participants in the 3 μg group (GMT 5·6 [95% CI 3·6–8·7]) versus 12 (50%) of 24 participants in the 6 μg group (7·7 [5·2–11·5]) versus none of 24 participants in the placebo group (2·0 [2·0–2·0]) at 14 days after the second dose, and six (25%) participants in the 3 μg group (5·4 [3·6–8·1] versus 20 (83%) in the 6 μg group (15·2 [11·2–20·7]) versus none in the placebo group (2·0 [2·0–2·0]) at 28 days after the second dose in the days 0 and 14 vaccination cohort; and 19 (79%) of 24 participants in the 3 μg group (16·0 [10·4–24·7]) versus 20 (83%) of 24 in the 6 μg group (25·9 [14·6–46·1) versus none of 23 in the placebo group (2·0 [2·0–2·0]) at 14 days after the second dose, and 20 (83%) in the 3 μg group (19·0 [13·2–27·4] versus 19 (79%) in the 6 μg group (29·6 [17·9–48·9]) versus one (4%) in the placebo group (2·2 [1·8–2·8]) at 28 days after the second dose in the days 0 and 28 vaccination cohort (table 2 , figure 3 ; appendix 2 p 19). The seroconversion rates of RBD-specific IgG were 20 (83%) of 24 participants in the 3 μg group (GMT 465·8 [95% CI 277·6–781·7] versus 24 (100%) of 24 participants in the 6 μg group (987·0 [647·8–1504·0]) versus two (8%) of 24 participants in the placebo group (84·8 [78·0–92·1]) at 14 days after the second dose, and 21 (88%) in the 3 μg group (465·8 [288·1–753·1]) versus 24 (100%) in the 6 μg group (1395·9 [955·2–2039·7]) versus two (8%) in the placebo group (89·8 [76·1–105·9]) at 28 days after the second dose in the days 0 and 14 vaccination cohort; and 24 (100%) of 24 participants in the 3 μg group (1365·1 [881·4–2086·4]) versus 24 (100%) of 24 participants in the 6 μg group (2152·7 [1446·1–3204·6]) versus none of 23 participants (80·0 [80·0-80·0]) in the placebo group at 14 days after the second dose, and 24 (100%) in the 3 μg group (1045·7 [721·6–1515·5]), versus 24 (100%) in the 6 μg group (1917·9 [1344·8–2735·2]) versus none in the placebo group (80·0 [80·0–80·0]) 28 days after the second dose in the days 0 and 28 vaccination cohort (table 2, figure 3; appendix 2 p 19). The dynamic changes of RBD-specific IgG, S-specific IgG, S-specific IgM, and neutralising antibodies to pseudovirus are shown in the appendix 2 (pp 19–23), showing that the antibody levels did not significantly increase until after the second dose of vaccine.
Table 2.
Seroconversion rates of neutralising antibodies to live SARS-CoV-2 and RBD-specific IgG
| 3 μg group | 6 μg group | Placebo group | p value* | |||
|---|---|---|---|---|---|---|
| Phase 1 | ||||||
| Days 0 and 14 vaccination cohort | ||||||
| Neutralising antibodies to live SARS-CoV-2 | ||||||
| Day 14 | 11/24 (45·8%; 25·6–67·2) | 12/24 (50·0%; 29·1–70·9) | 0/24 (0·0%; 0·0–14·3) | 0·77 | ||
| Day 28 | 6/24 (25·0%; 9·8–46·7) | 20/24 (83·3%; 62·6–95·3) | 0/24 (0·0%; 0·0–14·3) | <0·0001 | ||
| RBD-IgG | ||||||
