Abstract
During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, patients with defective immunity after chemo-immunotherapy due to hematological disorders showed prolonged symptoms and worse prognosis of coronavirus disease-2019 (COVID-19) pneumonia, probably due to inadequate adaptive immune response and noneffective viral clearance. We describe a single-center series of hematological immunocompromised patients undergoing passive immunization with hyperimmune plasma for persistent COVID-19 symptoms. In all cases, such treatment was well tolerated and contributed to clinical and radiological improvement and recovery; viral clearance was also achieved in a patients’ subset. Although requiring further investigation, these results suggest a specific role for hyperimmune plasma administration in hematological patients.
Keywords: COVID-19, hyperimmune plasma, immunodeficiency, onco-hematological patients
Introduction
The epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a major concern all over the world. To date, no specific therapeutic agents or vaccines for coronavirus disease-2019 (COVID-19) are available [1]. Hyperimmune plasma was exploited as an empirical treatment during outbreaks of Ebola virus in 2014 and other viral infections, such as SARS-CoV [2], H5N1 avian influenza [3], and H1N1 influenza [4], showing efficacy in terms of shorter hospital stay and lower mortality. In a case series of 5 critically ill patients with COVID-19 and acute respiratory distress syndrome (ARDS), a possible contribution to improved clinical status has been reported after the use of hyperimmune plasma [5]. A plausible explanation for its therapeutic efficacy relies in the putative activity of virus-specific neutralizing antibodies, which might result in direct clearance of COVID-19 [6–7].
Patients receiving chemo-immunotherapy as a treatment of lymphoproliferative or other hematological disorders usually develop prolonged, severe iatrogenic immunosuppression, eventually worsening the immune dysfunction already present at diagnosis in several patients. Treatment often includes the anti CD20 antibody rituximab, resulting in a delayed, marked hypogammaglobulinemia and/or lymphopenia. After the end of treatment, reconstitution of innate and adaptive immunity may take several months, causing an increased risk of opportunistic infections and hampering an efficient antimicrobial response.
During the outbreak of SARS-CoV-2 in Lombardy, we noticed an unusual persistency of COVID-19-related symptoms in some patients previously treated at our Institution with chemo-immunotherapy. Fever and/or respiratory symptoms lasted even more than 2 months and were consistently associated with continuous nasopharyngeal swab positivity. These reports are in keeping with other recent observations [8–10] and raised our interest in the administration of hyperimmune plasma from patients who had recovered after SARS-CoV2, aiming to providing virus-specific neutralizing antibodies in those patients unable to develop an adequate adaptive immunity.
Here, we describe a single-center preliminary experience of patients with immunologic deficiency after chemo-immunotherapy with persistent symptomatic infection due to SARS-CoV2 treated with hyperimmune plasma.
Methods
Patients
Seven patients with COVID-19 infection previously treated with chemo-immunotherapy due to hematological disorders and related immunodeficiency were identified between 30 March and 30 June 2020. Patients were regularly followed in our Center and their follow-up was updated at 31 October. All patients had a PCR-confirmed diagnosis of SARS-CoV2 and were hospitalized at our institution due to persistent (more than 6 weeks) clinical and radiological signs of interstitial pneumonia.
Treatment
Three infusions of hyperimmune plasma (each 210 mL) were given on an alternate day basis within a dedicated study protocol for patients considered more vulnerable due to an immunologic deficiency after chemo-immunotherapy for hematological neoplasms and to the persistence of infection symptoms.
All patients also received an empirical treatment for COVID-19 that included antibiotics, low molecular weight heparin, corticosteroid and hydroxychloroquine, as per institutional protocol. This study protocol was in accordance with the Declaration of Helsinki Institutional and was approved by the institutional review board.
Results
Cases
The clinical characteristics of the patients are summarized in Table 1.
Table 1.
Summary of clinical characteristics by individual patients.
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age, y | 48 | 60 | 60 | 43 | 70 | 69 | 60 |
| Sex | W | M | M | M | M | M | M |
| Hematological diagnosis | FL | FL | Indolent lymphoma | PMF | DLBCL | AML | CLL |
| Time since last chemo-immunotherapy [weeks] | 8 | 2 | 6 | 8 | 1 | 13 years | 48 |
| Type of chemo-immunotherapy | R-CHOP + R maintenance | RB + R maintenance | RB | alloHSCT | R-CHOP | alloHSCT | FCR |
| 1st line treatment for COVID-19 | |||||||
| Corticosteroid | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ |
| Hydroxychloroquine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| LMWH | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Antibiotics | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| - Gammaglobulin levels [mg/dL] | 330 | 380 | 210 | 610 | 180 | 380 | 400 |
| - Lymphocyte count [/mmc] | 1,220 | 120 | 250 | 200 | 430 | 170 | 1,290 |
| - CRP [mg/dL] | 6.5 | 10 | 0.1 | 6.9 | 7.5 | 6.8 | 3.7 |
| CRP after plasma [mg/dL] | 0.4 | 4.7 | 0.1 | 0.5 | 0.5 | 1.5 | 0.2 |
| COVID-19 symptoms resolved/improved | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Negativization of nasopharyngeal swab | ✓ | ✓ | NA | ✓ | ✓ | ✓ | ✓ |
| radiological improvement | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ |
| IgG anti-COVID post plasma infusion | ✓ | X | X | ✓ | X | X | ✓ |
X: not; ✓: yes; FL: follicular lymphoma; PMF: primary myelofibrosis; DLBCL: diffuse large B cell lymphoma; AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia; R: rituximab; RB: rituximab and bendamustine; alloHSCT: allogeneic hematopoietic stem cell transplant; FCR: fludarabine, cyclophosphamide and rituximab; LMWH: low-molecular-weight heparin; CRP: C reactive protein.
Patient 1, a 48-year-old female, with stage IV follicular lymphoma receiving treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) obtaining complete remission and still under rituximab maintenance. She was admitted on April 20th after a longer than 1-month history of fever and cough, progressively worsening till the appearance of dyspnea.
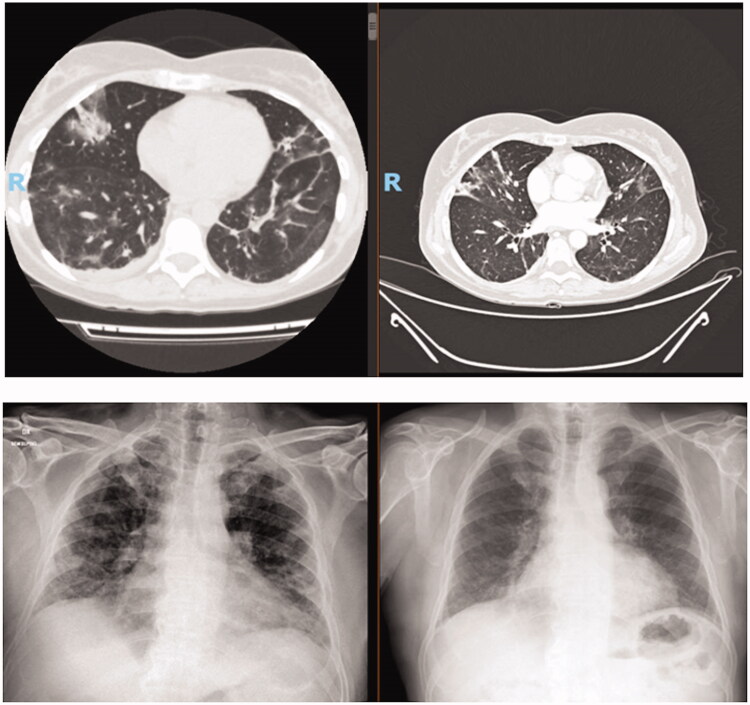
After a short course of noninvasive ventilation with CPAP alternated to low flow oxygen and empiric treatment as per institutional protocol, the patient rapidly recovered and could be discharged. Despite the initial improvement, high-grade fever and worsening dyspnea reappeared at withdrawal of corticosteroid therapy 10 days after the discharge. Therefore, the patient was readmitted to hospital being again in need of an intensive oxygen support (oxygen 10 l/min with venturi mask). A chest CT scan showed remarkable extension of lung infiltrates. As expected, nasopharyngeal swab still tested positive. Her blood tests showed deep hypogammaglobulinemia, compatible with her past history. This patient received 3 doses of plasma hyperimmune. All symptoms fully resolved in 2 days after the second infusion with oxygen weaning in a few days after the first infusion of plasma. A new chest CT scan repeated 5 days after the first plasma infusion documented extensive reduction and/or resolution of lung infiltrates (Figure 1). Patient was discharged the day after the last infusion. Moreover, a serological test performed after 30 days from last plasma administration showed positivity of anti-SARS-CoV2 IgG.