| Day 14 | 20/24 (83·3%; 62·6–95·3) | 24/24 (100%; 85·8–100) | 2/24 (8·3%; 1·0–27·0) | 0·11 | ||
| Day 28 | 21/24 (87·5%; 67·6–97·3) | 24/24 (100%; 85·8–100) | 2/24 (8·3%; 1·0–27·0) | 0·23 | ||
| Days 0 and 28 vaccination cohort | ||||||
| Neutralising antibodies to live SARS-CoV-2 | ||||||
| Day 14 | 19/24 (79·2%; 57·9–92·9) | 20/24 (83·3%; 62·6–95·3) | 0/23 (0·0%; 0·0–14·8) | 1·00 | ||
| Day 28 | 20/24 (83·3%; 62·6–95·3) | 19/24 (79·2%; 57·9–92·9) | 1/23 (4·4%; 0·1–22·0) | 1·00 | ||
| RBD-IgG | ||||||
| Day 14 | 24/24 (100%; 85·8–100) | 24/24 (100%; 85·8–100) | 0/23 (0·0%; 0·0–14·8) | 1·00 | ||
| Day 28 | 24/24 (100%; 85·8–100) | 24/24 (100%; 85·8–100) | 0/23 (0·0%; 0·0–14·8) | 1·00 | ||
| Phase 2 | ||||||
| Days 0 and 14 vaccination cohort | ||||||
| Neutralising antibodies to live SARS-CoV-2 | ||||||
| Day 14 | 109/118 (92·4%; 86·0–96·5) | 117/119 (98·3%; 94·1–99·8) | 2/60 (3·3%; 0·4–11·5) | 0·030 | ||
| Day 28 | 111/118 (94·1%; 88·2–97·6) | 117/118 (99·2%; 95·4–100) | 0/60 (0·0%; 0·0–6·0) | 0·066 | ||
| RBD-IgG | ||||||
| Day 14 | 111/115 (96·5%; 91·3–99·0) | 118/118 (100%; 96·9–100) | 0/56 (0·0%; 0·0–6·4) | 0·058 | ||
| Day 28 | 111/114 (97·4%; 92·5–99·5) | 118/118 (100%; 96·9–100) | 0/57 (0·0%; 0·0–6·3) | 0·12 | ||
| Days 0 and 28 vaccination cohort | ||||||
| Neutralising antibodies to live SARS-CoV-2 | ||||||
| Day 28 | 114/117 (97·4%; 92·7–99·5) | 118/118 (100%; 96·9–100) | 0/59 (0·0%; 0·0–6·1) | 0·12 | ||
| RBD-IgG | ||||||
| Day 28 | 116/117 (99·2%; 95·3–100) | 117/117 (100%; 96·9–100) | 4/59 (6·8%; 1·9–16·5) | 1·00 | ||
Data are n/N (%; 95% CI). Timepoints refer to the number of days since the second dose of vaccine in the schedule. RBD=receptor binding domain. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
p values are for comparisons between the 3 μg and 6 μg groups.
Figure 3.
Antibody titres of neutralising antibodies to live SARS-CoV-2 (A–D) and RBD-specific IgG (E–H) induced after two doses of CoronaVac or placebo given in the days 0 and 14 and days 0 and 28 vaccination cohorts, in the phase 1 and phase 2 trials
The error bars indicate the 95% CI of the GMT and the spots indicated the individual antibody titres, with the numbers above the spots showing the GMT estimate. Only p values for significant differences are shown on the figure, all p values for all data are in appendix 2 (p 19). GMT=geometric mean titre. RBD=receptor binding domain. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
At 14 days after the second dose of study drug, the average IFN-γ-positive SFCs per 100 000 cells were 7·4 (95% CI 3·9 to 11·1) in the 3 μg group, 3·9 (1·0 to 6·7) in the 6 μg group, and 1·5 (0·2 to 2·9) in the placebo group for the days 0 and 14 vaccination cohort; and 3·4 (0·9 to 5·7) in the 3 μg group, 1·2 (0·5 to 1·8) in the 6 μg group, and 1·2 (−0·1 to 2·5) in the placebo group for the days 0 and 28 vaccination cohort (appendix 2 pp 25–26).