Figure 1.
Radiological improvement before and after infusion of hyperimmune plasma in patients 1 and 7.
Patient 2, a 60-year-old man, who received first-line treatment for a follicular lymphoma with bendamustine and rituximab and still under maintenance with rituximab, was admitted on April 5th after experiencing diarrhea, cough and dyspnea for 1 week. His medical history included 1st-grade obesity, hypertension, ischemic heart disease and chronic atrial fibrillation. The patient quickly recovered after the initiation of empiric anti-COVID-19 treatment as per institutional protocol and low flow oxygen therapy; within 1 week, he was discharged with no more need of ventilatory support. However, shortness of breath reappeared soon after withdrawing corticosteroid therapy, requiring new admittance to hospital 15 days later. Naso-pharyngeal swab revealed persistency of COVID-19 and a CT lung scan demonstrated further spread and worsening of the interstitial lesions. Blood tests showed severe lymphopenia and hypogammaglobulinemia. Although dyspnea immediately improved after resuming low-dose prednisone, the patient was still unable to carry on without low flow oxygen support. Starting from May 15th, the patient received three doses of hyperimmune plasma with complete resolution of symptoms and oxygen weaning 2 days after the first dose. The patient was discharged on May 21st. Nasopharyngeal swab finally resulted negative after 4 weeks since last plasma infusion. Radiological check by lung CT scan performed after 6 weeks from plasma infusion showed improvement of interstitial lesions. One month after last hyperimmune plasma infusion, anti-SARS-CoV2 IgG still resulted negative.
Patient 3, a 60-year-old man, was diagnosed with an indolent non-Hodgkin’s lymphoma and for this reason he received rituximab and bendamustine as first line treatment from September 2019 till March 2020. In April 2020 he was admitted for persistent fever and dry cough, albeit not requiring oxygen support. His medical history was remarkable for ischemic cardiomyopathy and pulmonary thromboembolism. He underwent institutional treatment for SARS-COV2 pneumonia without benefit with a persistent fever and dry cough. He didn’t need oxygen therapy but he had a radiological worsening. For this reason treatment with hyperimmune plasma was started on May 15th observing a progressive resolution of symptoms leading to patient’s discharge after the third administration of plasma. Anti-SARS-CoV-2 antibodies results persisted negative after 1 month from the last plasma infusion.
Patient 4, a 43-year-old man, with a medical history of myocardial infarction and insulin-dependent diabetes, in November 2019 underwent haploidentical stem cell transplantation for Myelofibrosis. For this reason, as part of the conditioning regimen and the graft-versus-host-disease (GvHD) prophylaxis, he received several immunosuppressive drugs, including rituximab, cyclophosphamide, mycophenolate and calcineurin inhibitors. On 30 March 2020, he was admitted after a 1-week history of fever, diarrhea, cough and dyspnea and the diagnosis of COVID-19 was confirmed by molecular diagnosis of the nasal swab. The patient was treated according to the institutional protocol and low flow oxygen therapy, achieving rapid resolution of COVID-19 symptoms and after 2 weeks was transferred to a less intensive department considering the initial improvement. Unfortunately, fever and dyspnea rebound after 2 weeks with a persistent positivity of the naso-pharyngeal swab. A new CT lung scan demonstrated further extension of the previously reported interstitial lesions and due to a progressive worsening of dyspnea; he was re-admitted for more intensive assistance in a sub-intensive ward. During the re-admission he was treated with 3 doses of hyperimmune plasma in addition to low oxygen therapy (4 l/min with nasal prongs). After the second plasma infusion, a significant improvement of symptoms was observed with their complete resolution within 1 week. A serological test performed after 2 months documented the achievement of anti-SARS-CoV2 IgG positivity.
Patient 5, a 70-year-old man, with a large B-cell lymphoma completed 6 courses of rituximab plus CHOP on February 2020. Two weeks later he presented to the emergency room of our hospital with fever and dyspnea. Blood tests on admittance showed remarkable lymphopenia and hypogammaglobulinemia. SARS-COV2 pneumonia was confirmed by molecular diagnosis of the nasal swab. The patient was treated according to our institutional protocol and needed low flow oxygen support for a short time. After 2 weeks, he was discharged in good general conditions. Nevertheless, 10 days later, he came back to the emergency room because of reappearance of the initial symptoms. Naso-pharyngeal swab and bronchoalveolar lavage confirmed the COVID-19 persistent positivity and the lung CT scan showed a worsening of bilateral interstitial pneumonia. He needed oxygen ventilation with venturi mask al 8 l/min. After 3 infusions of hyperimmune plasma, a rapid clinical improvement was observed, along with the normalization of inflammatory index. He stopped oxygen therapy 4 days after the first plasma infusion and discharged 2 days after the third plasma infusion. Remarkably, a surveillance nasopharyngeal swab turned out negative after just 1 week since the last plasma administration. One month after the last infusion of plasma we observed a mild positive anti-SARS-CoV-2 IgG result, unfortunately not confirmed in the subsequent serological evaluations.
Patient 6, a 69-year-old man, in 2006 received an allogeneic stem cell transplantation for acute myeloid leukemia. After the transplant he received multiple lines of immunosuppressive drugs (rituximab and corticosteroids) for the treatment of chronic GvHD and for this reason he was severely hypogammaglobulinemic. In April 2020 a molecularly confirmed diagnosis of SARS-CoV2 interstitial pneumonia, was posed. Initially he did not require oxygen support and remained under mild supportive therapy (antibiotics and hydroxychloroquine) as an outpatient. After an initial improvement, the clinical picture progressively deteriorated, until he needed hospitalization due to severe dyspnea on May 20th. During his hospital stay, he was managed with the administration of a second line of broad spectrum antibiotics and iv immunoglobulins (400 mg/kg) replacement was provided without any clinical and radiological improvement. He needed oxygen therapy. Thus, hyperimmune plasma therapy was started on May 21st and we observed a quick clinical recovery. The patient was discharged without symptoms on May 28th. Nasopharyngeal swab repeated after 2 days from the third plasma infusion was negative, while anti-SARS-CoV2 IgG were negative after 1 month.
Patient 7, a 60-year-old man, suffering from chronic lymphocytic leukemia requiring a first line treatment with fludarabine, cyclophosphamide and rituximab 5 years before. Medical history included a noninsulin-dependent diabetes and hypertension. He was admitted to Hospital on April 2020 for fever and severe dyspnea requiring transfer to ICU to receive high-intensity ventilatory assistance with orotracheal intubation. After initial clinical recovery, patient was transferred to a sub-intensive care unit, but unfortunately he was sent back to ICU due to a re-worsening of respiratory failure. After 1 month of intensive care, the patient experienced a clinical improvement allowing his transfer to a rehabilitation clinic. Two weeks later he experienced a further episode of fever and dyspnea, deeming new admittance to hospital. Naso-pharyngeal swab was positive for COVID-19 and lung CT scan showed an initial worsening of interstitial pneumonia. Alternative sources of infection were excluded by bronchoalveolar lavage. He needed oxygen 10 l/min with venturi mask. From June 10th, he was treated with plasma hyperimmune and after 2 days since last plasma infusion, a remarkable clinical improvement was observed with oxygen weaning within a week. A chest radiography performed on 16th June showed a remarkable regression of lung infiltrates. Finally, anti-SARS-CoV2 IgG positivity was observed 2 months later.