In the phase 2 trial, the overall incidence of adverse reactions were 40 (33%) of 120 in the 3 μg group, 42 (35%) of 120 in the 6 μg group, and 13 (22%) of 60 in the placebo group for the days 0 and 14 vaccination cohort and 23 (19%) of 120 in the 3 μg group, 23 (19%) of 120 in the 6 μg group, and 11 (18%) of 60 in placebo group in the days 0 and 28 vaccination cohort, with no significant difference between the three groups for both schedules. However, the p value of comparison among the three groups was significant for the incidence of any injection-site symptoms (p=0·02) and injection-site pain (p=0·04; figure 2; appendix 2 pp 7–10). The most common symptom was injection-site pain, which occurred in 25 (21%) of 120 participants in the 3 μg group, 31 (26%) of 120 in the 6 μg group, and six (10%) of 60 in the placebo group for the days 0 and 14 vaccination cohort, and 12 (10%) of 120 in the 3 μg group, 13 (11%) of 120 in the 6 μg group, and six (10%) of 60 in the placebo group in the days 0 and 28 vaccination cohort. Most adverse reactions were mild (grade 1) in severity and the participants recovered within 48 h. No vaccine-related serious adverse events were noted within 28 days of the second dose of vaccine (figure 2; appendix 2 pp 7–10)
In the phase 2 trial, at baseline, none of the participants had any detectable neutralising antibodies. The seroconversion rates of neutralising antibodies to live SARS-CoV-2 were 109 (92%) of 118 participants in the 3 μg group (GMT 27·6 [95% CI 22·7–33·5]) versus 117 (98%) of 119 participants in the 6 μg group (34·5 [28·5–41·8] versus two (3%) of 60 participants in the placebo group (2·3 [2·0–2·5]) at 14 days after the second dose, and 111 (94%) of 118 in the 3 μg group (23·8 [20·5–27·7]) versus 117 (99%) of 118 in the 6 μg group (30·1 [26·1–34·7]) versus none of 60 in the placebo group (2·0 [2·0–2·0]) at 28 days after the second dose in the day 0 and 14 vaccination cohort; and 114 (97%) of 117 participants in the 3 μg group (44·1 [37·2–52·2]) versus 118 (100%) of 118 participants in the 6 μg group (65·4 [56·4–75·9]) versus none of 59 participants in the placebo group (2·0 [2·0–2·1]) at 28 days after the second dose in the days 0 and 28 vaccination cohort (table 2, figure 3). In post-hoc analyses, the neutralising antibody titres after the second dose of vaccine was lower in all participants who received the vaccine than was detected in 117 convalescent asymptomatic patients who had previously had COVID-19 (GMT 163·7 [95% CI 128·5–208·6]; table 2, figure 3; appendix 2 p 24). The seroconversion rates of RBD-specific IgG were 111 (97%) of 115 participants in the 3 μg group (GMT 1094·3 [95% CI 936·7–1278·4]) versus 118 (100%) of 118 participants in the 6 μg group (1365·4 [1160·4–1606·7]) versus none of 56 participants in the placebo group (81·0 [79·0–83·0]) at 14 days after the second dose and 111 (97%) of 114 in the 3 μg group (1053·7 [911·7–1217·7]) versus 118 (100%) of 118 in the 6 μg group (1318·2 [1156·9–1501·9]) versus none of 57 in the placebo group (80·0 [80·0–80·0]) at 28 days after the second dose in the day 0 and 14 vaccination cohort; and 116 (99%) of 117 in the 3 μg group (1783·6 [1519·3–2093·8]) versus 117 (100%) of 117 in the 6 μg group (2287·5 [2038·2–2567·3]) versus four (7%) of 59 in the placebo group (87·9 [79·7–96·9]) at 28 days after the second dose in the days 0 and 28 vaccination cohort (table 2, figure 3).
Based on the pooled data of the phase 1 and 2 trials (two vaccination cohorts pooled), the correlation coefficient between the neutralising antibody to live SARS-CoV-2 and RBD-specific IgG was 0·85 (95% CI 0·82–0·92) using the antibody titre at 28 days after the second dose of vaccine, and was 0·80 (0·75–0·86) using the titre 14 days after the second. The correlation coefficient between the neutralising antibody to live SARS-CoV-2 and the neutralising antibody to pseudovirus was 0·82 (0·76–0·88) using the antibody titre at 14 days after the second dose (no data taken at day 28). The correlation coefficient between the neutralising antibody to pseudovirus and RBD-specific IgG was 0·73 (0·66–0·80) using the antibody titre at 14 days after the second dose (no data taken at day 28; appendix 2 p 24).