Outcomes
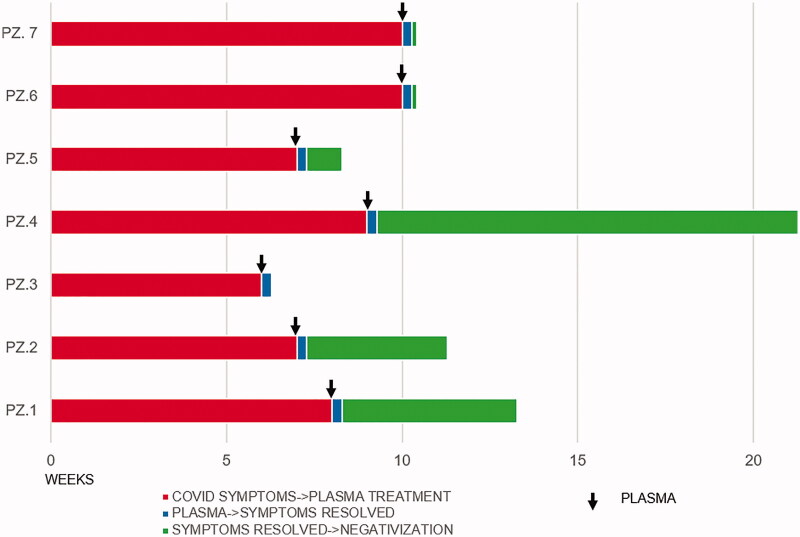
In all presented cases, patients showed a relevant immunocompromised state consistent with their previous medical history and faced long-lasting Sars-COV2-associated symptoms with documented persistency of viral infection despite state-of-the-art treatment, suggesting impaired ability to achieve long-term viral clearance based on their own immune response (Table 1 and Figure 2). Moreover, for the majority of them some features of clinical frailty were reported, due to age, comorbidities or progressive deterioration, which made undesirable the protraction of even non severe conditions related to the COVID-19 infection. In these patients, hyperimmune plasma was administered, aiming to provide virus-specific neutralizing antibodies. Overall, such treatment was well tolerated and in all cases resulted in a clinical benefit in terms of regression of fever, cough and/or dyspnea, less intensive oxygen requirement and rapid fall of the inflammatory marker CRP. Moreover, we succeeded in documenting viral clearance by nasopharyngeal swabs in 5 patients out of 7 (Figure 2). Finally, specific anti-SARS-CoV2 IgG positivity was observed for 3 out of 7 patients (Table 1).
Figure 2.
Summary of clinical course by individual patients and radiological improvement before and after infusion of hyperimmune plasma in patients 1 (upper quadrant) and 7 (lower quadrant).
Discussion
We report a case series of hematological patients with immunodeficiency due to previous chemo-immunotherapy or allogeneic stem cell transplantation and SARS-CoV-2 infection with persisting viremia. Most standard therapies for lymphomas include immunotherapy, such as rituximab, inducing a severe secondary immune deficiency, characterized by a B-cell depletion and decreased levels of serum immunoglobulins. Similarly, patients undergoing allogeneic stem cell transplantation show a pronounced immunological deficiency due to lympho-depletive chemotherapy and standard GvHD prophylaxis with calcineurin inhibitors that interfere with T and B lymphocytes function. The compromised immune system associated with hematological neoplasia might explain the higher risk of poor prognosis recently reported in patients with hematological malignancies and SARS-CoV-2 infection [11–12].
Hyperimmune plasma is a potential therapy successfully used in other infections such as SARS, influenza A or Ebola [13–15]. Based on these previous experiences, convalescent plasma therapy has been proposed also as potential treatment for patients with COVID-19 [16]. A plausible explanation for the benefit of hyperimmune plasma is to provide immunity by giving patients neutralizing antibodies for SARS-CoV-2. The results of the first randomized clinical trial of convalescent plasma for COVID-19 patients showed a greater efficacy of plasma use in less ill patients compared to critically ill patients [17]. This finding suggests a beneficial effect of convalescent plasma in terms of antibodies mediated viral elimination, with a limited effect on the organ damage due to the cytokine storm.
Our case series confirm that in patients with defects in immunity after chemo-immunotherapy and persistent symptoms of infection, but not advanced disease requiring mechanical ventilation, the use of hyperimmune plasma contributed to a prompt improvement and recovery of symptoms, eventually achieving a complete viral clearance and in some cases specific anti-SARS-CoV2 IgG antibody. In addition, we observed an immediate reduction of C-reactive protein (CRP), an inflammatory marker that reflects systemic inflammatory response typically observed in patients with COVID-19. The long-lasting duration of symptoms and the absence of benefit from previous treatments (including steroids, heparin and antiviral agents) suggest that antibodies from convalescent plasma might be indeed responsible for the therapeutic effect in our patients, although the late, additive effects of the several other therapies used prior to hyperimmune plasma cannot formally be ruled out. Moreover, it is not possible to establish whether patients might have recovered without the administration of passive immunotherapy. No significant adverse events attributable to plasma infusion were observed. Of interest, these results are fully in keeping with those of a very recent report of 17 patients showing that hyperimmune plasma may be an efficient and safe treatment in B-cell–depleted patients and protracted COVID-19 disease [18]. In conclusion, to the best of our knowledge this case series is novel in showing that hyperimmune plasma can be an effective and well tolerated therapy for patients with COVID-19 infection associated with immunodeficiency due to previous chemo-immunotherapy. As we acknowledge the limitations of this preliminary experience, further information needs to be acquired to know whether or not this type of passive immunotherapy could be specifically effective in this clinical setting.
Author contributions
SF, CC, and AW performed research, collected, analyzed and interpreted clinical data and wrote the manuscript. SF, AR, and FL designed and supervised the research. All authors revised the manuscript and approved the final version before submission.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Mair-Jenkins J, Saavedra-Campos M, Baillie K JK , et al. ; Convalescent Plasma Study Group The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS, Lee N, Chan PK, et al. . The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 2018;150:202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung IFN, To KKW, Lee CK, et al. . Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Influenza A (H1N1) Infection. Chest. 2013;144(2):464–473. [DOI] [PubMed] [Google Scholar]
- 5.Shen C, Wang Z, Zhao F, et al. . Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Xiong J, Bao L, et al. . Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen C, Chen J, Li R, et al. . A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci Transl Med. 2017;9(412):eaam5752. [DOI] [PubMed] [Google Scholar]
- 8.Shah V, Ko Ko T, Zuckerman M, et al. . Poor outcome and prolonged persistence of SARS-CoV-2 RNA in COVID-19 patients with hematological malignancies; King’s College Hospital experience. Br J Haematol. 2020;190(5):e279–e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tepasse P-R, Hafezi W, Lutz M, et al. . Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. 2020;190(2):185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi B, Manish M, Choudhary DC, Regan J, et al. . Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta V, Goel S, Kabarriti R, et al. . Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai M, Liu D, Liu M, et al. . Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnouf T, Seghatchian J.. Ebola virus convalescent blood products: where we are now and where we may need to go. Transfus Apher Sci. 2014;51(2):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung IF, To KK, Lee CK, et al. . Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clinical Infectious Diseases. 2011;52(4):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Wong R, Soo YOY, et al. . Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diorio C, Teachey DT, Bassiri H.. Convalescent plasma for COVID-19: an old therapy for a novel pathogen. The Hematologist. 2020;17(4). doi: 10.1182/hem.V17.4.10407 [DOI] [Google Scholar]
- 17.Li L, Zhang W, Hu Y, et al. . Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueso T, Pouderoux C, Perè H, et al. . Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]