Discussion
We found that two doses of CoronaVac at different concentrations and using different dosing schedules were well tolerated and moderately immunogenic in healthy adults aged 18–59 years. The incidence of adverse reactions in the 3 μg and 6 μg group were similar, indicating no dose-related safety concerns but more long-term follow-up is needed. Furthermore, most adverse reactions were mild, with the most common symptom being injection-site pain, which is in accordance with previous findings for another inactivated COVID-19 vaccine from Sinopharm (Beijing China).14 Compared with other COVID-19 vaccine candidates, such as viral-vectored vaccines or DNA or RNA vaccines, the occurrence of fever after vaccination with CoronaVac was relatively low.10, 11, 13
Over the course of the phase 1/2 trial, we changed the production process of the vaccine from the use of a cell factory process (which was used in our preclinical and phase 1 study to generate a 50 L culture of Vero cells) to use of a bioreactor for phase 2. The bioreactor process enabled use to optimise the process for growing cells, with precise control over cell culture parameters like dissolved oxygen, pH, and carbon dioxide and oxygen gas levels. We made this change to increase vaccine production capacity and meet biosafety requirements. Pre-clinical data for each phase trial (data not shown) indicated that the safety profiles of vaccines prepared via the new bioreactor process and old process are similar. Notably, immune responses in phase 2 were much better than those recorded in phase 1, with seroconversion rates over 90% in both the 3 μg and 6 μg groups. To investigate the reason for this change, we did a protein composition analysis of the purified inactivated SARS-CoV-2 virions and found that the bioreactor-produced vaccine had a higher redundancy of intact spike protein (molecular mass approximately 180 kDa) than did the vaccine produced via the cell factory process (appendix 2 p 27). Quantitative analysis showed that the intact spike protein accounted for approximately 3·7% of total protein mass of the vaccine used in phase 1 and approximately 7·0% of total protein mass of the vaccine used in phase 2 trials. Electron microscopic examination of the samples further verified that the average number of spikes per virion of the viral sample used in the phase 2 trial was almost double the number of spikes per virion of the sample used in phase 1 trial (appendix 2 p 27). These observations highlight the importance of developing an optimum manufacturing process and the integration of multidisciplinary techniques, such as genomics and structural biology to support a new era of precision vaccinology.
The immune response induced by 3 μg and 6 μg of vaccine in 0·5 mL of diluent per dose was similar in this study. As anticipated, after two doses of vaccine, immune responses induced by the days 0 and 28 vaccination schedule were larger than those induced by the days 0 and 14 vaccination schedule, regardless of the dose. However, quick antibody responses could be induced within a relatively short time by using a day 0 and 14 vaccination schedule, which might be suitable for emergency use and is of vital importance during the COVID-19 pandemic. Regarding the days 0 and 28 vaccination schedule, a more robust antibody response was generated and longer persistence could be expected than with the days 0 and 14 schedule, which supports potential routine use of the vaccine according to this schedule when the epidemic risk of COVID-19 is low. However, the actual immune persistence of the two schedules needs to be verified in future studies.
In the phase 2 trial, the level of neutralising antibodies included by the vaccine at day 28 after the last dose of vaccine ranged from a GMT of 23·8 to 65·4, depending on the vaccination schedule, which was lower than those of convalescent patients who previously had COVID-19 with an average GMT level of 163·7, tested by the same method in the same laboratory.19 However, we still think that CoronaVac could provide satisfying protection against COVID-19 on the basis of the following three reasons. First, from the experiences of other vaccines, such as the enterovirus 71 and varicella vaccines, most of the surrogate endpoints based on neutralising antibody titres have ranged from 8 to 24.20, 21 Second, our preclinical study15 indicated that the neutralising antibody titres of 1/24 elicited in macaque models conferred complete protection against SARS-CoV-2. Third, although several studies have found that antibody responses generated from natural infection with coronaviruses (eg, SARS-CoV-2, severe acute respiratory syndrome coronavirus, and Middle East respiratory syndrome coronavirus) might decrease substantially over time,22, 23, 24 reinfection in these patients has rarely been reported,25, 26, 27 which indicates that immunological memory might have an important role of prevention of re-infections. Therefore, the antibody level itself might not be the key for a successful COVID-19 vaccine, but rather the establishment of a recallable specific immune response to SARS-CoV-2. Furthermore, the efficacy of the investigational vaccine and its surrogate endpoint need to be determined in a future phase 3 trial. Additionally, comparability of our serum antibody results with those of other COVID-19 vaccine studies is restricted.
Two participants in the placebo group in the phase 1 trial and four in the placebo group in the phase 2 trial had seroconversion of anti-RBD IgG after vaccination, and one participant given placebo in the phase 1 trial and two in the phase 2 trial had seroconversion of neutralising antibodies after vaccination.
CoronaVac was well tolerated and induced humoral responses against SARS-CoV-2, which supported the approval of emergency use of CoronaVac in China, and three phase 3 clinical trials that are ongoing in Brazil (NCT04456595), Indonesia (NCT04508075), and Turkey (NCT04582344). Taking safety, immunogenicity, and production capacity into account, the low dose of 3 μg of CoronaVac in 0·5 mL of diluent, with a day 0 and 14 vaccination schedule, is being investigated in these ongoing trials. And the days 0 and 28 vaccination schedule with 3 μg of Coronavac in 0·5 mL of diluent will also be investigated in future phase 3 clinical trials. The protective efficacy of CoronaVac remains to be determined.
Our study had several limitations. First, we did not assess the T cell responses in the phase 2 trial; however, the response of type 1 T-helper cells and type 2 T-helper cells induced by CoronaVac will be studied in the ongoing phase 3 study in Brazil (NCT04456595). Second, we only reported immune response data for healthy adults, and did not include individuals from more susceptible groups in our study population (eg, older individuals [aged ≥60 years] or with comorbidities); and data on immune persistence is not yet available, which need to be further studied. Third, the calculated p values presented in this study cannot support any powerful statistical conclusions, and are only for reference and so should be interpreted with caution. Additionally, the T-cell responses measured by ELISpot were low in participants who were given vaccine, which provided no clear evidence that the vaccine induced T-cell responses. The assessment of immune reactions mediated by CD8 cells was not included in our study design, because inactivated vaccines are not thought to induce CD8 T-cell responses. Finally, the change in the manufacturing of vaccine batches for the phase 2 trial resulted in a higher level of the spike antigen contained in the vaccine than was used in the phase 1 trial. Although the change in manufacturing process was planned, the difference in antigenicity of the vaccines was not anticipated, and could potentially bring additional risks for the recipients of the vaccine. Fortunately, the safety profiles of the vaccines in the phase 1 and 2 trials were similar, although the vaccines for the phase 2 trial had substantially stronger immunogenicity than did the vaccines for phase 1 trial. However, the comparisons between the vaccine batches were also not an a-priori defined outcome or sufficiently powered.
In summary, CoronaVac was well tolerated and induced humoral responses against SARS-CoV-2, which suppored the approval of emergency use of CoronaVac in China and in three phase 3 studies. The protective efficacy of CoronaVac remains to be determined.
Data sharing
The individual participant-level data that underlie the results reported in this Article will be shared after deidentification (text, tables, figures, and appendices). This clinical trial is ongoing, and all the individual participant data cannot be available until after the immune persistence assessments have been done. The data will be available immediately after publication and finalisation of the complete clinical study report for at least 6 months. Supporting clinical documents including study protocol, statistical analysis plan, and the informed consent form will be available immediately after publication of this Article for at least 1 year. Information on how to access the supporting clinical documents is available online. Researchers who provide a scientifically sound proposal will be allowed to access the de-identified individual participant data. Proposals should be sent to the corresponding authors, at jszfc@vip.sina.com or gaoq@sinovac.com. These proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.
Acknowledgments
Acknowledgments
This study was funded by the National Key Research and Development Program (2020YFC0849600) and Beijing Science and Technology Program (Z201100005420023).
Contributors
YZ, GZ, HP, and CL were co-first authors of this manuscript. FZ was the principal investigator and HP was the coprincipal investigator of this trial. FZ, GZ, RT, and QG designed the trial and study protocol. YZ, YaH, and WH contributed to the literature search. All authors had access to data and GZ, FZ, and HP verified the data. WH, JL, XW wrote the first draft the manuscript. FZ, YZ, GZ, WY, YaH, and MY contributed to the data interpretation and revision of the manuscript. YuH monitored the trial. XC, XL, CJ, and YS were responsible for the site work including the recruitment, follow up, and data collection, and KC was the site coordinator. CL and ZC were responsible to the laboratory analysis.
Declaration of interests
QG is an employee of Sinovac Life Sciences. GZ, YaH, WH, WY, and YuH are employees of Sinovac Biotech. All other authors declare no competing interests.
Supplementary Materials
References
- 1.Walker PGT, Whittaker C, Watson OJ, et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369:413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2020. Coronavirus disease (COVID-19) situation report - 163.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200701-covid-19-sitrep-163.pdf?sfvrsn=c202f05b_2 [Google Scholar]
- 3.Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 4.Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26:1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . World Health Organization; Geneva: Oct 19, 2020. Draft landscape of COVID-19 candidate vaccines.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Google Scholar]
- 6.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 7.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580:576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 8.Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- 9.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. published July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu F-C, Li Y-H, Guan X-H, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30831-8. published online Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q, Bao L, Mao H, Wang L, Qin C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.China National Medical Products Administration Guidelines for grading standards of adverse events in clinical trials of preventive vaccines. 2019. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460.html
- 17.Li P, Liang Z, Gao Q, et al. Safety and immunogenicity of a novel human enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blinded, phase I clinical trial. Vaccine. 2012;30:3295–3303. doi: 10.1016/j.vaccine.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Nie J, Li Q, Wu J, et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Guo X, Xin, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa721. published online June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao B, Chen Z, Zeng G, et al. Efficacy, safety and immunogenicity of live attenuated varicella vaccine in healthy children in China: double-blind, randomized, placebo-controlled clinical trial. Clin Microbiol Infect. 2019;25:1026–1031. doi: 10.1016/j.cmi.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Jin P, Li J, Zhou Y, et al. Immunological surrogate endpoints to evaluate vaccine efficacy. Chin J Prev Med. 2015;49:1110–1114. (in Chinese). [PubMed] [Google Scholar]
- 22.Chen W, Xu Z, Mu J, et al. Antibody response and viraemia during the course of severe acute respiratory syndrome (SARS)-associated coronavirus infection. J Med Microbiol. 2004;53:435–438. doi: 10.1099/jmm.0.45561-0. [DOI] [PubMed] [Google Scholar]
- 23.Prévost J, Gasser R, Beaudoin-Bussières G, et al. Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne DC, Iblan I, Rha B, et al. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg Infect Dis. 2016;22:1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K, Lau JY-N, Yang L, Ma Z-G. SARS-CoV-2 reinfection in two patients who have recovered from COVID-19. Precis Clin Med. 2020 doi: 10.1093/pcmedi/pbaa031. published online Sept 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan PKS, Lui G, Hachim A, et al. Serologic responses in healthy adult with SARS-CoV-2 reinfection, Hong Kong, August 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2612.203833. published online Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual participant-level data that underlie the results reported in this Article will be shared after deidentification (text, tables, figures, and appendices). This clinical trial is ongoing, and all the individual participant data cannot be available until after the immune persistence assessments have been done. The data will be available immediately after publication and finalisation of the complete clinical study report for at least 6 months. Supporting clinical documents including study protocol, statistical analysis plan, and the informed consent form will be available immediately after publication of this Article for at least 1 year. Information on how to access the supporting clinical documents is available online. Researchers who provide a scientifically sound proposal will be allowed to access the de-identified individual participant data. Proposals should be sent to the corresponding authors, at jszfc@vip.sina.com or gaoq@sinovac.com. These proposals will be reviewed and approved by the sponsor, investigator, and collaborators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement.